Growth, Physiological Response, and Gill Health of Spotted Rose Snapper (Lutjanus guttatus) Reared at Different Salinities
Abstract
1. Introduction
2. Materials and Methods
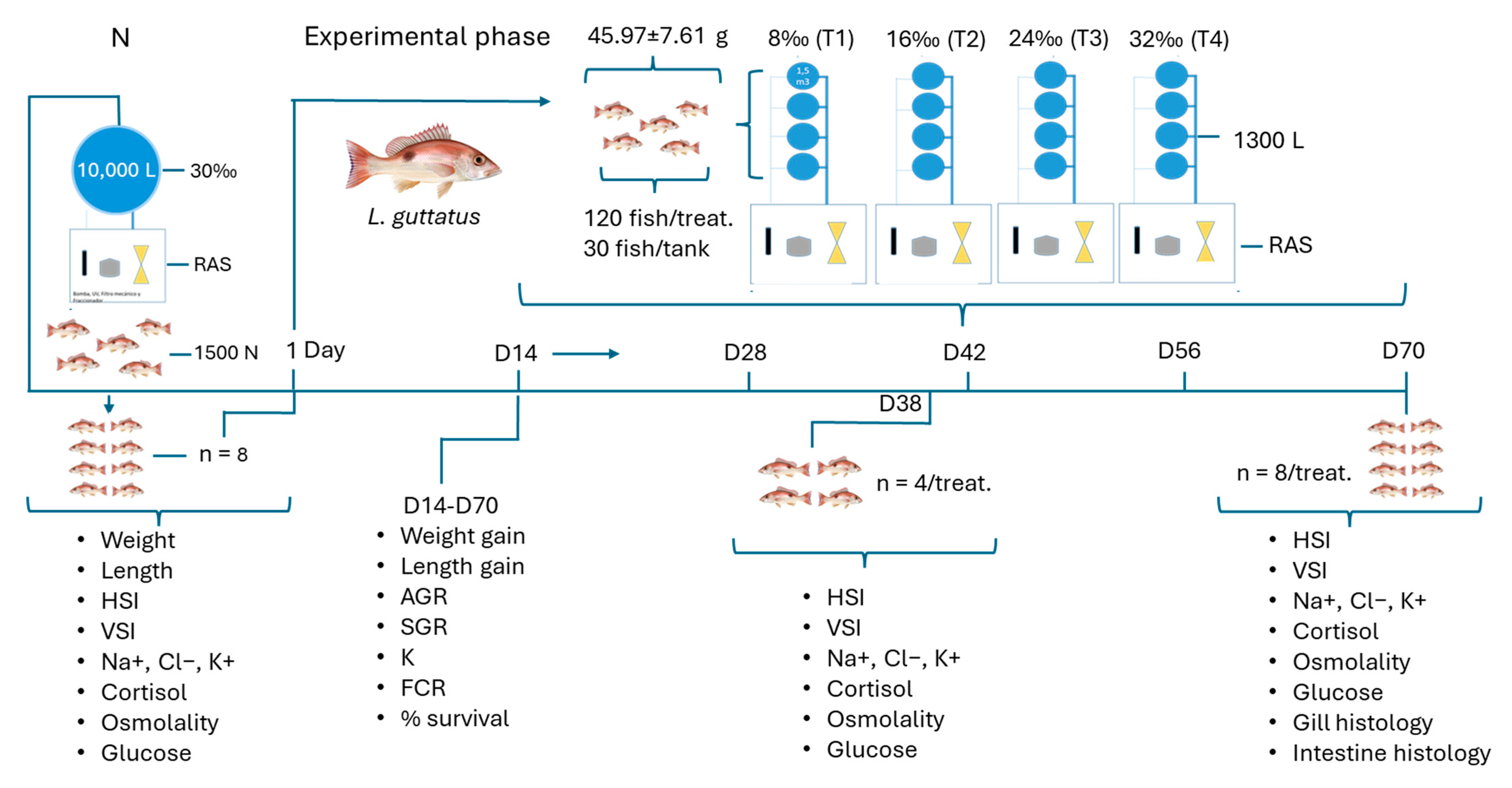
2.1. Experimental Design
2.2. Sampling and Analysis
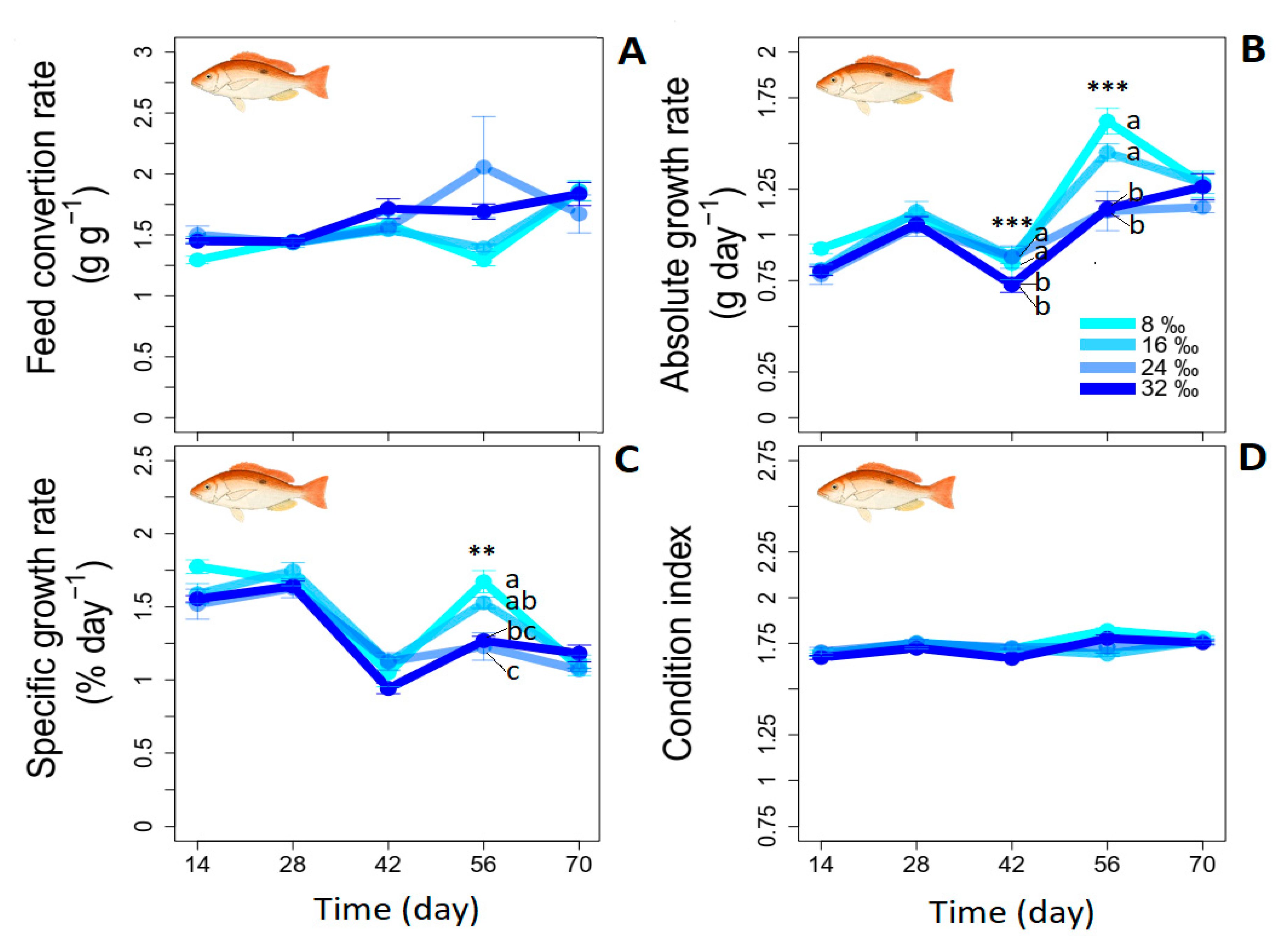
2.2.1. Growth and Survival Rates and Condition and Feed Conversion Ratios
- Absolute growth rate [26]:
- Specific growth rate [27]:
- Feed conversion ratio [28]:
- Survival rate [29]:
- Length-to-weight relationship [30]: ^b
- Fulton Condition Factor [31]:
2.2.2. Hepatosomatic Index (HSI) and Viscero-Somatic Index (VSI)
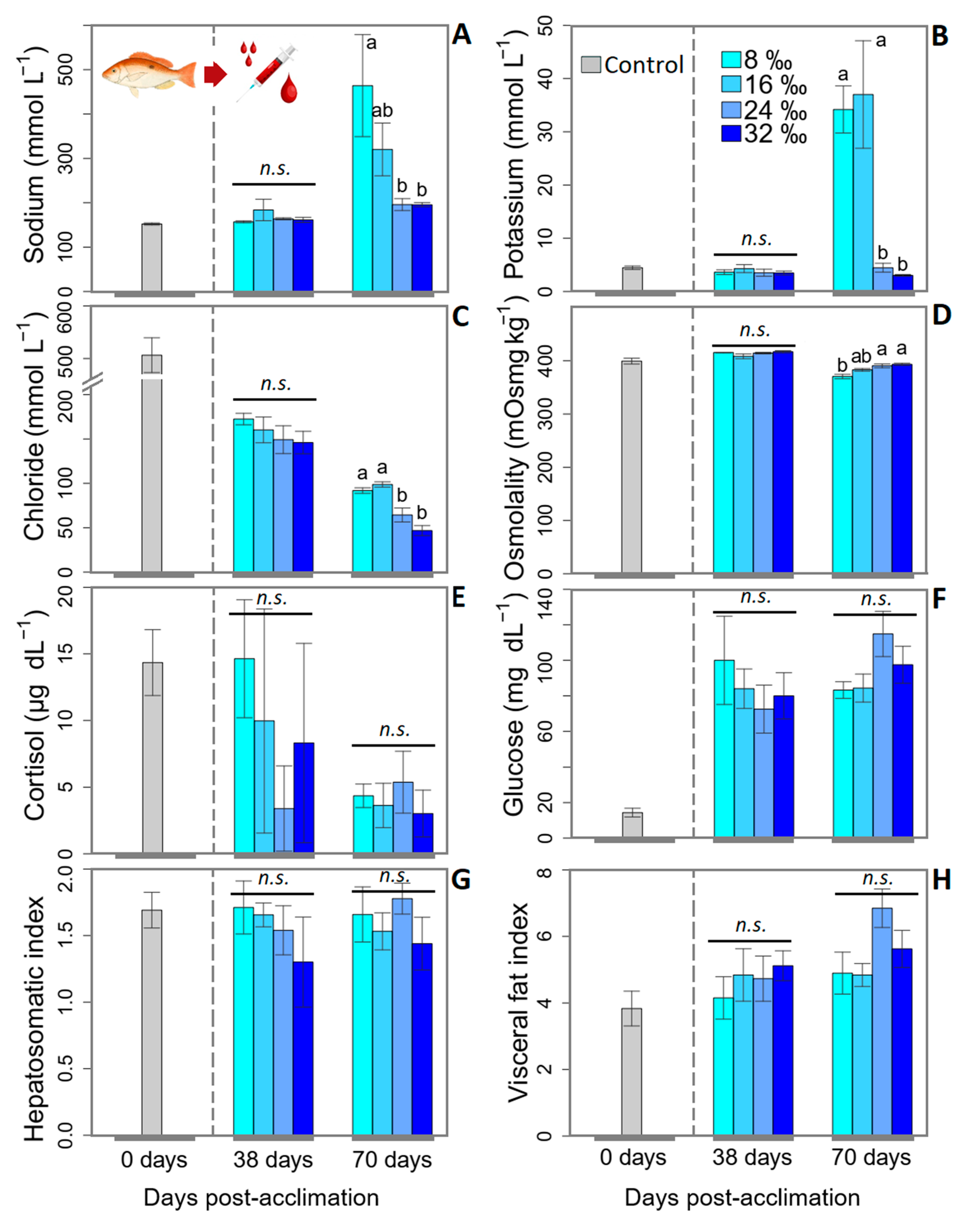
2.2.3. Blood Biochemical Tests
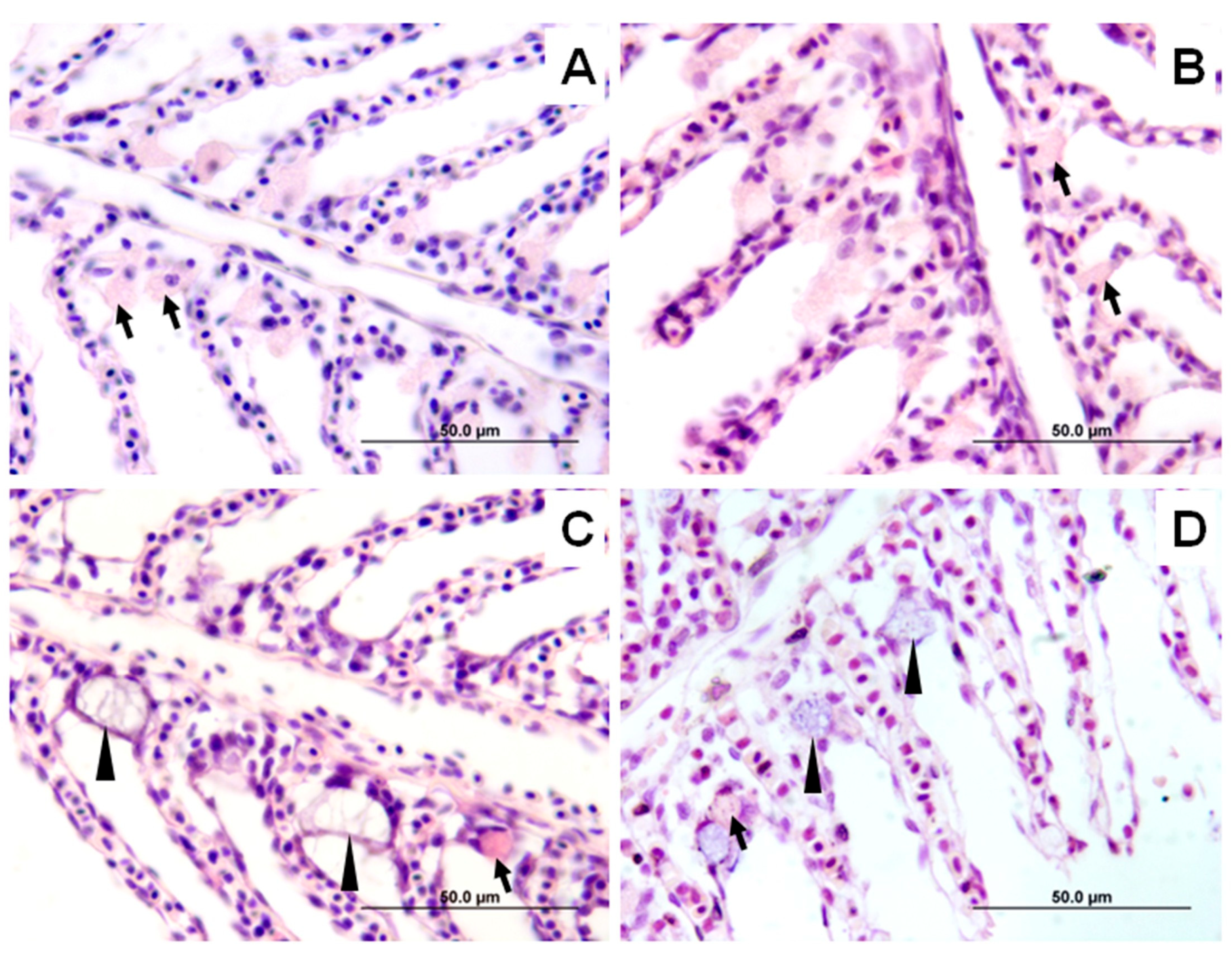
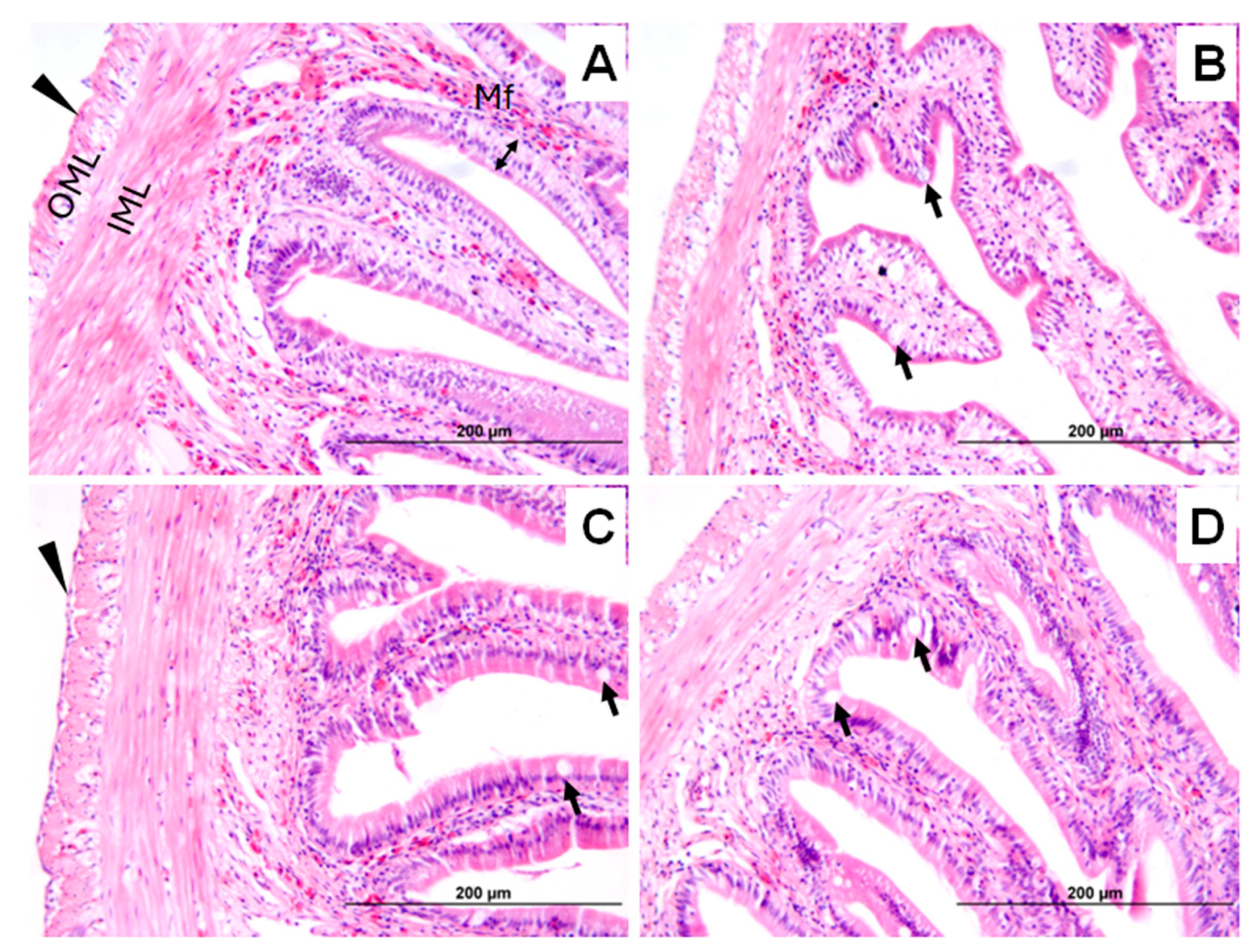
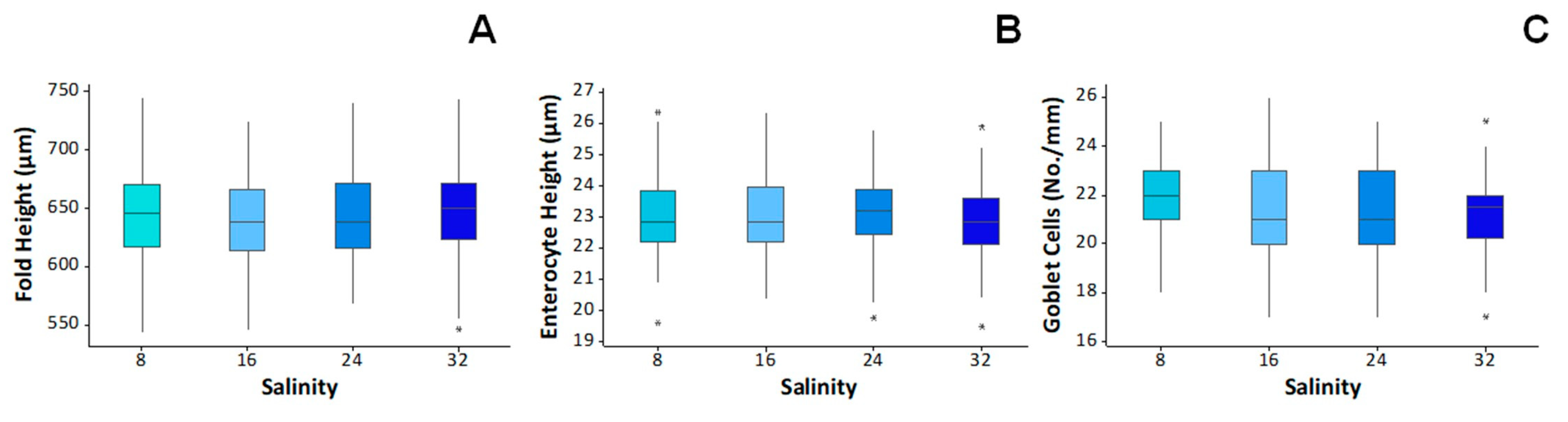
2.2.4. Histological Analysis
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oboh, A. Diversification of farmed fish species: A means to increase aquaculture production in Nigeria. Rev. Aquac. 2022, 14, 2089–2098. [Google Scholar] [CrossRef]
- Yue, G.H.; Tay, Y.X.; Wong, J.; Shen, Y.; Xia, J. Aquaculture species diversification in China. Aquac. Fish. 2023; early access. [Google Scholar] [CrossRef]
- Abou Anni, I.S.; Bianchini, A.; Barcarolli, I.F.; Junior, A.S.V.; Robaldo, R.B.; Tesser, M.B.; Sampaio, L.A. Salinity influence on growth, osmoregulation and energy turnover in juvenile pompano Trachinotus marginatus Cuvier 1832. Aquaculture 2016, 455, 63–72. [Google Scholar] [CrossRef]
- Wang, M.J.; Chu, T.Q.; Liu, F.; Zhan, W.; Lou, B.; Xu, W.T. Effects of salinity stress on antioxidant enzymes, non-specific immune enzymes and Na⁺/K⁺ ATPase activities of small yellow croaker (Larimichthys polyactis). Acta Oceanol. Sin. 2021, 43, 59–66. [Google Scholar]
- Zhang, C.X.; Zhou, L.; Ye, J.D.; Wang, L.; Huang, K.K.; Zhai, L.Q. Effects of acute salinity stress on the serum osmolality, serum ion concentrations, and ATPase activity in gill filaments of Japanese seabass (Lateolabrax japonicus) Fed with Diets Containing Different Magnesium Levels. J. Fish. China 2012, 36, 1425. [Google Scholar] [CrossRef]
- Chapman, F.A.; García, L.N.; Atencio, V.J.; Muñoz, R.J.; Silva, A.; Flores, H. Low-Salinity Acclimation of juvenile marine goliath grouper Epinephelus itajara. J. Appl. Aquac. 2014, 26, 179–186. [Google Scholar] [CrossRef]
- Wen, Z.Y.; Li, Y.; Bian, C.; Shi, Q.; Li, Y.Y. Characterization of two kcnk3 genes in rabbitfish (Siganus canaliculatus): Molecular cloning, distribution patterns and their potential roles in fatty acids metabolism and osmoregulation. Gen. Comp. Endocrinol. 2020, 296, 113546. [Google Scholar] [CrossRef]
- Blanco García, A.; Partridge, G.J.; Flik, G.; Roques, J.A.; Abbink, W. Ambient salinity and osmoregulation, energy metabolism and growth in juvenile yellowtail kingfish (Seriola lalandi Valenciennes 1833) in a recirculating aquaculture system. Aquac. Res. 2015, 46, 2789–2797. [Google Scholar] [CrossRef]
- Boeuf, G.; Payan, P. How should salinity influence fish growth? Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2001, 130, 411–423. [Google Scholar] [CrossRef]
- Mylonas, C.C.; Pavlidis, M.; Papandroulakis, N.; Zaiss, M.M.; Tsafarakis, D.; Papadakis, I.E.; Varsamos, S. Growth performance and osmoregulation in the shi drum (Umbrina cirrosa) adapted to different environmental salinities. Aquaculture 2009, 287, 203–210. [Google Scholar] [CrossRef]
- Arjona, F.J.; Vargas-Chacoff, L.; Ruiz-Jarabo, I.; del Río, M.P.M.; Mancera, J.M. Osmoregulatory response of Senegalese sole (Solea senegalensis) to changes in environmental salinity. Comp. Biochem. Physiol. A 2007, 148, 413–421. [Google Scholar] [CrossRef]
- Ruiz-Jarabo, I.; Márquez, P.; Vargas-Chacoff, L.; Martos-Sitcha, J.A.; Cárdenas, S.; Mancera, J.M. Narrowing the range of environmental salinities where juvenile meagre (Argyrosomus regius) can be cultured based on an osmoregulatory pilot study. Fishes 2018, 3, 48. [Google Scholar] [CrossRef]
- He, X.; Zhuang, P.; Zhang, L.; Xie, C. Osmoregulation in juvenile Chinese sturgeon (Acipenser sinensis Gray) during brackish water adaptation. Fish Physiol. Biochem. 2009, 35, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Bautista, J.; Rodríguez-Forero, A. Ionregulation in juvenile swordspine snook (Centropomus ensiferus, Poey, 1860) in relation to environmental salinity. J. Appl. Ichthyol. 2015, 31, 900–904. [Google Scholar] [CrossRef]
- Watson, C.J.; Nordi, W.M.; Esbaugh, A.J. Osmoregulation and branchial plasticity after acute freshwater transfer in red drum, Sciaenops ocellatus. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2014, 178, 82–89. [Google Scholar] [CrossRef]
- Calleja, F.; Guzmán, J.C.; Chavarría, H.A. Marine aquaculture in the Pacific coast of Costa Rica: Identifying the optimum areas for a sustainable development. Ocean Coast. Manag. 2022, 219, 106033. [Google Scholar] [CrossRef]
- Santos, G.; Ortiz-Gándara, I.; Del Castillo, A.; Arruti, A.; Gómez, P.; Ibáñez, R.; Ortiz, I. Intensified fish farming. Performance of electrochemical remediation of marine RAS waters. Sci. Total Environ. 2022, 847, 157368. [Google Scholar] [CrossRef]
- Fischer, W.; Krupp, F.; Schneider, W.; Sommer, C.; Carpenter, K.; Niem, V. Guía FAO para la Identificación de Especies para los Fines de la Pesca, Pacífico Centro-Oriental; Volume III, Vertebrates-Part 2; FAO: Rome, Italy, 1995; Available online: https://www.fao.org/4/v6250s/v6250s00.htm (accessed on 14 March 2025).
- Chacón-Guzmán, J.; Carvajal-Oses, M.; Herrera-Ulloa, Á. Optimization of larval culture for the production of juvenile spotted snapper Lutjanus guttatus in Costa Rica. Uniciencia 2021, 35, 10–26. [Google Scholar] [CrossRef]
- Abdo-de la Parra, M.I.; Rodríguez-Ibarra, L.E.; Rodríguez-Montes de Oca, G.; Velasco-Blanco, G.; Ibarra-Casto, L. Estado actual del cultivo de larvas del pargo flamenco (Lutjanus guttatus). Lat. Am. J. Aquat. Res. 2015, 43, 415–423. [Google Scholar] [CrossRef]
- Ibarra-Castro, L.; Ochoa-Bojórquez, M.O.; Sánchez-Téllez, J.L.; Rojo-Cebreros, A.H.; Álvarez-Lajonchère, L. A new efficient method for the mass production of juvenile spotted rose snapper Lutjanus guttatus. Aquac. Rep. 2020, 18, 100550. [Google Scholar] [CrossRef]
- Correa-Herrera, T.; Jiménez-Segura, L.F. Reproductive biology of Lutjanus guttatus (Perciformes: Lutjanidae) in Utría National Park, Colombian Pacific. Rev. Biol. Trop. 2013, 61, 829–840. [Google Scholar] [CrossRef][Green Version]
- Alcalá-Carrillo, M.; Castillo-Vargasmachuca, S.G.; Ponce-Palafox, J.T. Efectos de la temperatura y salinidad sobre el crecimiento y supervivencia de juveniles de pargo Lutjanus guttatus. Lat. Am. J. Aquat. Res. 2016, 44, 159–164. [Google Scholar] [CrossRef]
- Castillo-Vargasmachuca, S.; Ponce-Palafox, J.; Arambul-Muñoz, E.; López-Gómez, C.; Arredondo-Figueroa, J.L.; Spanopoulos-Hernández, M. The combined effects of salinity and temperature on the proximate composition and energetic value of spotted rose snapper Lutjanus guttatus (Steindachner, 1869). Lat. Am. J. Aquat. Res. 2017, 45, 1054–1058. [Google Scholar] [CrossRef]
- Bullard, S.A.; Benz, G.W.; Overstreet, R.M.; Williams, E.H.; Hemdal, J. Six new host records and an updated list of wild hosts for Neobenedenia melleni (MacCallum) (Monogenea: Capsalidae). Comp. Parasitol. 2000, 67, 190–196. [Google Scholar]
- Hopkins, K.D. Reporting fish growth: C. J. World Aquac. Soc. 1992, 23, 173–179. [Google Scholar] [CrossRef]
- Ricker, W.E. Growth rates and models. In Fish Physiology; Hoar, W.S., Randall, D.J., Eds.; Academic Press: New York, NY, USA, 1979; Volume 8, pp. 677–743. [Google Scholar]
- Tacon, A.G.J. Standard Methods for the Nutrition and Feeding of Farmed Fish and Shrimp; FAO: Rome, Italy, 1990. [Google Scholar]
- Zar, J.H. Biostatistical Analysis; Pearson Education India: Delhi, India, 1999. [Google Scholar]
- Ricker, W.E. Linear regressions in fishery research. J. Fish. Res. Board Can. 1973, 30, 409–434. [Google Scholar] [CrossRef]
- Fulton, T.W. The rate of growth of fishes. Twenty-Second Annu. Rep. 1904, 22, 141–241. [Google Scholar]
- Jan, M.; Jan, N. Studies on the fecundity (F), gonadosomatic index (GSI) and hepatosomatic index (HSI) of Salmo trutta fario (Brown trout) at Kokernag trout fish farm, Anantnag, Jammu and Kashmir. Int. J. Fish. Aquat. Stud. 2017, 5, 170–173. [Google Scholar]
- Bell, J.G.; Pratoomyot, J.; Strachan, F.; Henderson, R.J.; Fontanillas, R.; Hebard, A.; Tocher, D.R. Growth, flesh adiposity and fatty acid composition of Atlantic salmon (Salmo salar) families with contrasting flesh adiposity: Effects of replacement of dietary fish oil with vegetable oils. Aquaculture 2010, 306, 225–232. [Google Scholar] [CrossRef]
- Tietz, N.W. Clinical Guide to Laboratory Tests, 3rd ed.; W.B. Saunders Company: Philadelphia, PA, USA, 1995. [Google Scholar]
- Skeggs, L.T.; Hochstrasser, H. Colorimetric determination of chloride with mercuric thiocyanate. Clin. Chem. 1964, 10, 918–926. [Google Scholar] [CrossRef]
- Higgs, P.; Costa, M.; Freke, A.; Papasouliotis, K. Measurement of thyroxine and cortisol in canine and feline blood samples using two immunoassay analysers. J. Small Anim. Pract. 2014, 55, 153–159. [Google Scholar] [CrossRef]
- Herra-Vargas, S.; Brenes-Mora, E.; Baldi, M.; Bouza-Mora, L.; Huertas-Segura, R.M.; Castro-Ramírez, L.; Suárez-Esquivel, M. Parámetros sanguíneos y perfil de hormonas reproductivas de hembras de Choloepus hoffmanni en cautiverio. Rev. Biol. Trop. 2018, 66, 280–292. [Google Scholar] [CrossRef]
- Wutich, A.; Rosinger, A.Y.; Stoler, J.; Jepson, W.; Brewis, A. Measuring human water needs. Am. J. Hum. Biol. 2020, 32, e23350. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.M.; Pannu, S.; Liu, S.; Burckhardt, J.C.; Hughes, T.; Van Treuren, W.; Tropini, C. Single-strain behavior predicts responses to environmental pH and osmolality in the gut microbiota. MBio 2023, 14, e00753-23. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org (accessed on 12 July 2024).
- Glover, D.C.; DeVries, D.R.; Wright, R.A. Effects of temperature, salinity and body size on routine metabolism of coastal largemouth bass Micropterus salmoides. J. Fish Biol. 2012, 81, 1463–1478. [Google Scholar] [CrossRef] [PubMed]
- Weirich, C.R.; Wills, P.S.; Baptiste, R.M.; Woodward, P.N.; Riche, M.A. Production characteristics and body composition of Florida pompano reared to market size at two different densities in low-salinity recirculating aquaculture systems. N. Am. J. Aquacult. 2009, 71, 165–173. [Google Scholar] [CrossRef]
- Abdel-Rahim, M.M.; Lotfy, A.M.; Toutou, M.M.; Aly, H.A.; Sallam, G.R.; Abdelaty, B.S.; Helal, A.M. Effects of salinity level on the survival, growth, feed utilization, carcass composition, haematological and serum biochemical changes of juvenile meagre (Argyrosomus regius) (Asso, 1801) grown in ground saltwater. Aquac. Res. 2020, 51, 1038–1050. [Google Scholar] [CrossRef]
- Gil, E.A.B.; Sinisterra, J.A.A. Crecimiento en jaulas del pargo lunarejo Lutjanus guttatus (Steindachner, 1869) con dos tipos de dieta en Bahía Málaga, municipio de Buenaventura, Colombia. Entramado 2010, 6, 12–23. [Google Scholar]
- Castillo-Vargasmachuca, S.; Ponce-Palafox, J.T.; Ortíz, E.C.; Arredondo-Figueroa, J.L. Effect of the initial stocking body weight on growth of spotted rose snapper Lutjanus guttatus (Steindachner, 1869) in marine floating cages. Rev. Biol. Mar. Oceanogr. 2007, 42, 261–267. [Google Scholar] [CrossRef]
- Wootton, R.J. Ecology of Teleost Fishes; Fish and Fisheries Series 1; Chapman and Hall: London, UK, 1990. Available online: https://trove.nla.gov.au/work/16614123 (accessed on 8 January 2025).
- Furspan, P.; Prange, H.D.; Greenwald, L. Energetics and osmoregulation in the catfish Ictalurus nebulosus and I. punctatus. Comp. Biochem. Physiol. A 1984, 77, 773–778. [Google Scholar] [CrossRef]
- Morgan, J.D.; Iwama, G.K. Energy cost of NaCl transport in isolated gills of cutthroat trout. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1999, 277, R631–R639. [Google Scholar] [CrossRef][Green Version]
- Galkanda-Arachchige, H.S.C.; Davis, R.P.; Nazeer, S.; Ibarra-Castro, L.; Davis, D.A. Effect of salinity on growth, survival, and serum osmolality of red snapper, Lutjanus campechanus. Fish Physiol. Biochem. 2021, 47, 1687–1696. [Google Scholar] [CrossRef]
- Marcogliese, D.J. The impact of climate change on the parasites and infectious diseases of aquatic animals. Rev. Sci. Tech. 2008, 27, 467–484. [Google Scholar] [CrossRef]
- Altinok, I.; Grizzle, J.M. Effects of salinity on Yersinia ruckeri infection of rainbow trout and brown trout. J. Aquat. Anim. Health 2001, 13, 334–339. [Google Scholar] [CrossRef]
- Ziegeweid, J.R.; Black, M.C. Hematocrit and plasma osmolality values of young-of-year shortnose sturgeon following acute exposures to combinations of salinity and temperature. Fish Physiol. Biochem. 2010, 36, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Lisboa, V.; Barcarolli, I.F.; Sampaio, L.A.; Bianchini, A. Effect of salinity on survival, growth and biochemical parameters in juvenile Lebranch mullet Mugil liza (Perciformes: Mugilidae). Neotrop. Ichthyol. 2015, 13, 447–452. [Google Scholar] [CrossRef]
- Liu, B.; Guo, H.Y.; Zhu, K.C.; Guo, L.; Liu, B.S.; Zhang, N.; Zhang, D.C. Growth, physiological, and molecular responses of golden pompano Trachinotus ovatus (Linnaeus, 1758) reared at different salinities. Fish Physiol. Biochem. 2019, 45, 1879–1893. [Google Scholar] [CrossRef]
- Mozanzadeh, M.T.; Safari, O.; Oosooli, R.; Mehrjooyan, S.; Najafabadi, M.Z.; Hoseini, S.J.; Monem, J. The effect of salinity on growth performance, digestive and antioxidant enzymes, humoral immunity and stress indices in two euryhaline fish species: Yellowfin seabream (Acanthopagrus latus) and Asian seabass (Lates calcarifer). Aquaculture 2021, 534, 736329. [Google Scholar] [CrossRef]
- Resley, M.J.; Webb, K.A., Jr.; Holt, G.J. Growth and survival of juvenile cobia, Rachycentron canadum, at different salinities in a recirculating aquaculture system. Aquaculture 2006, 253, 398–407. [Google Scholar] [CrossRef]
- Katsika, L.; Huesca Flores, M.; Kotzamanis, Y.; Estevez, A.; Chatzifotis, S. Understanding the interaction effects between dietary lipid content and rearing temperature on growth performance, feed utilization, and fat deposition of sea bass (Dicentrarchus labrax). Animals 2021, 1, 392. [Google Scholar] [CrossRef]
- Varsamos, S.; Nebel, C.; Charmantier, G. Ontogeny of osmoregulation in postembryonic fish: A review. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2005, 141, 401–429. [Google Scholar] [CrossRef]
- McCormick, S.D. Endocrine control of osmoregulation in teleost fish. Am. Zool. 2001, 41, 781–794. [Google Scholar] [CrossRef]
- Ruiz-Jarabo, I.; Tinoco, A.B.; Vargas-Chacoff, L.; Martos-Sitcha, J.A.; Rodríguez-Rúa, A.; Cárdenas, S.; Mancera, J.M. Environmental salinity affects growth and metabolism in fingerling meagre (Argyrosomus regius). Fishes 2019, 4, 6. [Google Scholar] [CrossRef]
- Barton, B.A. Stress in fishes: A diversity of responses with particular reference to changes in circulating corticosteroids. Integr. Comp. Biol. 2002, 42, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Nash, R.D.; Valencia, A.H.; Geffen, A.J. The origin of Fulton’s condition factor—Setting the record straight. Fisheries 2006, 31, 236–238. [Google Scholar]
- Takei, Y.; Hwang, P.P. Homeostatic responses to osmotic stress. In Fish Physiology; Academic Press: San Diego, CA, USA, 2016; Volume 35, pp. 207–249. [Google Scholar] [CrossRef]
- Maetz, J. Aspects of adaptation to hypo-osmotic and hyperosmotic environments. In Biochemical and Biophysical Perspectives in Marine Biology; Malins, D.C., Sargent, J.R., Eds.; Academic Press: London, UK, 1974; pp. 1–167. [Google Scholar]
- Evans, D.H.; Claiborne, J.B. Osmotic and ionic regulation in fishes. In Osmotic and Ionic Regulation; CRC Press: Boca Raton, FL, USA, 2008; pp. 295–366. [Google Scholar]
- Kültz, D. Physiological mechanisms used by fish to cope with salinity stress. J. Exp. Biol. 2015, 218, 1907–1914. [Google Scholar] [CrossRef]
- Sangiao-Alvarellos, S.; Laiz-Carrion, R.; Guzmán, J.M.; Martín del Río, M.P.; Míguez, J.M.; Mancera, J.M.; Soengas, J.L. Acclimation of S. aurata to various salinities alters energy metabolism of osmoregulatory and nonosmoregulatory organs. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2003, 285, R897–R907. [Google Scholar] [CrossRef]
- Tang, C.H.; Lai, D.Y.; Lee, T.H. Effects of salinity acclimation on Na⁺/K⁺–ATPase responses and FXYD11 expression in the gills and kidneys of the Japanese eel (Anguilla japonica). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2012, 163, 302–310. [Google Scholar] [CrossRef]
- Marshall, W.S. Osmoregulation in estuarine and intertidal fishes. In Fish Physiology; Academic Press: London, UK, 2012; Volume 32, pp. 395–434. [Google Scholar] [CrossRef]
- Takvam, M.; Wood, C.M.; Kryvi, H.; Nilsen, T.O. Ion transporters and osmoregulation in the kidney of teleost fishes as a function of salinity. Front. Physiol. 2021, 12, 664588. [Google Scholar] [CrossRef]
- Takei, Y.; Balment, R.J. The neuroendocrine regulation of fluid intake and fluid balance. In Fish Physiology; Academic Press: London, UK, 2009; Volume 28, pp. 365–419. [Google Scholar] [CrossRef]
- Evans, D.H.; Piermarini, P.M.; Choe, K.P. The multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol Rev. 2005, 85, 97–177. [Google Scholar] [CrossRef]
- Hiroi, J.; McCormick, S.D. Variation in salinity tolerance, gill Na+/K+-ATPase, Na+/K+/2Cl− cotransporter and mitochondria-rich cell distribution in three salmonids Salvelinus namaycush, Salvelinus fontinalis and Salmo salar. J. Exp. Biol. 2007, 210, 1015–1024. [Google Scholar] [CrossRef]
- Marshall, W.S. Na+, Cl−, Ca2+ and Zn2+ transport by fish gills: Retrospective review and prospective synthesis. J. Exp. Zool. 2002, 293, 264–283. [Google Scholar] [CrossRef]
- Shephard, K.L. Functions for fish mucus. Rev. Fish Biol. Fisher. 1994, 4, 401–429. [Google Scholar] [CrossRef]
- Roberts, S.; Powell, M.D. Comparative ionic flux and gill mucous cell histochemistry: Effects of salinity and disease status in Atlantic salmon (Salmo salar L.). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2003, 134, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shen, Y.; Bao, Y.; Wu, Z.; Yang, B.; Jiao, L.; Zhang, C.; Tocher, D.R.; Zhou, Q.; Jin, M. Physiological responses and adaptive strategies to acute low-salinity environmental stress of the euryhaline marine fish black seabream (Acanthopagrus schlegelii). Aquat. Toxicol. 2022, 554, 738117. [Google Scholar] [CrossRef]
- García-Vargas, F.; Fajer-Ávila, E.J.; Lamothe-Argumedo, M.R. Two new species of Dactylogyridae (Monogenoidea) on rose spotted snapper, Lutjanus guttatus (Osteichthyes: Lutjanidae), from the coasts of Nayarit and Sinaloa, Mexico. Zootaxa 2008, 1729, 61–68. [Google Scholar] [CrossRef]
- Montoya-Mendoza, J.; Jiménez-Badillo, L.; Salgado-Maldonado, G.; Mendoza-Franco, E.F. Helminth Parasites of the Red Snapper, Lutjanus campechanus (Perciformes: Lutjanidae) from the Reef Santiaguillo, Veracruz, Mexico. J. Parasitol. 2014, 100, 868–872. [Google Scholar] [CrossRef]
- Buchmann, K.; Bresciani, J. Parasitic infections in pond-reared rainbow trout (Oncorhynchus mykiss) in Denmark. Dis. Aquat. Organ. 1997, 28, 125–138. [Google Scholar] [CrossRef]
- Chong, R. Monogenean Infections. In Aquaculture Pathophysiology; Kibenge, F., Baldisserotto, B., Chong, R., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 517–526. [Google Scholar]
- Ogawa, K.; Yokoyama, H. Parasitic diseases of cultured marine fish in Japan. Fish Pathol. 1998, 33, 303–309. [Google Scholar] [CrossRef]
- Grano-Maldonado, M.I.; Aguirre-Villaseñor, H.; Betancourt-Lozano, M.; Fajer-Avila, E.J. In vitro effect of low salinity on egg hatching and larval survival of Heterobothrium ecuadori (Monogenea) infecting bullseye puffer fish Sphoeroides annulatus. Aquac. Res. 2015, 46, 1522–1526. [Google Scholar] [CrossRef]
- Grosell, M.; Wood, C.M.; Wilson, R.W.; Bury, N.R.; Hogstrand, C.; Rankin, J.C.; Jensen, F.B. Bicarbonate secretion plays a role in chloride and water absorption of the European flounder intestine. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R936–R946. [Google Scholar] [CrossRef]
- Wilson, R.W.; Grosell, M. Intestinal bicarbonate secretion in marine teleost fish—Source of bicarbonate, pH sensitivity, and consequences for whole animal acid–base and calcium homeostasis. Biochim. Biophys. Acta BBA-Biomembr. 2003, 1618, 163–174. [Google Scholar] [CrossRef]
- Lin, G.; Li, S.; Huang, J.; Gao, D.; Lu, J. Hypoosmotic stress induced functional alternations of intestinal barrierintegrity, inflammatory reactions, and neurotransmission along gut-brain axisin the yellowfin seabream (Acanthopagrus latus). Fish Physiol. Biochem. 2021, 47, 1725–1738. [Google Scholar] [CrossRef]
- Huo, X.; Zhang, Q.; Chang, J.; Yang, G.; He, S.; Yang, C.; Liang, X.; Zhang, Y.; Su, J. Nanopeptide C-I20 as a novel feed additive effectively alleviates detrimental impacts of soybean meal on mandarin fish by improving the intestinal mucosal barrier. Front. Immunol. 2023, 14, 1197767. [Google Scholar] [CrossRef]
- Brijs, J.; Hennig, G.W.; Gräns, A.; Dekens, E.; Axelsson, M.; Olsson, C. Exposure to seawater increases intestinal motility in euryhaline rainbow trout (Oncorhynchus mykiss). J. Exp. Biol. 2017, 220, 2397–2408. [Google Scholar] [CrossRef]


| Salinity Treatments (‰) | |||||
|---|---|---|---|---|---|
| 8 | 16 | 24 | 32 | p Model | |
| Initial weight (g) | 45.9 ± 0.7 | 45.7 ± 0.7 | 46.2 ± 0.7 | 46.1 ± 0.7 | n.s. |
| Final weight (g) | 126.8 a ± 2.6 | 123.4 ab ± 2.4 | 116.0 b ± 2.3 | 116.0 b ± 2.3 | ** |
| Initial total length (mm) | 140.5 ± 0.7 | 139.0 ± 0.7 | 140.0 ± 0.7 | 140.4 ± 0.8 | n.s. |
| Final total length (mm) | 192.4 a ± 1.2 | 191.3 ab ± 1.2 | 187.6 b ± 1.2 | 187.7 b ± 1.2 | ** |
| Weight gain (%) | 176.2 a ± 0.0 | 170.4 ab ± 0.0 | 151.1 b ± 0.1 | 151.6 b ± 0.0 | * |
| AGR (g day−1) | 1.2 ± 0.0 | 1.1 ± 0.0 | 1.0 ± 0.6 | 1.0 ± 0.0 | n.s. |
| SGR (% BW day−1) | 1.5 ± 0.0 | 1.4 ± 0.0 | 1.3 ± 0.1 | 1.3 ± 0.0 | n.s. |
| Survival (%) | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | n.s. |
| FCR | 1.9 ± 0.0 | 2.00 ± 0.0 | 2.1 ± 0.1 | 2.1± 0.1 | n.s. |
| HSI | 1.7 ± 0.2 | 1.5 ± 0.1 | 1.8 ± 0.1 | 1.5 ± 0.2 | n.s. |
| VSI | 4.9 ± 0.6 | 4.8 ± 0.3 | 6.9 ± 0.6 | 5.6 ± 0.6 | n.s. |
| K | 1.8 ± 0.0 | 1.8 ± 0.0 | 1.8 ± 0.0 | 1.8 ± 0.0 | n.s. |
| Salinity | |||||
|---|---|---|---|---|---|
| Gill Variables | 8 | 16 | 24 | 32 | p Model |
| Ionocyte count | 123.3 ± 4.8 c | 122.4 ± 4.9 c | 82.6 ± 4.2 a | 90.4 ± 4.4 b | *** |
| Ionocyte Size | 8.0 ± 0.7 d | 7.4 ± 0.7 c | 4.9 ± 0.4 a | 5.2 ± 0.4 b | *** |
| Goblet Cell Count | 92.3 ± 12.3 b | 71.4 ± 12.5 a | 153.9 ± 7.9 c | 154.9 ± 7.9 c | *** |
| Goblet Cell Size | 5.6 ± 0.4 b | 5.4 ± 0.4 a | 9.0 ± 0.6 c | 9.0 ± 0.6 c | *** |
| Aneurysms (%) | 25.0 ab | 62.5 b | 62.5 b | 0 a | * |
| Hyperplasia (%) | 0 a | 62.5 b | 100 b | 100 b | *** |
| Parasitized fish (%/No. Monogenean) | 0 a (0) | 37.5 ab (8) | 87.5 b (22) | 50 ab (18) | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chacón-Guzmán, J.; Jiménez-Montealegre, R.; Duncan, N.; Calvo-Elizondo, E.; Valverde-Chavarría, S.; Pérez-Molina, J.P.; Rodríguez-Forero, A.; Segura-Badilla, J.; Soto-Alvarado, E.; Corrales, T.; et al. Growth, Physiological Response, and Gill Health of Spotted Rose Snapper (Lutjanus guttatus) Reared at Different Salinities. Fishes 2025, 10, 472. https://doi.org/10.3390/fishes10090472
Chacón-Guzmán J, Jiménez-Montealegre R, Duncan N, Calvo-Elizondo E, Valverde-Chavarría S, Pérez-Molina JP, Rodríguez-Forero A, Segura-Badilla J, Soto-Alvarado E, Corrales T, et al. Growth, Physiological Response, and Gill Health of Spotted Rose Snapper (Lutjanus guttatus) Reared at Different Salinities. Fishes. 2025; 10(9):472. https://doi.org/10.3390/fishes10090472
Chicago/Turabian StyleChacón-Guzmán, Jonathan, Ricardo Jiménez-Montealegre, Neil Duncan, Elman Calvo-Elizondo, Silvia Valverde-Chavarría, Junior Pastor Pérez-Molina, Adriana Rodríguez-Forero, Javier Segura-Badilla, Enoc Soto-Alvarado, Tifanny Corrales, and et al. 2025. "Growth, Physiological Response, and Gill Health of Spotted Rose Snapper (Lutjanus guttatus) Reared at Different Salinities" Fishes 10, no. 9: 472. https://doi.org/10.3390/fishes10090472
APA StyleChacón-Guzmán, J., Jiménez-Montealegre, R., Duncan, N., Calvo-Elizondo, E., Valverde-Chavarría, S., Pérez-Molina, J. P., Rodríguez-Forero, A., Segura-Badilla, J., Soto-Alvarado, E., Corrales, T., Víquez, C., Suárez-Esquivel, M., Castro-Ramírez, L., Cruz-Quintana, Y., & Gisbert, E. (2025). Growth, Physiological Response, and Gill Health of Spotted Rose Snapper (Lutjanus guttatus) Reared at Different Salinities. Fishes, 10(9), 472. https://doi.org/10.3390/fishes10090472








