Fish Alone Are Not Enough: Zoobenthos Improves Water Quality Assessment in Impacted Rivers
Abstract
1. Introduction
- (1)
- The regulating ecosystem service related to maintaining water quality is expected to be more pronounced in headwater streams (low-order rivers) compared to larger, higher-order rivers [31]. Accordingly, we anticipated a gradual decline in water quality and ecological integrity downstream, with higher quality classes in the upstream reaches of the Bistrița River.
- (2)
- Given their sensitivity to pollutants and complex ecological roles, fish serve as valuable indicators of environmental health, functioning as keystone ecological indicators [13]. Because they respond to environmental changes over larger spatial and temporal scales than invertebrates or algae, we expected ichthyofauna to provide an accurate representation of ecosystem integrity within the study area.
2. Materials and Methods
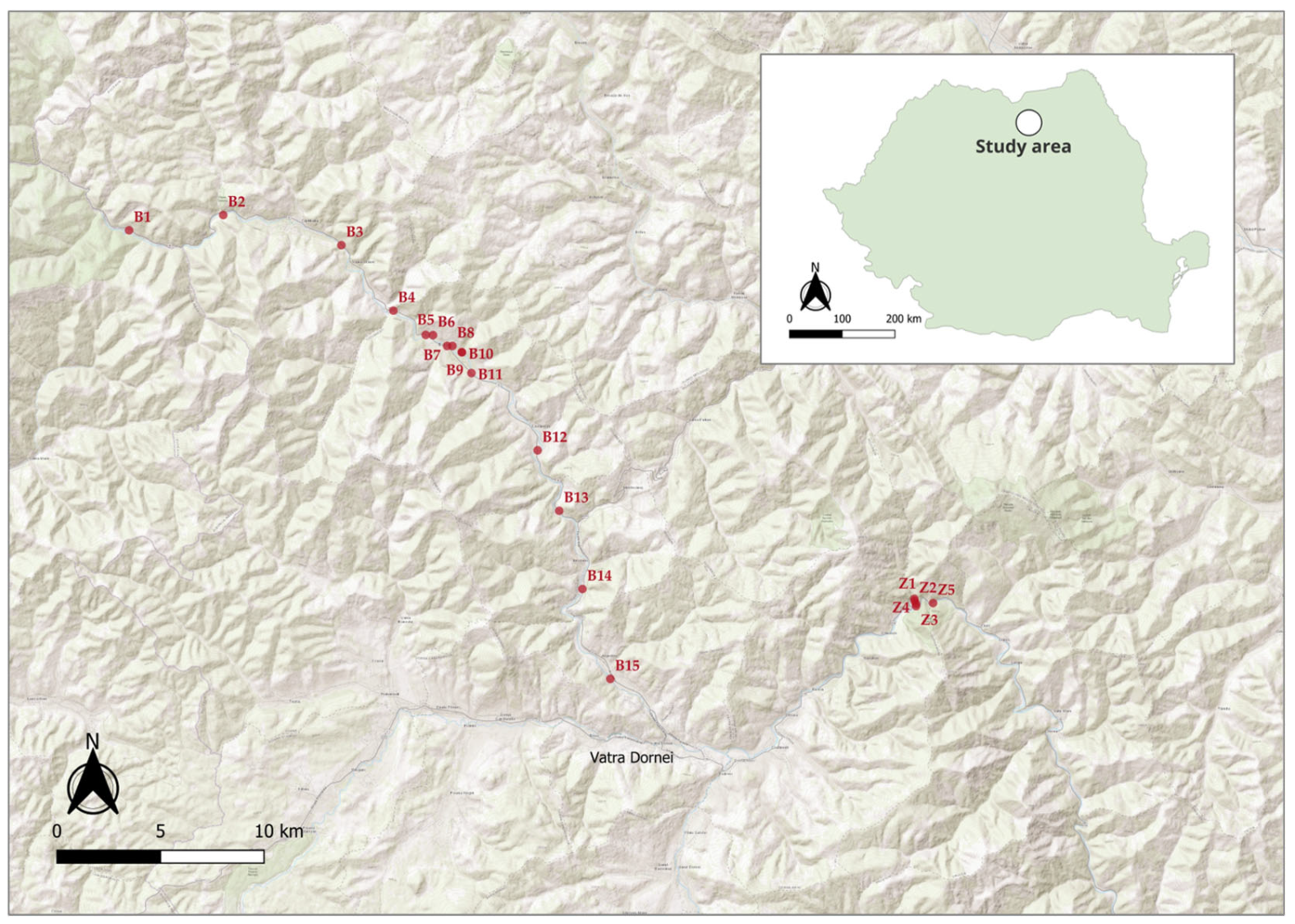
2.1. Study Area
2.2. Abiotic Parameters and Impact Assessment
2.3. Sampling and Processing of Biotic Communities
2.4. Statistical Analyses and Calculation of Biotic Indices
3. Results
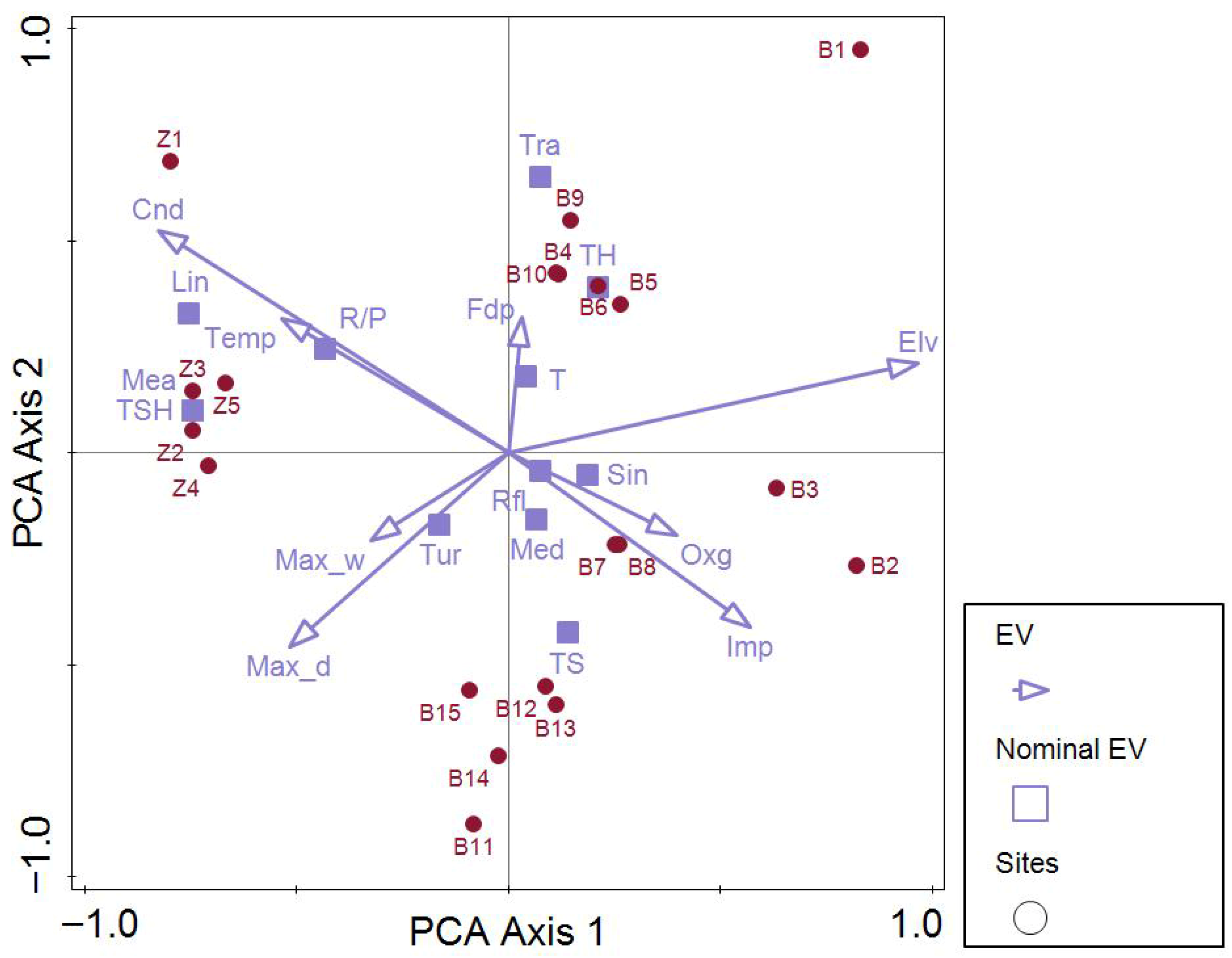
3.1. Assessment of Pressures and Impacts
3.2. Abiotic Parameters
3.3. Fish Communities
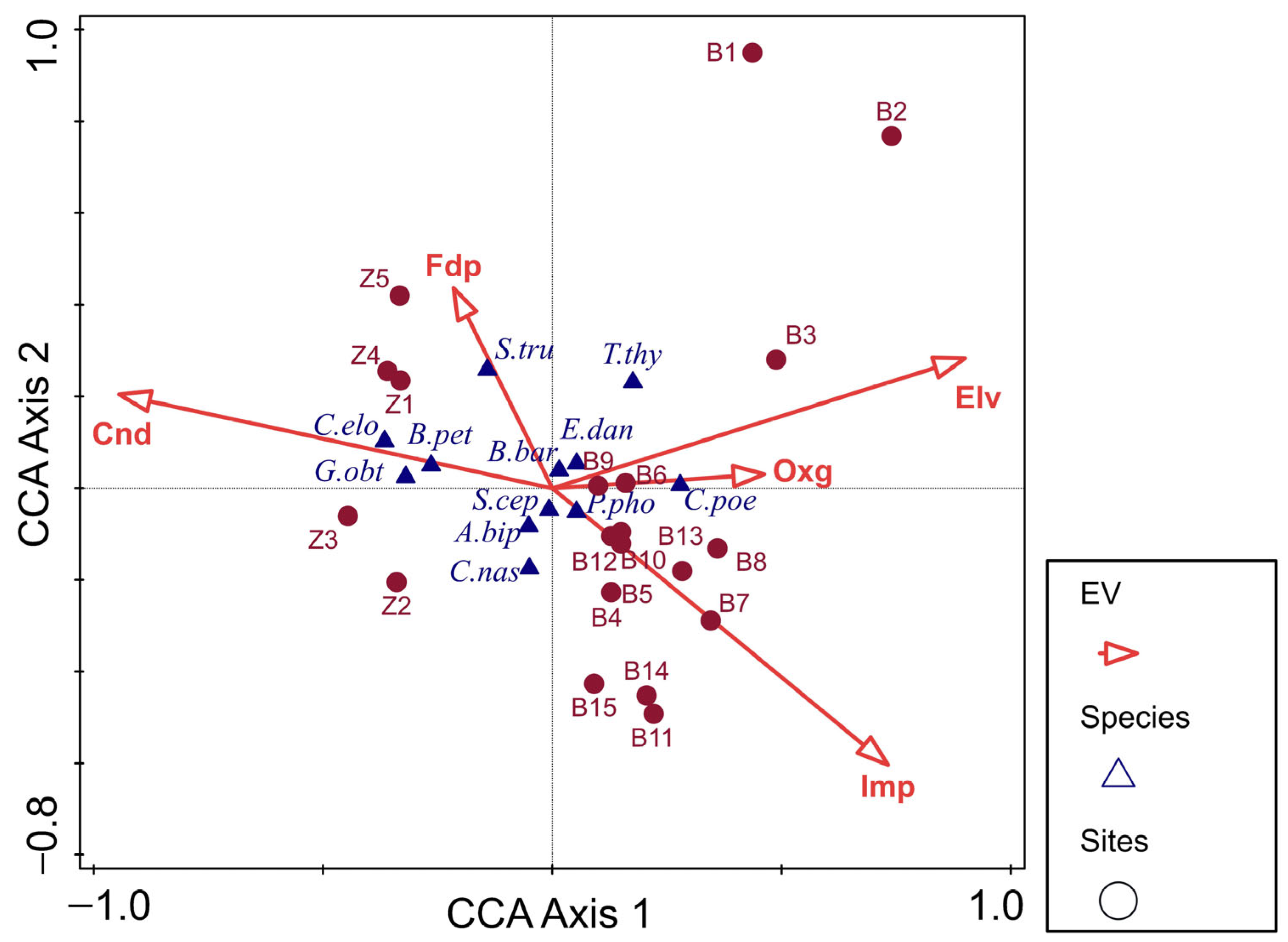
3.4. Zoobenthos and Diatom Communities
4. Discussion
4.1. Pressure and Impact Assessment in the Study Area
4.2. Fish Communities
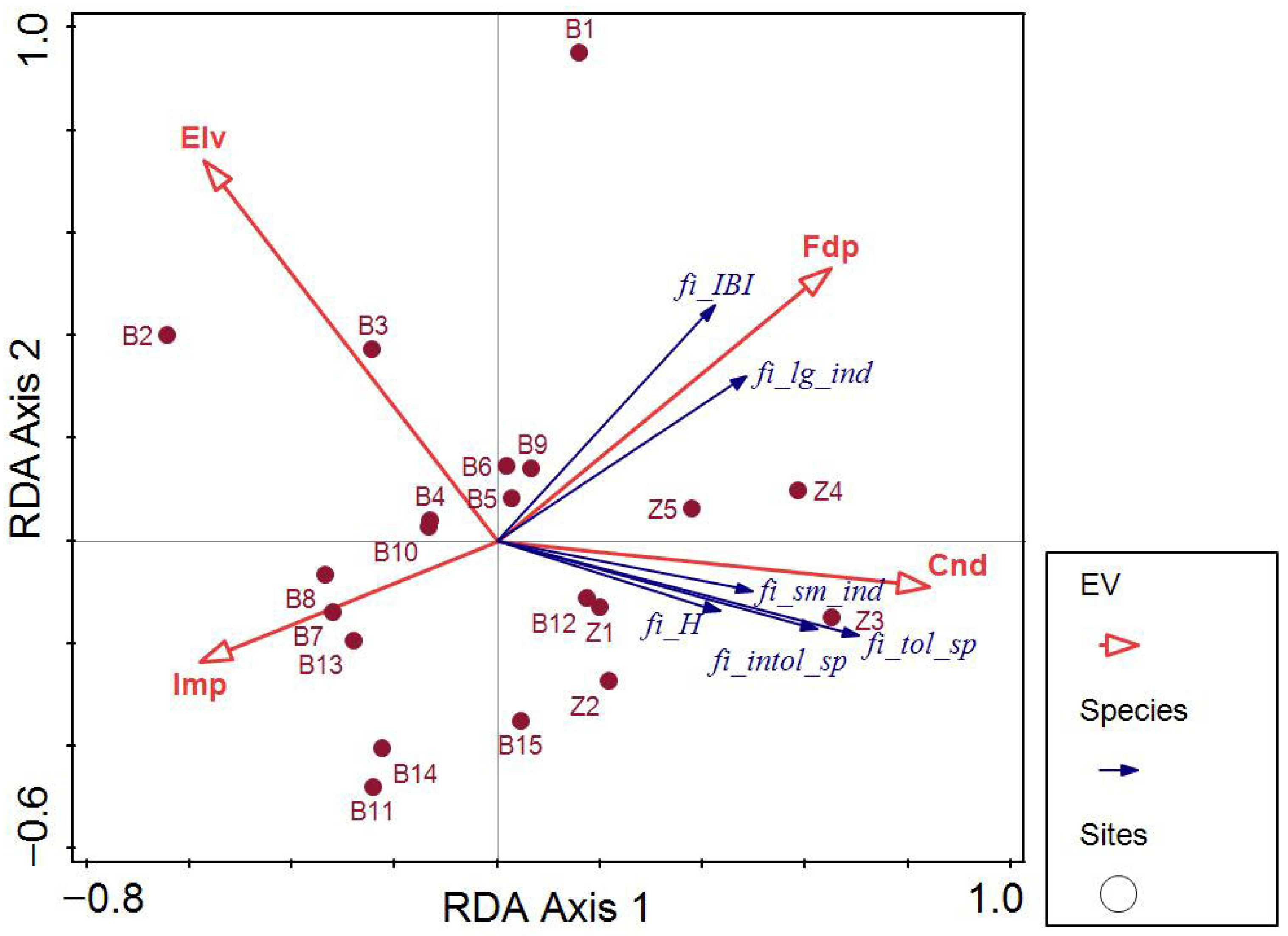
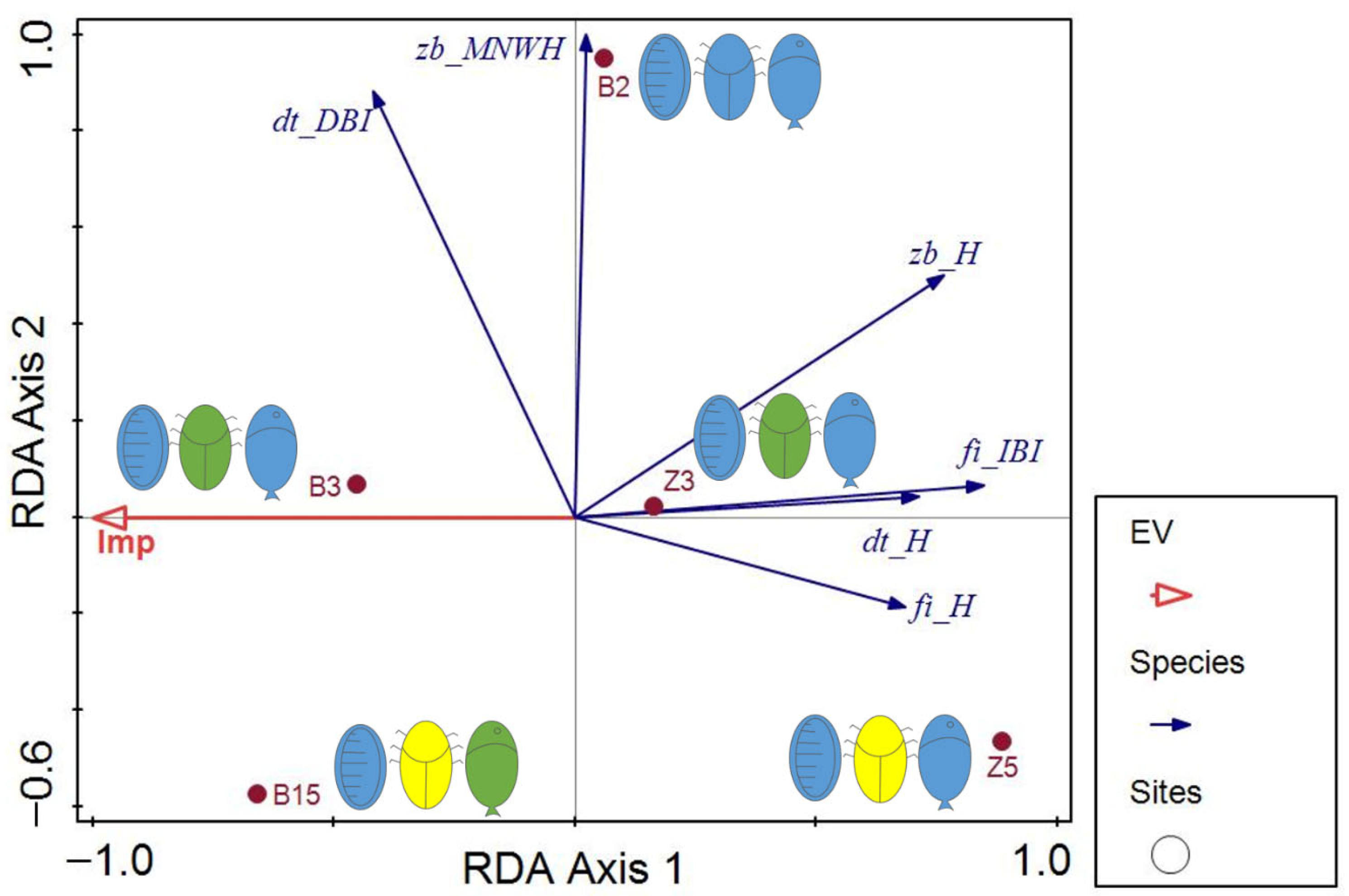
4.3. Comparative Analyses Involving Fish, Zoobenthos and Diatoms
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yates, A.G.; Brua, R.B.; Culp, J.M.; Aguiar, F.C.; Ajayan, A.P.; Aspin, T.; Bundschuh, M.; Calderón, M.R.; Csabai, Z.; Dallas, H.; et al. Charting a Course for Freshwater Biomonitoring: The Grand Challenges Identified by the Global Scientific Community. Ecol. Indic. 2025, 176, 113646. [Google Scholar] [CrossRef]
- Keck, F.; Peller, T.; Alther, R.; Barouillet, C.; Blackman, R.; Capo, E.; Chonova, T.; Couton, M.; Fehlinger, L.; Kirschner, D.; et al. The Global Human Impact on Biodiversity. Nature 2025, 641, 395–400. [Google Scholar] [CrossRef]
- Cantonati, M.; Poikane, S.; Pringle, C.M.; Stevens, L.E.; Turak, E.; Heino, J.; Richardson, J.S.; Bolpagni, R.; Borrini, A.; Cid, N.; et al. Characteristics, Main Impacts, and Stewardship of Natural and Artificial Freshwater Environments: Consequences for Biodiversity Conservation. Water 2020, 12, 260. [Google Scholar] [CrossRef]
- Simon, T.P. Introduction: Biological Integrity and Use of Ecological Health Concepts for Application to Water Resource Characterization. In Assessing the Sustainability and Biological Integrity of Water Resources Using Fish Communities; Simon, T.P., Ed.; CRC Press: Boca Raton, FL, USA, 2020; pp. 3–16. [Google Scholar] [CrossRef]
- Holt, E.A.; Miller, S.W. Bioindicators: Using Organisms to Measure Environmental Impacts. Nature 2011, 3, 8–13. [Google Scholar]
- Chapman, D. Water Quality Assessments: A Guide to the Use of Biota, Sediments and Water in Environmental Monitoring, 2nd ed.; CRC Press: London, UK, 2021. [Google Scholar] [CrossRef]
- Zacharias, I.; Liakou, P.; Biliani, I. A Review of the Status of Surface European Waters Twenty Years after WFD Introduction. Environ. Process. 2020, 7, 1023–1039. [Google Scholar] [CrossRef]
- Resh, V.H. Which Group Is Best? Attributes of Different Biological Assemblages Used in Freshwater Biomonitoring Programs. Environ. Monit. Assess. 2008, 138, 131–138. [Google Scholar] [CrossRef]
- Dedić, A.; Gerhardt, A.; Kelly, M.G.; Stanić-Koštroman, S.; Šiljeg, M.; Kalamujić Stroil, B.; Kamberović, J.; Mateljak, Z.; Pešić, V.; Vucković, I.; et al. Innovative Methods and Approaches for WFD: Ideas to Fill Knowledge Gaps in Science and Policy. Water Solut. 2020, 3, 30–42. [Google Scholar]
- Feio, M.J.; Hughes, R.M.; Callisto, M.; Nichols, S.J.; Odume, O.N.; Quintella, B.R.; Kuemmerlen, M.; Aguiar, F.C.; Almeida, S.F.P.; Alonso-EguíaLis, P.; et al. The Biological Assessment and Rehabilitation of the World’s Rivers: An Overview. Water 2021, 13, 371. [Google Scholar] [CrossRef]
- Villéger, S.; Brosse, S.; Mouchet, M.; Mouillot, D.; Vanni, M.J. Functional Ecology of Fish: Current Approaches and Future Challenges. Aquat. Sci. 2017, 79, 783–801. [Google Scholar] [CrossRef]
- Granado-Lorencio, C.; Guisande, C.; Pelayo-Villamil, P.; Manjarrés-Hernández, A.; García-Roselló, E.; Heine, J.; Pérez-Costas, E.; González-Vilas, L.; González-Dacosta, J.; Lobo, J.M. Diversity Dimensions of Freshwater Fish Species around the World. J. Geogr. Inf. Syst. 2021, 13, 1–18. [Google Scholar] [CrossRef]
- Chovanec, A.; Hofer, R.; Schiemer, F. Chapter 18 Fish as Bioindicators. In Trace Metals and other Contaminants in the Environment; Elsevier: Amsterdam, The Netherlands, 2003; Volume 6, pp. 639–676. [Google Scholar] [CrossRef]
- Roset, N.; Grenouillet, G.; Goffaux, D.; Pont, D.; Kestemont, P. A Review of Existing Fish Assemblage Indicators and Methodologies. Fish. Manag. Ecol. 2007, 14, 393–405. [Google Scholar] [CrossRef]
- Karr, J.R. Assessment of Biotic Integrity Using Fish Communities. Fisheries 1981, 6, 21–27. [Google Scholar] [CrossRef]
- Karr, J.R.; Fausch, K.D.; Angermeier, P.L.; Yant, P.R.; Schlosser, I.J. Assessing Biological Integrity in Running Waters. A Method and Its Rationale; Illinois Natural History Survey, Champaign, Special Publication: Champaign, IL, USA, 1986; Volume 5, pp. 1–28. [Google Scholar]
- Ruaro, R.; Gubiani, É.A. A Scientometric Assessment of 30 Years of the Index of Biotic Integrity in Aquatic Ecosystems: Applications and Main Flaws. Ecol. Indic. 2013, 29, 105–110. [Google Scholar] [CrossRef]
- Vadas, R.L.; Hughes, R.M.; Bae, Y.J.; Baek, M.J.; Gonzáles, O.C.B.; Callisto, M.; Carvalho, D.R.D.; Chen, K.; Ferreira, M.T.; Fierro, P.; et al. Assemblage-Based Biomonitoring of Freshwater Ecosystem Health via Multimetric Indices: A Critical Review and Suggestions for Improving Their Applicability. Water Biol. Secur. 2022, 1, 100054. [Google Scholar] [CrossRef]
- Karr, J.R.; Larson, E.R.; Chu, E.W. Ecological Integrity Is Both Real and Valuable. Conservat. Sci. Pract. 2022, 4, e583. [Google Scholar] [CrossRef]
- Simon, T.P.; Evans, N.T. Environmental Quality Assessment Using Stream Fishes. In Ecosystem Function; Hauer, F.R., Lamberti, G.A., Eds.; Methods in stream ecology; Elsevier: London, UK; Academic Press: San Diego, CA, USA, 2017; pp. 319–334. [Google Scholar]
- Allan, J.D.; Castillo, M.M.; Capps, K.A. Trophic Relationships. In Stream Ecology: Structure and Function of Running Waters; Springer: Cham, Switzerland, 2021; pp. 247–284. [Google Scholar]
- Rico-Sánchez, A.E.; Rodríguez-Romero, A.J.; Sedeño-Díaz, J.E.; López-López, E.; Sundermann, A. Aquatic Macroinvertebrate Assemblages in Rivers Influenced by Mining Activities. Sci. Rep. 2022, 12, 3209. [Google Scholar] [CrossRef]
- Vitecek, S.; Johnson, R.; Poikane, S. Assessing the Ecological Status of European Rivers and Lakes Using Benthic Invertebrate Communities: A Practical Catalogue of Metrics and Methods. Water 2021, 13, 346. [Google Scholar] [CrossRef]
- Sama, A.H.; Yuzir, M.A.; Azman, S. Water Quality Assessment Using Selected Macroinvertebrate Based Indices and Water Quality Index of Sungai Air Hitam Selangor. Trop. Aqua. Soil Pollut. 2024, 4, 143–156. [Google Scholar] [CrossRef]
- Munfarida, I.; Munir, M. Comparative Assessment of River Water Quality Based on Macro-Invertebrate Community Structures as Biological Indicators in Three Rivers of East Java; Bogor, Indonesia. AIP Conf. Proc. 2023, 2913, 040001. [Google Scholar] [CrossRef]
- Allan, J.D.; Castillo, M.M.; Capps, K.A. Primary Producers. In Stream Ecology: Structure and Function of Running Waters; Springer: Cham, Switzerland, 2021; pp. 141–176. [Google Scholar]
- Masouras, A.; Karaouzas, I.; Dimitriou, E.; Tsirtsis, G.; Smeti, E. Benthic Diatoms in River Biomonitoring—Present and Future Perspectives within the Water Framework Directive. Water 2021, 13, 478. [Google Scholar] [CrossRef]
- Madhankumar, M.; Venkatachalapathy, R.A. Biological Diatom Indices Based Preliminary Assessment of Water Quality of Thamirabarani River, Southern India. Int. J. Environ. Anal. Chem. 2024, 104, 9370–9382. [Google Scholar] [CrossRef]
- Yan, G.; Yin, X.; Wang, X.; Huang, M. Can Relative Abundance of Diatoms (RAD) Serve as an Indicator for the Water Quality Assessment in River-Connected Lakes? A Case Study at Dongting Lake. Environ. Sci. Eur. 2024, 36, 106. [Google Scholar] [CrossRef]
- Lobo, E.A.; Heinrich, C.G.; Schuch, M.; Wetzel, C.E.; Ector, L. Diatoms as Bioindicators in Rivers. In River Algae; Necchi, O., Jr., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 245–271. [Google Scholar] [CrossRef]
- Yeakley, A.J.; Ervin, D.; Chang, H.; Granek, E.F.; Dujon, V.; Shandas, V.; Brown, D. Ecosystem Services of Streams and Rivers. In River Science; Gilvear, D.J., Greenwood, M.T., Thoms, M.C., Wood, P.J., Eds.; Wiley: Hoboken, NJ, USA, 2016; pp. 335–352. [Google Scholar] [CrossRef]
- Maftei, A.E.; Iancu, O.G.; Buzgar, N. Assessment of Minor Elements Contamination in Bistriţa River Sediments (Upstream of Izvorul Muntelui Lake, Romania) with the Implication of Mining Activity. J. Geochem. Explor. 2014, 145, 25–34. [Google Scholar] [CrossRef]
- Maftei, A.E.; Dill, H.G.; Buzatu, A.; Iancu, O.G.; Buzgar, N.; Andráš, P. Chemical and Mineralogical Composition of Fluvial Sediments (Bistriţa River, Romania): Geogenic vs. Anthropogenic Input into Rivers on Its Way through Mining Areas. Geochemistry 2018, 78, 385–395. [Google Scholar] [CrossRef]
- Gheţeu, D.; Neagu, A.; Miron, I.; Erhan, M. Functional Communities of Benthic Macroinvertebrates from the Superior Catchment Area of the Bistriţa River. Sci. Ann. UAIC 2006, LII, 59–66. [Google Scholar]
- Battes, K.W. Influence of the Hydrotechnical Development and of Bistriţa River’s Pollution on the Natural Fish Population. SSRB 1999, 4, 283–294. [Google Scholar]
- Stoica, I.; Battes, K.W.; Pricope, F.; Prisecaru, M. Research on the Status of the Fish Communities of the Upper Course of Bistriţa River. SSRB 2013, 22, 66–73. [Google Scholar]
- Romanescu, G.; Bounegru, O. Ice Dams and Backwaters as Hydrological Risk Phenomena—Case Study: The Bistriţa River; Upstream of the Izvorul Muntelui Lake (Romania); WIT Transactions on Ecology and the Environment: Dubrovnik, Croatia, 2012; pp. 167–178. [Google Scholar] [CrossRef]
- NATURA 2000 Expert Viewer. 2025. Available online: https://natura2000.eea.europa.eu/ (accessed on 23 July 2025).
- QGIS Development Team QGIS Geographic Information System. Open Source Geospatial Foundation Project. 2024. Available online: http://qgis.org (accessed on 14 September 2025).
- Paisley, M.F.; Trigg, D.J.; Walley, W.J. Revision of the Biological Monitoring Working Party (BMWP) Score System: Derivation of Present-only and Abundance-related Scores from Field Data. River Res. Appl. 2014, 30, 887–904. [Google Scholar] [CrossRef]
- Gibson, R.B.; Fonseca, A. Trade-Offs in Impact Assessment Design and Implementation. In Handbook of Environmental Impact Assessment; Research handbooks on impact assessment series; Edward Elgar Publishing: Cheltenham, UK; Northampton, MA, USA, 2022; pp. 233–257. [Google Scholar] [CrossRef]
- Kottelat, M.; Freyhof, J. Handbook of European Freshwater Fishes; Publications Kottelat, Cornol and Freyhof, Berlin: Cornol, Switerland, 2007; pp. 1–646. [Google Scholar]
- Iftime, A.; Oţel, V. An Annotated Systematical Checklist of the Romanian Ichthyofauna. Zootaxa 2025, 5654, 1–64. [Google Scholar] [CrossRef]
- Begić, V.; Sertić Perić, M.; Hančić, S.; Marchiotti, I.; Gabud, T.; Šestak Panižić, I.; Radanović, I.; Korać, P. Effectiveness of Five Different Solutions for Preserving Aquatic Insects Commonly Used in Morphological and Stream Ecology Studies. Biologia 2022, 78, 1011–1026. [Google Scholar] [CrossRef]
- Blanco, S. Diatom Taxonomy and Identification Keys. In Modern Trends in Diatom Identification: Fundamentals and Applications; Cristóbal, G., Blanco, S., Bueno, G., Eds.; Springer International Publishing: Cham, Switerland, 2020; pp. 25–38. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M.; AlgaeBase. World-Wide Electronic Publication, University of Galway. 2025. Available online: https://www.algaebase.org (accessed on 7 August 2025).
- Kriska, G. Freshwater Invertebrates in Central Europe: A Field Guide; Springer International Publishing: Cham, Switerland, 2022; pp. 1–527. [Google Scholar] [CrossRef]
- GBIF Secretariat. Backbone Taxonomy. Checklist Dataset. 2023. Available online: https://doi.org/10.15468/39omei (accessed on 8 June 2025).
- Šmilauer, P.; Lepš, J. Multivariate Analysis of Ecological Data Using Canoco 5, 2nd ed.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; pp. 1–362. [Google Scholar]
- ter Braak, C.J.F.; Šmilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination, Version 5.0; Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025; Available online: https://www.R-project.org (accessed on 21 July 2025).
- Peterson, B.G.; Carl, P. PerformanceAnalytics: Econometric Tools for Performance and Risk Analysis. 2024. Available online: https://CRAN.R-project.org/package=PerformanceAnalytics (accessed on 21 July 2025).
- Coste, M.; Boutry, S.; Tison-Rosebery, J.; Delmas, F. Improvements of the Biological Diatom Index (BDI): Description and Efficiency of the New Version (BDI-2006). Ecol. Indic. 2009, 9, 621–650. [Google Scholar] [CrossRef]
- Maraşlıoğlu, F.; Bektaş, S.; Özen, A. Comparative Performance of Physicochemical-Based and Diatom-Based Metrics in Assessing the Water Quality of Mert Stream, Turkey. J. Ecol. Eng. 2020, 21, 18–31. [Google Scholar] [CrossRef]
- Ghetti, P.F. Manuale Di Applicazione Indice Biotico Esteso (IBE). I Macroinvertebrati Nel Controllo Della Qualità Degli Ambienti di Acque Correnti; Provincia Autonoma di Trento, Agenzia provinciale per la protezione dell’ambiente: Trento, Italy, 1997; pp. 1–22. (In Italian) [Google Scholar]
- Kelly, M.G.; Adams, C.; Graves, A.C.; Jamieson, J.; Krokowski, J.; Lycett, E.; Murray-Bligh, J.; Pritchard, S.; Wilkins, C. The Trophic Diatom Index: A User’s Manual; Environmental Agency: Bristol, UK, 2001; pp. 1–135. [Google Scholar]
- Gheţeu, D.; Aoncioaie, C.; Neagu, A.; Miron, I. Aspects of the Anthropic Impact and Pollution from the Superior Catchment Area of Bistriţa River. Sci. Ann. UAIC 2007, LIII, 251–256. [Google Scholar]
- Logez, M.; Bady, P.; Melcher, A.; Pont, D. A Continental-scale Analysis of Fish Assemblage Functional Structure in European Rivers. Ecography 2013, 36, 80–91. [Google Scholar] [CrossRef]
- Acreman, M.; Hughes, K.A.; Arthington, A.H.; Tickner, D.; Dueñas, M. Protected Areas and Freshwater Biodiversity: A Novel Systematic Review Distils Eight Lessons for Effective Conservation. Conserv. Lett. 2020, 13, e12684. [Google Scholar] [CrossRef]
- Stefanidis, K.; Oikonomou, A.; Stoumboudi, M.; Dimitriou, E.; Skoulikidis, N. Do Water Bodies Show Better Ecological Status in Natura 2000 Protected Areas Than Non-Protected Ones?—The Case of Greece. Water 2021, 13, 3007. [Google Scholar] [CrossRef]
- De Mello, K.; Taniwaki, R.H.; Macedo, D.R.; Leal, C.G.; Randhir, T.O. Biomonitoring for Watershed Protection from a Multiscale Land-Use Perspective. Diversity 2023, 15, 636. [Google Scholar] [CrossRef]
- Bănăduc, D.; Curtean-Bănăduc, A.; Barinova, S.; Lozano, V.; Afanasyev, S.; Leite, T.; Branco, P.; Gomez Isaza, D.; Geist, J.; Tegos, A.; et al. Multi-Interacting Natural and Anthropogenic Stressors on Freshwater Ecosystems: Their Current Status and Future Prospects for 21st Century. Water 2024, 16, 1483. [Google Scholar] [CrossRef]
- Stoffels, R.J.; Rehwinkel, R.A.; Price, A.E.; Fagan, W.F. Dynamics of Fish Dispersal during River-Floodplain Connectivity and Its Implications for Community Assembly. Aquat. Sci. 2016, 78, 355–365. [Google Scholar] [CrossRef]
- Williams, J.E.; Gregory, S.; Wildman, R. Fish Assemblage Structure and Habitat Relationships of a Large Floodplain River in Western North America. River Res. Apps. 2024, 40, 809–820. [Google Scholar] [CrossRef]
- Ihuț, A.; Zitek, A.; Weiss, S.J.; Ratschan, C.; Holzer, G.; Kaufmann, T.; Cocan, D.; Constantinescu, R.; Mireșan, V. Danube Salmon (Hucho hucho) in Central and South Eastern Europe: A review for the development of an international program for the rehabilitation and conservation of Danube Salmon populations. Bul. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Anim. Sci. Biotechnol. 2014, 71, 86–101. [Google Scholar] [CrossRef]
- Curtean-Bănăduc, A.; Marić, S.; Gábor, G.; Didenko, A.; Rey Planellas, S.; Bănăduc, D. Hucho Hucho (Linnaeus, 1758): Last Natural Viable Population in the Eastern Carpathians—Conservation Elements. Turk. J. Zool. 2019, 43, 215–223. [Google Scholar] [CrossRef]
- Cocan, D.; Mireşan, V.; Constantinescu, R.; Popescu, F.; Uiuiu, P.; Ihuţ, A.; Nicula, A.S.; Laţiu, C. Ichthyofaunal Diversity of Ruscova River—A Danube Salmon (Hucho hucho, Linnaeus 1758) Spawning Tributary; Proceedings on the Multidisciplinary Conference on Sustainable Development; Filodiritto Editore: Bologna, Italy, 2020; pp. 251–260. [Google Scholar]
- Danet, A.; Giam, X.; Olden, J.D.; Comte, L. Past and Recent Anthropogenic Pressures Drive Rapid Changes in Riverine Fish Communities. Nat. Ecol. Evol. 2024, 8, 442–453. [Google Scholar] [CrossRef]
- Kirk, M.A.; Rahel, F.J.; Laughlin, D.C. Environmental Filters of Freshwater Fish Community Assembly along Elevation and Latitudinal Gradients. Global Ecol. Biogeogr. 2022, 31, 470–485. [Google Scholar] [CrossRef]
- Hering, D.; Johnson, R.K.; Kramm, S.; Schmutz, S.; Szoszkiewicz, K.; Verdonschot, P.F.M. Assessment of European Streams with Diatoms, Macrophytes, Macroinvertebrates and Fish: A Comparative Metric-based Analysis of Organism Response to Stress. Freshw. Biol. 2006, 51, 1757–1785. [Google Scholar] [CrossRef]
- De Castro-Català, N.; Dolédec, S.; Kalogianni, E.; Skoulikidis, N.T.; Paunovic, M.; Vasiljević, B.; Sabater, S.; Tornés, E.; Muñoz, I. Unravelling the Effects of Multiple Stressors on Diatom and Macroinvertebrate Communities in European River Basins Using Structural and Functional Approaches. Sci. Total Environ. 2020, 742, 140543. [Google Scholar] [CrossRef]
- Wright, I.A.; Ryan, M.M. Impact of Mining and Industrial Pollution on Stream Macroinvertebrates: Importance of Taxonomic Resolution, Water Geochemistry and EPT Indices for Impact Detection. Hydrobiologia 2016, 772, 103–115. [Google Scholar] [CrossRef]
- De Jonge, M.; Vandevijver, B.; Blust, R.; Bervoets, L. Responses of Aquatic Organisms to Metal Pollution in a Lowland River in Flanders: A Comparison of Diatoms and Macroinvertebrates. Sci. Total Environ. 2008, 407, 615–629. [Google Scholar] [CrossRef]
- Markert, N.; Guhl, B.; Feld, C.K. Water Quality Deterioration Remains a Major Stressor for Macroinvertebrate, Diatom and Fish Communities in German Rivers. Sci. Total Environ. 2024, 907, 167994. [Google Scholar] [CrossRef]
- Wu, J.; Mao, R.; Li, M.; Xia, J.; Song, J.; Cheng, D.; Sun, H. Assessment of Aquatic Ecological Health Based on Determination of Biological Community Variability of Fish and Macroinvertebrates in the Weihe River Basin, China. J. Environ. Manag. 2020, 267, 110651. [Google Scholar] [CrossRef]
- Fierro, P.; Valdovinos, C.; Vargas-Chacoff, L.; Bertrán, C.; Arismendi, I. Macroinvertebrates and Fishes as Bioindicators of Stream Water Pollution. In Water Quality; Tutu, H., Ed.; InTech: London, UK, 2017. [Google Scholar] [CrossRef]
- Cheimonopoulou, M.T.; Bobori, D.C.; Theocharopoulos, I.; Lazaridou, M. Assessing Ecological Water Quality with Macroinvertebrates and Fish: A Case Study from a Small Mediterranean River. Environ. Manag. 2011, 47, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Herlihy, A.T.; Sifneos, J.C.; Hughes, R.M.; Peck, D.V.; Mitchell, R.M. The Relation of Lotic Fish and Benthic Macroinvertebrate Condition Indices to Environmental Factors across the Conterminous USA. Ecol. Indic. 2020, 112, 105958. [Google Scholar] [CrossRef] [PubMed]
| Pressures | Code | Impact(s) Caused | Details | Location | Importance |
|---|---|---|---|---|---|
| Barriers across the waterway | BAR | No longitudinal connectivity along the river, isolation of fish populations | Zugreni weir. | Z2, Z3, Z4 | 0.2 |
| Barriers: Embankment | EMB | No lateral connectivity between the river and the riparian zone. Impacts in the riparian zone, like decreased food resources, lack of riparian vegetation, lack of suitable hiding places for wildlife. | Most embankments along the Bistrița Aurie River were gabions. Concrete embankments in Ciocănești locality (47°28′58.14″ N, 25°16′34.68″ E). | B5, B7, B11, B14, B15, Z4 | 0.2 |
| Diffuse pollution due to agriculture and forestry, including pisciculture | PAF | Diffuse leaks of possible toxic elements in the hyporheic zone. | The toxic elements that might find their way into the river include pesticides used in agriculture, biocides used in fighting the attacks of Hylobius abietis (Insecta, Curculionidae), different substances coming from the local fish farms. | B1–B15 | 0.3 |
| Diffuse pollution due to domestic waste waters | PDW | Diffuse leaks of possible toxic elements in the hyporheic zone. | Caused by undersized/no sewage in human settlements near the Bistrița Aurie River in ROSAC0010, together with industrial or waste deposits located in the river proximity. In ROSAC0196, only the sites located downstream of the touristic chalet Zugreni might be affected. Organic pollution was also described by [57] | B1–B15, Z2–Z3 | 0.5 |
| Diffuse pollution due to mining activities | PMA | Input of heavy metals into the river (Fe, Mn, Cu, Cr, Zn), even if the mines themselves are closed | The manganese exploitation from Dadu, Oiţa and Tolovanu, the iron and manganese exploitation from Arșița-Iacobeni, or the manganese and polimetalic sulphures exploration from Gândacu -Suhărzelul Mare, now closed, were all located between Cârlibaba and Iacobeni [32,33] | B3–B15 | 0.5 |
| Poaching | POA | Declining fish population. Habitat destruction. | Large species like Hucho hucho were affected | B1–B15, Z1–Z5 | 0.1 |
| Resource exploitation—forest | REF | Indirect impacts on river communities, siltation | Decreased vegetation buffer in the catchment area leads to higher sediment input in the river, affecting the spawning grounds and food resources of fishes. Log processing techniques can affect water quality by increasing turbidity (e.g., the transport of logs, known as “corhănit” in the area) or by accumulation of by-products from the sawmills located near the river (e.g., Valea Stânei) | B1–B15, Z1–Z5 | 0.1 |
| Resource exploitation—sediments | RES | Sediment removal. Habitat destruction. | Not a common activity in the area. | Z1 | 0.1 |
| Uncontrolled waste disposal | UWD | Indirect impacts on river communities, possible toxic leaks. | Accumulation of microplastics, non-biodegradable wastes and possible toxic effects of harmful substances from the discarded bottles. Aesthetic issues. | B1–B15, Z1–Z5 | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoica, I.; Battes, K.P.; Șuteu Ciorca, A.-M.; Cîmpean, M. Fish Alone Are Not Enough: Zoobenthos Improves Water Quality Assessment in Impacted Rivers. Fishes 2025, 10, 467. https://doi.org/10.3390/fishes10090467
Stoica I, Battes KP, Șuteu Ciorca A-M, Cîmpean M. Fish Alone Are Not Enough: Zoobenthos Improves Water Quality Assessment in Impacted Rivers. Fishes. 2025; 10(9):467. https://doi.org/10.3390/fishes10090467
Chicago/Turabian StyleStoica, Ionuț, Karina P. Battes, Anca-Mihaela Șuteu Ciorca, and Mirela Cîmpean. 2025. "Fish Alone Are Not Enough: Zoobenthos Improves Water Quality Assessment in Impacted Rivers" Fishes 10, no. 9: 467. https://doi.org/10.3390/fishes10090467
APA StyleStoica, I., Battes, K. P., Șuteu Ciorca, A.-M., & Cîmpean, M. (2025). Fish Alone Are Not Enough: Zoobenthos Improves Water Quality Assessment in Impacted Rivers. Fishes, 10(9), 467. https://doi.org/10.3390/fishes10090467







