Review on Toxicity Effect of Emerging Contaminants on Trans-/Multi-Generational Fish
Abstract
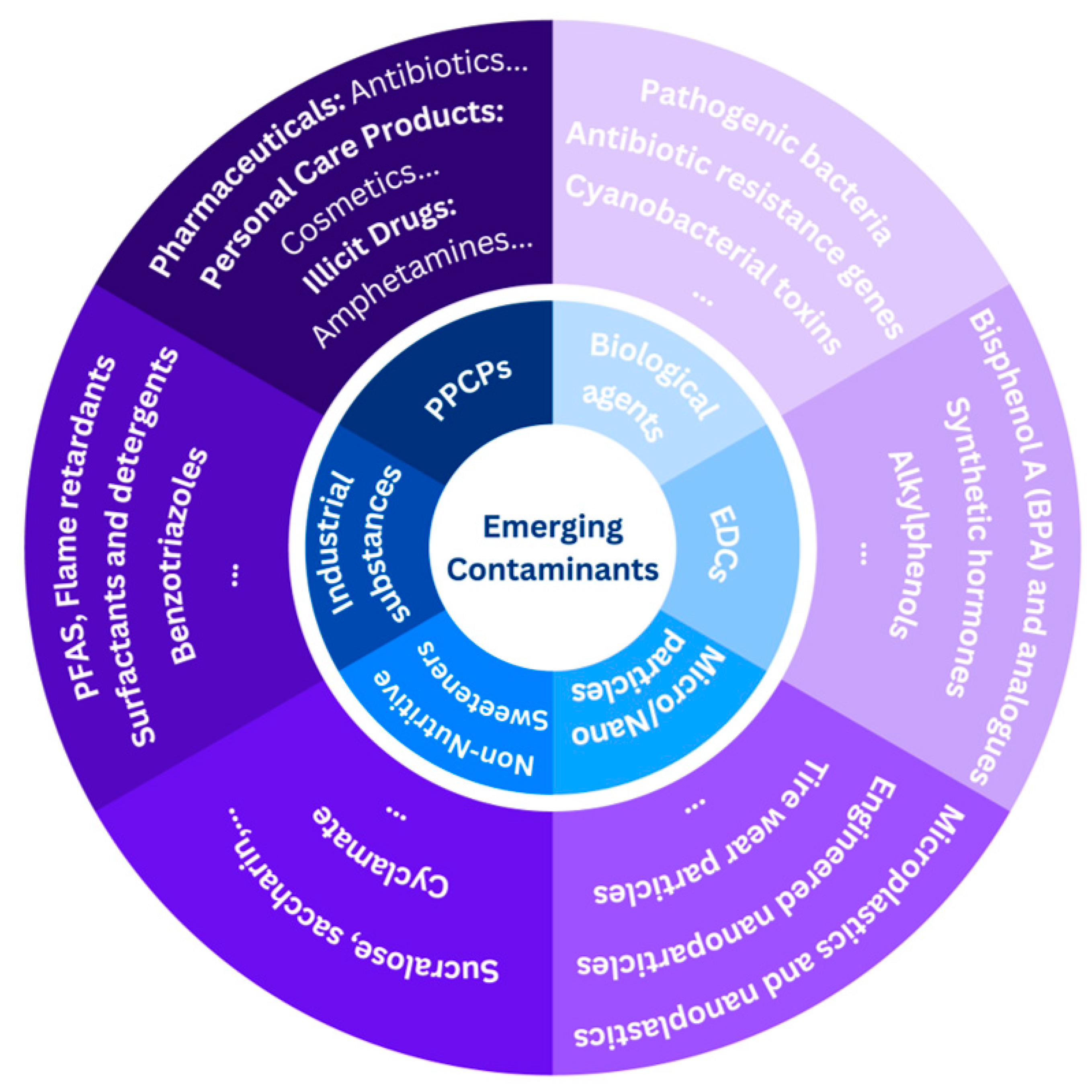
1. Introduction
2. The Toxic Action of Typical ECs
2.1. Micro/Nano Plastics (MNPs)
2.2. Pharmaceutical and Personal Care Products (PPCPs)
2.3. Endocrine Disrupting Chemicals (EDCs)
2.4. Mixture Effects
| ECs | Fish Species | Exposure Dose | Exposure Duration | Generations | Endpoint in Offspring | Reference |
|---|---|---|---|---|---|---|
| Single ECs on trans-/multi-generational fish | ||||||
| Microplastics | fathead minnows (Pimephales promelas) | 100, 2000 particles·L−1 (150–500 μm) | P: 178 dpf F1: 12 dpf | P, F1 | P: Affect the growth, lipid storage, and external coloration. F1: In the low-concentration treatment (100 particles·L−1), microplastics can delayed onset of spawning, reduced egg survival rate, and a higher malformation rate in the offspring. | [37] |
| fathead minnows (Pimephales promelas) | 100, 2000 particles·L−1 (150–500 μm) | P: 178 dpf F1: 12 dpf | P, F1 | F1: Methylation was more significant in parental males than in parental females and F1 offspring. | [38] | |
| Carbamazepine | Zebrafish (Danio rerio) | 10 μg/L | P: 6 weeks | P, F1, F2, F3, F4 | P: Reproductive output, courtship and aggressive behaviors, 11-ketotestosterone (11 KT), and sperm morphology decreased. F1-F4 (only paternal fish exposed): 11 KT, reproductive output, altered courtship, aggression, and sperm morphology decreased. | [55] |
| OP | viviparous eelpout (Zoarces viviparus) | 25, 100 μg·L−1 | P: 17 and 35 dpf | P, F1 | F1: the mortality of embryos increased, and the body length and weight decreased; the expression levels of estrogen receptors and vitellogenin of F1 embryos were increased; abnormal gonadal differentiation occurred. | [63] |
| PFOS | Zebrafish (Danio rerio) | 5, 50, 250 μg·L−1 | P: 150 dpf F1: 7 dpf | P, F1 | F1: The mortality rate of F1 larvae was 100% at 250 μg·L−1; the locomotor activity of F1 larvae were abnormally enhanced in 5 μg·L−1. | [66] |
| BPA | rare minnow (Gobiocypris rarus) | 15, 225 μg·L−1 | P: 21 dpf | P, F1 | P: induced poor quality of the embryos. F1: The fertilization, hatching, and spontaneous coiling rates decreased; the malformation rate increased; ossification in craniofacial cartilage were delayed; embryonic heart rate increased. | [60] |
| NP | Zebrafish (Danio rerio) | 2, 20, 200 μg·L−1 | P: 32 dpf F1: 120 dpf F2: 140 dpf F3: 7 dpf (recovery) | P, F1, F2, F3 | P: the expression of fshβ and lhβ were upregulated; F1: inhibit the growth of zebrafish and cause gonadal damage. the expression of fshβ and lhβ were upregulated. fshr, lhr, and esr were decreased. F2: inhibit the growth of zebrafish and cause gonadal damage. exhibits stronger tolerance to high concentrations of NP (20 and 200 μg·L−1), but higher sensitivity to low-concentration NP (2 μg·L−1) F3: Fertility ratio and hatching ratio were decreased. | [6] |
| Combined ECs on trans-/multi-generational fish | ||||||
| Microplastics and EE2 | fathead minnows (Pimephales promelas) | MPs, MP + EE2 (10 ng·L−1), MP + EE2 (50 ng·L−1) | P: 44 dpf F1: 14 or 21 dpf | P, F1 | F1: reduced embryos locomotor activity; stronger swimming ability. | [65] |
| Microplastics and salinity | marine medaka (Oryzias melastigma) | 180 μg·L−1 polystyrene ( 5.0 μm), 5‰ and 25‰ salinity | F1: 25 dph F2: 120 dph (recovery) | F0 (unexposed), F1, F2 (recovery) | F1: high salinity treatment increases in MDA content, TAC, CAT activity and cortisol levels. Additionally, also upregulated the mRNA levels of antioxidant genes (nrf2, keap1a, keap1b, sod1, cat, mt2) and HPI axis genes (crhr1, crhr2, pomca, mc2r, cyp11c1). Reduced the growth performance. | [39] |
| Nanoplastics and bisphenol | Zebrafish (Danio rerio) | BPA 20 μg·L−1, NPs 1 mg·L−1, NPs 1 mg·L−1 + BPA 20 μg·L−1 (N + B) | F0: 28 dpf F1: 4 months (recovery) | F0, F1 | F0: N + B groups induced the gut damage and microbiota dysbiosis; Differentially expressed metabolites were mainly enriched in purine metabolism, mTOR, FoxO pathways, and all enriched in mTOR in control/NPs/N + B and control/BPA/N + B groups. The N + B group had the most such metabolites; nanoparticle + BPA co-exposure exacerbated F0 zebrafish liver metabolic disturbance, with mTOR possibly the mechanism. F1: Dead egg rate was increased at NPs, and N + B groups; the TC and TG level increased at N + B groups. | [36] |
| Bifenthrin, Levonorgestrel, ethinylestradiolt, renbolone | inland silverside (Menidia beryllina) | bifenthrin (3.02 ng·L−1), EE2 (6.79 ng·L−1), levonorgestrel ( 9.27 ng·L−1), trenbolone ( 9.60 ng·L−1). | F0: 8–21 dph F1: unexposed F2: unexposed | F0, F1, F2 | F0, F1, F2: Through RRBS, which were attributed to strict inheritance of DNA methylation alterations and dysregulation of epigenetic control mechanisms. differential methylation was more frequent in gene bodies than in promoter regions across all treatments and generations | [64] |
| Antibiotic | CAM, CIP, CTC, EYR, ENR, LCM, NOR, OFL, OTC, ROX, SDZ, SMX, SMZ, TC, trimethoprim | 0, 1, and 100 μg·L−1 | F0: 150 dpf F1: eggs | F0, F1 | F1: All antibiotics were detected in F1 eggs in 1 and 100 μg·L−1 treatment. Cardiotoxicity, and the apoptosis of cardiac cells. let-7a-5p regulated the cardiac hypertrophy signaling pathway. | [57] |
2.5. Population-Level Implications of Multigenerational Effects
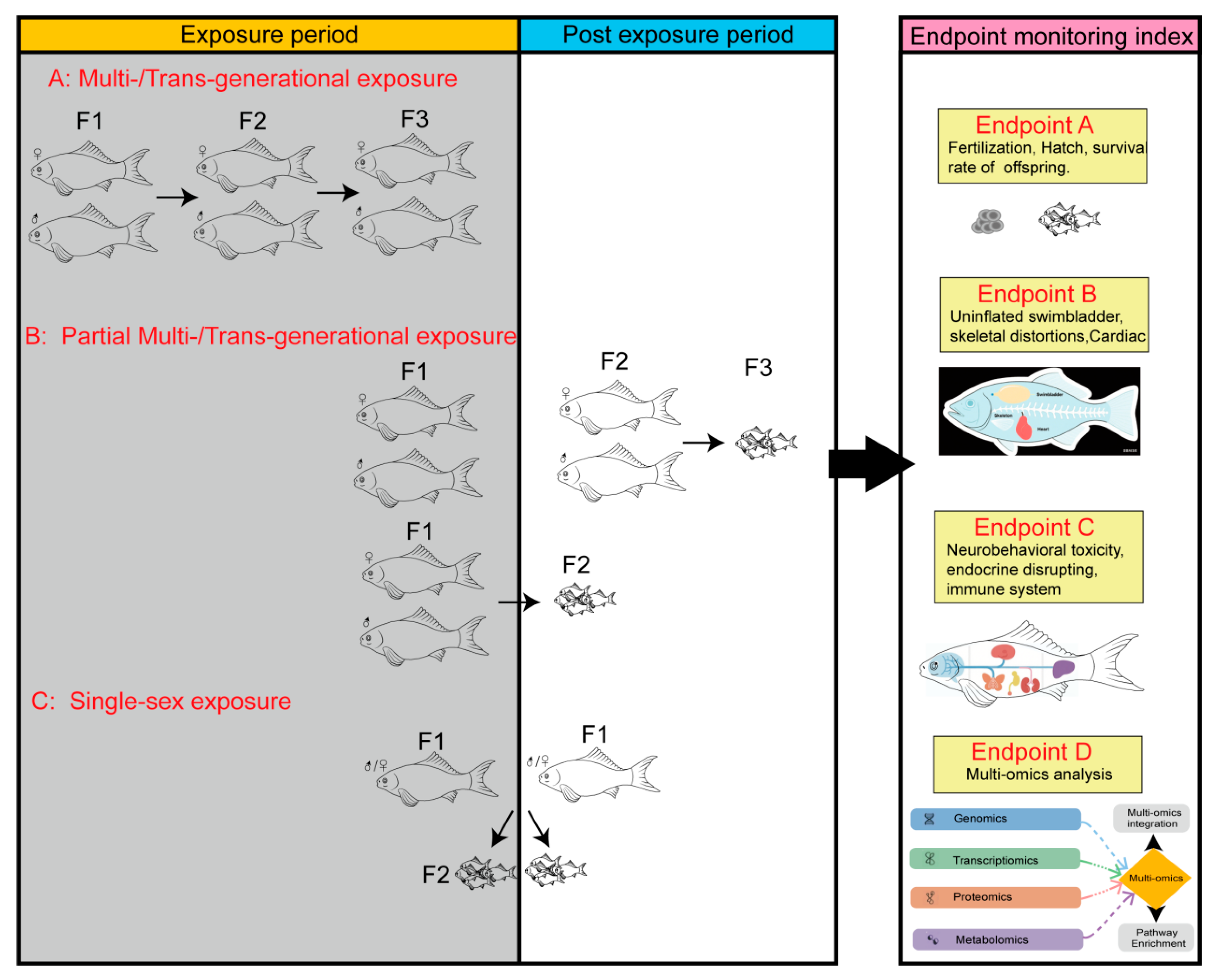
3. Mechanisms of the Trans-/Multi-Generations Toxicity of ECs on Fishes
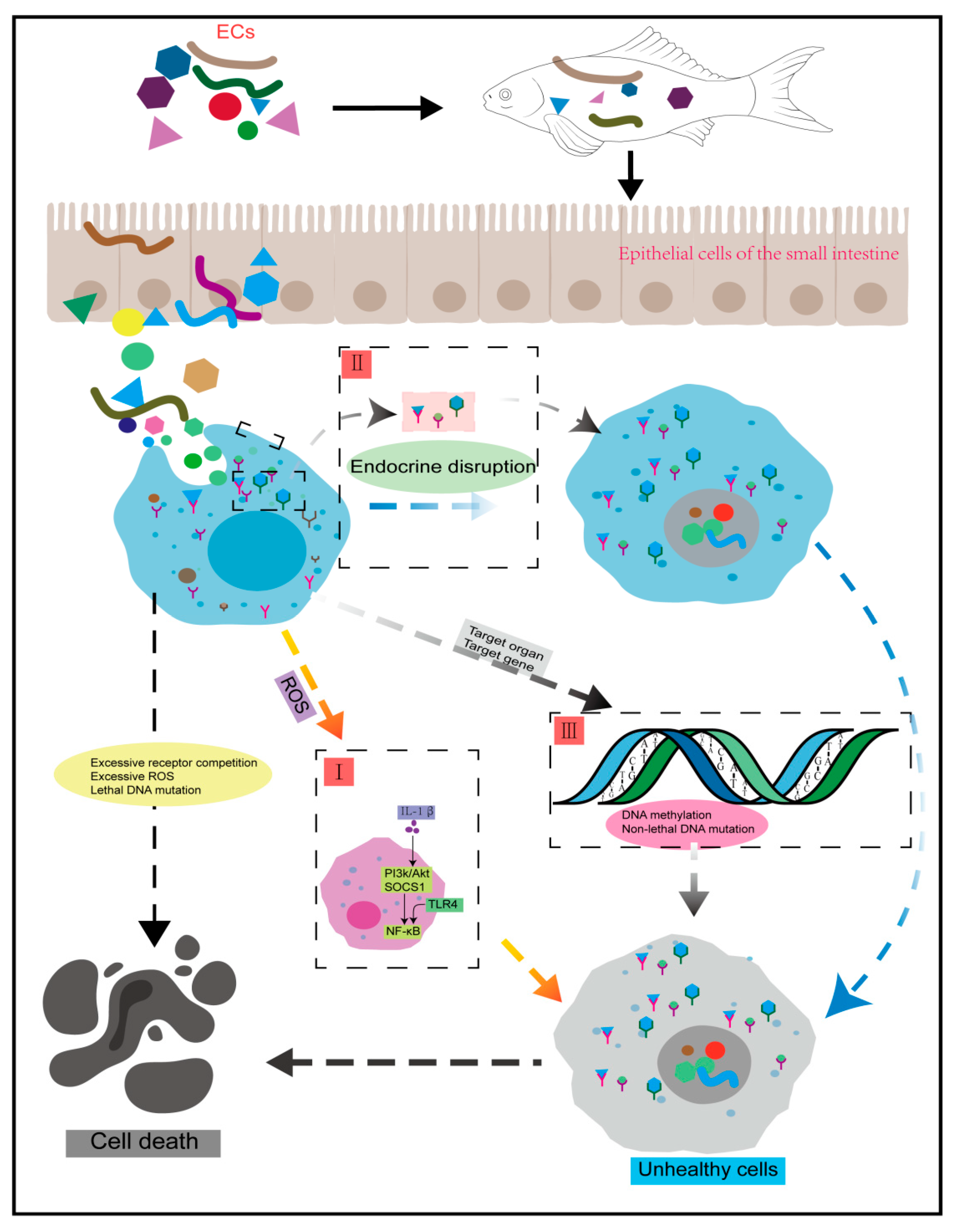
3.1. Trans-Generational Transmission, Bioaccumulation and Oxidative Damage
3.2. Endocrine Interference and Signaling Pathways Disorder
3.3. The Transgenerational Inheritance of Epigenetic Modifications
3.3.1. Epigenetic Modifications
3.3.2. Critical Developmental Windows
3.3.3. Methodological Approaches
3.3.4. Transmit Contaminant-Induced Marks
3.3.5. Mechanisms Enabling Contaminant Effects to Persist Through F3
4. Prospects and Challenges of ML and AI for Toxicity of ECs to Multi-Generational Fish
5. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANNs | artificial neural networks |
| AR | androgen receptor |
| ARGs | antibiotic resistance genes |
| BaP | benzo[a]pyrene |
| BPA | bisphenol A |
| CAM | clarithromycin |
| CAT | catalase |
| Cd | cadmium |
| CIP | ciprofloxacin |
| CTC | chlortetracycline |
| DF | deep forest |
| DMLs | differentially Methylated Loci |
| DMRs | differentially methylated regions |
| ECs | emerging contaminants |
| EDCRG | EDC-responsive genes |
| EDCs | endocrine-disrupting chemicals |
| EE2 | 17α-ethinylestradiol |
| ENR | enrofloxacin |
| ER | estrogen receptor |
| EYR | erythromycin |
| FAS | fatty acid synthase |
| FoxO | factor forkhead box |
| GAT | graph attention network |
| GPx | glutathione peroxidase |
| GR | glutathione reductase |
| HPG | hypothalamic-pituitary-gonadal |
| HPI | hypothalamic-pituitary-interrenal |
| IL-1β | interleukin-1β |
| IL-6 | interleukin-6 |
| LCM | lincomycin |
| MDA | Malondialdehyde |
| MeHg | methylmercury |
| MEHP | mono-(2-ethylhexyl) phthalate |
| MGEs | mobile genetic elements |
| miRNA | microRNA |
| ML | Machine Learning |
| MNPs | microplastics and nanoplastics |
| MPs | microplastics |
| mRNA | messenger RNA |
| mTOR | mechanistic target of rapamycin |
| NF-κB | nuclear factor κB |
| NOR | norfloxacin |
| NP | nonylphenol |
| NPs | nanoplastics |
| Nrf2 | NF-E2-related factor 2 |
| OFL | ofloxacin |
| OP | octylphenol |
| OTC | oxytetracycline |
| PBMT | persistence, bioaccumulation, mobility, and toxicity |
| PFAS | polyfluoroalkyl substances |
| PFOA | perfluorooctanoic acid |
| PFOS | perfluorooctane sulfonate |
| PMT | persistent, mobile, and toxic |
| POPs | Persistent Organic Pollutants |
| PPCPs | pharmaceutical and personal care products |
| QSAR | quantitative structure-activity relationship |
| ROS | reactive oxygen species |
| ROX | roxithromycin |
| RRBS | reduced representation bisulfite sequencing |
| SDZ | sulfadiazine |
| SMX | sulfamethoxazole |
| SMZ | sulfamethazine |
| TC | tetracycline |
| TL | transfer learning |
| TMP | trimethoprim |
| TNF-α | tumor necrosis factor-α |
| vPvM | very persistent and very mobile |
References
- Morin-Crini, N.; Lichtfouse, E.; Liu, G.; Balaram, V.; Ribeiro, A.R.L.; Lu, Z.; Stock, F.; Carmona, E.; Teixeira, M.R.; Picos-Corrales, L.A.; et al. Worldwide cases of water pollution by emerging contaminants: A review. Environ. Chem. Lett. 2022, 20, 2311–2338. [Google Scholar] [CrossRef]
- Puri, M.; Gandhi, K.; Kumar, M.S. Emerging environmental contaminants: A global perspective on policies and regulations. J. Environ. Manag. 2023, 332, 117344. [Google Scholar] [CrossRef]
- Liu, J.L.; Wong, M.H. Pharmaceuticals and personal care products (PPCPs): A review on environmental contamination in China. Environ. Int. 2013, 59, 208–224. [Google Scholar] [CrossRef] [PubMed]
- Rissman, E.F.; Adli, M. Minireview: Transgenerational epigenetic inheritance: Focus on endocrine disrupting compounds. Endocrinology 2014, 155, 2770–2780. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shen, X.; Jiang, W.; Xi, Y.; Li, S. Comprehensive review of emerging contaminants: Detection technologies, environmental impact, and management strategies. Ecotoxicol. Environ. Saf. 2024, 278, 116420. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Chen, Q.; Zhu, B.; Zhao, H.; Duan, S. Multigenerational reproduction and developmental toxicity, and HPG axis gene expression study on environmentally-relevant concentrations of nonylphenol in zebrafish. Sci. Total Environ. 2021, 764, 144259. [Google Scholar] [CrossRef]
- Junaid, M.; Siddiqui, J.A.; Liu, S.; Lan, R.; Abbas, Z.; Chen, G.; Wang, J. Adverse multigeneration combined impacts of micro(nano)plastics and emerging pollutants in the aquatic environment. Sci. Total Environ. 2023, 882, 163679. [Google Scholar] [CrossRef]
- Sun, D.; Chen, Q.; Zhu, B.; Lan, Y.; Duan, S. Long-Term Exposure to Benzo[a]Pyrene Affects Sexual Differentiation and Embryos Toxicity in Three Generations of Marine Medaka (Oryzias Melastigma). Int. J. Environ. Res. Public Health 2020, 17, 970. [Google Scholar] [CrossRef]
- Chakraborty, S.; Dissanayake, M.; Godwin, J.; Wang, X.; Bhandari, R.K. Ancestral BPA exposure caused defects in the liver of medaka for four generations. Sci. Total Environ. 2023, 856, 159067. [Google Scholar] [CrossRef]
- Chakraborty, S.; Anand, S.; Coe, S.; Reh, B.; Bhandari, R.K. The PCOS-NAFLD Multidisease Phenotype Occurred in Medaka Fish Four Generations after the Removal of Bisphenol A Exposure. Environ. Sci. Technol. 2023, 57, 12602–12619. [Google Scholar] [CrossRef]
- Deviller, G.; Lundy, L.; Fatta-Kassinos, D. Recommendations to derive quality standards for chemical pollutants in reclaimed water intended for reuse in agricultural irrigation. Chemosphere 2020, 240, 124911. [Google Scholar] [CrossRef]
- Richardson, S.D.; Manasfi, T. Water Analysis: Emerging Contaminants and Current Issues. Anal. Chem. 2024, 96, 8184–8219. [Google Scholar] [CrossRef] [PubMed]
- Kappenberg, F.; Duda, J.C.; Schurmeyer, L.; Gul, O.; Brecklinghaus, T.; Hengstler, J.G.; Schorning, K.; Rahnenfuhrer, J. Guidance for statistical design and analysis of toxicological dose-response experiments, based on a comprehensive literature review. Arch. Toxicol. 2023, 97, 2741–2761. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Khatri, M.; Puri, S. Toxicological evaluation of metal oxide nanoparticles and mixed exposures at low doses using zebra fish and THP1 cell line. Environ. Toxicol. 2019, 34, 375–387. [Google Scholar] [CrossRef]
- Ribeiro, M.J.; Scott-Fordsmand, J.J.; Amorim, M.J.B. Multigenerational exposure to cobalt (CoCl(2)) and WCCo nanoparticles in Enchytraeus crypticus. Nanotoxicology 2019, 13, 751–760. [Google Scholar] [CrossRef]
- Carvalho, R.N.; Arukwe, A.; Ait-Aissa, S.; Bado-Nilles, A.; Balzamo, S.; Baun, A.; Belkin, S.; Blaha, L.; Brion, F.; Conti, D.; et al. Mixtures of chemical pollutants at European legislation safety concentrations: How safe are they? Toxicol. Sci. 2014, 141, 218–233. [Google Scholar] [CrossRef]
- Li, H.; Zeng, L.; Wang, C.; Shi, C.; Li, Y.; Peng, Y.; Chen, H.; Zhang, J.; Cheng, B.; Chen, C.; et al. Review of the toxicity and potential molecular mechanisms of parental or successive exposure to environmental pollutants in the model organism Caenorhabditis elegans. Environ. Pollut. 2022, 311, 119927. [Google Scholar] [CrossRef]
- Carvalho, L.; Oya-Silva, L.F.; Perussolo, M.C.; Oliveira Guaita, G.; Moreira Brito, J.C.; Evans, A.A.; Prodocimo, M.M.; Cestari, M.M.; Braga, T.T.; Silva de Assis, H.C. Experimentally exposed toxic effects of long-term exposure to environmentally relevant concentrations of CIP in males and females of the silver catfish Rhamdia quelen. Chemosphere 2023, 336, 139216. [Google Scholar] [CrossRef]
- Xin, F.; Susiarjo, M.; Bartolomei, M.S. Multigenerational and transgenerational effects of endocrine disrupting chemicals: A role for altered epigenetic regulation? Semin. Cell Dev. Biol. 2015, 43, 66–75. [Google Scholar] [CrossRef]
- DeCourten, B.M.; Forbes, J.P.; Roark, H.K.; Burns, N.P.; Major, K.M.; White, J.W.; Li, J.; Mehinto, A.C.; Connon, R.E.; Brander, S.M. Multigenerational and Transgenerational Effects of Environmentally Relevant Concentrations of Endocrine Disruptors in an Estuarine Fish Model. Environ. Sci. Technol. 2020, 54, 13849–13860. [Google Scholar] [CrossRef]
- Bergmann, M.; Allen, S.; Krumpen, T.; Allen, D. High Levels of Microplastics in the Arctic Sea Ice Alga Melosira arctica, a Vector to Ice-Associated and Benthic Food Webs. Environ. Sci. Technol. 2023, 57, 6799–6807. [Google Scholar] [CrossRef] [PubMed]
- Jolaosho, T.L.; Rasaq, M.F.; Omotoye, E.V.; Araomo, O.V.; Adekoya, O.S.; Abolaji, O.Y.; Hungbo, J.J. Microplastics in freshwater and marine ecosystems: Occurrence, characterization, sources, distribution dynamics, fate, transport processes, potential mitigation strategies, and policy interventions. Ecotoxicol. Environ. Saf. 2025, 294, 118036. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M.; Hoellein, T. The global odyssey of plastic pollution. Science 2020, 368, 1184–1185. [Google Scholar] [CrossRef] [PubMed]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Marine pollution. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Reboa, A.; Cutroneo, L.; Consani, S.; Geneselli, I.; Petrillo, M.; Besio, G.; Capello, M. Mugilidae fish as bioindicator for monitoring plastic pollution: Comparison between a commercial port and a fishpond (north-western Mediterranean Sea). Mar. Pollut. Bull. 2022, 177, 113531. [Google Scholar] [CrossRef]
- Kang, H.M.; Byeon, E.; Jeong, H.; Kim, M.S.; Chen, Q.; Lee, J.S. Different effects of nano- and microplastics on oxidative status and gut microbiota in the marine medaka Oryzias melastigma. J. Hazard. Mater. 2021, 405, 124207. [Google Scholar] [CrossRef]
- Kim, L.; Cui, R.; Il Kwak, J.; An, Y.J. Trophic transfer of nanoplastics through a microalgae-crustacean-small yellow croaker food chain: Inhibition of digestive enzyme activity in fish. J. Hazard. Mater. 2022, 440, 129715. [Google Scholar] [CrossRef]
- Bai, Z.; He, Y.; Hu, G.; Cheng, L.; Wang, M. Microplastics at an environmentally relevant dose enhance mercury toxicity in a marine copepod under multigenerational exposure: Multi-omics perspective. J. Hazard. Mater. 2024, 478, 135529. [Google Scholar] [CrossRef]
- Tanaka, K.; Takada, H. Microplastic fragments and microbeads in digestive tracts of planktivorous fish from urban coastal waters. Sci. Rep. 2016, 6, 34351. [Google Scholar] [CrossRef]
- Hamer, J.; Gutow, L.; Kohler, A.; Saborowski, R. Fate of microplastics in the marine isopod Idotea emarginata. Environ. Sci. Technol. 2014, 48, 13451–13458. [Google Scholar] [CrossRef]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Galloway, T.S. The impact of polystyrene microplastics on feeding, function and fecundity in the marine copepod Calanus helgolandicus. Environ. Sci. Technol. 2015, 49, 1130–1137. [Google Scholar] [CrossRef]
- Yang, H.; Xiong, H.; Mi, K.; Xue, W.; Wei, W.; Zhang, Y. Toxicity comparison of nano-sized and micron-sized microplastics to Goldfish Carassius auratus Larvae. J. Hazard. Mater. 2020, 388, 122058. [Google Scholar] [CrossRef]
- Sokmen, T.O.; Sulukan, E.; Turkoglu, M.; Baran, A.; Ozkaraca, M.; Ceyhun, S.B. Polystyrene nanoplastics (20 nm) are able to bioaccumulate and cause oxidative DNA damages in the brain tissue of zebrafish embryo (Danio rerio). Neurotoxicology 2020, 77, 51–59. [Google Scholar] [CrossRef]
- Ding, J.; Huang, Y.; Liu, S.; Zhang, S.; Zou, H.; Wang, Z.; Zhu, W.; Geng, J. Toxicological effects of nano- and micro-polystyrene plastics on red tilapia: Are larger plastic particles more harmless? J. Hazard. Mater. 2020, 396, 122693. [Google Scholar] [CrossRef]
- Liu, Z.; Li, L.; Sun, B.; Ding, Y.; Lv, Y.; Wu, Q.; Zhao, S.; Zhang, X.; Shen, T. Transgenerational effects of Nanoplastics and bisphenol A on Zebrafish lipid metabolism: Disruption of the gut Microbiota-liver axis via mTOR pathway. Aquat. Toxicol. 2025, 284, 107401. [Google Scholar] [CrossRef] [PubMed]
- Bucci, K.; Bayoumi, M.; Stevack, K.; Watson-Leung, T.; Rochman, C.M. Microplastics may induce food dilution and endocrine disrupting effects in fathead minnows (Pimephales promelas), and decrease offspring quality. Environ. Pollut. 2024, 345, 123551. [Google Scholar] [CrossRef] [PubMed]
- Wade, M.J.; Bucci, K.; Rochman, C.M.; Meek, M.H. Microplastic exposure is associated with epigenomic effects in the model organism Pimephales promelas (fathead minnow). J. Hered. 2025, 116, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, X.; Gao, L.; Zhang, H.T.; Li, J.; Ye, Y.; Zhu, Q.L.; Zheng, J.L.; Yan, X. Transgenerational effects of microplastics on Nrf2 signaling, GH/IGF, and HPI axis in marine medaka Oryzias melastigma under different salinities. Sci. Total Environ. 2024, 906, 167170. [Google Scholar] [CrossRef]
- Molnarova, L.; Halesova, T.; Tomesova, D.; Vaclavikova, M.; Bosakova, Z. Monitoring Pharmaceuticals and Personal Care Products in Healthcare Effluent Wastewater Samples and the Effectiveness of Drug Removal in Wastewater Treatment Plants Using the UHPLC-MS/MS Method. Molecules 2024, 29, 1480. [Google Scholar] [CrossRef]
- Lesser, L.E.; Mora, A.; Moreau, C.; Mahlknecht, J.; Hernandez-Antonio, A.; Ramirez, A.I.; Barrios-Pina, H. Survey of 218 organic contaminants in groundwater derived from the world’s largest untreated wastewater irrigation system: Mezquital Valley, Mexico. Chemosphere 2018, 198, 510–521. [Google Scholar] [CrossRef]
- K’Oreje, K.O.; Kandie, F.J.; Vergeynst, L.; Abira, M.A.; Van Langenhove, H.; Okoth, M.; Demeestere, K. Occurrence, fate and removal of pharmaceuticals, personal care products and pesticides in wastewater stabilization ponds and receiving rivers in the Nzoia Basin, Kenya. Sci. Total Environ. 2018, 637–638, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. The occurrence of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs in surface water in South Wales, UK. Water Res. 2008, 42, 3498–3518. [Google Scholar] [CrossRef]
- Perez-Alvarez, I.; Islas-Flores, H.; Gomez-Olivan, L.M.; Barcelo, D.; Lopez De Alda, M.; Perez Solsona, S.; Sanchez-Aceves, L.; SanJuan-Reyes, N.; Galar-Martinez, M. Determination of metals and pharmaceutical compounds released in hospital wastewater from Toluca, Mexico, and evaluation of their toxic impact. Environ. Pollut. 2018, 240, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.D.; Cho, J.; Kim, I.S.; Vanderford, B.J.; Snyder, S.A. Occurrence and removal of pharmaceuticals and endocrine disruptors in South Korean surface, drinking, and waste waters. Water Res. 2007, 41, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Yu, B.; Hu, Y.; Liu, Z.; Zhao, K.; Li, C.; Li, M.; Lyu, C.; Lu, H.; Zhong, S.; et al. Occurrence and Health Risk Assessment of Sulfonamide Antibiotics in Different Freshwater Fish in Northeast China. Toxics 2023, 11, 835. [Google Scholar] [CrossRef]
- Ye, C.; Shi, J.; Zhang, X.; Qin, L.; Jiang, Z.; Wang, J.; Li, Y.; Liu, B. Occurrence and bioaccumulation of sulfonamide antibiotics in different fish species from Hangbu-Fengle River, Southeast China. Environ. Sci. Pollut. Res. Int. 2021, 28, 44111–44123. [Google Scholar] [CrossRef]
- Fent, K.; Weston, A.A.; Caminada, D. Ecotoxicology of human pharmaceuticals. Aquat. Toxicol. 2006, 76, 122–159. [Google Scholar] [CrossRef]
- Ogunwole, G.A.; Saliu, J.K.; Osuala, F.I.; Odunjo, F.O. Chronic levels of ibuprofen induces haematoxic and histopathology damage in the gills, liver, and kidney of the African sharptooth catfish (Clarias gariepinus). Environ. Sci. Pollut. Res. Int. 2021, 28, 25603–25613. [Google Scholar] [CrossRef]
- Kidd, K.A.; Blanchfield, P.J.; Mills, K.H.; Palace, V.P.; Evans, R.E.; Lazorchak, J.M.; Flick, R.W. Collapse of a fish population after exposure to a synthetic estrogen. Proc. Natl. Acad. Sci. USA 2007, 104, 8897–8901. [Google Scholar] [CrossRef]
- Shi, F.; Huang, Y.; Yang, M.; Lu, Z.; Li, Y.; Zhan, F.; Lin, L.; Qin, Z. Antibiotic-induced alternations in gut microflora are associated with the suppression of immune-related pathways in grass carp (Ctenopharyngodon idellus). Front. Immunol. 2022, 13, 970125. [Google Scholar] [CrossRef]
- Au-Yeung, C.; Lam, K.L.; Choi, M.H.; Chan, K.W.; Cheung, Y.S.; Tsui, Y.L.; Mo, W.Y. Impact of Prophylactic Antibiotic Use in Ornamental Fish Tanks on Microbial Communities and Pathogen Selection in Carriage Water in Hong Kong Retail Shops. Microorganisms 2024, 12, 1184. [Google Scholar] [CrossRef]
- Hamid, N.; Junaid, M.; Wang, Y.; Pu, S.Y.; Jia, P.P.; Pei, D.S. Chronic exposure to PPCPs mixture at environmentally relevant concentrations (ERCs) altered carbohydrate and lipid metabolism through gut and liver toxicity in zebrafish. Environ. Pollut. 2021, 273, 116494. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, R.K.; vom Saal, F.S.; Tillitt, D.E. Transgenerational effects from early developmental exposures to bisphenol A or 17alpha-ethinylestradiol in medaka, Oryzias latipes. Sci. Rep. 2015, 5, 9303. [Google Scholar] [CrossRef] [PubMed]
- Fraz, S.; Lee, A.H.; Pollard, S.; Srinivasan, K.; Vermani, A.; David, E.; Wilson, J.Y. Paternal Exposure to Carbamazepine Impacts Zebrafish Offspring Reproduction Over Multiple Generations. Environ. Sci. Technol. 2019, 53, 12734–12743. [Google Scholar] [CrossRef] [PubMed]
- Hammill, K.M.; Fraz, S.; Lee, A.H.; Wilson, J.Y. The effects of parental carbamazepine and gemfibrozil exposure on sexual differentiation in zebrafish (Danio rerio). Environ. Toxicol. Chem. 2018, 37, 1696–1706. [Google Scholar] [CrossRef]
- Xuan, R.; Qiu, W.; Zhou, Y.; Magnuson, J.T.; Luo, S.; Greer, J.B.; Xu, B.; Liu, J.; Xu, E.G.; Schlenk, D.; et al. Parental transfer of an antibiotic mixture induces cardiotoxicity in early life-stage zebrafish: A cross-generational study. Sci. Total Environ. 2022, 849, 157726. [Google Scholar] [CrossRef]
- Xiao, Y.; Han, D.; Currell, M.; Song, X.; Zhang, Y. Review of Endocrine Disrupting Compounds (EDCs) in China’s water environments: Implications for environmental fate, transport and health risks. Water Res. 2023, 245, 120645. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, S.; Yu, P.; Wang, D.; Hu, B.; Zheng, P.; Zhang, M. A bibliometric analysis of emerging contaminants (ECs) (2001-2021): Evolution of hotspots and research trends. Sci. Total Environ. 2024, 907, 168116. [Google Scholar] [CrossRef]
- Fan, X.; Wu, L.; Hou, T.; He, J.; Wang, C.; Liu, Y.; Wang, Z. Maternal Bisphenol A exposure impaired endochondral ossification in craniofacial cartilage of rare minnow (Gobiocypris rarus) offspring. Ecotoxicol. Environ. Saf. 2018, 163, 514–520. [Google Scholar] [CrossRef]
- Scsukova, S.; Rollerova, E.; Bujnakova Mlynarcikova, A. Impact of endocrine disrupting chemicals on onset and development of female reproductive disorders and hormone-related cancer. Reprod. Biol. 2016, 16, 243–254. [Google Scholar] [CrossRef]
- Maqbool, F.; Mostafalou, S.; Bahadar, H.; Abdollahi, M. Review of endocrine disorders associated with environmental toxicants and possible involved mechanisms. Life Sci. 2016, 145, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, T.H.; Andreassen, T.K.; Pedersen, S.N.; Van der Ven, L.T.; Bjerregaard, P.; Korsgaard, B. Effects of waterborne exposure of octylphenol and oestrogen on pregnant viviparous eelpout (Zoarces viviparus) and her embryos in ovario. J. Exp. Biol. 2002, 205, 3857–3876. [Google Scholar] [CrossRef] [PubMed]
- Major, K.M.; DeCourten, B.M.; Li, J.; Britton, M.; Settles, M.L.; Mehinto, A.C.; Connon, R.E.; Brander, S.M. Early Life Exposure to Environmentally Relevant Levels of Endocrine Disruptors Drive Multigenerational and Transgenerational Epigenetic Changes in a Fish Model. Front. Mar. Sci. 2020, 7, 2020. [Google Scholar] [CrossRef]
- Persinger, M.; Ward, J. Evaluation of cross-generational exposure to microplastics and co-occurring contaminants on embryonic and larval behavior in fathead minnows, Pimephales promelas. PeerJ 2025, 13, e19927. [Google Scholar] [CrossRef]
- Wang, M.; Chen, J.; Lin, K.; Chen, Y.; Hu, W.; Tanguay, R.L.; Huang, C.; Dong, Q. Chronic zebrafish PFOS exposure alters sex ratio and maternal related effects in F1 offspring. Environ. Toxicol. Chem. 2011, 30, 2073–2080. [Google Scholar] [CrossRef]
- Pierron, F.; Lorioux, S.; Heroin, D.; Daffe, G.; Etcheverria, B.; Cachot, J.; Morin, B.; Dufour, S.; Gonzalez, P. Transgenerational epigenetic sex determination: Environment experienced by female fish affects offspring sex ratio. Environ. Pollut. 2021, 277, 116864. [Google Scholar] [CrossRef]
- Nilsen, E.; Smalling, K.L.; Ahrens, L.; Gros, M.; Miglioranza, K.S.B.; Pico, Y.; Schoenfuss, H.L. Critical review: Grand challenges in assessing the adverse effects of contaminants of emerging concern on aquatic food webs. Environ. Toxicol. Chem. 2019, 38, 46–60. [Google Scholar] [CrossRef]
- Cheng, H.; Lv, C.; Li, J.; Wu, D.; Zhan, X.; Song, Y.; Zhao, N.; Jin, H. Bioaccumulation and biomagnification of emerging poly- and perfluoroalkyl substances in marine organisms. Sci. Total Environ. 2022, 851, 158117. [Google Scholar] [CrossRef]
- Aubert, N.; Ameller, T.; Legrand, J.J. Systemic exposure to parabens: Pharmacokinetics, tissue distribution, excretion balance and plasma metabolites of [14C]-methyl-, propyl- and butylparaben in rats after oral, topical or subcutaneous administration. Food Chem. Toxicol. 2012, 50, 445–454. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Zhou, R.R.; Lu, G.H.; Yan, Z.H.; Jiang, R.R.; Sun, Y.; Zhang, P. Interactive transgenerational effects of polystyrene nanoplastics and ethylhexyl salicylate on zebrafish. Environ. Sci.-Nano 2021, 8, 146–159. [Google Scholar] [CrossRef]
- Li, Z.H.; Zlabek, V.; Velisek, J.; Grabic, R.; Machova, J.; Randak, T. Modulation of antioxidant defence system in brain of rainbow trout (Oncorhynchus mykiss) after chronic carbamazepine treatment. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2010, 151, 137–141. [Google Scholar] [CrossRef]
- Pitt, J.A.; Trevisan, R.; Massarsky, A.; Kozal, J.S.; Levin, E.D.; Di Giulio, R.T. Maternal transfer of nanoplastics to offspring in zebrafish (Danio rerio): A case study with nanopolystyrene. Sci. Total Environ. 2018, 643, 324–334. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Shared principles in NF-kappaB signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef] [PubMed]
- Bowden, T.J. Modulation of the immune system of fish by their environment. Fish. Shellfish. Immunol. 2008, 25, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Milla, S.; Depiereux, S.; Kestemont, P. The effects of estrogenic and androgenic endocrine disruptors on the immune system of fish: A review. Ecotoxicology 2011, 20, 305–319. [Google Scholar] [CrossRef] [PubMed]
- Jobling, S.; Williams, R.; Johnson, A.; Taylor, A.; Gross-Sorokin, M.; Nolan, M.; Tyler, C.R.; van Aerle, R.; Santos, E.; Brighty, G. Predicted exposures to steroid estrogens in U.K. rivers correlate with widespread sexual disruption in wild fish populations. Environ. Health Perspect. 2006, 114 (Suppl. 1), 32–39. [Google Scholar] [CrossRef]
- Morshead, M.L.; Jensen, K.M.; Ankley, G.T.; Vliet, S.; LaLone, C.A.; Aller, A.V.; Watanabe, K.H.; Villeneuve, D.L. Putative adverse outcome pathway development based on physiological responses of female fathead minnows to model estrogen versus androgen receptor agonists. Aquat. Toxicol. 2023, 261, 106607. [Google Scholar] [CrossRef]
- Matsushima, A.; Teramoto, T.; Kakuta, Y. Crystal structure of endocrine-disrupting chemical bisphenol A and estrogen-related receptor gamma. J. Biochem. 2022, 171, 23–25. [Google Scholar] [CrossRef]
- Georgin, J.; Franco, D.S.P.; Manzar, M.S.; Meili, L.; El Messaoudi, N. A critical and comprehensive review of the current status of 17beta-estradiol hormone remediation through adsorption technology. Environ. Sci. Pollut. Res. Int. 2024, 31, 24679–24712. [Google Scholar] [CrossRef] [PubMed]
- Sciorio, R.; Greco, P.F.; Greco, E.; Tramontano, L.; Elshaer, F.M.; Fleming, S. Potential effects of environmental toxicants on sperm quality and potential risk for fertility in humans. Front. Endocrinol. 2025, 16, 1545593. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Li, J.; Wang, Y.; Song, J.; Ni, A.; Fang, L.; Xi, M.; Qian, Q.; Wang, Z.; Wang, H. Deciphering the molecular mediators of triclosan-induced lipid accumulation: Intervention via short-chain fatty acids and miR-101a. Environ. Pollut. 2024, 343, 123153. [Google Scholar] [CrossRef]
- Liu, J.; Lu, L.; Song, H.; Liu, S.; Liu, G.; Lou, B.; Shi, W. Effects of triclosan on lipid metabolism and underlying mechanisms in the cyprinid fish Squalidus argentatus. Sci. Total Environ. 2024, 951, 175627. [Google Scholar] [CrossRef] [PubMed]
- Thapa, S.; Lv, M.; Xu, H. Acetylcholinesterase: A Primary Target for Drugs and Insecticides. Mini Rev. Med. Chem. 2017, 17, 1665–1676. [Google Scholar] [CrossRef]
- Burch, E.; Hussein, M.A.; Zaki, M.; Kamal, L.T.; Zaki, G.; Shoeib, T.; Dawood, M.; Sewilam, H.; Abdelnaser, A. Assessing the Effects of Pesticides on Aquacultured Fish and Ecosystems: A Comprehensive Environmental Health Review. Fishes 2025, 10, 223. [Google Scholar] [CrossRef]
- Berg, V.; Kraugerud, M.; Nourizadeh-Lillabadi, R.; Olsvik, P.A.; Skare, J.U.; Alestrom, P.; Ropstad, E.; Zimmer, K.E.; Lyche, J.L. Endocrine effects of real-life mixtures of persistent organic pollutants (POP) in experimental models and wild fish. J. Toxicol. Environ. Health A 2016, 79, 538–548. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, H.; Liao, C.; Diao, X.; Zhen, J.; Chen, L.; Xue, Q. Toxicology mechanism of the persistent organic pollutants (POPs) in fish through AhR pathway. Toxicol. Mech. Methods 2010, 20, 279–286. [Google Scholar] [CrossRef]
- Tohyama, S.; Miyagawa, S.; Lange, A.; Ogino, Y.; Mizutani, T.; Tatarazako, N.; Katsu, Y.; Ihara, M.; Tanaka, H.; Ishibashi, H.; et al. Understanding the molecular basis for differences in responses of fish estrogen receptor subtypes to environmental estrogens. Environ. Sci. Technol. 2015, 49, 7439–7447. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, L.P.; Shen, Y.Q. A systematic review of advances in intestinal microflora of fish. Fish. Physiol. Biochem. 2021, 47, 2041–2053. [Google Scholar] [CrossRef]
- Kim, S.; Stroski, K.M.; Killeen, G.; Smitherman, C.; Simcik, M.F.; Brooks, B.W. 8:8 Perfluoroalkyl phosphinic acid affects neurobehavioral development, thyroid disruption, and DNA methylation in developing zebrafish. Sci. Total Environ. 2020, 736, 139600. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Han, E.J.; Ahn, G.; Kwak, I.S. Effects of combined stressors to cadmium and high temperature on antioxidant defense, apoptotic cell death, and DNA methylation in zebrafish (Danio rerio) embryos. Sci. Total Environ. 2020, 716, 137130. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Gao, Y. DNA methylation and gene expression alterations in zebrafish embryos exposed to cadmium. Environ. Sci. Pollut. Res. Int. 2021, 28, 30101–30110. [Google Scholar] [CrossRef] [PubMed]
- Pierron, F.; Daffe, G.; Daramy, F.; Heroin, D.; Barre, A.; Bouchez, O.; Clerendeau, C.; Romero-Ramirez, A.; Nikolski, M. Transgenerational endocrine disruptor effects of cadmium in zebrafish and contribution of standing epigenetic variation to adaptation. J. Hazard. Mater. 2023, 455, 131579. [Google Scholar] [CrossRef]
- Kamstra, J.H.; Sales, L.B.; Alestrom, P.; Legler, J. Differential DNA methylation at conserved non-genic elements and evidence for transgenerational inheritance following developmental exposure to mono(2-ethylhexyl) phthalate and 5-azacytidine in zebrafish. Epigenetics Chromatin 2017, 10, 20. [Google Scholar] [CrossRef]
- Wan, T.; Mo, J.; Au, D.W.; Qin, X.; Tam, N.Y.; Kong, R.Y.; Seemann, F. The role of DNA methylation on gene expression in the vertebrae of ancestrally benzo[a]pyrene exposed F1 and F3 male medaka. Epigenetics 2023, 18, 2222246. [Google Scholar] [CrossRef]
- Wang, S.Y.; Lau, K.; Lai, K.P.; Zhang, J.W.; Tse, A.C.; Li, J.W.; Tong, Y.; Chan, T.F.; Wong, C.K.; Chiu, J.M.; et al. Hypoxia causes transgenerational impairments in reproduction of fish. Nat. Commun. 2016, 7, 12114. [Google Scholar] [CrossRef]
- Skvortsova, K.; Tarbashevich, K.; Stehling, M.; Lister, R.; Irimia, M.; Raz, E.; Bogdanovic, O. Retention of paternal DNA methylome in the developing zebrafish germline. Nat. Commun. 2019, 10, 3054. [Google Scholar] [CrossRef]
- Wang, X.; Bhandari, R.K. The dynamics of DNA methylation during epigenetic reprogramming of primordial germ cells in medaka (Oryzias latipes). Epigenetics 2020, 15, 483–498. [Google Scholar] [CrossRef]
- Beck, D.; Ben Maamar, M.; Skinner, M.K. Genome-wide CpG density and DNA methylation analysis method (MeDIP, RRBS, and WGBS) comparisons. Epigenetics 2022, 17, 518–530. [Google Scholar] [CrossRef]
- Carvan, M.J., 3rd; Kalluvila, T.A.; Klingler, R.H.; Larson, J.K.; Pickens, M.; Mora-Zamorano, F.X.; Connaughton, V.P.; Sadler-Riggleman, I.; Beck, D.; Skinner, M.K. Mercury-induced epigenetic transgenerational inheritance of abnormal neurobehavior is correlated with sperm epimutations in zebrafish. PLoS ONE 2017, 12, e0176155. [Google Scholar] [CrossRef]
- Iwanami, N.; Lawir, D.F.; Sikora, K.; O’Meara, C.; Takeshita, K.; Schorpp, M.; Boehm, T. Transgenerational inheritance of impaired larval T cell development in zebrafish. Nat. Commun. 2020, 11, 4505. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Zhang, R.P.; Wan, F.; Guo, D.Y.; Wang, P.; Xiang, L.X.; Shao, J.Z. TET2 plays an essential role in erythropoiesis by regulating lineage-specific genes via DNA oxidative demethylation in a zebrafish model. Mol. Cell Biol. 2014, 34, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- Cavasotto, C.N.; Scardino, V. Machine Learning Toxicity Prediction: Latest Advances by Toxicity End Point. ACS Omega 2022, 7, 47536–47546. [Google Scholar] [CrossRef] [PubMed]
- Vo, A.H.; Van Vleet, T.R.; Gupta, R.R.; Liguori, M.J.; Rao, M.S. An Overview of Machine Learning and Big Data for Drug Toxicity Evaluation. Chem. Res. Toxicol. 2020, 33, 20–37. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, G. Machine Learning Based Toxicity Prediction: From Chemical Structural Description to Transcriptome Analysis. Int. J. Mol. Sci. 2018, 19, 2358. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, Y.; Xu, Z.; Feng, C.; Dong, Z.; Fan, W.; Leung, K.M.Y.; Wu, F. Predicting the Site-Specific Toxicity of Metals to Fishes Using a New Machine Learning-Based Approach. Environ. Sci. Technol. 2025, 59, 14881–14891. [Google Scholar] [CrossRef]
- Sun, T.; Wei, C.; Liu, Y.; Ren, Y. Explainable machine learning models for predicting the acute toxicity of pesticides to sheepshead minnow (Cyprinodon variegatus). Sci. Total Environ. 2024, 957, 177399. [Google Scholar] [CrossRef]
- Costa, D.F.D.; Zanardini, M.; Sanches, E.A.; de Souza, A.R.S.; da Silva Rodrigues, M.; de Moraes, A.C.N.; Habibi, H.R.; Nobrega, R.H. Androgenic overactivation and epigenetic remodeling drive intergenerational toxicity of bisphenol S in zebrafish. Ecotoxicol. Environ. Saf. 2025, 303, 118831. [Google Scholar] [CrossRef]
- Zhang, B.F.; Wu, X.Z.; Wang, J.X.; Liao, Y.L.; Cai, Z.X.; Liu, Y.; Pei, D.S. Neurotoxicity of chronic nano-neodymium oxide exposure in zebrafish: Behavioral and molecular insights. J. Hazard. Mater. 2025, 495, 138879. [Google Scholar] [CrossRef]
- Feng, J.X.; Li, P.; Liu, Y.; Liu, L.; Li, Z.H. A latest progress in the study of fish behavior: Cross-generational effects of behavior under pollution pressure and new technologies for behavior monitoring. Environ. Sci. Pollut. Res. Int. 2024, 31, 11529–11542. [Google Scholar] [CrossRef] [PubMed]
- Sheffield, T.Y.; Judson, R.S. Ensemble QSAR Modeling to Predict Multispecies Fish Toxicity Lethal Concentrations and Points of Departure. Environ. Sci. Technol. 2019, 53, 12793–12802. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, M.; Kall, S.; Svedberg, P.; Inda-Diaz, J.S.; Molander, S.; Coria, J.; Backhaus, T.; Kristiansson, E. Transformers enable accurate prediction of acute and chronic chemical toxicity in aquatic organisms. Sci. Adv. 2024, 10, eadk6669. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Huang, G.; Chi, M.; Shi, Y.; Jiang, J.; Feng, C.; Yan, Z.; Xu, Z. Prediction of chemical reproductive toxicity to aquatic species using a machine learning model: An application in an ecological risk assessment of the Yangtze River, China. Sci. Total Environ. 2021, 796, 148901. [Google Scholar] [CrossRef]
- Liu, J.; Guo, W.; Dong, F.; Aungst, J.; Fitzpatrick, S.; Patterson, T.A.; Hong, H. Machine learning models for rat multigeneration reproductive toxicity prediction. Front. Pharmacol. 2022, 13, 1018226. [Google Scholar] [CrossRef]
- Haque, M.M.; Holder, L.B.; Skinner, M.K. Genome-Wide Locations of Potential Epimutations Associated with Environmentally Induced Epigenetic Transgenerational Inheritance of Disease Using a Sequential Machine Learning Prediction Approach. PLoS ONE 2015, 10, e0142274. [Google Scholar] [CrossRef]
- Mavaie, P.; Holder, L.; Skinner, M. Identifying unique exposure-specific transgenerational differentially DNA methylated region epimutations in the genome using hybrid deep learning prediction models. Environ. Epigenet 2023, 9, dvad007. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, D.; Huang, Y.; Chen, S.; Duan, M. Review on Toxicity Effect of Emerging Contaminants on Trans-/Multi-Generational Fish. Fishes 2025, 10, 535. https://doi.org/10.3390/fishes10110535
Sun D, Huang Y, Chen S, Duan M. Review on Toxicity Effect of Emerging Contaminants on Trans-/Multi-Generational Fish. Fishes. 2025; 10(11):535. https://doi.org/10.3390/fishes10110535
Chicago/Turabian StyleSun, Dong, Yuna Huang, Shuyuan Chen, and Meina Duan. 2025. "Review on Toxicity Effect of Emerging Contaminants on Trans-/Multi-Generational Fish" Fishes 10, no. 11: 535. https://doi.org/10.3390/fishes10110535
APA StyleSun, D., Huang, Y., Chen, S., & Duan, M. (2025). Review on Toxicity Effect of Emerging Contaminants on Trans-/Multi-Generational Fish. Fishes, 10(11), 535. https://doi.org/10.3390/fishes10110535






