Preliminary Assessment of Age and Growth of the Red Swamp Crayfish Procambarus clarkii [Girard, 1852] in the River Nile in Egypt by Direct and Indirect Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Laboratory Experiment
2.1.1. Carapace Length Frequency Analysis
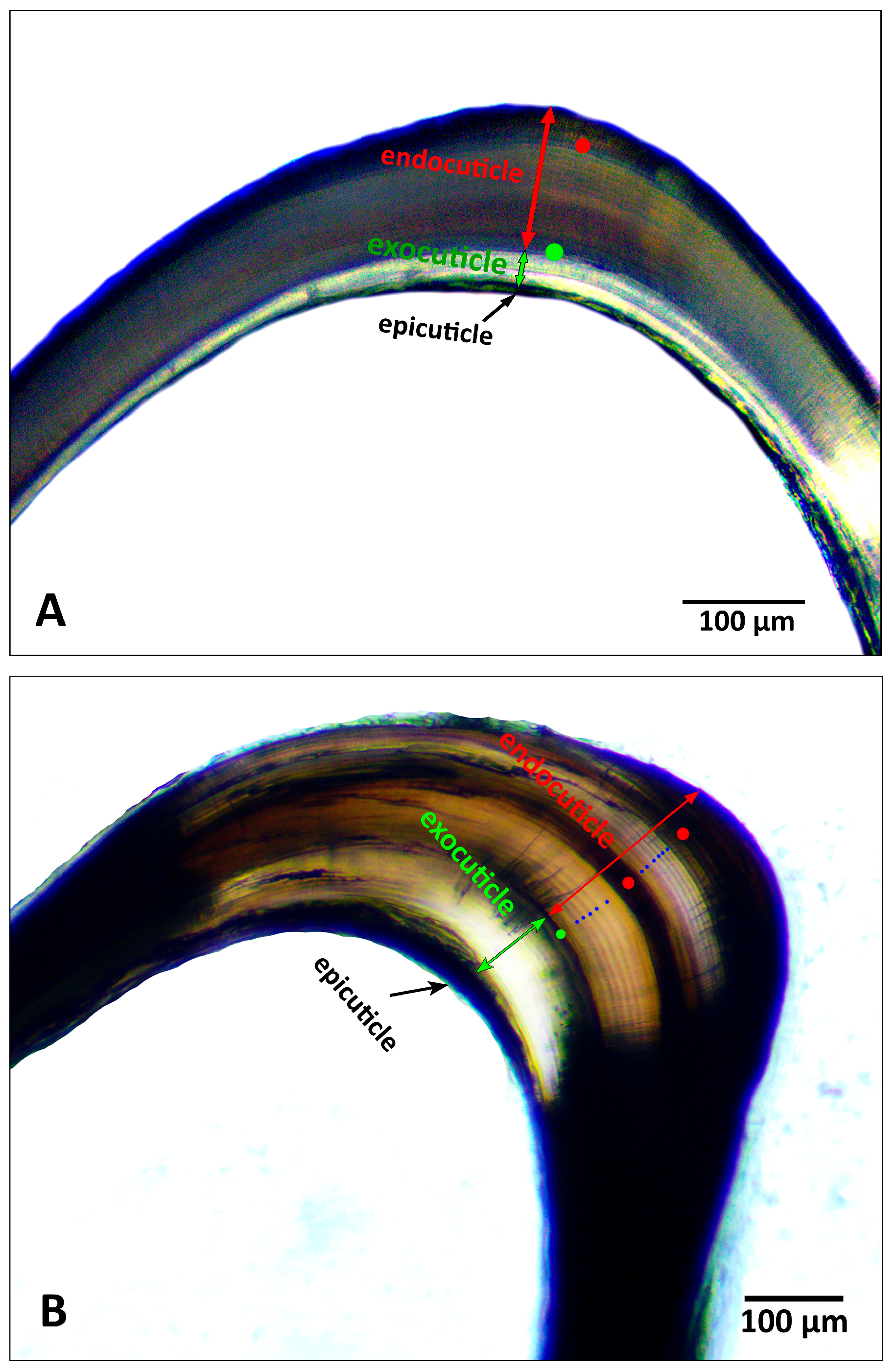
2.1.2. Age Determination via Gastric Mill Ossicles Processing
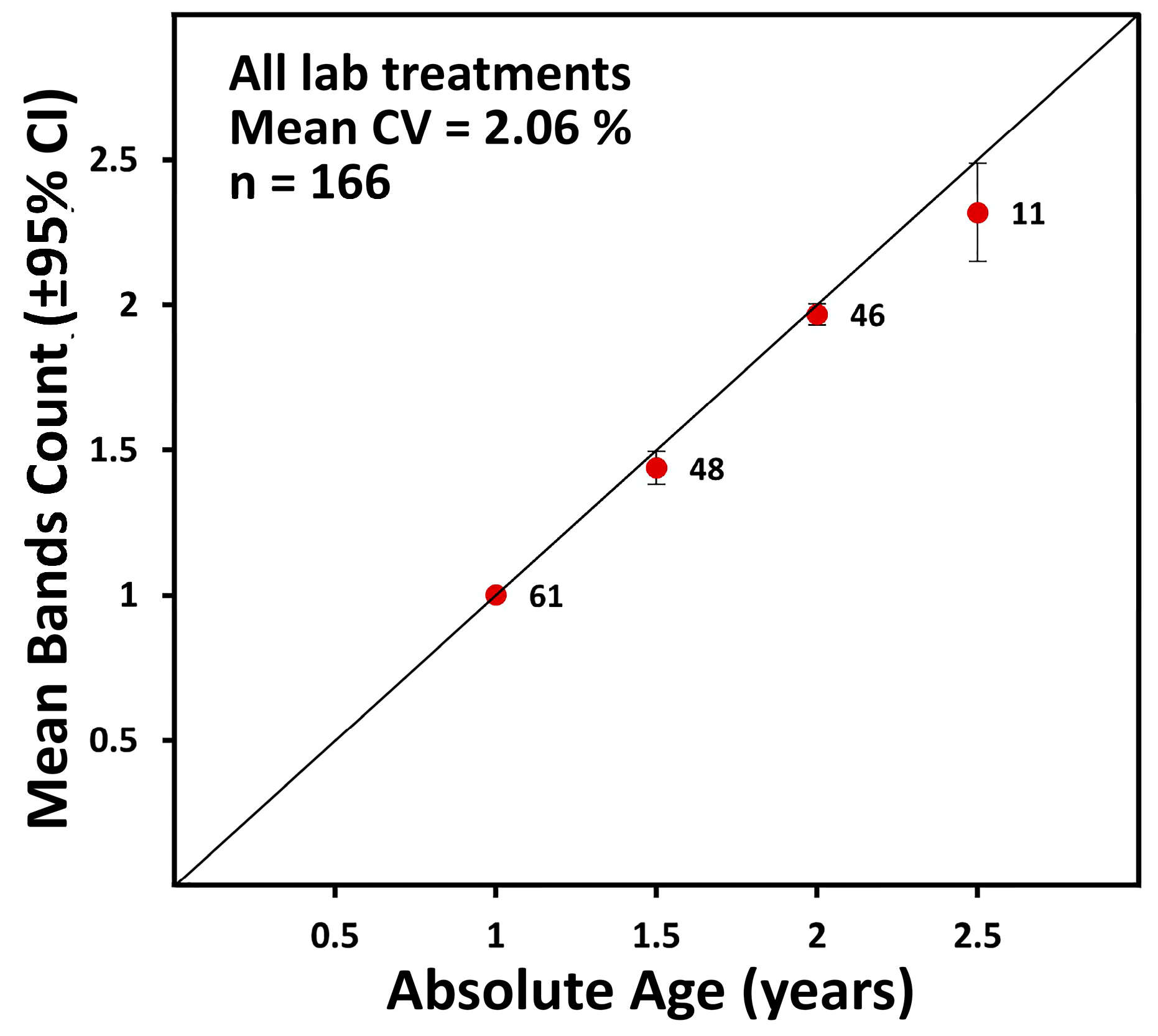
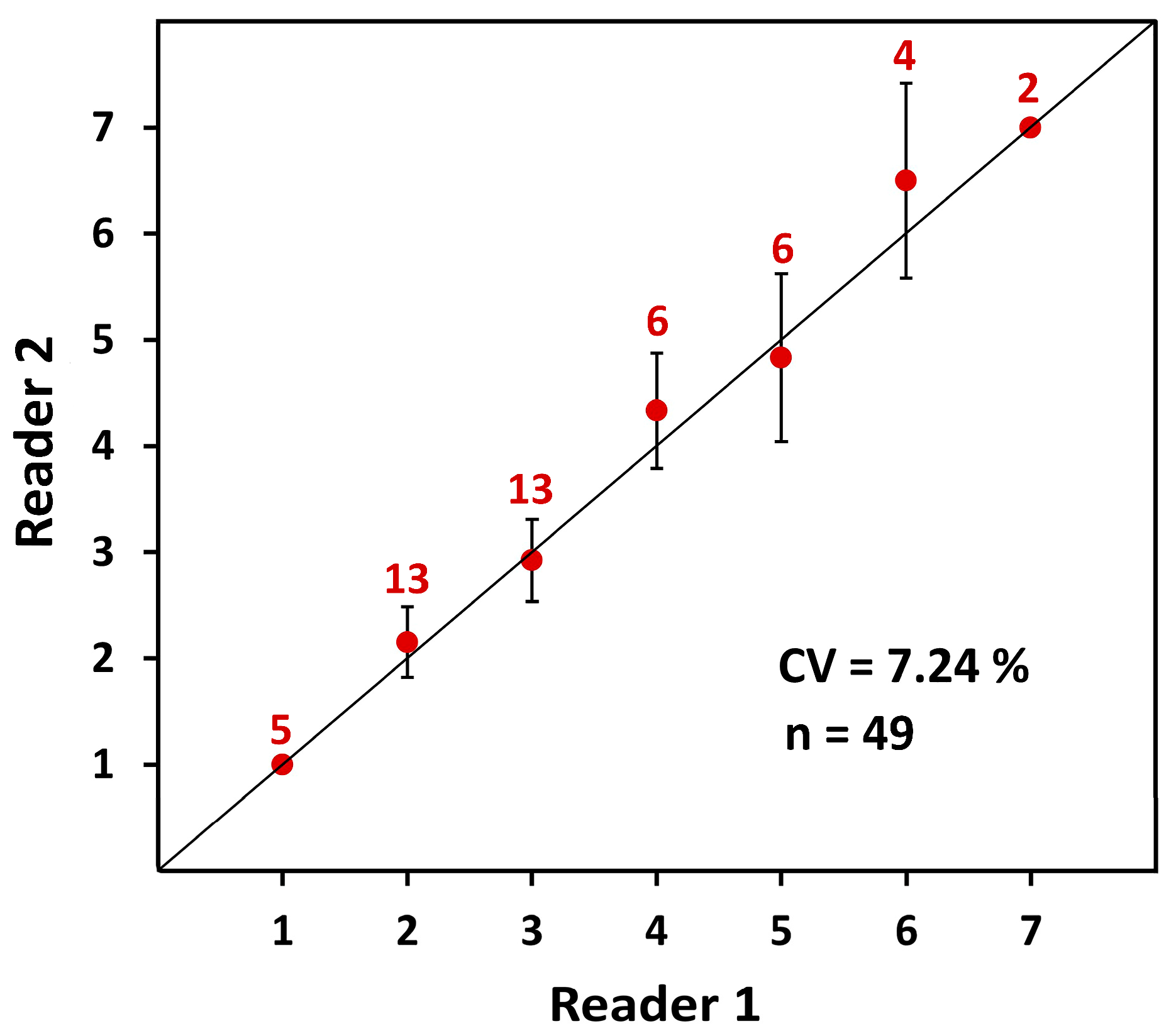
2.1.3. Accuracy (Age Validation) and Precision
2.1.4. Estimation of Von Bertalanffy Growth Curves
2.2. Field Work
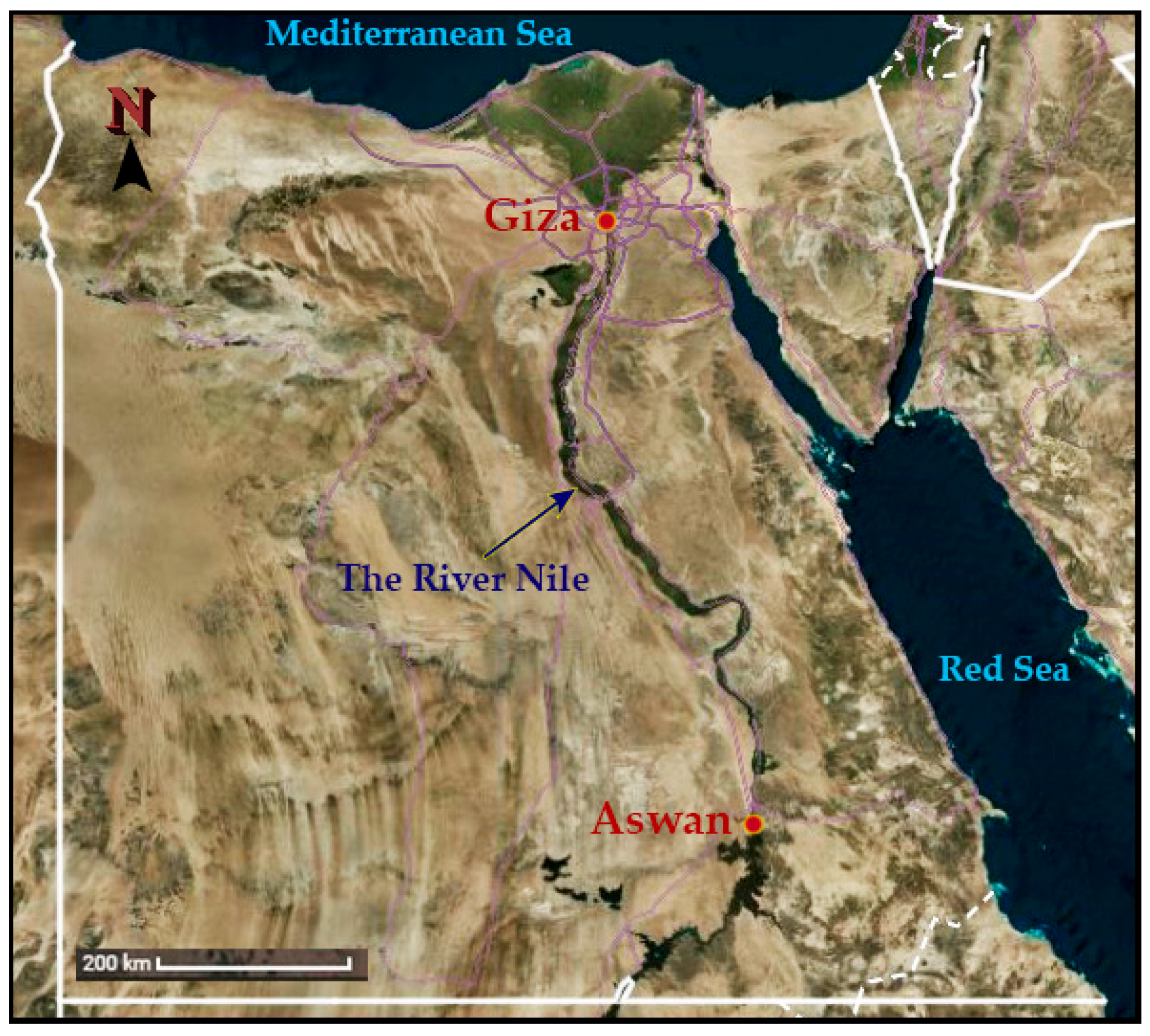
2.2.1. Sites of Study
2.2.2. Sampling
2.2.3. Age Determination
Direct Method
Indirect Method
2.2.4. Size and Age–at–Sexual Maturity
3. Results
3.1. Laboratory Experiment
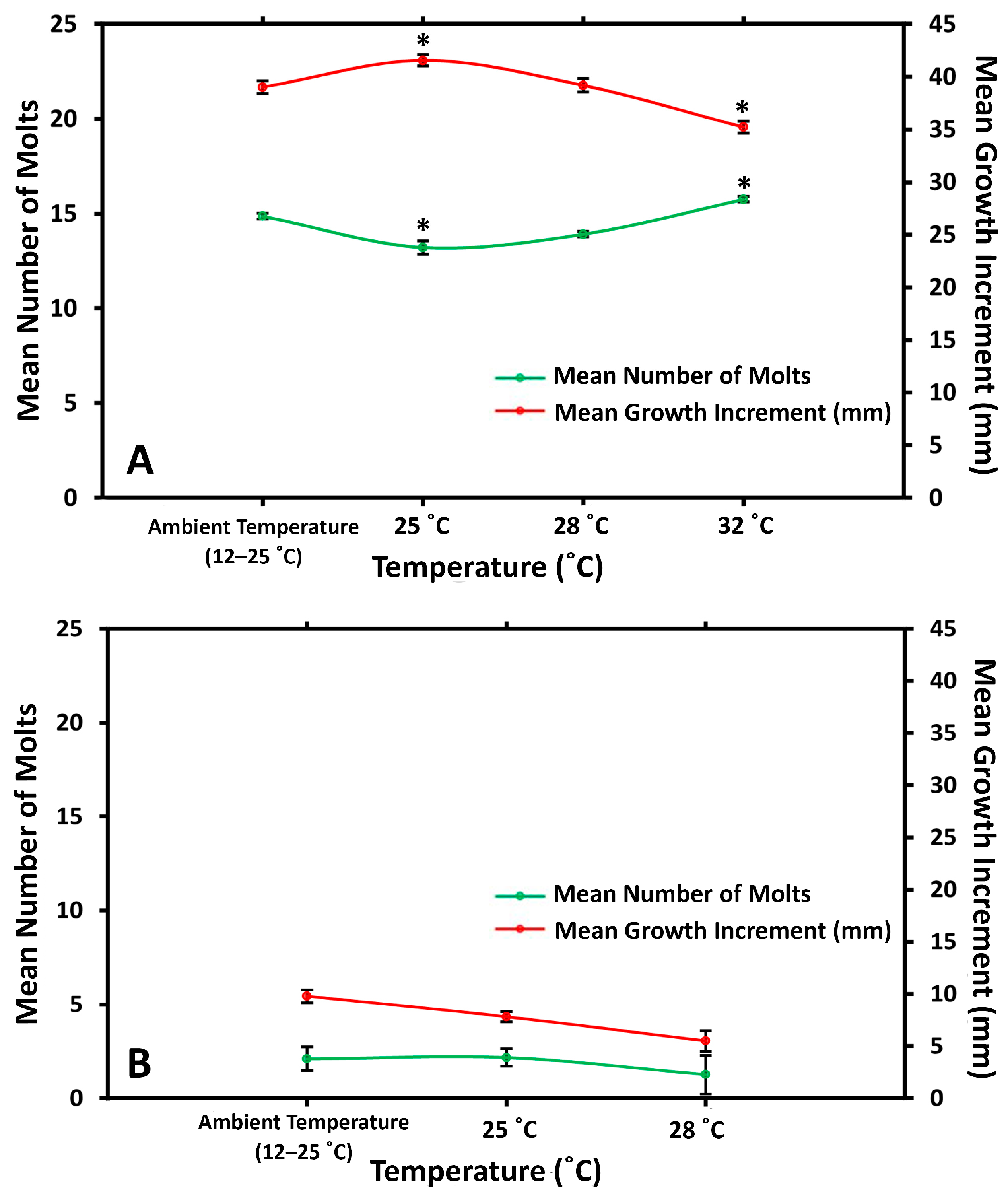
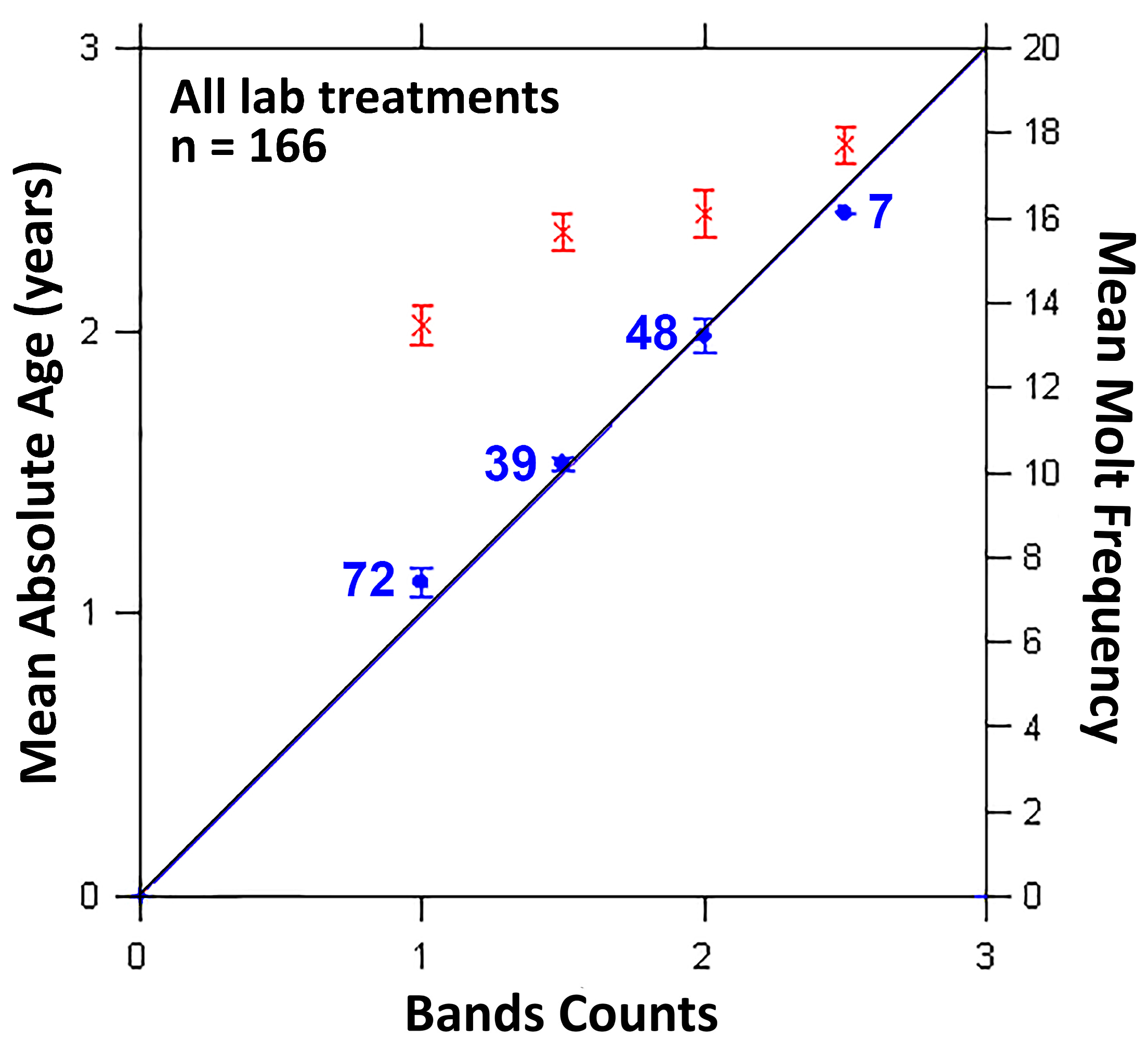
3.1.1. Molt Frequency
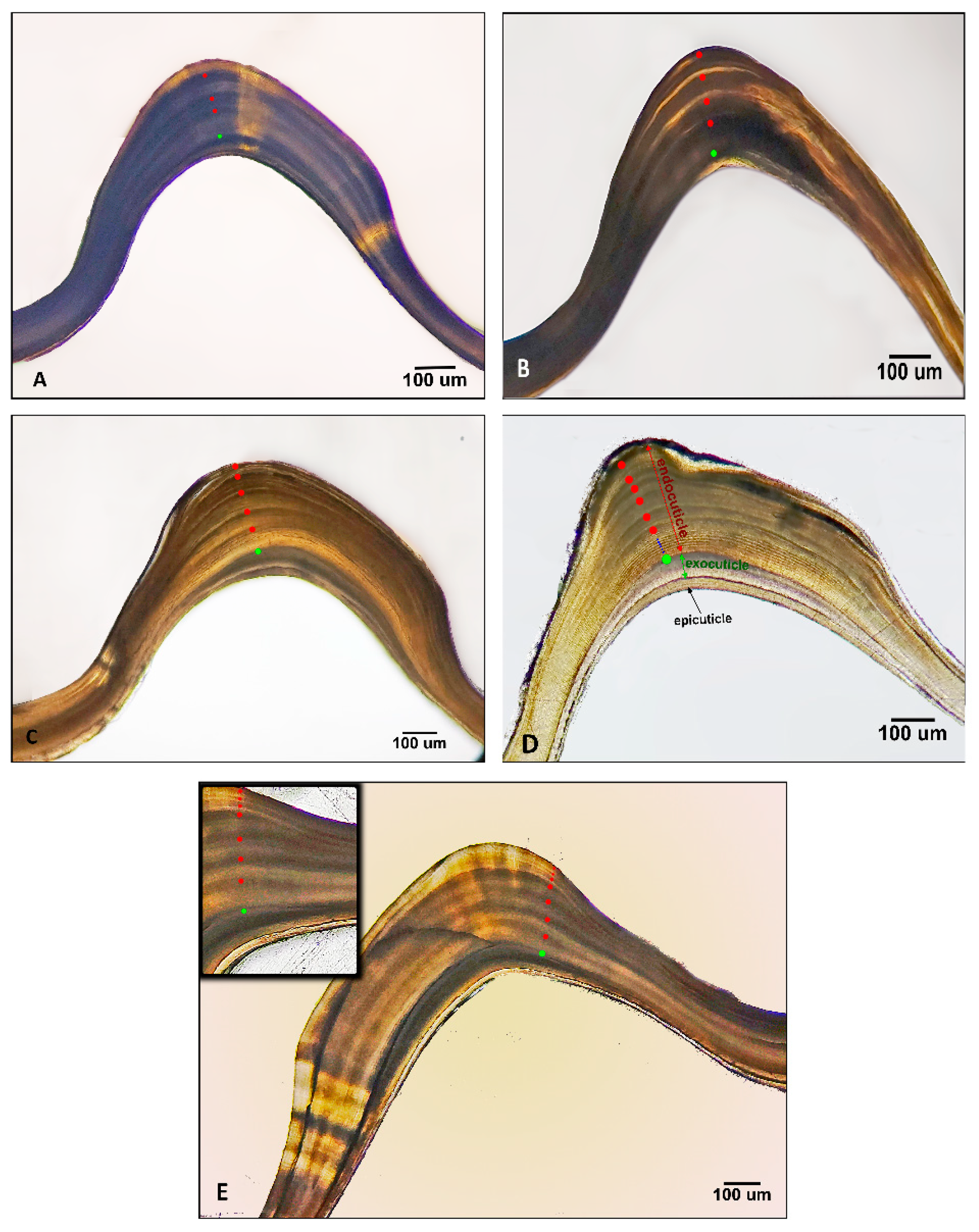
3.1.2. Gastric Mill Processing and Growth Bands Validation
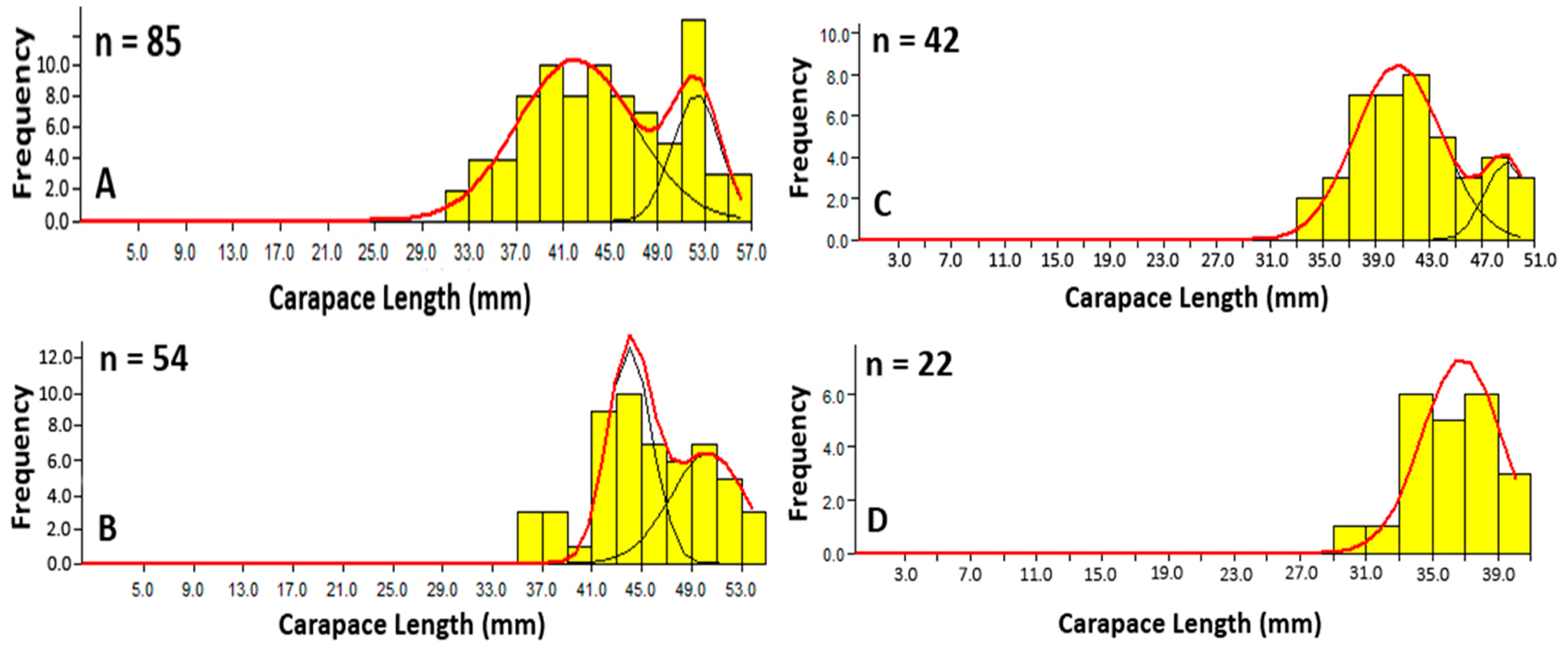
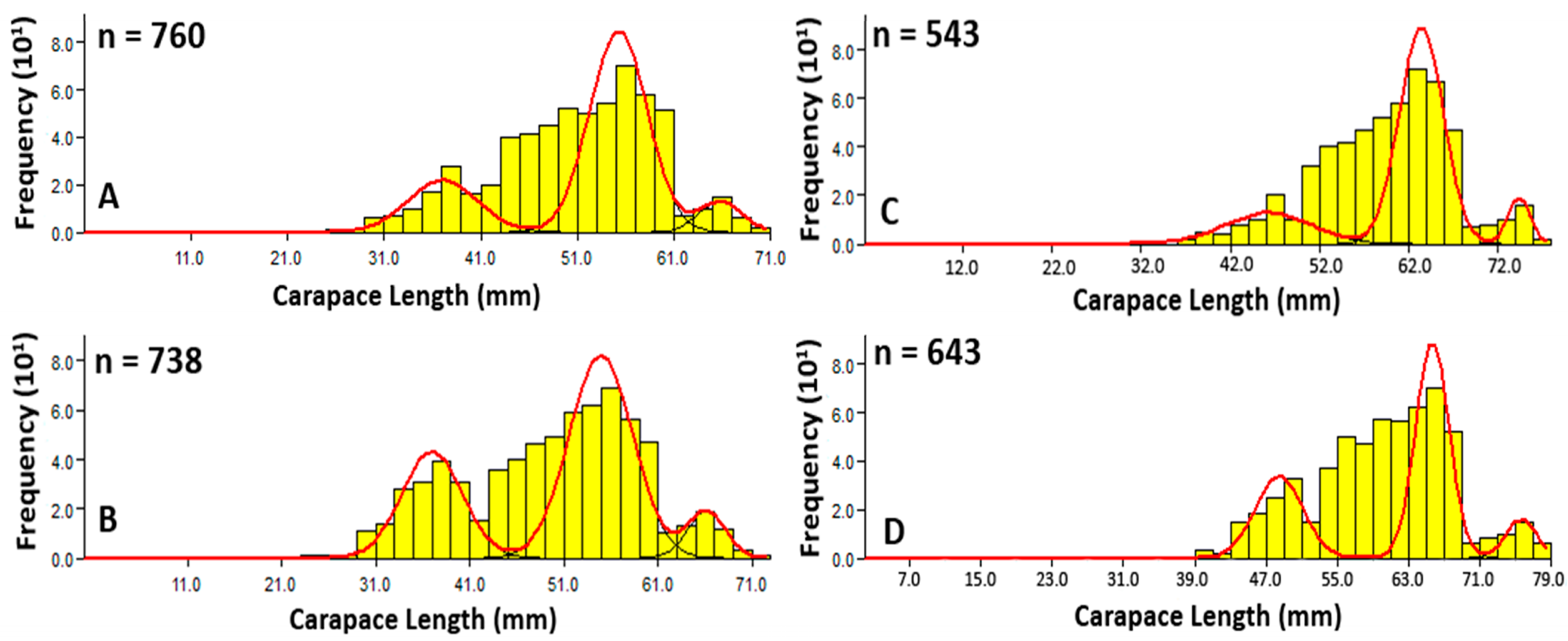
3.1.3. Carapace Length Frequency Analysis
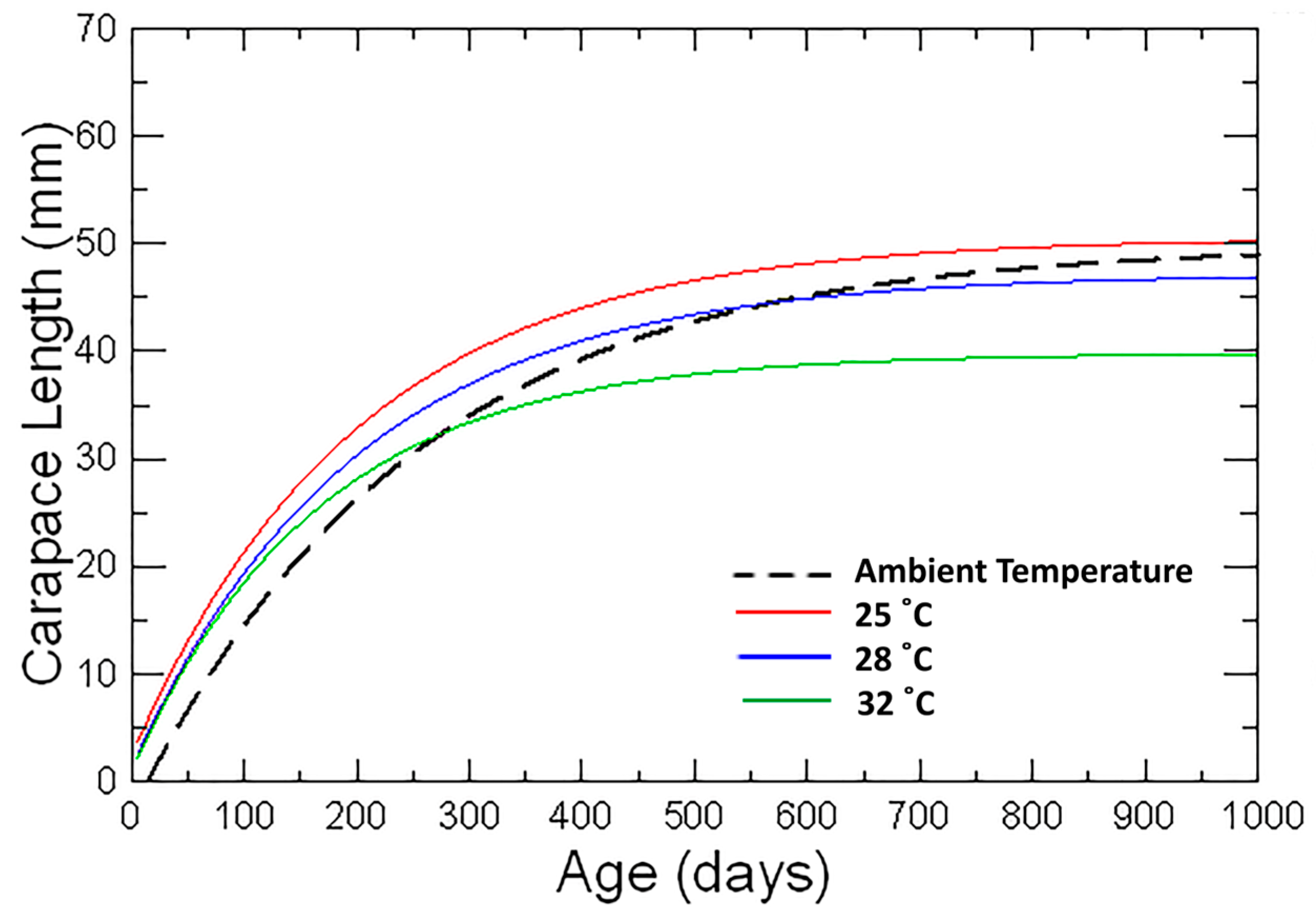
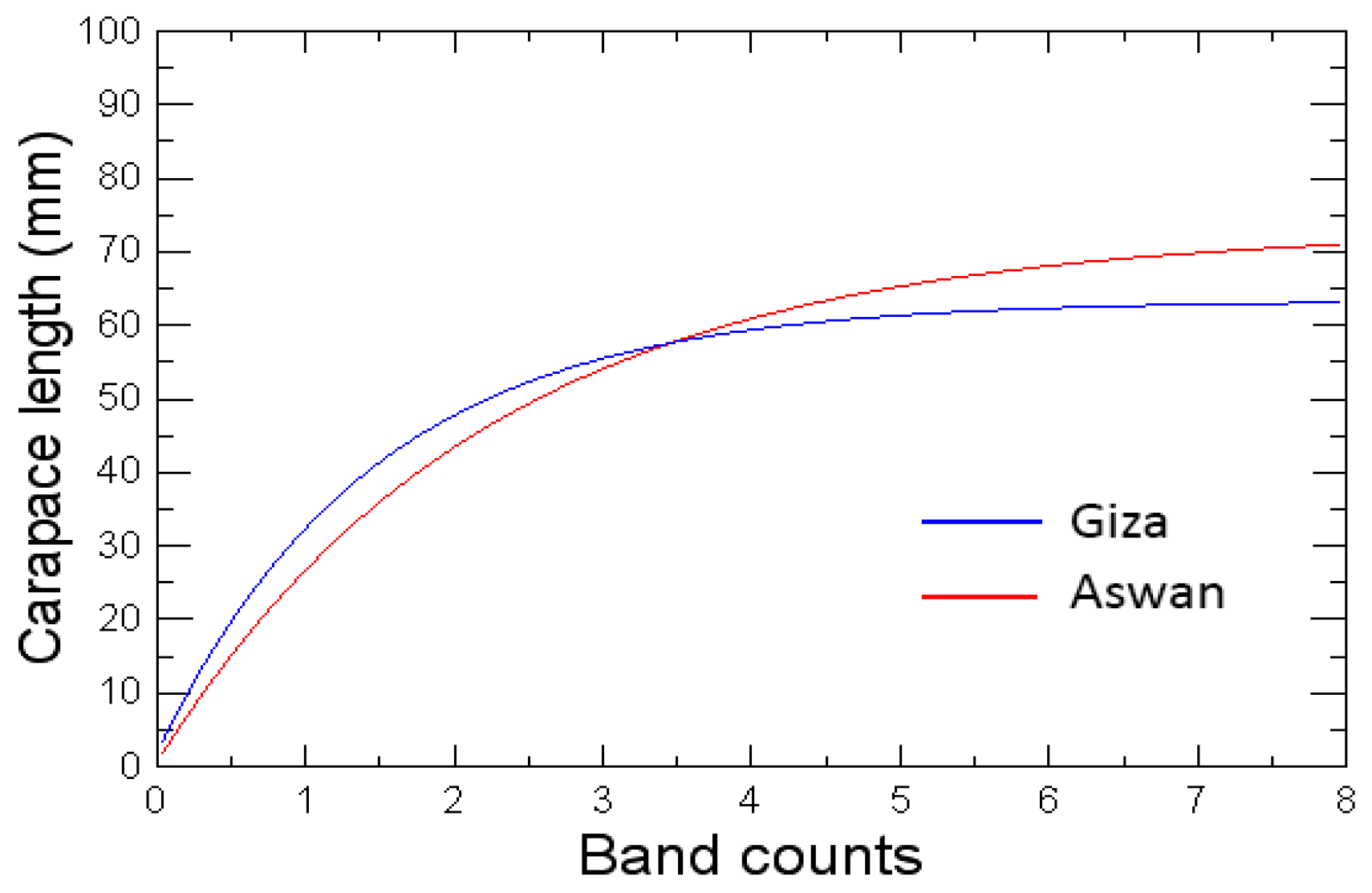
3.1.4. Estimation of Von Bertalanffy Growth Curves
3.2. Field Work
3.2.1. Age Determination
Direct Method
Indirect Method
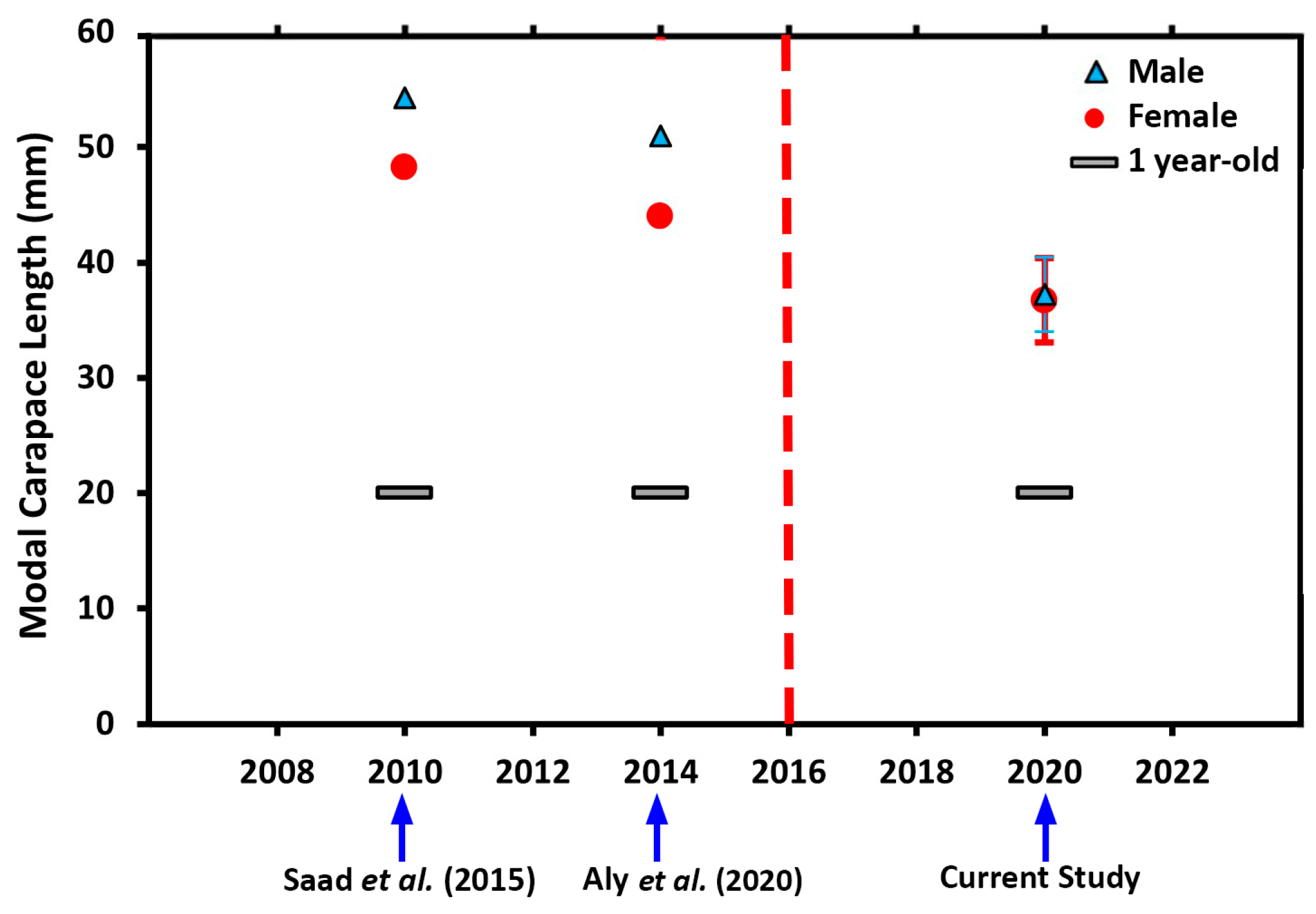
3.2.2. Size and Age–at–Sexual Maturity
4. Discussion
4.1. Direct Age Determination and Validation
4.1.1. Direct Age Determination
4.1.2. Age Validation
4.2. Indirect Age Determination
4.3. Growth Rate in Relation to Temperature
4.4. Size and Age at Sexual Maturity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Capinha, C.; Anastácio, P. Assessing the Environmental Requirements of Invaders Using Ensembles of Distribution Models: Environmental Requirements from Ensembles of Models. Divers. Distrib. 2011, 17, 13–24. [Google Scholar] [CrossRef]
- Lodge, D.M.; Taylor, C.A.; Holdich, D.M.; Skurdal, J. Nonindigenous Crayfishes Threaten North American Freshwater Biodiversity: Lessons from Europe. Fisheries 2000, 25, 7–20. [Google Scholar] [CrossRef]
- Lane, S.J.; Fujioka, M. The Impact of Changes in Irrigation Practices on the Distribution of Foraging Egrets and Herons (Ardeidae) in the Rice Fields of Central Japan. Biol. Conserv. 1998, 83, 221–230. [Google Scholar] [CrossRef]
- Galeotti, P.; Rubolini, D.; Fea, G.; Ghia, D.; Nardi, P.A.; Gherardi, F.; Fasola, M. Female Freshwater Crayfish Adjust Egg and Clutch Size in Relation to Multiple Male Traits. Proc. R. Soc. B Biol. Sci. 2006, 273, 1105–1110. [Google Scholar] [CrossRef]
- Loureiro, T.G.; Anastácio, P.M.; Bueno, S.L.S.; Araujo, P.B.; Souty-Grosset, C.; Almerão, M.P. Distribution, Introduction Pathway, and Invasion Risk Analysis of the North American Crayfish Procambarus clarkii (Decapoda: Cambaridae) in Southeast Brazil. J. Crustac. Biol. 2015, 35, 88–96. [Google Scholar] [CrossRef]
- Oficialdegui, F.J.; Clavero, M.; Sánchez, M.I.; Green, A.J.; Boyero, L.; Michot, T.C.; Klose, K.; Kawai, T.; Lejeusne, C. Unravelling the Global Invasion Routes of a Worldwide Invader, the Red Swamp Crayfish (Procambarus clarkii). Freshw. Biol. 2019, 64, 1382–1400. [Google Scholar] [CrossRef]
- Clark, W.H.; Ralston, G.L. First Record of Crayfish from Baja California, Mexico (Decapoda, Astacidea). Crustaceana 1976, 30, 106–107. [Google Scholar] [CrossRef]
- Azofeifa-Solano, J.C.; Villalobos-Rojas, F.; Romero-Chaves, R.; Wehrtmann, I.S. Modeling the Habitat Suitability of Two Exotic Freshwater Crayfishes in Mesoamerica and the Caribbean: Cherax quadricarinatus (von Martens, 1868) and Procambarus clarkii Girard, 1852 (Decapoda: Astacidea: Parastacidae, Cambaridae). J. Crustac. Biol. 2023, 43, ruad059. [Google Scholar] [CrossRef]
- Gutiérrez-Yurrita, P.J.; Montes, C. Bioenergetics of Juveniles of Red Swamp Crayfish (Procambarus clarkii). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001, 130, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Scalici, M.; Chiesa, S.; Scuderi, S.; Celauro, D.; Gibertini, G. Population Structure and Dynamics of Procambarus clarkii (Girard, 1852) in a Mediterranean Brackish Wetland (Central Italy). Biol. Invasions 2010, 12, 1415–1425. [Google Scholar] [CrossRef]
- Matsuzaki, S.S.; Terui, A.; Kodama, K.; Tada, M.; Yoshida, T.; Washitani, I. Influence of Connectivity, Habitat Quality and Invasive Species on Egg and Larval Distributions and Local Abundance of Crucian Carp in Japanese Agricultural Landscapes. Biol. Conserv. 2011, 144, 2081–2087. [Google Scholar] [CrossRef]
- Putra, M.D.; Bláha, M.; Wardiatno, Y.; Krisanti, M.; Yonvitner; Jerikho, R.; Kamal, M.M.; Mojžišová, M.; Bystřický, P.K.; Kouba, A.; et al. Procambarus clarkii (Girard, 1852) and Crayfish Plague as New Threats for Biodiversity in Indonesia. Aquat. Conserv. Mar. Freshw. Ecosyst. 2018, 28, 1434–1440. [Google Scholar] [CrossRef]
- Ibrahim, A.; Khalil, M. The Red Swamp Crayfish in Egypt, 1st ed.; the Center of Research and Studies of Protectorates, Ain Shams University: Cairo, Egypt, 2009. [Google Scholar]
- Yahkoub, Y.B.; Fekhaoui, M.; El Qoraychy, I.; Yahyaoui, A. Current State of Knowledge on Louisiana Crawfish (Procambarus clarkii Girard, 1852) in Morocco. Aquac. Aquar. Conserv. Legis. 2019, 12, 618–628. [Google Scholar]
- Geiger, W.; Alcorlo, P.; Baltanás, A.; Montes, C. Impact of an Introduced Crustacean on the Trophic Webs of Mediterranean Wetlands. Biol. Invasions 2005, 7, 49–73. [Google Scholar] [CrossRef]
- Cruz, M.J.; Rebelo, R. Colonization of Freshwater Habitats by an Introduced Crayfish, Procambarus clarkii, in Southwest Iberian Peninsula. Hydrobiologia 2007, 575, 191–201. [Google Scholar] [CrossRef]
- Chucholl, C. Population Ecology of an Alien “Warm Water” Crayfish (Procambarus clarkii) in a New Cold Habitat. Knowl. Manag. Aquat. Ecosyst. 2011, 29, 401. [Google Scholar] [CrossRef]
- Mazza, G.; Tricarico, E.; Genovesi, P.; Gherardi, F. Biological Invaders Are Threats to Human Health: An Overview. Ethol. Ecol. Evol. 2014, 26, 112–129. [Google Scholar] [CrossRef]
- Lemmers, P.; Van Der Kroon, R.; Van Kleef, H.H.; Verhees, J.J.F.; Van Der Velde, G.; Leuven, R.S.E.W. Limiting Burrowing Activity and Overland Dispersal of the Invasive Alien Red Swamp Crayfish Procambarus clarkii by Sophisticated Design of Watercourses. Ecol. Eng. 2022, 185, 106787. [Google Scholar] [CrossRef]
- Haubrock, P.J.; Inghilesi, A.F.; Mazza, G.; Bendoni, M.; Solari, L.; Tricarico, E. Burrowing Activity of Procambarus clarkii on Levees: Analysing Behaviour and Burrow Structure. Wetl. Ecol. Manag. 2019, 27, 497–511. [Google Scholar] [CrossRef]
- Ibrahim, A.M.; Khalil, M.T.; Mubarak, F. On the Feeding Behavior of the Exotic Crayfish Procambarus clarkii in Egypt and Its Prospects in the Biocontrol of Local Vector Snails. J. Union Arab. Biol. Cairo 1995, 4, 321–340. [Google Scholar]
- Tolba, R. The Red Swamp Crayfish Procambarus clarkit (Decapoda; Cambaridae) as Bio-Indicator for Otal Water Quality Including Cu and Cd Pollution. Egypt. J. Aquat. Biol. Fish. 1999, 3, 59–71. [Google Scholar] [CrossRef]
- Ibrahim, A.; Khalil, M.; Mubarak, M. Ecological Studies on the Exotic Crayfishes Procambarus clarkii (Girard 1852) and P. Zoangulus Hobbs & Hobbs 1990, in the River Nile Egypt. J.-Egypt. Ger. Soc. Zool. 1996, 20, 167–186. [Google Scholar]
- Ibrahim, A.; Khalil, M.; Mubark, M. Ecological Studies on the Exotic Crayfishes Procambarus clarkii and Procambarus Zonangulus in the River Nile. Int. J. Ecol. Env. Sci. 1997, 23, 217–228. [Google Scholar]
- Soliman, G.; El-Assal, F.; El-Deen, M.; Hamdi, S. The Reproductive Biology of the Red Swamp Crayfish P. clarkii (Girard, 1852) (Decapoda: Cambridae) in the River Nile, Egypt. Egypt. J. Zool. 1998, 30, 311–325. [Google Scholar]
- Soliman, G.; El-Assal, F.; El-Deen, M.S.; Hamdy, S. Habitat, Distribution and Behaviour of the Red Swamp Crawfish Procambarus clarkii (Girard, 1852) (Decapoda: Combaridae) in the River Nile, Egypt. Egypt. J. Zool. 1998, 30, 297–310. [Google Scholar]
- Hamdi, S. Studies on the Red Swamp Crayfish Procambarus clarkii (Girard, 1852) (Decapoda: Camrbaridae) in the River Nile. Master’s Thesis, Cairo University, Giza, Egypt, 1994. [Google Scholar]
- Kilada, R.W.; Roddick, D.; Mombourquette, K. Age Determination, Validation, Growth and Minimum Size of Sexual Maturity of the Greenland smoothcockle (Serripes groenlandicus, Bruguiere, 1789) in Eastern Canada. J. Shellfish Res. 2007, 26, 443–450. [Google Scholar] [CrossRef]
- Campana, S.E. Accuracy, Precision and Quality Control in Age Determination, Including a Review of the Use and Abuse of Age Validation Methods. J. Fish Biol. 2001, 59, 197–242. [Google Scholar] [CrossRef]
- Campana, S.E.; Marks, L.; Joyce, W.; Kohler, N.E. Effects of Recreational and Commercial Fishing on Blue Sharks (Prionace glauca) in Atlantic Canada, with Inferences on the North Atlantic Population. Can. J. Fish. Aquat. Sci. 2006, 63, 670–682. [Google Scholar] [CrossRef]
- Hartnoll, R.G.; Paul, R.G.K. The Embryonic Development of Attached and Isolated Eggs of Carcinus maenas. Int. J. Invertebr. Reprod. 1982, 5, 247–252. [Google Scholar] [CrossRef]
- Hartnoll, R.G. Growth in Crustacea—Twenty Years On. In Advances in Decapod Crustacean Research; Paula, J.P.M., Flores, A.A.V., Fransen, C.H.J.M., Eds.; Springer: Dordrecht, The Netherlands, 2001; pp. 111–122. ISBN 978-90-481-5688-7. [Google Scholar]
- Kilada, R.; Driscoll, J.G. Age Determination in Crustaceans: A Review. Hydrobiologia 2017, 799, 21–36. [Google Scholar] [CrossRef]
- Sheehy, M.R.J. Potential of Morphological Lipofuscin Age-Pigment as an Index of Crustacean Age. Mar. Biol. 1990, 107, 439–442. [Google Scholar] [CrossRef]
- Kilada, R.; Sainte-Marie, B.; Rochette, R.; Davis, N.; Vanier, C.; Campana, S. Direct Determination of Age in Shrimps, Crabs, and Lobsters. Can. J. Fish. Aquat. Sci. 2012, 69, 1728–1733. [Google Scholar] [CrossRef]
- Leland, J.C.; Coughran, J.; Bucher, D.J. A Preliminary Investigation into the Potential Value of Gastric Mills for Ageing Crustaceans. In New Frontiers in Crustacean Biology; Brill: Leiden, The Netherlands, 2011; pp. 57–68. ISBN 90-474-2771-8. [Google Scholar]
- Gnanalingam, G.; Butler, M.J.; Matthews, T.R.; Hutchinson, E.; Kilada, R. Directly Ageing the Caribbean Spiny Lobster, Panulirus Argus with Validated Band Counts from Gastric Mill Ossicles. ICES J. Mar. Sci. 2019, 76, 442–451. [Google Scholar] [CrossRef]
- Mullowney, D.; O’Connell, N.; Kilada, R.; Rochette, R. Refining Age at Legal-Size Estimation in the Newfoundland & Labrador Populations of the Snow Crab Chionoecetes opilio (Fabricius, 1788) (Decapoda: Brachyura: Oregoniidae). J. Crustac. Biol. 2023, 43, ruad067. [Google Scholar] [CrossRef]
- Huntsberger, C.J.; Kilada, R.; Chen, Y.; Wahle, R.A. Integrating Different Aging Methods to Model the Dynamics of Hard-to-Age Crab Growth: Age at Size Estimates for the Jonah crab (Cancer Borealis). Fish. Res. 2024, 276, 107061. [Google Scholar] [CrossRef]
- Vatcher, H.E.; Roer, R.D.; Dillaman, R.M. Structure, Molting, and Mineralization of the Dorsal Ossicle Complex in the Gastric Mill of the Blue Crab, C Allinectes sapidus: Structure of blue crab ossicle and tooth. J. Morphol. 2015, 276, 1358–1367. [Google Scholar] [CrossRef] [PubMed]
- Kilada, R.; Acuña, E. Direct Age Determination by Growth Band Counts of Three Commercially Important Crustacean Species in Chile. Fish. Res. 2015, 170, 134–143. [Google Scholar] [CrossRef]
- Mouser, J.B.; Glover, J.; Brewer, S.K. Gastric Mill Age Estimates for Ringed Crayfish Faxonius neglectus neglectus (Faxon) and the Influence of Temperature on Band Formation. Freshw. Crayfish 2020, 25, 59–67. [Google Scholar] [CrossRef]
- Huntsberger, C.J.; Kilada, R.; Ambrose, W.G.; Wahle, R.A. Age-at-Size Relationships of the American Lobster (Homarus americanus) from Three Contrasting Thermal Regimes Using Gastric Mill Band Counts as a Direct Aging Technique. Can. J. Fish. Aquat. Sci. 2020, 77, 1733–1740. [Google Scholar] [CrossRef]
- Sheridan, M.; O’Connor, I.; Henderson, A.C. Investigating the Effect of Molting on Gastric Mill Structure in Norway Lobster (Nephrops norvegicus) and Its Potential as a Direct Ageing Tool. J. Exp. Mar. Biol. Ecol. 2016, 484, 16–22. [Google Scholar] [CrossRef]
- Becker, C.; Dick, J.T.A.; Cunningham, E.M.; Schmitt, C.; Sigwart, J.D. The Crustacean Cuticle Does Not Record Chronological Age: New Evidence from the Gastric Mill Ossicles. Arthropod Struct. Dev. 2018, 47, 498–512. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, M.; O’Connor, I. Evidence of Complete Gastric Mill Ossicle Loss at Ecdysis in the European Green Crab Carcinus maenas (Linnaeus, 1758) (Decapoda: Brachyura: Carcinidae). J. Crustac. Biol. 2018, 38, 435–442. [Google Scholar] [CrossRef]
- Sheridan, M.; Durán, J.; Gil, M.D.M.; Pastor, E.; O’Connor, I. Can Crustacean Cuticle Preserve a Record of Chronological Age? Investigating the Utility of Decapod Crustacean Eyestalks for Age Determination of Two European Spider Crab Species. Fish. Res. 2020, 224, 105467. [Google Scholar] [CrossRef]
- He, N.; Ruan, G.; Liu, Y. Effects of Sustained High-Temperature Stress on Growth, Performance, Digestive Enzyme Activity and Immune Indexes of Crayfish, Procambarus clarkii. Acta Hydrobiol. Sin. 2022, 46, 395–402. [Google Scholar]
- Romaire, R.P.; Lutz, C.G. Population Dynamics of Procambarus clarkii (Girard) and Procambarus acutus acutus (Girard) (Decapoda: Cambaridae) in Commercial Ponds. Aquaculture 1989, 81, 253–274. [Google Scholar] [CrossRef]
- Taylor, R.C. Crayfish (Procambarus spiculifer) Growth Rate and Final Thermal Preferendum. J. Therm. Biol. 1990, 15, 79–81. [Google Scholar] [CrossRef]
- Huner, J.V.; Barr, J.E. Red Swamp Crawfish: Biology and Exploitation, 3rd ed.; Louisiana Sea Grant College Program, Louisiana State University: Baton Rouge, LA, USA, 1991. [Google Scholar]
- Aquiloni, L.; Gherardi, F. Evidence of Female Cryptic Choice in Crayfish. Biol. Lett. 2008, 4, 163–165. [Google Scholar] [CrossRef]
- Anastacio, P.M.; Parente, V.S.; Correia, A.M. Crayfish Effects on Seeds and Seedlings: Identification and Quantification of Damage. Freshw. Biol. 2005, 50, 697–704. [Google Scholar] [CrossRef]
- Badr, A. Fulfilling the Potentials of Residential Solar Energy in Egypt. Build. Eng. 2024, 2, 1510. [Google Scholar] [CrossRef]
- Oliveira, J.; Fabião, A. Growth Responses of Juvenile Red Swamp Crayfish, Procambarus clarkii Girard, to Several Diets under Controlled Conditions. Aquac. Res. 1998, 29, 123–129. [Google Scholar] [CrossRef]
- Jover, M.; Fernández-Carmona, J.; Del Río, M.C.; Soler, M. Effect of Feeding Cooked-Extruded Diets, Containing Different Levels of Protein, Lipid and Carbohydrate on Growth of Red Swamp Crayfish (Procambarus clarkii). Aquaculture 1999, 178, 127–137. [Google Scholar] [CrossRef]
- Gutiérrez-Yurrita, P.J.; Del Olmo, C.M. Population Dynamics of Juveniles of Red Swamp Crayfish (Procambarus clarkii) under Controlled Conditions. Freshw Crayfish 2004, 14, 180–189. [Google Scholar]
- Leland, J.C.; Bucher, D.J.; Coughran, J. Direct Age Determination of a Subtropical Freshwater Crayfish (Redclaw, Cherax quadricarinatus) Using Ossicular Growth Marks. PLoS ONE 2015, 10, e0134966. [Google Scholar] [CrossRef] [PubMed]
- Kilada, R.; Ibrahim, N.K. Preliminary Investigation of Direct Age Determination Using Band Counts in the Gastric Mill of the Blue Swimmer Crab (Portunus PelagicusLinnaeus, 1758) in Two Salt-Water Lakes in the Eastern Mediterranean. J. Crustac. Biol. 2016, 36, 119–128. [Google Scholar] [CrossRef]
- Rebert, A.L.; Kruse, G.H.; Webb, J.B.; Tamone, S.L.; Oxman, D.; McNeel, K.W. Evaluation of a Direct Age Estimation Method for Terminally Molted Male Snow Crab Chionoecetes opilio (Fabricius 1788) (Decapoda: Brachyura: Oregoniidae). J. Crustac. Biol. 2020, 40, 549–555. [Google Scholar] [CrossRef]
- Chakraborty, R.D.; Gayathri, A.P.; Purushothaman, P.; Kuberan, G.; Maheswarudu, G.; Abdussamad, E.M. Preliminary Investigation of Age Analysis in Crustacean Species from the Indian Coast, Using Growth Bands. Crustaceana 2022, 95, 605–614. [Google Scholar] [CrossRef]
- Nagy, A.; El-Zeiny, A.; Elshaier, M.; Sowilem, M.; Atwa, W. Water Quality Assessment of Mosquito Breeding Water Localities in the Nile Valley of Giza Governorate. J. Environ. Sci. Mansoura Univ. 2021, 50, 1–10. [Google Scholar] [CrossRef]
- Ashour, M.A.; El Degwee, Y.A.; Hashem, R.H.; Abdou, A.A.; Abu-Zaid, T.S. The Extent to Which the Available Water Resources in Upper Egypt Can Be Affected by Climate Change. Limnol. Rev. 2024, 24, 164–177. [Google Scholar] [CrossRef]
- Taketomi, Y.; Nishikawa, S.; Koga, S. Testis and Androgenic Gland During Development of External Sexual Characteristics of the Crayfish Procambarus clarkii. J. Crustac. Biol. 1996, 16, 24–34. [Google Scholar] [CrossRef]
- Ando, H.; Makioka, T. Structure of the Ovary and Mode of Oogenesis in a Freshwater Crayfish, Procambarus clarkii (Girard). Zoolog. Sci. 1998, 15, 893–901. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhao, W.; Tang, Z.; Huang, L.; Zhu, X.; Liang, X.; Yan, A.; Lu, Z.; Yu, Y.; Tang, D.; et al. Comparative Transcriptomic Analysis of the Different Developmental Stages of Ovary in Red Swamp Crayfish Procambarus clarkii. BMC Genom. 2021, 22, 199. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.E.-H.; Hassan, M. Anatomical and Histological Studies on the Male Reproductive System of the Red Swamp Crayfish Procambarus clarkii. Egypt. J. Aquat. Biol. Fish. 2010, 14, 87–100. [Google Scholar] [CrossRef]
- Hutchinson, C.E.; TenBrink, T.T. Age Determination of the Yellow Irish Lord: Management Implications as a Result of New Estimates of Maximum Age. N. Am. J. Fish. Manag. 2011, 31, 1116–1122. [Google Scholar] [CrossRef]
- Kilada, R.; Agnalt, A.-L.; Hammeken Arboe, N.; Bjarnason, S.; Burmeister, A.; Farestveit, E.; Gíslason, Ó.S.; Guðlaugsdóttir, A.; Guðmundsdóttir, D.; Jónasson, J.P.; et al. Feasibility of Using Growth Band Counts in Age Determination of Four Crustacean Species in the Northern Atlantic. J. Crustac. Biol. 2015, 35, 499–503. [Google Scholar] [CrossRef]
- Kilada, R.; Webb, J.B.; McNeel, K.W.; Slater, L.M.; Smith, Q.; Ferguson, J. Preliminary Assessment of a Direct Age Determination Method for 3 Commercially Important Crustaceans from Alaska. Fish. Bull. 2016, 115, 42–49. [Google Scholar] [CrossRef]
- Hartnoll, R. Strategies of Crustacean Growth. Mem. Aust. Mus. 1983, 18, 121–131. [Google Scholar] [CrossRef]
- Gong, J.; Yu, K.; Shu, L.; Ye, H.; Li, S.; Zeng, C. Evaluating the Effects of Temperature, Salinity, Starvation and Autotomy on Molting Success, Molting Interval and Expression of Ecdysone Receptor in Early Juvenile Mud Crabs, Scylla Paramamosain. J. Exp. Mar. Biol. Ecol. 2015, 464, 11–17. [Google Scholar] [CrossRef]
- Gencer, Ö. Survival and Moulting Rates of Larvae of Callinectes Sapidus Rathbun, 1896 under Different Environmental Circumstances (Brachyura, Portunidae). Crustaceana 2023, 96, 1135–1150. [Google Scholar] [CrossRef]
- Hutchinson, E.; Matthews, T.R.; Ross, E.; Hagedorn, S.; Butler, M.J. Gastric Mill Ossicles Record Chronological Age in the Caribbean Spiny Lobster (Panulirus argus). Fish. Res. 2024, 277, 107083. [Google Scholar] [CrossRef]
- Price, J.O.; Payne, J.F. Size, Age, and Population Dynamics in an R-Selected Population of Orconectes Neglectus Chaenodactylus Williams (Decapoda, Cambaridae). Crustaceana 1984, 46, 29–38. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, J.; Rui, J.; Ni, Z.; Shu, C. Growth Marks in Eyestalks of Portunus Trituberculatus: A Development of Technique and Evidence of Molting. Acta Oceanol. Sin. 2021, 40, 84–91. [Google Scholar] [CrossRef]
- Kilada, R.; Reiss, C.S.; Kawaguchi, S.; King, R.A.; Matsuda, T.; Ichii, T. Validation of Band Counts in Eyestalks for the Determination of Age of Antarctic Krill, Euphausia Superba. PLoS ONE 2017, 12, e0171773. [Google Scholar] [CrossRef] [PubMed]
- Fowler, A. Validation of Annual Growth Increments in the Otoliths of a Small, Tropical Coral Reef Fish. Mar. Ecol. Prog. Ser. 1990, 64, 25–38. [Google Scholar] [CrossRef]
- Amato, C.G.; Waugh, D.A.; Feldmann, R.M.; Schweitzer, C.E. Effect of Calcification on Cuticle Density in Decapods: A Key to Lifestyle. J. Crustac. Biol. 2008, 28, 587–595. [Google Scholar] [CrossRef]
- Saad, A.E.-H.; Mehanna, S.; Khalil, M.; Said, M. Population Dynamics of the Freshwater Crayfish Procambarus clarkii (Girard,1852) in the River Nile, Egypt. Egypt. J. Aquat. Biol. Fish. 2015, 19, 101–116. [Google Scholar] [CrossRef][Green Version]
- Chiesa, S.; Scalici, M.; Gibertin, G. Occurrence of Allochthonous Freshwater Crayfishes in Latium (Central Italy). Bull. Fr. Pêche Piscic. 2006, 380–381, 883–902. [Google Scholar] [CrossRef]
- Frutiger, A.; Borner, S.; Büsser, T.; Eggen, R.; Müller, R.; Müller, S.; Wasmer, H. How to Control Unwanted Populations of Procambarus clarkii in Central Europe. Freshw. Crayfish 1999, 12, 714–726. [Google Scholar]
- Huner, J. Procambarus. In Biology of Freshwater Crayfish; Blackwell Science Ltd.: Oxford, UK, 2002; pp. 541–584. [Google Scholar]
- Scalici, M.; Gherardi, F. Structure and Dynamics of an Invasive Population of the Red Swamp Crayfish (Procambarus clarkii) in a Mediterranean Wetland. Hydrobiologia 2007, 583, 309–319. [Google Scholar] [CrossRef]
- Guerra, J.; Niño, A. Ecology of Red Swamp Crayfish (Procambarus clarkii, Girard) in the Central Meseta of Spain. Freshw. Crayfish 1995, 8, 179–200. [Google Scholar]
- Ligas, A. Population Dynamics of Procambarus clarkii (Girard, 1852) (Decapoda, Astacidea, Cambaridae) from Southern Tuscany (Italy). Crustaceana 2008, 81, 601–609. [Google Scholar] [CrossRef]
- Parrack, M.L.; Cummings, N.J. Errors in Transforming Length Samples to Age Frequencies without Age Samples. Fish. Res. 2003, 63, 235–243. [Google Scholar] [CrossRef]
- Heery, E.C.; Berkson, J. Systematic Errors in Length Frequency Data and Their Effect on Age-Structured Stock Assessment Models and Management. Trans. Am. Fish. Soc. 2009, 138, 218–232. [Google Scholar] [CrossRef]
- Aly, W.; El-Far, A.; Fetouh, M.A. Some Fisheries and Biological Aspects of the Crayfish Procambarus clarkii (Girard, 1852) in the River Nile, Egypt. Egypt. J. Aquat. Biol. Fish. 2020, 24, 33–42. [Google Scholar] [CrossRef]
- Saeed, M.; Kilada, R.; Saad, A.E.-H.; Mehanna, S.F.; Abeer, S.A.; Khalil, M.T. Comparative Study on Genetic Variation and Nutritional Value of Freshwater Crayfish Procambarus clarkii in the River Nile, Egypt. Egypt. J. Aquat. Biol. Fish. 2024, 28, 2131–2151. [Google Scholar] [CrossRef]
- Aiken, D.E. The Crayfish Orconectes Virilis: Survival in a Region with Severe Winter Conditions. Can. J. Zool. 1968, 46, 207–211. [Google Scholar] [CrossRef]
- Carmona-Osalde, C.; Rodriguez-Serna, M.; Olvera-Novoa, M.A.; Gutierrez-Yurrita, P.J. Gonadal Development, Spawning, Growth and Survival of the Crayfish Procambarus Llamasi at Three Different Water Temperatures. Aquaculture 2004, 232, 305–316. [Google Scholar] [CrossRef]
- Vernberg, W.B.; Vernberg, F.J. Freshwater Adaptations. Environ. Adapt. 1983, 8, 335. [Google Scholar]
- Jin, S.; Jacquin, L.; Huang, F.; Xiong, M.; Li, R.; Lek, S.; Li, W.; Liu, J.; Zhang, T. Optimizing Reproductive Performance and Embryonic Development of Red Swamp Crayfish Procambarus clarkii by Manipulating Water Temperature. Aquaculture 2019, 510, 32–42. [Google Scholar] [CrossRef]
- Wenner, A.M.; Fusaro, C.; Oaten, A. Size at Onset of Sexual Maturity and Growth Rate in Crustacean Populations. Can. J. Zool. 1974, 52, 1095–1106. [Google Scholar] [CrossRef]
- Huner, J. Observations on the Life Histories of Recreationally Important Crawfishes in Temporary Habitats. Proc. Louis. Acad. Sci. 1975, 38, 20–24. [Google Scholar]
- Huner, J. Exploitation of Freshwater Crayfishes in North-America. Fisheries 1978, 3, 2–5. [Google Scholar]
- Jin, S.; Jacquin, L.; Xiong, M.; Li, R.; Lek, S.; Li, W.; Zhang, T. Reproductive Pattern and Population Dynamics of Commercial Red Swamp Crayfish (Procambarus clarkii) from China: Implications for Sustainable Aquaculture Management. PeerJ 2019, 7, e6214. [Google Scholar] [CrossRef]
- Huner, J.; Romaire, R. Size Maturity as a Means of Comparing Populations of Procambarus clarkii (Girard)(Crustacea, Decapoda) from Different Habitats. Freshw. Crayfish 1978, 4, 53–64. [Google Scholar]
- Penn, G.H. A Study of the Life History of the Louisiana Red-Crawfish, Cambarus Clarkii Girard. Ecology 1943, 24, 1–18. [Google Scholar] [CrossRef]
- Suko, T. Studies on the Development of the Crayfish. I. The Development of Secondary Sex Characters in Appendages. Sci. Rep. Saitama Univ. B 1953, 1, 77–96. [Google Scholar]
- Huner, J.V.; Barr, J.E. Red Swamp Crawfish: Biology and Exploitation, revised ed.; Louisiana Sea Grant College Program, Louisiana State University: Baton Rouge, LA, USA, 1984. [Google Scholar]
- Lutz, C.; Wolters, W. Growth and Yield of Red Swamp Crawfish and White River Crawfish Stocked Separately and in Combination. Crawfish Tales 1987, 6, 20–21. [Google Scholar]
- Correia, A.M.; Costa, A.C. Introduction of the Red Swamp Crayfish, Procambarus clarkii (Crustacea: Decapoda) in São Miguel, Azores, Portugal. Arquipél. Ciênc. Biológicas E Mar. Life Mar. Sci. 1994, 12, 67–73. [Google Scholar]
- Oluoch, A.O. Breeding Biology of the Louisiana Red Swamp Crayfish Procambarus clarkii Girard in Lake Naivasha, Kenya. Hydrobiologia 1990, 208, 85–92. [Google Scholar] [CrossRef]
- Correia, A. Population Dynamics of Procambarus clarkii (Crustacea: Decapoda) in Portugal. Freshw. Crayfish 1995, 8, 276–290. [Google Scholar]
- Sommer, T.; Goldman, C. The Crayfish Procambarus clarkii from California Ricefields: Ecology, Problems and Potential for Harvest. Freshw. Crayfish 1983, 5, 418–428. [Google Scholar]
- Catlin, N. Age Validation, Size-at-Age, Size-at-Maturity, and Age-at-Maturity in Snow Crab (Chionoecetes Opilio) from Different Thermal Regimes in Newfoundland. Bachelor’ Thesis, University of New Brunswick, Fredericton, NB, Canada, 2020. [Google Scholar]
- Bluhm, B.A.; Kilada, R.; Ambrose, W.; Renaud, P.E.; Sundet, J.H. First Record of Cuticle Bands in the Stomach Ossicles of the Red King Crab Paralithodes Camtschaticus (Tilesius, 1815) (Decapoda: Anomura: Lithodidae) from Norway. J. Crustac. Biol. 2019, 39, 703–710. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeed, M.; Kilada, R.; Mehanna, S.; Saad, A.; Khalil, M. Preliminary Assessment of Age and Growth of the Red Swamp Crayfish Procambarus clarkii [Girard, 1852] in the River Nile in Egypt by Direct and Indirect Methods. Fishes 2025, 10, 453. https://doi.org/10.3390/fishes10090453
Saeed M, Kilada R, Mehanna S, Saad A, Khalil M. Preliminary Assessment of Age and Growth of the Red Swamp Crayfish Procambarus clarkii [Girard, 1852] in the River Nile in Egypt by Direct and Indirect Methods. Fishes. 2025; 10(9):453. https://doi.org/10.3390/fishes10090453
Chicago/Turabian StyleSaeed, Mohamed, Raouf Kilada, Sahar Mehanna, Abdelhalim Saad, and Magdy Khalil. 2025. "Preliminary Assessment of Age and Growth of the Red Swamp Crayfish Procambarus clarkii [Girard, 1852] in the River Nile in Egypt by Direct and Indirect Methods" Fishes 10, no. 9: 453. https://doi.org/10.3390/fishes10090453
APA StyleSaeed, M., Kilada, R., Mehanna, S., Saad, A., & Khalil, M. (2025). Preliminary Assessment of Age and Growth of the Red Swamp Crayfish Procambarus clarkii [Girard, 1852] in the River Nile in Egypt by Direct and Indirect Methods. Fishes, 10(9), 453. https://doi.org/10.3390/fishes10090453








