Lipid and Fatty Acid Composition of Low-Value Mediterranean Fish in Winter and Spring for Discard Valorization
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Crude Lipid Determination and Fatty Acid Composition Analysis
2.3. Statistical Analysis and Modeling
3. Results
3.1. Crude Lipid Content
3.2. Fatty Acid Composition
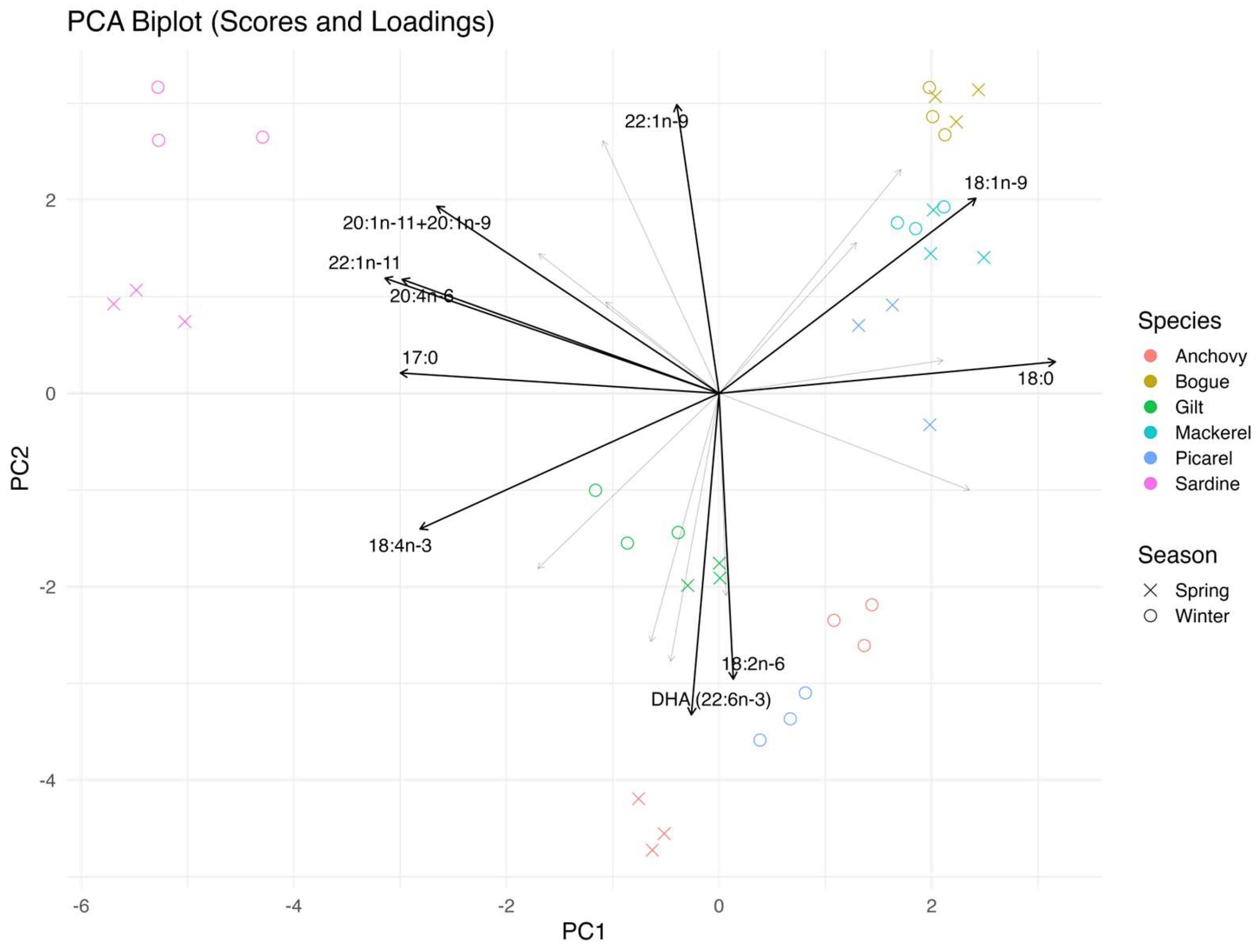
3.3. Principal Component Analysis
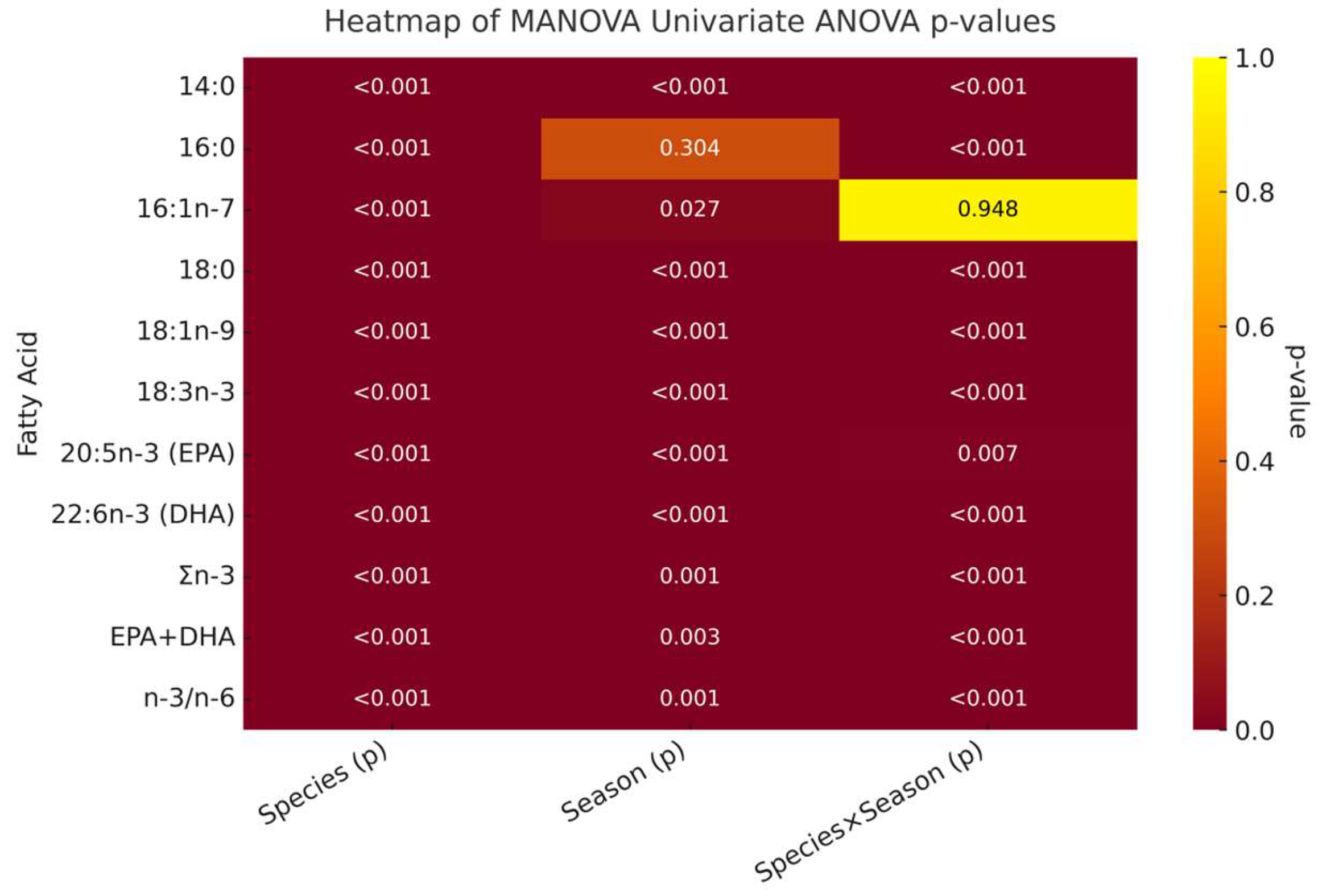
3.4. Multivariate Analysis of Variance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of Variance |
| AOCS | American Oil Chemists’ Society |
| DHA | Docosahexaenoic Acid |
| EPA | Eicosapentaenoic Acid |
| EU | European Union |
| FAO | Food and Agriculture Organization |
| FAME | Fatty Acid Methyl Ester |
| FID | Flame Ionization Detector |
| GC | Gas Chromatography |
| LC-PUFA | Long-Chain Polyunsaturated Fatty Acids |
| MANOVA | Multivariate Analysis of Variance |
| MUFA | Monounsaturated Fatty Acids |
| ND | Not Detected (below detection limit) |
| NS | Not Significant |
| PCA | Principal Component Analysis |
| PUFA | Polyunsaturated Fatty Acids |
| SFA | Saturated Fatty Acids |
Appendix A
| Species (Scientific Name) | Winter Prey Items | Spring Prey Items | References |
|---|---|---|---|
| European pilchard (Sardina pilchardus) | Larvae (<10 mm SL): tintinnid protozoans (e.g., Codonellopsis sp., ~48%), copepod nauplii (~46%). Larger larvae (10–16 mm SL): calanoid copepod post-nauplii (mainly Clausocalanus spp., ~53%), copepod nauplii (~22%), plus cyclopoid and harpacticoid copepods, particulate organic matter, protozoan cysts | Copepods (e.g., Clausocalanus, Oncaea, Paracalanus), crustacean larvae, teleost eggs, dinoflagellates (Ceratium, Lingulodinium), tintinnids | [54,55,56] |
| Anchovy (Engraulis encrasicolus) | Diets of larvae (<9 mm SL) are dominated by copepod nauplii and tintinnid protozoans, with contributions from appendicularians and small cladocerans. Adults and juveniles feed mainly on copepods (Oncaea mediterranea, O. venusta, Microsetella rosea) and decapod larvae. | Larger larvae (>9 mm SL) and juveniles shift towards copepod post-nauplii (e.g., Clausocalanus, Candacia, Temora spp.), cladocerans (Evadne spp.), euphausiids and fish eggs. Seasonal shifts correspond to changes in zooplankton availability and spawning-related energy demands. | [56,57,58] |
| Curled picarel (Centracanthus cirrus) | Primarily small zooplankton—mainly copepods—along with occasional mysid shrimps and fish larvae. | Diet remains consistent year-round, dominated by copepods, mysids and fish larvae * | [59] |
| Gilt sardine (Sardinella aurita) | Primarily zooplanktonic crustaceans (mainly copepods), along with euphausiids, amphipods, decapod larvae, teleost eggs and larvae and siphonophores. Small individuals feed mostly on copepods and other microplankton. | Diet shifts to include larger zooplankton such as hyperiid amphipods, mysids, euphausiids, siphonophores and teleost larvae and eggs, while copepods remain important prey. Larvae (<8 mm) feed mainly on copepod nauplii and Evadne spp., whereas larger larvae consume more copepod postnauplii. | [34,35] |
| Horse mackerel (Trachurus mediterraneus) | Diet mainly consists of copepods (e.g., Corycaeus sp., Oncaea media, Euterpina acutifrons, Oithona nana), decapod larvae, bivalve larvae and small teleost larvae. | Increased consumption of polychaetes (Neanthes fucata, Platynereis dumerilii), decapod larvae, amphipods, isopods and euphausiids, with copepods still dominant. Fish eggs and larvae also contribute during spawning periods. | [60,61,62] |
| Bogue (Boops boops) | Omnivorous diet dominated by crustaceans (e.g., copepods), benthic organisms, small mollusks and seagrass fragments. | Similar diet with increased occurrence of gelatinous zooplankton (e.g., Pelagia noctiluca), fish larvae and seasonal phytoplankton. | [36,37,63] |
References
- FAO. The State of World Fisheries and Aquaculture 2020. In Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar]
- FAO. Code of Conduct for Responsible Fisheries; FAO: Rome, Italy, 1995. [Google Scholar]
- EU. Regulation (EU) No 1380/2013 of the European Parliament and of the Council of 11 December 2013 on the Common Fisheries Policy, Amending Council Regulations (EC) No 1954/2003 and (EC) No 1224/2009 and Repealing Council Regulations (EC) No 2371/2002 and (EC) No 639/2004 and Council Decision 2004/585/EC. 2013. Available online: https://eur-lex.europa.eu/eli/reg/2013/1380/oj/eng (accessed on 24 August 2025).
- Roda, P.; Gilman, E.; Huntington, T.; Kennelly, S.J.; Suuronen, P.; Chaloupka, M.; Medley, P. A Third Assessment of Global Marine Fisheries Discards; FAO: Rome, Italy, 2019. [Google Scholar]
- FAO. International Guidelines on Bycatch Management and Reduction of Discards; FAO: Rome, Italy, 2011. [Google Scholar]
- Tsagarakis, K.; Palialexis, A.; Vassilopoulou, V. Mediterranean fishery discards: Review of the existing knowledge. ICES J. Mar. Sci. 2014, 71, 1219–1234. [Google Scholar] [CrossRef]
- FAO. Monitoring Discards in Mediterranean and Black Sea Fisheries: Methodology for Data Collection; FAO Fsheries and Aquaculture Technical Paper No. 639; FAO: Rome, Italy, 2019. [Google Scholar]
- Roussos, E.; Triantaphyllidis, G.; Ilia, V.; Tsagarakis, K.; Machias, A.; Tziveleka, L.A.; Roussis, V.; Ioannou, E.; Kotzamanis, Y. Status of Fishery Discards and By-Products in Greece and Potential Valorization Scenarios towards a National Exploitation Master Plan. Mar. Drugs 2024, 22, 264. [Google Scholar] [CrossRef] [PubMed]
- Mutalipassi, M.; Esposito, R.; Ruocco, N.; Viel, T.; Costantini, M.; Zupo, V. Bioactive Compounds of Nutraceutical Value from Fishery and Aquaculture Discards. Foods 2021, 10, 1495. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, J.A.; Fernández-Compás, A.; Blanco, M.; Rodríguez-Amado, I.; Moreno, H.; Borderías, J.; Pérez-Martín, R.I. Development of bioprocesses for the integral valorisation of fish discards. Biochem. Eng. J. 2019, 144, 198–208. [Google Scholar] [CrossRef]
- Nag, M.; Lahiri, D.; Dey, A.; Sarkar, T.; Pati, S.; Joshi, S.; Bunawan, H.; Mohammed, A.; Edinur, H.A.; Ghosh, S.; et al. Seafood Discards: A Potent Source of Enzymes and Biomacromolecules With Nutritional and Nutraceutical Significance. Front. Nutr. 2022, 9, 879929. [Google Scholar] [CrossRef]
- Calder, P.C. Very long-chain n-3 fatty acids and human health: Fact, fiction and the future. Proc. Nutr. Soc. 2018, 77, 52–72. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Essential fatty acids in health and chronic disease. Am. J. Clin. Nutr. 1999, 70, 560s–569s. [Google Scholar] [CrossRef]
- Shepherd, J.; Bachis, E. Changing Supply and Demand for Fish Oil. Aquac. Econ. Manag. 2014, 18, 395–416. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2024. In Blue Transformation in Action; FAO: Rome, Italy, 2024. [Google Scholar]
- Ozogul, Y.; Polat, A.; Uçak, İ.; Ozogul, F. Seasonal fat and fatty acids variations of seven marine fish species from the Mediterranean Sea. Eur. J. Lipid Sci. Technol. 2011, 113, 1491–1498. [Google Scholar] [CrossRef]
- Bandarra, N.M.; Batista, I.; Nunes, M.L.; Empis, J.M.; Christie, W.W. Seasonal Changes in Lipid Composition of Sardine (Sardina pilchardus). J. Food Sci. 1997, 62, 40–42. [Google Scholar] [CrossRef]
- Ben Rebah, F.; Abdelmouleh, A.; Kammoun, W.; Yezza, A. Seasonal variation of lipid content and fatty acid composition of Sardinella aurita from the Tunisian coast. J. Mar. Biol. Assoc. United Kingd. 2009, 90, 569–573. [Google Scholar] [CrossRef]
- Zlatanos, S.; Laskaridis, K. Seasonal variation in the fatty acid composition of three Mediterranean fish—sardine (Sardina pilchardus), anchovy (Engraulis encrasicholus) and picarel (Spicara smaris). Food Chem. 2007, 103, 725–728. [Google Scholar] [CrossRef]
- Bariche, M. Field Identification Guide to the Living Marine Resources of the Eastern and Southern Mediterranean; FAO: Rome, Italy, 2012. [Google Scholar]
- Folch, J.; Lees, M.; Sloane-Stanley, G. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Christie, W.W. Preparation of ester derivatives of fatty acids for chromatographic analysis. Adv. Lipid Methodol. 1993, 2, 69–111. [Google Scholar]
- García-Moreno, P.J.; Pérez-Gálvez, R.; Morales-Medina, R.; Guadix, A.; Guadix, E.M. Discarded species in the west Mediterranean sea as sources of omega-3 PUFA. Eur. J. Lipid Sci. Technol. 2013, 115, 982–989. [Google Scholar] [CrossRef]
- Suárez, M.D.; Sáez, M.I.; Rincón Cervera, M.Á.; Hidalgo, L.; Guil-Guerrero, J.L. Discarded fish on the Spanish Mediterranean coast: Influence of season on fatty acids profiles. Mediterr. Mar. Sci. 2021, 22, 232–245. [Google Scholar] [CrossRef]
- Sargent, J.; Tocher, D.; Bell, J. The lipids. In Fish Nutrition, 3rd ed.; Halver, J., Hardy, R., Eds.; Academic Press: San Diego, CA, USA, 2002; pp. 181–257. [Google Scholar]
- Tocher, D.R. Metabolism and functions of lipids and fatty acids in teleost fish. Rev. Fish. Sci. 2003, 11, 107–184. [Google Scholar] [CrossRef]
- Kaur, G.; Cameron-Smith, D.; Garg, M.; Sinclair, A.J. Docosapentaenoic acid (22:5n-3): A review of its biological effects. Prog. Lipid Res. 2011, 50, 28–34. [Google Scholar] [CrossRef]
- Vila-Belmonte, M.A.R.; Bou, R.; Lloret, E.; Lloret, J. Fatty acid content and profile of round sardinella (Sardinella aurita), an expanding thermophilic species in the NW Mediterranean. Mediterr. Mar. Sci. 2024, 25, 300–310. [Google Scholar] [CrossRef]
- Biton-Porsmoguer, S.; Bou, R.; Lloret, E.; Alcaide, M.; Lloret, J. Fatty acid composition and parasitism of European sardine (Sardina pilchardus) and anchovy (Engraulis encrasicolus) populations in the northern Catalan Sea in the context of changing environmental conditions. Conserv. Physiol. 2020, 8, coaa121. [Google Scholar] [CrossRef]
- Muns-Pujadas, L.; Constenla, M.; Dallarés, S.; Soler-Membrives, A.; Padrós, F.; Sala, R. The round sardinella (Sardinella aurita Valenciennes, 1847) from the NW Mediterranean: A healthy and safe choice for human consumption. Mediterr. Mar. Sci. 2025, 26, 16–29. [Google Scholar] [CrossRef]
- Ucar, Y. Elemental Compositions and Fatty Acid Profiles of Bogue Fish (Boops boops) From Mediterranean Coast: A Comprehensive Evaluation of the Potential Effects on Human Health. Biol. Trace Elem. Res. 2020, 196, 272–284. [Google Scholar] [CrossRef]
- Orban, E.; Di Lena, G.; Nevigato, T.; Masci, M.; Casini, I.; Caproni, R. Proximate, unsaponifiable lipid and fatty acid composition of bogue (Boops boops) and horse mackerel (Trachurus trachurus) from the Italian trawl fishery. J. Food Compos. Anal. 2011, 24, 1110–1116. [Google Scholar] [CrossRef]
- Koca, H.U.; Sürengil, G.; Aktaş, Ö.; Pak, F.; Koca, S.B. Seasonal and Reproductive Period Changes in Nutrient composition of Nemipterus randalli (Russell, 1986) and Boops boops (Linnaeus, 1758) from Northwest Mediterranean, Türkiye. Acta Aquat. Turc. 2025, 21, 1–14. [Google Scholar] [CrossRef]
- Lomiri, S.; Scacco, U.; Mostarda, E.; Andaloro, F. Size-related and temporal variation in the diet of the round sardinella, Sardinella aurita (Valenciennes, 1847), in the central Mediterranean Sea. J. Appl. Ichthyol. 2008, 24, 539–545. [Google Scholar] [CrossRef]
- Morote, E.; Olivar, M.P.; Villate, F.; Uriarte, I. Diet of round sardinella, Sardinella aurita, larvae in relation to plankton availability in the NW Mediterranean. J. Plankton Res. 2008, 30, 807–816. [Google Scholar] [CrossRef]
- Dobroslavić, T.; Zlatović, A.; Bartulović, V.; Lučić, D.; Glamuzina, B. Diet overlap of juvenile salema (Sarpa salpa), bogue (Boops boops) and common two-banded sea bream (Diplodus vulgaris) in the south-eastern Adriatic. J. Appl. Ichthyol. 2013, 29, 181–185. [Google Scholar] [CrossRef]
- Milisenda, G.; Rosa, S.; Fuentes, V.L.; Boero, F.; Guglielmo, L.; Purcell, J.E.; Piraino, S. Jellyfish as prey: Frequency of predation and selective foraging of Boops boops (Vertebrata, Actinopterygii) on the mauve stinger Pelagia noctiluca (Cnidaria, Scyphozoa). PLoS ONE 2014, 9, e94600. [Google Scholar] [CrossRef]
- Giogios, I.; Grigorakis, K.; Nengas, I.; Papasolomontos, S.; Papaioannou, N.; Alexis, M.N. Fatty acid composition and volatile compounds of selected marine oils and meals. J. Sci. Food Agric. 2008, 89, 88–100. [Google Scholar] [CrossRef]
- Karsli, B. Comparative analysis of the fatty acid composition of commercially available fish oil supplements in Turkey: Public health risks and benefits. J. Food Compos. Anal. 2021, 103, 104105. [Google Scholar] [CrossRef]
- Simopoulos, A.P.; DiNicolantonio, J.J. The importance of a balanced omega-6 to omega-3 ratio in the prevention and management of obesity. Open Heart 2016, 3, e000385. [Google Scholar] [CrossRef]
- Hao, L.; Chen, C.Y.; Nie, Y.H.; Kaliannan, K.; Kang, J.X. Differential Interventional Effects of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on High Fat Diet-Induced Obesity and Hepatic Pathology. Int. J. Mol. Sci. 2023, 24, 17261. [Google Scholar] [CrossRef]
- Bishehkolaei, M.; Pathak, Y. Influence of omega n-6/n-3 ratio on cardiovascular disease and nutritional interventions. Hum. Nutr. Metab. 2024, 37, 200275. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef] [PubMed]
- Legendre, P.; Legendre, L. Complex ecological data sets. In Numerical Ecology; Developments in Environmental Modelling; Elsevier: Amsterdam, The Netherlands, 2012; pp. 1–57. [Google Scholar]
- Graeve, M.; Greenacre, M.J. The selection and analysis of fatty acid ratios: A new approach for the univariate and multivariate analysis of fatty acid trophic markers in marine pelagic organisms. Limnol. Oceanogr. Methods 2020, 18, 196–210. [Google Scholar] [CrossRef]
- Parrish, C.C. Lipids in Marine Ecosystems. ISRN Oceanogr. 2013, 2013, 1–16. [Google Scholar] [CrossRef]
- Sánchez-García, A.B.; Zárate-Santana, Z.; Patino-Alonso, C. A Multivariate Analysis with MANOVA-Biplot of Learning Approaches in Health Science Students. Soc. Sci. 2025, 14, 403. [Google Scholar] [CrossRef]
- Marino, R.; Albenzio, M.; Della Malva, A.; Racioppo, A.; Speranza, B.; Bevilacqua, A. Valorization of fish from the Adriatic Sea: Nutritional properties and shelf life prolongation of Aphia minuta through essential oils. Front. Nutr. 2024, 11, 1454228. [Google Scholar] [CrossRef]
- Tacon, A.G.J.; Metian, M. Global overview on the use of fish meal and fish oil in industrially compounded aquafeeds: Trends and future prospects. Aquaculture 2008, 285, 146–158. [Google Scholar] [CrossRef]
- Caruso, G.; Floris, R.; Serangeli, C.; Di Paola, L. Fishery Wastes as a Yet Undiscovered Treasure from the Sea: Biomolecules Sources, Extraction Methods and Valorization. Mar. Drugs 2020, 18, 622. [Google Scholar] [CrossRef]
- Pérez-Martín, R.I.; Antelo, L.T.; Vázquez, J.A.; Mirón, J. An on-land management and valorisation approach for biomass associated with landing obligation compliance. Mar. Policy 2020, 116, 103506. [Google Scholar] [CrossRef]
- Herceg Romanić, S.; Jovanović, G.; Mustać, B.; Stojanović-Đinović, J.; Stojić, A.; Čadež, T.; Popović, A. Fatty acids, persistent organic pollutants, and trace elements in small pelagic fish from the eastern Mediterranean Sea. Mar. Pollut. Bull. 2021, 170, 112654. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.M.; Coelho, M.P.; Gil, M.M.; Pita, C.; Silva, P.M. Changing the way we look to fisheries’ discards. Reg. Stud. Mar. Sci. 2024, 71, 103434. [Google Scholar] [CrossRef]
- Borme, D.; Legovini, S.; de Olazabal, A.; Tirelli, V. Diet of Adult Sardine Sardina pilchardus in the Gulf of Trieste, Northern Adriatic Sea. J. Mar. Sci. Eng. 2022, 10, 1012. [Google Scholar] [CrossRef]
- Chen, C.-T.; Carlotti, F.; Harmelin-Vivien, M.; Guilloux, L.; Bănaru, D. Temporal variation in prey selection by adult European sardine (Sardina pilchardus) in the NW Mediterranean Sea. Prog. Oceanogr. 2021, 196, 102617. [Google Scholar] [CrossRef]
- Morote, E.; Olivar, M.P.; Villate, F.; Uriarte, I. A comparison of anchovy (Engraulis encrasicolus) and sardine (Sardina pilchardus) larvae feeding in the Northwest Mediterranean: Influence of prey availability and ontogeny. ICES J. Mar. Sci. 2010, 67, 897–908. [Google Scholar] [CrossRef]
- Bacha, M.; Amara, R. Spatial, temporal and ontogenetic variation in diet of anchovy (Engraulis encrasicolus) on the Algerian coast (SW Mediterranean). Estuar. Coast. Shelf Sci. 2009, 85, 257–264. [Google Scholar] [CrossRef]
- Costalago, D.; Palomera, I.; Tirelli, V. Seasonal comparison of the diets of juvenile European anchovy Engraulis encrasicolus and sardine Sardina pilchardus in the Gulf of Lions. J. Sea Res. 2014, 89, 64–72. [Google Scholar] [CrossRef]
- Petrova, T.N.; Koulish, A.V.; Klimova, T.N. On the Reproduction of the Curled Picarel Centracanthus cirrus (Sparidae) in the Black Sea. J. Ichthyol. 2025, 65, 164–168. [Google Scholar] [CrossRef]
- Bayhan, B.; Sever, T.M.; Kara, A. Diet composition of the Mediterranean horse mackerel, Trachurus mediterraneus (Steindachner, 1868) (Osteichthyes: Carangidae), from the Aegean Sea. Belg. J. Zool. 2013, 143, 15–22. [Google Scholar] [CrossRef]
- Georgieva, Y.G.; Daskalov, G.M.; Klayn, S.L.; Stefanova, K.B.; Stefanova, E.S. Seasonal diet and feeding strategy of horse mackerel Trachurus mediterraneus (Steindachner, 1868) (Perciformes: Carangidae) in the South-Western Black Sea. Acta Zool. Bulg. 2019, 71, 201–210. [Google Scholar]
- Koç, H.T.; Erdoğan, Z. Feeding Habits of the Mediterranean Horse Mackerel. Nat. Eng. Sci. 2019, 4, 182–193. [Google Scholar] [CrossRef]
- El-Maremie, H.; El-Mor, M. Feeding Habits of the Bogue, Boops boops (Linnaeus, 1758) (Teleostei: Sparidae) in Benghazi Coast, Eastern Libya. J. Life Sci. 2015, 9, 189–196. [Google Scholar] [CrossRef]
| Winter | Spring | p | |
|---|---|---|---|
| Sardina pilchardus | 4.0 ± 0.08 | 11.0 ± 0.23 | *** |
| Engraulis encrasicolus | 2.0 ± 0.11 | 2.5 ± 0.13 | * |
| Centracanthus cirrus | 1.7 ± 0.09 | 7.2 ± 0.10 | * |
| Saridenella aurita | 5.5 ± 0.29 | 5.2 ± 0.11 | NS |
| Trachurus mediterraneus | 2.9 ± 0.13 | 5.6 ± 0.33 | *** |
| Boops boops | 1.9 ± 0.07 | 8.4 ± 0.23 | *** |
| Sardina pilchardus | Engraulis encrasicolus | Centracanthus cirrus | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Winter | Spring | p | Winter | Spring | p | Winter | Spring | p | |
| 14:0 | 5.69 ± 0.316 | 5.99 ± 0.055 | NS | 2.77 ± 0.129 | 3.32 ± 0.122 | ** | 3.23 ± 0.233 | 3.10 ± 0.151 | NS |
| 16:0 | 18.49 ± 0.742 | 16.99 ± 0.251 | * | 22.58 ± 0.560 | 21.77 ± 1.059 | NS | 19.30 ± 0.518 | 21.50 ± 0.648 | * |
| 16:1n-7 | 7.32 ± 0.249 | 6.54 ± 0.131 | ** | 3.51 ± 0.120 | 3.33 ± 0.116 | NS | 3.81 ± 0.145 | 4.71 ± 0.123 | ** |
| 17:0 | 0.83 ± 0.014 | 0.54 ± 0.014 | *** | 0.00 ± 0.000 | 0.45 ± 0.022 | *** | 0.31 ± 0.005 | 0.16 ± 0.016 | *** |
| 18:0 | 4.00 ± 0.322 | 3.58 ± 0.097 | NS | 5.71 ± 0.316 | 5.17 ± 0.150 | NS | 6.88 ± 0.229 | 5.82 ± 0.332 | * |
| 18:1n-9 | 5.20 ± 0.248 | 8.06 ± 0.065 | *** | 7.65 ± 0.065 | 7.45 ± 0.406 | NS | 8.45 ± 0.161 | 16.38 ± 0.521 | *** |
| 18:1n-7 | 2.78 ± 0.130 | 2.12 ± 0.008 | ** | 2.76 ± 0.070 | 2.41 ± 0.126 | * | 2.10 ± 0.021 | 2.49 ± 0.071 | ** |
| 18:2n-6 | 0.98 ± 0.021 | 1.25 ± 0.023 | *** | 1.37 ± 0.067 | 1.88 ± 0.094 | ** | 1.73 ± 0.019 | 1.06 ± 0.027 | *** |
| 18:3n-3 | 0.64 ± 0.000 | 1.10 ± 0.024 | *** | 0.97 ± 0.015 | 1.77 ± 0.068 | *** | 1.11 ± 0.049 | 0.74 ± 0.015 | *** |
| 18:4n-3 | 2.08 ± 0.051 | 3.28 ± 0.082 | *** | 2.16 ± 0.029 | 2.49 ± 0.137 | * | 1.63 ± 0.200 | 0.77 ± 0.021 | ** |
| 20:0 | 0.32 ± 0.008 | 0.48 ± 0.013 | *** | 0.58 ± 0.085 | 0.66 ± 0.053 | NS | 0.99 ± 0.070 | 0.23 ± 0.019 | *** |
| 20:1n-11 + 20:1n-9 | 5.62 ± 0.448 | 5.31 ± 0.271 | NS | 0.69 ± 0.050 | 1.13 ± 0.310 | NS | 1.20 ± 0.172 | 1.91 ± 0.091 | ** |
| 20:2n-6 | 0.08 ± 0.032 | 0.00 ± 0.000 | * | ND | ND | NS | 0.00 ± 0.000 | 0.06 ± 0.002 | *** |
| 20:4n-6 | 0.21 ± 0.108 | 0.24 ± 0.022 | NS | ND | ND | NS | 0.00 ± 0.000 | 0.04 ± 0.003 | *** |
| 20:4n-3 | 1.02 ± 0.362 | 0.47 ± 0.023 | NS | 1.30 ± 0.081 | 0.79 ± 0.062 | ** | 1.50 ± 0.042 | 0.93 ± 0.105 | ** |
| 20:5n-3 | 7.95 ± 0.213 | 7.17 ± 0.094 | ** | 8.03 ± 0.294 | 7.09 ± 0.306 | * | 6.54 ± 0.051 | 5.39 ± 0.288 | ** |
| 22:1n-11 | 7.23 ± 0.866 | 5.95 ± 0.401 | NS | 0.35 ± 0.070 | 0.85 ± 0.032 | *** | 0.18 ± 0.024 | 0.24 ± 0.012 | * |
| 22:1n-9 | 0.38 ± 0.025 | 0.40 ± 0.003 | NS | 0.21 ± 0.058 | 0.22 ± 0.045 | NS | 0.25 ± 0.017 | 0.24 ± 0.036 | NS |
| 22:5n-3 | 1.31 ± 0.160 | 1.62 ± 0.244 | NS | 1.22 ± 0.144 | 1.24 ± 0.411 | NS | 1.18 ± 0.042 | 2.54 ± 0.301 | ** |
| 22:6n-3 | 13.59 ± 0.954 | 16.06 ± 0.193 | * | 20.36 ± 0.990 | 23.41 ± 1.154 | * | 22.03 ± 0.218 | 18.37 ± 1.105 | ** |
| 24:1n-9 | 1.47 ± 0.144 | 1.45 ± 0.126 | NS | 1.71 ± 0.025 | 2.35 ± 0.563 | NS | 2.33 ± 0.040 | 1.84 ± 0.329 | NS |
| SFA | 29.33 ± 1.294 | 27.57 ± 0.304 | NS | 31.64 ± 0.929 | 31.36 ± 1.321 | NS | 30.71 ± 0.883 | 30.80 ± 1.028 | NS |
| MUFA | 30.01 ± 1.606 | 29.82 ± 0.669 | NS | 16.88 ± 0.046 | 17.73 ± 0.583 | NS | 18.31 ± 0.231 | 27.80 ± 0.929 | *** |
| Σn-3 | 26.59 ± 1.589 | 29.68 ± 0.188 | * | 34.04 ± 1.391 | 36.80 ± 1.332 | NS | 33.98 ± 0.458 | 28.74 ± 1.384 | ** |
| Σn-3 LC PUFA | 23.87 ± 1.558 | 25.31 ± 0.293 | NS | 30.91 ± 1.386 | 32.53 ± 1.173 | NS | 31.24 ± 0.230 | 27.23 ± 1.349 | ** |
| Σn-6 | 1.27 ± 0.152 | 1.49 ± 0.017 | NS | 1.37 ± 0.067 | 1.88 ± 0.094 | ** | 1.73 ± 0.019 | 1.16 ± 0.031 | *** |
| EPA + DHA | 21.54 ± 1.166 | 23.23 ± 0.197 | NS | 28.39 ± 1.281 | 30.51 ± 1.452 | NS | 28.56 ± 0.226 | 23.75 ± 1.383 | ** |
| SFA/MUFA | 0.98 ± 0.038 | 0.92 ± 0.030 | NS | 1.87 ± 0.052 | 1.77 ± 0.054 | NS | 1.68 ± 0.033 | 1.18 ± 0.164 | ** |
| Σn-3/Σn-6 | 20.95 + 1.215 | 19.92 + 0.355 | NS | 24.84 + 0.205 | 19.54 + 0.710 | *** | 19.70 + 0.459 | 24.87 + 0.902 | ** |
| Saridenella aurita | Trachurus mediterraneus | Boops boops | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Winter | Spring | p | Winter | Spring | p | Winter | Spring | p | |
| 14:0 | 6.84 ± 0.117 | 4.96 ± 0.238 | *** | 3.70 ± 0.072 | 3.04 ± 0.061 | *** | 5.69 ± 0.086 | 5.54 ± 0.156 | NS |
| 16:0 | 21.17 ± 0.911 | 19.98 ± 0.758 | NS | 19.56 ± 0.397 | 20.44 ± 0.227 | * | 20.23 ± 0.169 | 21.92 ± 0.262 | ** |
| 16:1n-7 | 6.02 ± 0.035 | 5.02 ± 0.223 | ** | 5.65 ± 0.182 | 4.92 ± 0.148 | ** | 7.32 ± 0.155 | 6.97 ± 0.080 | * |
| 17:0 | 0.54 ± 0.014 | 0.36 ± 0.009 | *** | 0.23 ± 0.050 | 0.13 ± 0.031 | NS | 0.26 ± 0.025 | 0.19 ± 0.014 | * |
| 18:0 | 5.51 ± 0.275 | 5.13 ± 0.178 | NS | 6.05 ± 0.178 | 6.68 ± 0.280 | * | 7.10 ± 0.182 | 6.96 ± 0.162 | NS |
| 18:1n-9 | 6.53 ± 0.244 | 6.99 ± 0.437 | NS | 19.72 ± 0.255 | 19.70 ± 0.858 | NS | 16.33 ± 0.125 | 16.56 ± 0.042 | * |
| 18:1n-7 | 2.60 ± 0.062 | 2.43 ± 0.081 | * | 2.77 ± 0.105 | 2.92 ± 0.020 | NS | 3.28 ± 0.087 | 3.20 ± 0.018 | NS |
| 18:2n-6 | 1.86 ± 0.119 | 1.58 ± 0.046 | * | 1.19 ± 0.017 | 1.24 ± 0.023 | * | 1.24 ± 0.046 | 1.19 ± 0.033 | NS |
| 18:3n-3 | 1.14 ± 0.015 | 1.05 ± 0.052 | * | 0.87 ± 0.053 | 0.80 ± 0.022 | NS | 0.89 ± 0.006 | 0.80 ± 0.008 | *** |
| 18:4n-3 | 1.82 ± 0.038 | 1.64 ± 0.070 | * | 0.98 ± 0.026 | 0.96 ± 0.056 | NS | 1.10 ± 0.101 | 1.08 ± 0.034 | NS |
| 20:0 | 0.71 ± 0.135 | 0.34 ± 0.013 | ** | 0.34 ± 0.012 | 0.35 ± 0.025 | NS | 0.54 ± 0.025 | 0.48 ± 0.050 | NS |
| 20:1n-11 + 20:1n-9 | 0.70 ± 0.040 | 0.64 ± 0.085 | NS | 2.28 ± 0.060 | 2.00 ± 0.103 | * | 1.82 ± 0.051 | 1.65 ± 0.079 | * |
| 20:2n-6 | 0.07 ± 0.029 | ND | * | 0.00 ± 0.000 | 0.02 ± 0.029 | NS | 0.00 ± 0.000 | 0.02 ± 0.040 | NS |
| 20:4n-6 | 0.08 ± 0.049 | ND | * | 0.00 ± 0.000 | 0.06 ± 0.048 | NS | 0.00 ± 0.000 | 0.01 ± 0.024 | NS |
| 20:4n-3 | 1.74 ± 0.059 | 1.39 ± 0.021 | ** | 1.70 ± 0.046 | 1.34 ± 0.074 | ** | 1.61 ± 0.040 | 1.41 ± 0.055 | ** |
| 20:5n-3 | 9.82 ± 0.461 | 9.81 ± 0.364 | NS | 5.57 ± 0.200 | 5.20 ± 0.177 | NS | 5.18 ± 0.045 | 4.86 ± 0.254 | NS |
| 22:1n-11 | 0.09 ± 0.024 | 0.36 ± 0.149 | * | 0.58 ± 0.093 | 0.81 ± 0.029 | * | 0.19 ± 0.015 | 0.22 ± 0.021 | NS |
| 22:1n-9 | 0.24 ± 0.012 | 0.20 ± 0.023 | NS | 0.33 ± 0.015 | 0.34 ± 0.010 | NS | 0.43 ± 0.047 | 0.45 ± 0.025 | NS |
| 22:5n-3 | 1.56 ± 0.775 | 2.23 ± 0.759 | NS | 2.04 ± 0.071 | 2.15 ± 0.152 | NS | 1.93 ± 0.055 | 2.16 ± 0.325 | NS |
| 22:6n-3 | 16.48 ± 1.324 | 22.10 ± 0.488 | ** | 13.71 ± 0.392 | 15.65 ± 0.461 | ** | 10.04 ± 0.067 | 10.72 ± 0.549 | NS |
| 24:1n-9 | 1.21 ± 0.504 | 1.57 ± 0.543 | NS | 1.01 ± 0.035 | 1.22 ± 0.068 | ** | 0.80 ± 0.035 | 0.89 ± 0.029 | * |
| SFA | 34.77 ± 1.096 | 30.77 ± 1.108 | * | 29.87 ± 0.499 | 30.66 ± 0.170 | NS | 33.81 ± 0.265 | 35.08 ± 0.533 | * |
| MUFA | 17.39 ± 0.495 | 17.21 ± 0.525 | NS | 32.33 ± 0.319 | 31.92 ± 1.050 | NS | 30.18 ± 0.196 | 29.94 ± 0.245 | NS |
| Σn-3 | 32.55 ± 1.386 | 38.22 ± 0.895 | ** | 24.87 ± 0.121 | 26.10 ± 0.870 | NS | 20.75 ± 0.282 | 21.04 ± 0.575 | NS |
| Σn-3 LC PUFA | 29.60 ± 1.342 | 35.53 ± 1.014 | ** | 23.02 ± 0.196 | 24.34 ± 0.800 | NS | 18.76 ± 0.187 | 19.15 ± 0.589 | NS |
| Σn-6 | 2.01 ± 0.041 | 1.58 ± 0.046 | *** | 1.19 ± 0.017 | 1.32 ± 0.046 | * | 1.24 ± 0.046 | 1.23 ± 0.082 | NS |
| EPA + DHA | 26.30 ± 1.766 | 31.91 ± 0.379 | ** | 19.28 ± 0.238 | 20.85 ± 0.635 | * | 15.21 ± 0.093 | 15.58 ± 0.801 | NS |
| SFA/MUFA | 2.00 ± 0.088 | 1.79 ± 0.069 | * | 0.92 ± 0.014 | 0.96 ± 0.027 | NS | 1.12 ± 0.007 | 1.17 ± 0.017 | ** |
| Σn-3/Σn-6 | 16.16 + 0.679 | 24.14 + 1.221 | ** | 20.91 + 0.388 | 19.85 + 1.134 | NS | 16.74 + 0.402 | 17.16 + 1.419 | NS |
| Species | PCA Separation (Winter vs. Spring) | t-Test Support | Interpretation |
|---|---|---|---|
| Sardina pilchardus | Clear (PC1) | Strong | Pronounced winter–spring variation |
| Engraulis encrasicolus | Moderate | Partial | Directional winter–spring trend, less robust |
| Centracanthus cirrus | Clear, opposite shift | Strong | Unique winter–spring dynamics |
| Sardinella aurita | None | Weak/None | Stable fatty acid profile |
| Trachurus mediterraneus | Clear (PC1) | Strong | Strong winter–spring variation |
| Boops boops | Overlapping | Weak/None | Minimal winter–spring difference |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsopelakos, A.; Miliou, H. Lipid and Fatty Acid Composition of Low-Value Mediterranean Fish in Winter and Spring for Discard Valorization. Fishes 2025, 10, 454. https://doi.org/10.3390/fishes10090454
Tsopelakos A, Miliou H. Lipid and Fatty Acid Composition of Low-Value Mediterranean Fish in Winter and Spring for Discard Valorization. Fishes. 2025; 10(9):454. https://doi.org/10.3390/fishes10090454
Chicago/Turabian StyleTsopelakos, Aristeidis, and Helen Miliou. 2025. "Lipid and Fatty Acid Composition of Low-Value Mediterranean Fish in Winter and Spring for Discard Valorization" Fishes 10, no. 9: 454. https://doi.org/10.3390/fishes10090454
APA StyleTsopelakos, A., & Miliou, H. (2025). Lipid and Fatty Acid Composition of Low-Value Mediterranean Fish in Winter and Spring for Discard Valorization. Fishes, 10(9), 454. https://doi.org/10.3390/fishes10090454








