Antioxidant Responses of the Pacific Abalone Haliotis discus hannai to Turbidity Changes
Abstract
1. Introduction
2. Materials and Methods
2.1. Abalones
2.2. Suspended Sediment
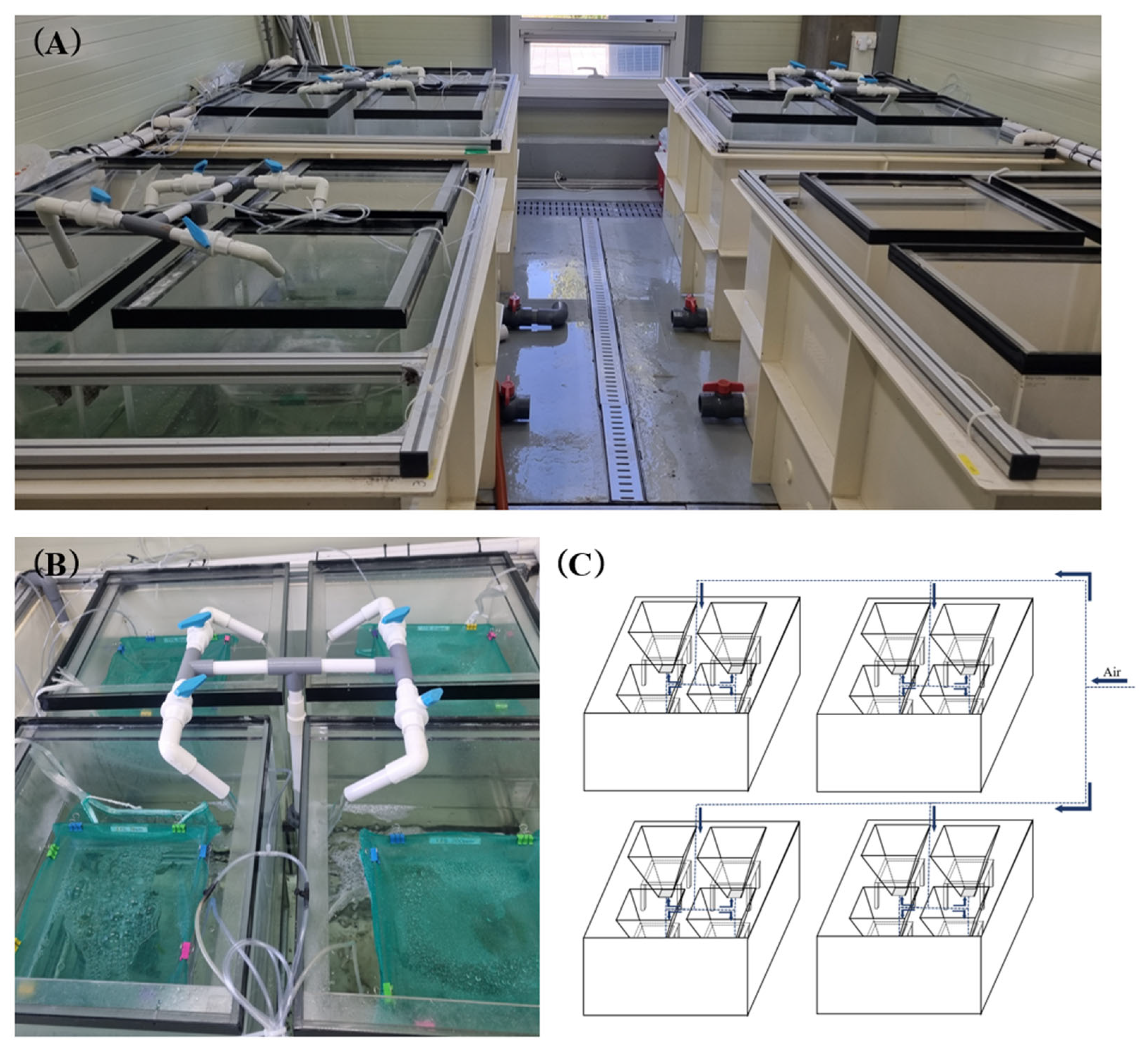
2.3. Experimental Design
2.4. H2O2 Activity, Total Antioxidant Capacity (TAC) Assays, and MDA Contents
2.5. Antioxidant Enzyme Activities
2.6. Statistical Analysis
3. Results
3.1. Survival Rate
3.2. H2O2 Activity and MDA Content
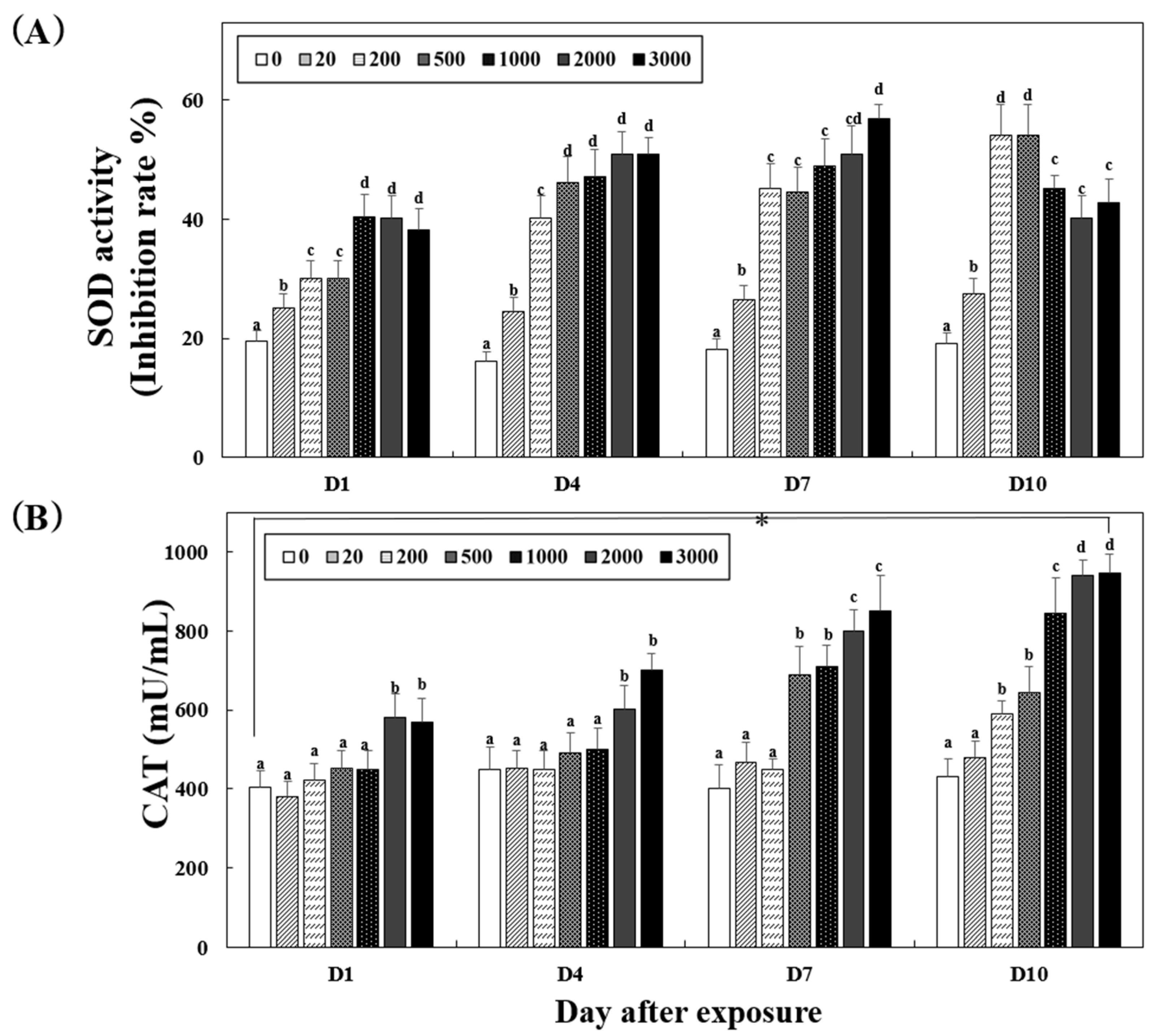
3.3. Antioxidant Enzymatic Activities
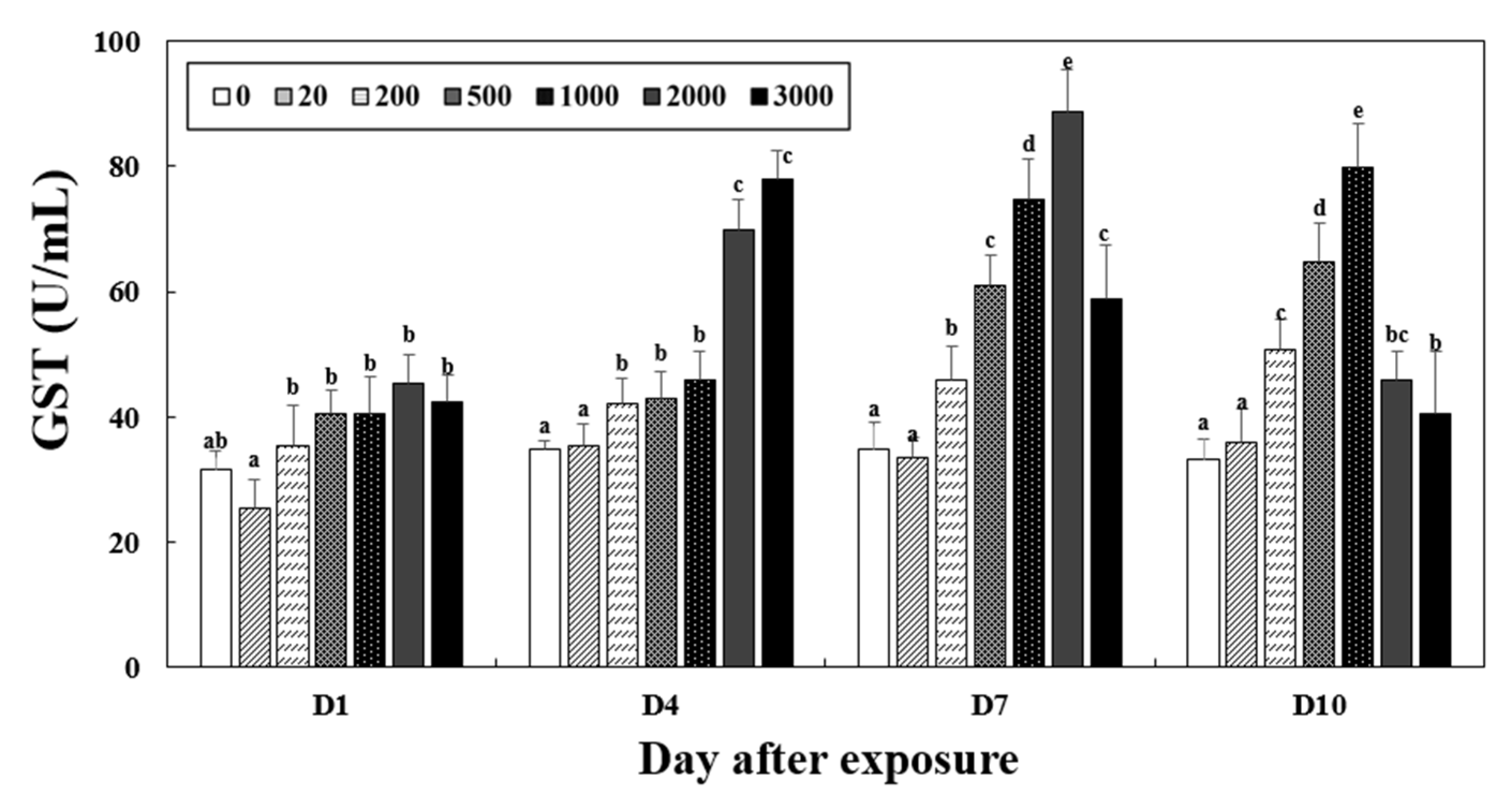
3.4. Detoxification Enzymatic Activities
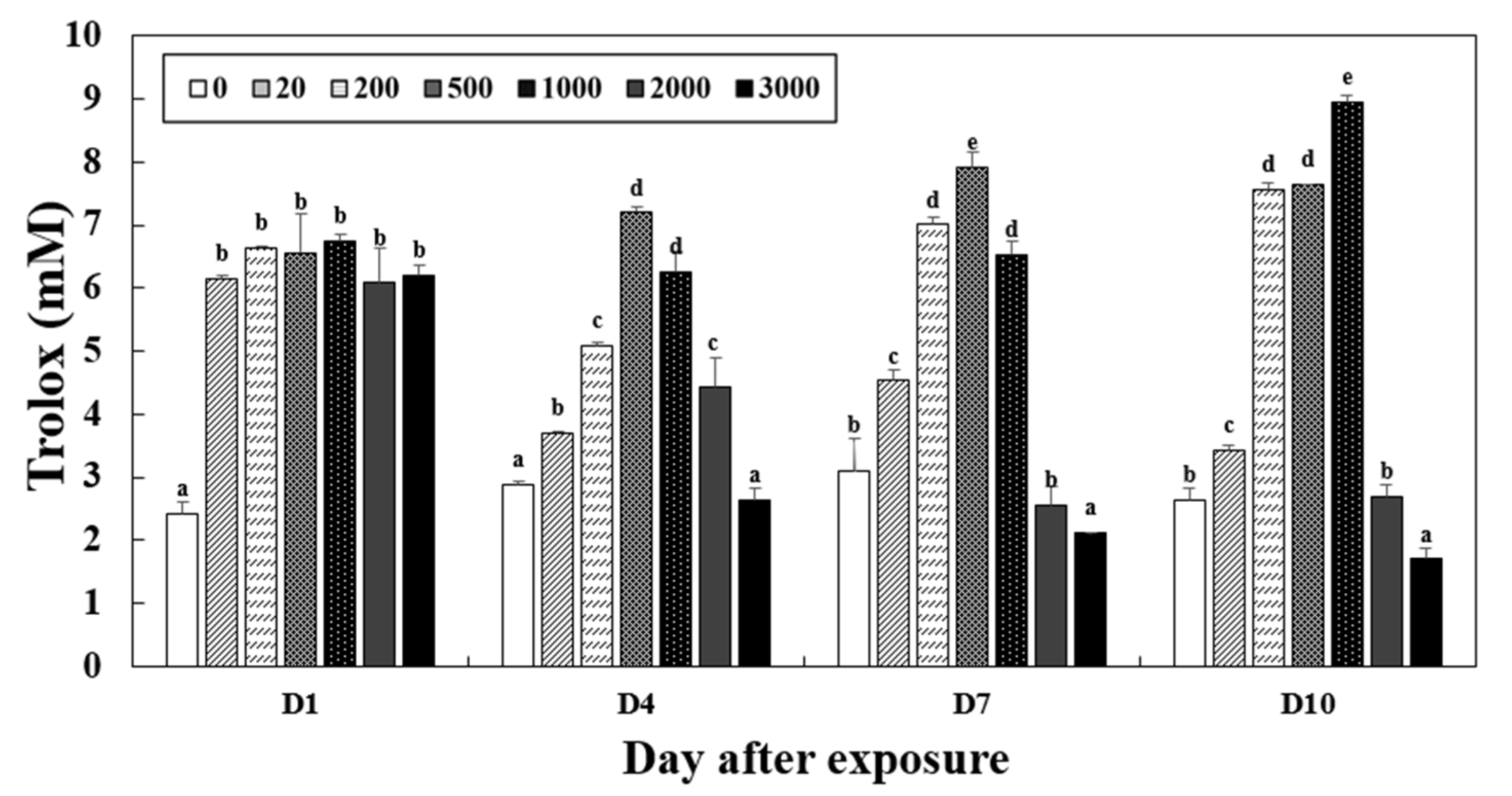
3.5. Total Antioxidant Capacity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neff, J.M.; Bothner, M.H.; Maciolek, N.J.; Grassle, J.F. Impacts of exploratory drilling for oil and gas on the benthic environment of Georges Bank. Mar. Environ. Res. 1989, 27, 77–114. [Google Scholar] [CrossRef]
- Cranford, P.J.; Gordon, D.C., Jr.; Lee, K.; Armsworthy, S.L.; Tremblay, G.H. Chronic toxicity and physical disturbance effects of water- and oil-based drilling fluids and some major constituents on adult sea scallops (Placopecten magellanicus). Mar. Environ. Res. 1999, 48, 225–256. [Google Scholar] [CrossRef]
- Elkatatny, S.; Kamal, M.S.; Alakbari, F.; Mahmoud, M. Optimizing the rheological properties of water-based drilling fluid using clays and nanoparticles for drilling horizontal and multi-lateral wells. Appl. Rheol. 2018, 28, 201843606. [Google Scholar] [CrossRef]
- Strachan, M.F.; Kingston, P.F. A comparative study on the effects of barite, ilmenite and bentonite on four suspension feeding bivalves. Mar. Pollut. Bull. 2012, 64, 2029–2038. [Google Scholar] [CrossRef] [PubMed]
- Cranford, P.J.; Gordon, D.C., Jr. The influence of dilute clay suspensions on sea scallop (Placopecten magellanicus) feeding activity and tissue growth. Nethe. J. Sea Res. 1992, 30, 107–120. [Google Scholar] [CrossRef]
- Lee, Y.C.; Jin, E.S.; Jung, S.W.; Kim, Y.M.; Chang, K.S.; Yang, J.W.; Kim, S.W.; Kim, Y.O.; Shin, H.J. Utilizing the algicidal activity of aminoclay as a practical treatment for toxic red tides. Sci. Rep. 2013, 3, 1292. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, X.; Yu, Z.M.; Zhang, P.P.; Cao, X.H.; Yuan, Y.Q. Impact assessment of modified clay on embryo-larval stages of turbot Scophthalmus maximus L. J. Oceanol. Limnol. 2019, 37, 1051–1061. [Google Scholar] [CrossRef]
- Archambault, M.C.; Bricelj, V.M.; Grant, J.; Anderson, D.M. Effects of suspended and sedimented clays on juvenile hard clams, Mercenaria mercenaria, within the context of harmful algal bloom mitigation. Mar Biol. 2004, 144, 553–565. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, W.; Mai, K.; Xu, W.; Zhong, X. Effects of dietary zinc on gene expression of antioxidant enzymes and heat shock proteins in hepatopancreas of abalone Haliotis discus hannai. Comp. Biochem. Physiol. C Toxicol. Pharmacol 2011, 154, 1–6. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhou, Z.; Wang, L.; Shi, X.; Wang, J.; Yue, F.; Yi, Q.; Yang, C.; Song, L. The immunomodulation of inducible nitric oxide in scallop Chlamys farreri. Fish Shellfish Immunol. 2013, 34, 100–108. [Google Scholar] [CrossRef]
- Carnevali, S.; Petruzzelli, S.; Longoni, B.; Vanacore, R.; Barale, R.; Cipollini, M.; Scatena, F.; Paggiaro, P.; Celi, A.; Giuntini, C. Cigarette smoke extract induces oxidative stress and apoptosis in human lung fibroblasts. Am. J. Physiol. Lung Cell Mol. Physiol. 2003, 284, 955–963. [Google Scholar] [CrossRef]
- Roch, P. Defense mechanisms and disease prevention in farmed marine invertebrates. Aquaculture 1999, 172, 125–145. [Google Scholar] [CrossRef]
- Park, C.J.; Kim, S.Y. Abalone aquaculture in Korea. J. Shellfish. Res. 2013, 32, 17–19. [Google Scholar] [CrossRef]
- Park, M.; Shin, S.K.; Do, Y.H.; Yarish, C.; Kim, J.K. Application of open water integrated multi-trophic aquaculture to intensive monoculture: A review of the current status and challenges in Korea. Aquaculture 2018, 497, 174–183. [Google Scholar] [CrossRef]
- Song, S.Y.; Park, D.H.; Lee, S.H.; Lim, H.K.; Park, J.W.; Jeong, C.R.; Kim, S.J.; Cho, S.S. Purification of phenoloxidase from Haliotis discus hannai and its anti-inflammatory activity in vitro. Fish Shellfish Immun. 2023, 137, 108741. [Google Scholar] [CrossRef]
- Debnath, T.; Mijan, M.A.; Kim, D.H.; Jo, J.E.; Kim, Y.O.; Lee, J.J.; Pyo, H.J.; Lim, B.O. Anti-Inflammatory Effects of Haliotis discus hannai I no on Dextran Sulfate Sodium-Induced Colitis in Mice. J. Food Biochem. 2015, 39, 209–217. [Google Scholar] [CrossRef]
- Neff, J.M. Composition, environmental fates, and biological effects of water-based drilling muds and cuttings discharged to the marine environment: A synthesis and annotated bibliography. In Proceedings of the Offshore Operators Committee, Singapore, 20–21 September 2005. [Google Scholar]
- Li, Z.; Jiang, M. A Numerical Study for the Drilling Fluid’s Discharge in South China Sea. In Proceedings of the ISOPE International Ocean and Polar Engineering Conference ISOPE, Honolulu, HI, USA, 16–21 June 2019; p. ISOPE-I-19-498. [Google Scholar]
- Lee, S.-H.; Jang, S.-C.; Yoon, H.-S. Spatial Distribution Characteristics of Transparency and Suspended Solids in Busan Coastal Waters. J. Korean Soc. Mar. Environ. Energy 2022, 25, 171–182. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Yoon, S.J.; Park, G.S. Ecotoxicological effects of the increased suspended solids on marine benthic organisms. J. Environ. Sci. Int. 2011, 20, 1383–1394. [Google Scholar] [CrossRef]
- Gao, Y.H.; Yu, Z.M.; Song, X.X.; Cao, X.H. Impact of modified clays on the infant oyster (Crassostrea gigas). Mar. Sci. Bull. 2007, 26, 53–60, (In Chinese with English Abstract). [Google Scholar]
- Zhang, Y.; Song, X.; Shen, H.; Cao, X.; Yuan, Y.; Wu, Z.; Yu, Z. The effects of modified clay on abalone (Haliotis discus hannai) based on laboratory and field experiments. Environ. Toxicol. Chem. 2020, 39, 2065–2075. [Google Scholar] [CrossRef]
- Wang, G.J.; Xie, J.; Yu, D.G.; Wu, L.; Hu, Z.Y. Physiological responses of abalone Haliotis diversicolor to suspended sediment stress. J. Dalian. Fish Univ. 2007, 22, 352–356, (In Chinese with English Abstract). [Google Scholar]
- Newcombe, C.P.; MacDonald, D.D. Effects of suspended sediments on aquatic ecosystems. N. Am. J. Fish. Manag. 1991, 11, 72–82. [Google Scholar] [CrossRef]
- Wilber, D.H.; Clarke, D.G. Biological effects of suspended sediments: A review of suspended sediment impacts on fish and shellfish with relation to dredging activities in estuaries. N. Am. J. Fish. Manag. 2001, 21, 855–875. [Google Scholar] [CrossRef]
- Hess, S.; Prescott, L.J.; Hoey, A.S.; McMahon, S.A.; Wenger, A.S.; Rummer, J.L. Species-specific impacts of suspended sediments on gill structure and function in coral reef fishes. Proc. R. Soc. B Biol. Sci. 2017, 284, 20171279. [Google Scholar] [CrossRef] [PubMed]
- Lesser, M.P. Oxidative stress in marine environments: Biochemistry and physiological ecology. Annu. Rev. Physiol. 2006, 68, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kim, J.A.; Lee, D.W.; Park, Y.S.; Kim, J.H.; Choi, C.Y. Oxidative stress and apoptosis in disk abalone (Haliotis discus hannai) caused by water temperature and pH changes. Antioxidants 2023, 12, 1003. [Google Scholar] [CrossRef]
- Cid, A.; Picado, A.; Correia, J.B.; Chaves, R.; Silva, H.; Caldeira, J.; Diniz, M.S. Oxidative stress and histological changes following exposure to diamond nanoparticles in the freshwater Asian clam Corbicula fluminea (Müller, 1774). J. Hazard. Mater. 2015, 284, 27–34. [Google Scholar] [CrossRef]
- Ni, H.; Peng, L.; Gao, X.; Ji, H.; Ma, J.; Li, Y.; Jiang, S. Effects of maduramicin on adult zebrafish (Danio rerio): Acute toxicity, tissue damage and oxidative stress. Ecotoxicol. Environ. Saf. 2019, 168, 249–259. [Google Scholar] [CrossRef]
- Khan, B.; Adeleye, A.S.; Burgess, R.M.; Russo, S.M.; Ho, K.T. Effects of graphene oxide nanomaterial exposures on the marine bivalve, Crassostrea virginica. Aquat. Toxicol. 2019, 216, 105297. [Google Scholar] [CrossRef]
- Kim, J.A.; Kim, M.J.; Choi, J.Y.; Park, Y.S.; Kim, J.H.; Choi, C.Y. Exposure to bisphenol A and fiber-type microplastics induce oxidative stress and cell damage in disk abalone Haliotis discus hannai: Bioaccumulation and toxicity. Fish Shellfish Immunol. 2024, 144, 109277. [Google Scholar] [CrossRef]
- Li, X.; Lin, L.; Luan, T.; Yang, L.; Lan, C. Effects of landfill leachate effluent and bisphenol A on glutathione and glutathione-related enzymes in the gills and digestive glands of the freshwater snail Bellamya purificata. Chemosphere 2008, 70, 1903–1909. [Google Scholar] [CrossRef]
- Apak, R.; Ozyurek, M.; Guclu, K.; Çapanoğlu, E. Antioxidant activity/capacity measurement. 1. Classification, physicochemical principles, mechanisms, and electron transfer (ET)-based assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef]
- Haida, Z.; Hakiman, M. A comprehensive review on the determination of enzymatic assay and nonenzymatic antioxidant activities. Food Sci. Nutr. 2019, 7, 1555–1563. [Google Scholar] [CrossRef]
- Santovito, G.; Trentin, E.; Gobbi, I.; Bisaccia, P.; Tallandini, L.; Irato, P. Non-enzymatic antioxidant responses of Mytilus galloprovincialis: Insights into the physiological role against metal-induced oxidative stress. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 240, 108909. [Google Scholar] [CrossRef]
- Sedeño-Díaz, J.E.; López-López, E. Oxidative stress in Physella acuta: An integrative response of exposure to water from two rivers of Atlantic Mexican slope. Front. Physiol. 2022, 13, 932537. [Google Scholar] [CrossRef]

| Bentonite (mg/L) | Survival Rate (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | Day 10 | |
| 0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 |
| 20 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 96.± 4.2 * |
| 200 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 99.7 ± 0.3 * | 99.7 ± 0.3 * | 93.1 ± 3.0 ** |
| 500 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 96.6 ± 2.4 * | 96.0 ± 4.0 * | 96.0 ± 4.0 * |
| 1000 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 96.5 ± 1.2 * | 96.5 ± 1.2 * | 95.8 ± 2.0 * |
| 2000 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 93.3 ± 3.3 ** | 93.3 ± 4.2 ** | 92.2 ± 4.5 ** |
| 3000 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 90.0 ± 1.1 ** | 90.0 ± 4.2 ** | 90.0 ± 4.2 ** |
| H2O2 | MDA | SOD | CAT | GST | TAC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source | df | F | p | F | p | F | p | F | p | F | p | F | p |
| Time | 3 | 34.215 | 0.045 | 30.144 | 0.039 | 28.397 | 0.012 | 30.984 | 0.033 | 24.196 | 0.419 | 31.904 | 0.187 |
| Bentonite | 6 | 3.495 | 0.039 | 1.352 | 0.044 | 2.064 | 0.102 | 4.511 | 0.012 | 3.010 | 0.042 | 2.901 | 0.046 |
| T × B | 18 | 0.784 | 0.195 | 0.894 | 0.395 | 0.954 | 0.688 | 0.598 | 0.049 | 0.606 | 0.207 | 0.732 | 0.721 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.A.; Choi, D.M.; Jung, Y.-H.; Park, H.-S.; Kim, T.; Jang, S.-I.; Lee, D.-W. Antioxidant Responses of the Pacific Abalone Haliotis discus hannai to Turbidity Changes. Fishes 2025, 10, 455. https://doi.org/10.3390/fishes10090455
Song JA, Choi DM, Jung Y-H, Park H-S, Kim T, Jang S-I, Lee D-W. Antioxidant Responses of the Pacific Abalone Haliotis discus hannai to Turbidity Changes. Fishes. 2025; 10(9):455. https://doi.org/10.3390/fishes10090455
Chicago/Turabian StyleSong, Jin Ah, Dong Mun Choi, Yun-Hwan Jung, Heung-Sik Park, Taihun Kim, Seog-Il Jang, and Dae-Won Lee. 2025. "Antioxidant Responses of the Pacific Abalone Haliotis discus hannai to Turbidity Changes" Fishes 10, no. 9: 455. https://doi.org/10.3390/fishes10090455
APA StyleSong, J. A., Choi, D. M., Jung, Y.-H., Park, H.-S., Kim, T., Jang, S.-I., & Lee, D.-W. (2025). Antioxidant Responses of the Pacific Abalone Haliotis discus hannai to Turbidity Changes. Fishes, 10(9), 455. https://doi.org/10.3390/fishes10090455






