Effect of Dandelion Root Extract on Growth, Biochemical Indices, Antioxidant Capacity, Intestinal Histomorphology and Microbiota in Micropterus salmoides
Abstract
1. Introduction
2. Materials and Methods
2.1. Feed Preparation
2.2. Fish Rearing and Sample Collection
2.3. Growth Performance
2.4. Biochemical Indicators
2.5. Antioxidant Capacity
2.6. Intestinal Histology
2.7. Intestinal Microbiota
2.8. Statistical Analysis
3. Results
3.1. Effect of DRE on Growth Performance of Largemouth Bass
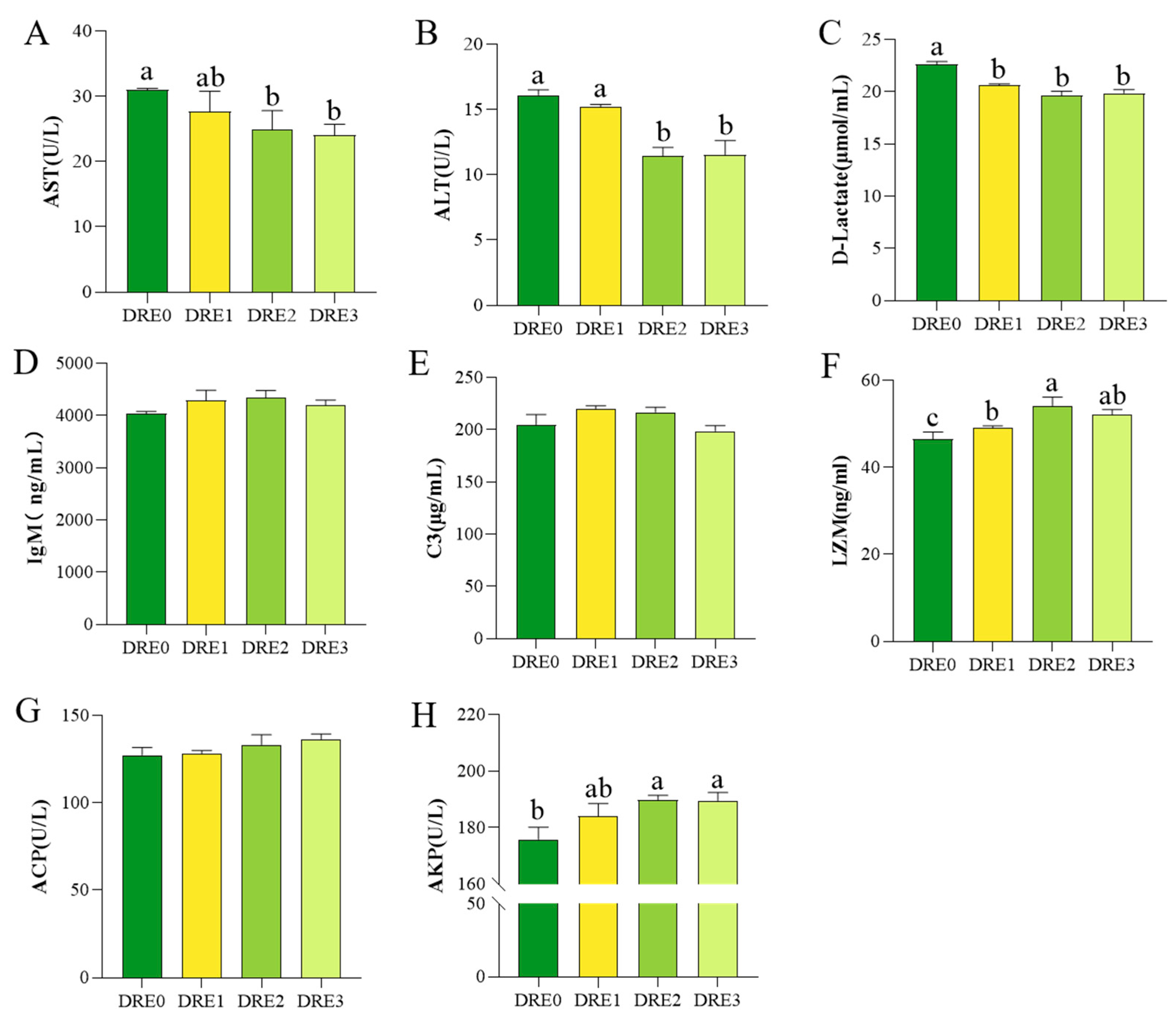
3.2. Effect of DRE on Serum Biochemical Parameters of Largemouth Bass
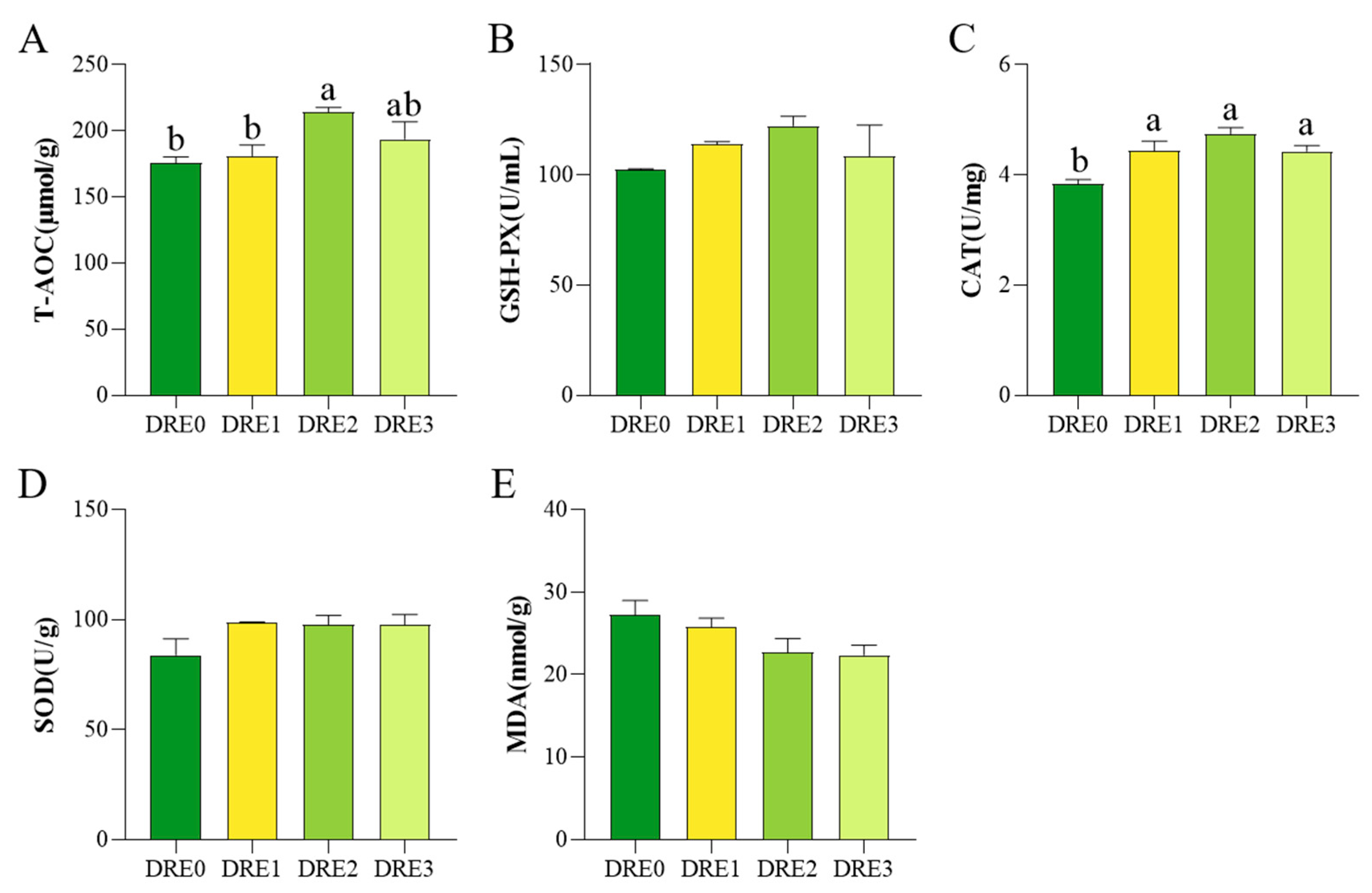
3.3. Effect of DRE on Antioxidant Indexes in the Liver of Largemouth Bass
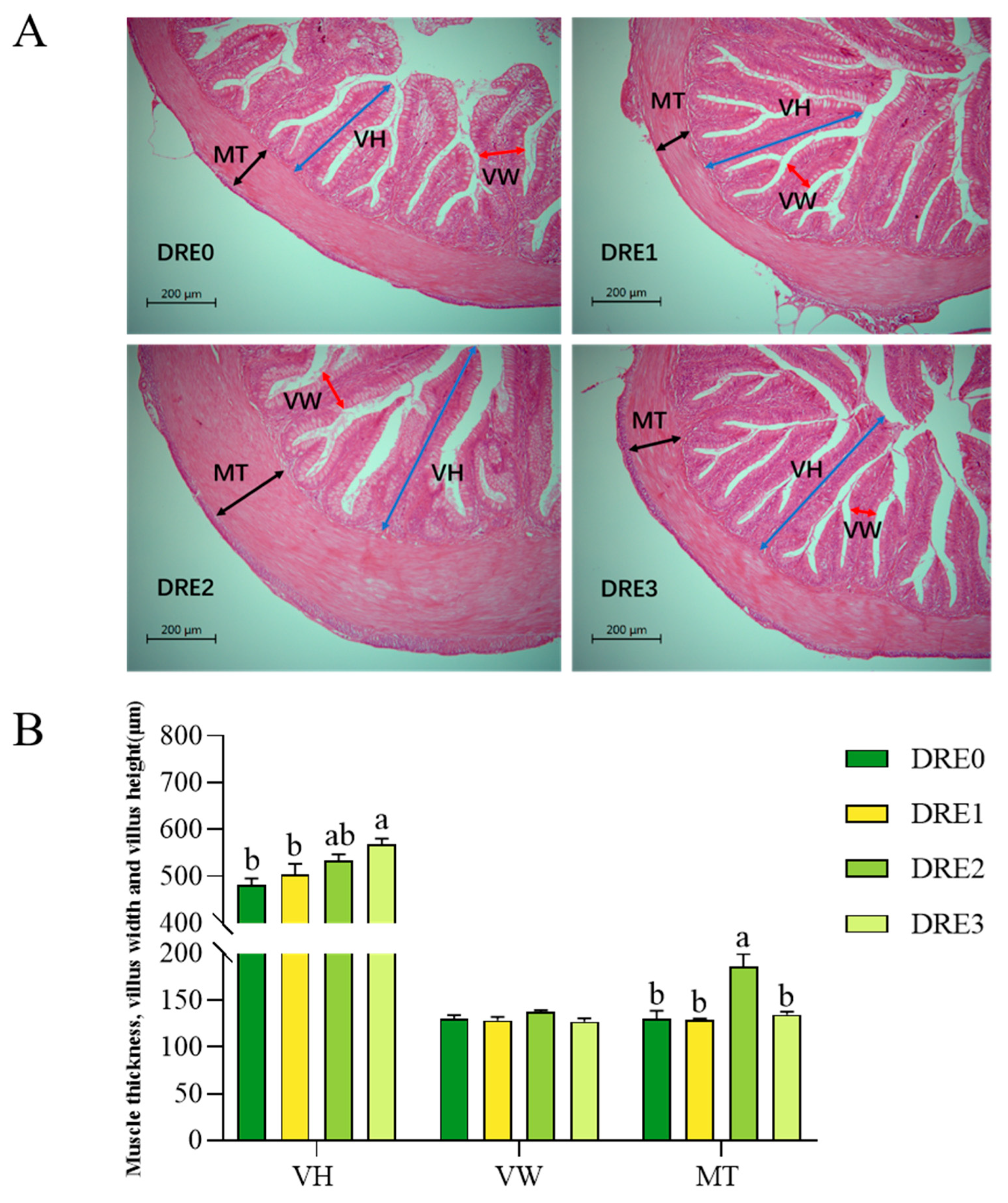
3.4. Effect of DRE on the Intestinal Morphology of Largemouth Bass
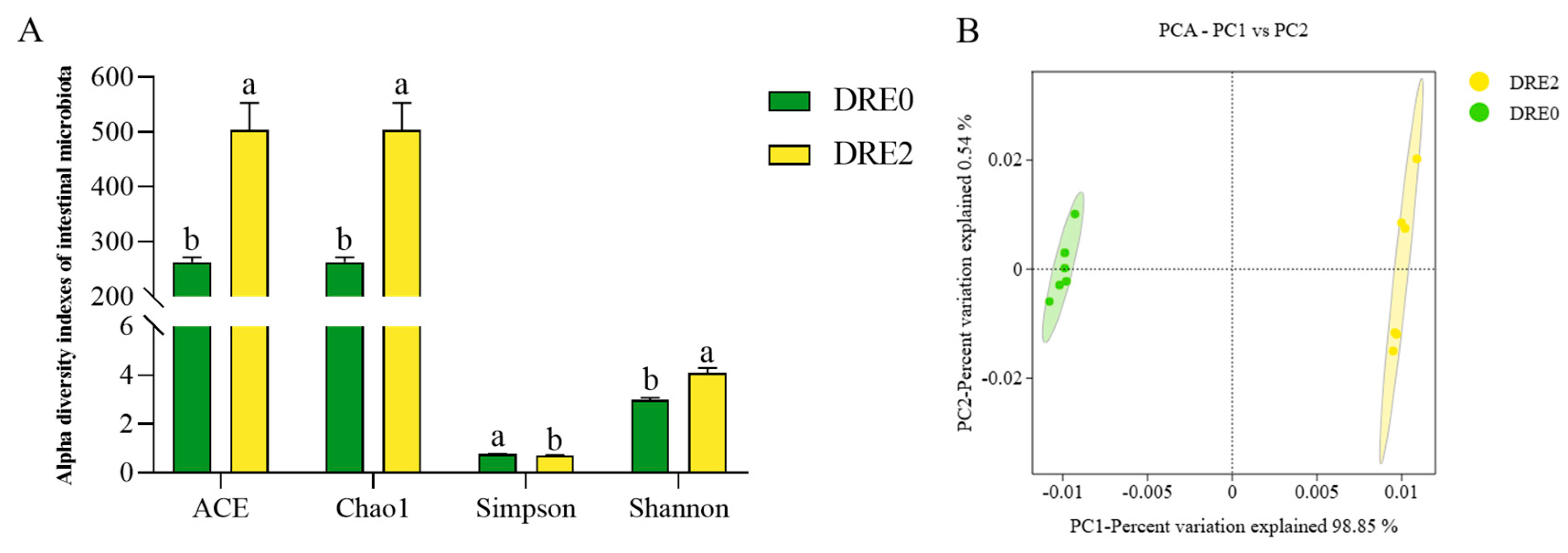
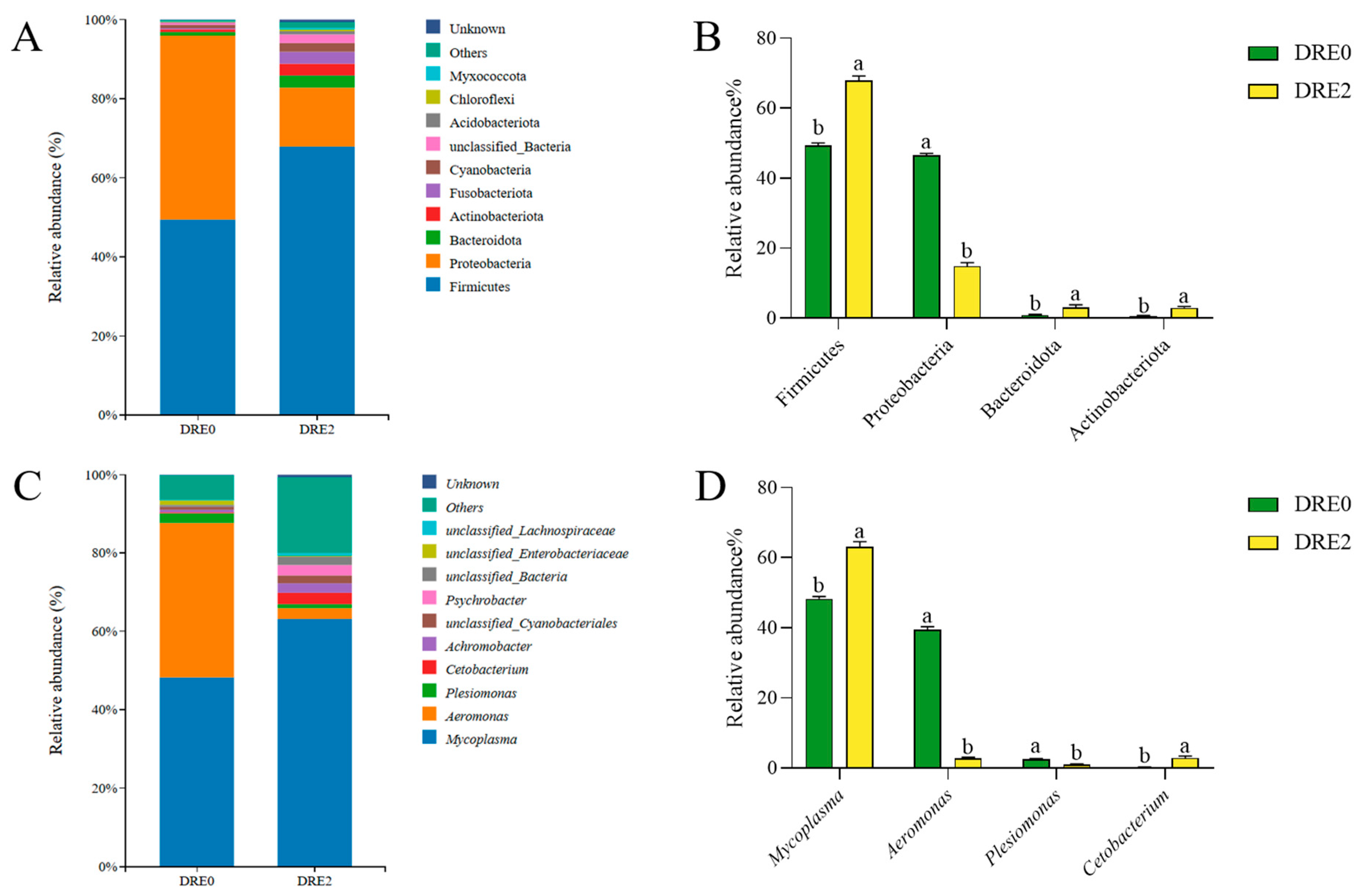
3.5. Effect of DRE on the Intestinal Microbiota of Largemouth Bass
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flores-Méndez, L.C.; Lizárraga-Velázquez, C.E.; Sánchez-Gutiérrez, E.Y.; Arrizon, J.; Leyva-López, N.; Hernández, C. Study of the Effect of Dietary Agavin Supplementation in Blood Parameters and Antioxidant Enzymes of Juvenile Nile Tilapia (Oreochromis niloticus) under Stress Conditions. Fishes 2022, 7, 340. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Metwally, A.E.-S.; El-Sharawy, M.E.; Atta, A.M.; Elbialy, Z.I.; Abdel-Latif, H.M.R.; Paray, B.A. The Role of β-Glucan in the Growth, Intestinal Morphometry, and Immune-Related Gene and Heat Shock Protein Expressions of Nile Tilapia (Oreochromis niloticus) under Different Stocking Densities. Aquaculture 2020, 523, 735205. [Google Scholar] [CrossRef]
- Cabello, F.C.; Godfrey, H.P.; Buschmann, A.H.; Dölz, H.J. Aquaculture as yet Another Environmental Gateway to the Development and Globalisation of Antimicrobial Resistance. Lancet 2016, 16, e127–e133. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Lv, Z.; Zhang, Z.; Han, Y.; Liu, Z.; Zhang, H. A Review of Antibiotics, Antibiotic Resistant Bacteria, and Resistance Genes in Aquaculture: Occurrence, Contamination, and Transmission. Toxics 2023, 11, 420. [Google Scholar] [CrossRef]
- Fan, M.; Zhang, X.; Song, H.; Zhang, Y. Dandelion (Taraxacum Genus): A Review of Chemical Constituents and Pharmacological Effects. Molecules 2023, 28, 5022. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Roberts, T.H.; Matthews, K.R.; Bezerra, C.F.; Morais-Braga, M.F.B.; Coutinho, H.D.M.; Sharopov, F.; Salehi, B.; Yousaf, Z.; Sharifi-Rad, M.; et al. Ethnobotany of the Genus Taraxacum-Phytochemicals and Antimicrobial Activity. Phytother. Res. 2018, 32, 2131–2145. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Miao, M.; Song, Y.; Fang, X.; Zhang, J.; Zhang, Y.; Miao, J. Total Flavonoids of Taraxacum Mongolicum Inhibit Non-Small Cell Lung Cancer by Regulating Immune Function. J. Ethnopharmacol. 2021, 281, 114514. [Google Scholar] [CrossRef]
- Lis, B.; Rolnik, A.; Jedrejek, D.; Soluch, A.; Stochmal, A.; Olas, B. Dandelion (Taraxacum officinale L.) Root Components Exhibit Anti-Oxidative and Antiplatelet Action in an in Vitro Study. J. Funct. Foods 2019, 59, 16–24. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Shi, P.; Li, P.; Fu, Y.; Tan, G.; Zhou, J.; Zeng, J.; Huang, P. Modulation of Acute Intestinal Inflammation by Dandelion Polysaccharides: An In-Depth Analysis of Antioxidative, Anti-Inflammatory Effects and Gut Microbiota Regulation. Int. J. Mol. Sci. 2024, 25, 1429. [Google Scholar] [CrossRef]
- Qiao, Q.; Song, X.; Zhang, C.; Jiang, C.; Jiang, R. Structure and Immunostimulating Activity of Polysaccharides Derived from the Roots and Leaves of Dandelion. Chem. Biol. Technol. Agric. 2024, 11, 51. [Google Scholar] [CrossRef]
- Cai, L.; Wan, D.; Yi, F.; Luan, L. Purification, Preliminary Characterization and Hepatoprotective Effects of Polysaccharides from Dandelion Root. Molecules 2017, 22, 1409. [Google Scholar] [CrossRef]
- Li, W.; Luo, F.; Wu, X.; Fan, B.; Yang, M.; Zhong, W.; Guan, D.; Wang, F.; Wang, Q. Anti-Inflammatory Effects and Mechanisms of Dandelion in RAW264.7 Macrophages and Zebrafish Larvae. Front. Pharmacol. 2022, 13, 906927. [Google Scholar] [CrossRef] [PubMed]
- Hosseini Shekarabi, S.P.; Mostafavi, Z.S.; Mehrgan, M.S.; Islami, H.R. Dietary Supplementation with Dandelion (Taraxacum officinale) Flower Extract Provides Immunostimulation and Resistance against Streptococcus Iniae Infection in Rainbow Trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2021, 118, 180–187. [Google Scholar] [CrossRef]
- Xue, S.; Xia, B.; Zou, Y.; Li, L.; Zhang, B.; Shen, Z.; Xiang, Y.; Han, Y.; Chen, W. Dandelion Extract on Growth Performance, Immunity, Stress and Infection Resistance in Common Carp. Aquac. Rep. 2022, 26, 101330. [Google Scholar] [CrossRef]
- He, G.; Sun, H.; Liao, R.; Wei, Y.; Zhang, T.; Chen, Y.; Lin, S. Effects of Herbal Extracts (Foeniculum vulgare and Artemisia annua) on Growth, Liver Antioxidant Capacity, Intestinal Morphology and Microorganism of Juvenile Largemouth Bass, Micropterus salmoides. Aquac. Rep. 2022, 23, 101081. [Google Scholar] [CrossRef]
- Tan, X.; Sun, Z. Dietary Dandelion Extract Improved Growth Performance, Immunity, Intestinal Morphology and Microbiota Composition of Golden Pompano Trachinotus ovatus. Aquac. Rep. 2020, 18, 100491. [Google Scholar] [CrossRef]
- Salem, M.O.A.; Salem, T.A.; Yürüten Özdemir, K.; Sönmez, A.Y.; Bilen, S.; Güney, K. Antioxidant Enzyme Activities and Immune Responses in Rainbow Trout (Onchorhynchus mykiss) Juveniles Fed Diets Supplemented with Dandelion (Taraxacum officinalis) and Lichen (Usnea barbata) Extracts. Fish Physiol. Biochem. 2021, 47, 1053–1062. [Google Scholar] [CrossRef]
- Li, Q.; Fu, M.; Zhu, S.; Liu, J.; Li, Y.; Xue, Z.; Zhou, Z.; Yu, L. Effects of Dietary Hydroxy-Cinnamic Acid Derivatives on Growth, Muscle, and Intestinal Parameters of Tilapia (Oreochromis niloticus). Fish Physiol. Biochem. 2025, 51, 4. [Google Scholar] [CrossRef]
- Meng, X.; Cai, H.; Li, H.; You, F.; Jiang, A.; Hu, W.; Li, K.; Zhang, X.; Zhang, Y.; Chang, X.; et al. Clostridium Butyricum-Fermented Chinese Herbal Medicine Enhances the Immunity by Modulating the Intestinal Microflora of Largemouth Bass (Micropterus salmoides). Aquaculture 2023, 562, 738768. [Google Scholar] [CrossRef]
- Samolińska, W.; Grela, E.R.; Kowalczuk-Vasilev, E.; Kiczorowska, B.; Klebaniuk, R.; Hanczakowska, E. Evaluation of Garlic and Dandelion Supplementation on the Growth Performance, Carcass Traits, and Fatty Acid Composition of Growing-Finishing Pigs. Anim. Feed Sci. Technol. 2020, 259, 114316. [Google Scholar] [CrossRef]
- Mao, J.; Wang, Y.; Wang, W.; Duan, T.; Yin, N.; Guo, T.; Guo, H.; Liu, N.; An, X.; Qi, J. Effects of Taraxacum mongolicum Hand.-Mazz. (dandelion) on growth performance, expression of genes coding for tight junction protein and mucin, microbiota composition and short chain fatty acids in ileum of broiler chickens. BMC Vet. Res. 2022, 18, 180. [Google Scholar] [CrossRef]
- Mostafavi, Z.S.; Shekarabi, S.P.H.; Mehrgan, M.S.; Islami, H.R. Amelioration of Growth Performance, Physio-Metabolic Responses, and Antioxidant Defense System in Rainbow Trout, Oncorhynchus mykiss, Using Dietary Dandelion, Taraxacum officinale, Flower Extract. Aquaculture 2022, 546, 737296. [Google Scholar] [CrossRef]
- Tan, X.; Sun, Z.; Zhou, C.; Huang, Z.; Tan, L.; Xun, P.; Huang, Q.; Lin, H.; Ye, C.; Wang, A. Effects of Dietary Dandelion Extract on Intestinal Morphology, Antioxidant Status, Immune Function and Physical Barrier Function of Juvenile Golden Pompano Trachinotus ovatus. Fish Shellfish Immunol. 2018, 73, 197–206. [Google Scholar] [CrossRef]
- Giri, S.S.; Kim, S.G.; Woo, K.J.; Jung, W.J.; Lee, S.B.; Lee, Y.M.; Jo, S.J.; Kim, J.H.; Park, S.C. Impact of Dandelion Polysaccharides on Growth and Immunity Response in Common Carp Cyprinus carpio. Fish Shellfish Immunol. 2022, 128, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Tan, X.; Xu, M.; Liu, Q.; Ye, H.; Zou, C.; Zhou, Y.; Su, N.; Chen, L.; Wang, A.; et al. Effects of Dietary Dandelion Extracts on Growth Performance, Liver Histology, Immune-Related Gene Expression and CCl4 Resistance of Hybrid Grouper (Epinephelus lanceolatus♂ × Epinephelus fuscoguttatus♀). Fish Shellfish Immunol. 2019, 88, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Cheng, Y.; Qian, S.; Zhang, W.; Huang, M.; Yang, S.; Fei, H. An Evaluation of Laminarin Additive in the Diets of Juvenile Largemouth Bass (Micropterus salmoides): Growth, Antioxidant Capacity, Immune Response and Intestinal Microbiota. Animals 2023, 13, 459. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, K.; Zhong, H.; Ma, Y.; Guo, Z.; Tang, Z.; Liang, J.; Luo, Y.; Su, Z.; Wang, L. Effects of Lycium Barbarum Polysaccharides on Immunological Parameters, Apoptosis, and Growth Performance of Nile Tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2020, 97, 509–514. [Google Scholar] [CrossRef]
- Mashoof, S.; Criscitiello, M.F. Fish Immunoglobulins. Biology 2016, 5, 45. [Google Scholar] [CrossRef]
- Lulijwa, R.; Young, T.; Symonds, J.E.; Walker, S.P.; Delorme, N.J.; Alfaro, A.C. Uncoupling Thermotolerance and Growth Performance in Chinook Salmon: Blood Biochemistry and Immune Capacity. Metabolites 2021, 11, 547. [Google Scholar] [CrossRef]
- Chen, W.; Xia, B.; Zou, Y.; Xiang, Y.; Shen, Z.; Xue, S. Protective Effect of Dietary Dandelion (Taraxacum officinale) Extract on Common Carp under Acute Ammonia Stress. Aquac. Rep. 2023, 30, 101609. [Google Scholar] [CrossRef]
- Du, J.-H.; Xu, M.-Y.; Wang, Y.; Lei, Z.; Yu, Z.; Li, M.-Y. Evaluation of Taraxacum mongolicum Flavonoids in Diets for Channa Argus Based on Growth Performance, Immune Responses, Apoptosis and Antioxidant Defense System under Lipopolysaccharide Stress. Fish Shellfish Immunol. 2022, 131, 1224–1233. [Google Scholar] [CrossRef]
- Madeira, D.; Narciso, L.; Cabral, H.N.; Vinagre, C.; Diniz, M.S. Influence of Temperature in Thermal and Oxidative Stress Responses in Estuarine Fish. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2013, 166, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Shi, A.; Ma, H.; Shi, X.; Zhou, W.; Pan, W.; Song, Y.; Chen, Q.; Yu, X.; Niu, C.; Yang, Y.; et al. Effects of Microbe-Derived Antioxidants on Growth, Digestive and Aminotransferase Activities, and Antioxidant Capacities in the Hepatopancreas of Eriocheir Sinensis under Ammonia Nitrogen Stress. Aquac. Fish. 2024, 9, 957–966. [Google Scholar] [CrossRef]
- Storey, K.B. Oxidative Stress: Animal Adaptations in Nature. Braz. J. Med. Biol. Res. 1996, 29, 1715–1733. [Google Scholar] [PubMed]
- Chen, P.; Zhu, Y.; Wu, X.; Gu, X.; Xue, M.; Liang, X. Metabolic Adaptation to High-Starch Diet in Largemouth Bass (Micropterus salmoides) Was Associated with the Restoration of Metabolic Functions via Inflammation, Bile Acid Synthesis and Energy Metabolism. Br. J. Nutr. 2022, 129, 381–394. [Google Scholar] [CrossRef]
- Kocot, A.M.; Jarocka-Cyrta, E.; Drabińska, N. Overview of the Importance of Biotics in Gut Barrier Integrity. Int. J. Mol. Sci. 2022, 23, 2896. [Google Scholar] [CrossRef]
- Nadal, A.L.; Ikeda-Ohtsubo, W.; Sipkema, D.; Peggs, D.; McGurk, C.; Forlenza, M.; Wiegertjes, G.F.; Brugman, S. Feed, Microbiota, and Gut Immunity: Using the Zebrafish Model to Understand Fish Health. Front. Immunol. 2020, 11, 114. [Google Scholar] [CrossRef] [PubMed]
- Takiishi, T.; Fenero, C.I.M.; Câmara, N.O.S. Intestinal Barrier and Gut Microbiota: Shaping Our Immune Responses throughout Life. Tissue Barriers 2017, 5, e1373208. [Google Scholar] [CrossRef]
- Köse, Ö.; Karabulut, H.A.; Er, A. Dandelion Root Extract in Trout Feed and Its Effects on the Physiological Performance of Oncorhynchus mykiss and Resistance to Lactococcus garvieae Infection. Ann. Anim. Sci. 2024, 24, 161–177. [Google Scholar] [CrossRef]
- He, M.; Li, X.; Poolsawat, L.; Guo, Z.; Yao, W.; Zhang, C.; Leng, X. Effects of Fish Meal Replaced by Fermented Soybean Meal on Growth Performance, Intestinal Histology and Microbiota of Largemouth Bass (Micropterus salmoides). Aquac. Nutr. 2020, 26, 1058–1071. [Google Scholar] [CrossRef]
- Liang, Y.; Duan, H.; Zhang, P.; Han, H.; Gao, F.; Li, Y.; Xu, Z. Extraction and Isolation of the Active Ingredients of Dandelion and Its Antifungal Activity against Candida Albicans. Mol. Med. Rep. 2019, 21, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Sun, J.; Li, Y. Evaluation of Toxicity, Bacteriostatic, Analgesic, Anti-Inflammatory, and Antipyretic Activities of Huangqin-Honghua-Pugongying-Jinyinhua Extract. Vet. Sci. 2021, 8, 330. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-M.; Gao, W.-R.; Liang, P.; Cai, G.-H.; Yang, H.-L.; Lin, J.-B.; Sun, Y.-Z. Pleurotus Eryngii Root Waste and Soybean Meal Co-Fermented Protein Improved the Growth, Immunity, Liver and Intestinal Health of Largemouth Bass (Micropterus salmoides). Fish Shellfish Immunol. 2024, 149, 109551. [Google Scholar] [CrossRef] [PubMed]
- Round, J.L.; Mazmanian, S.K. The Gut Microbiota Shapes Intestinal Immune Responses during Health and Disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef]
| Ingredient | Content |
|---|---|
| Domestic fish meal 1 | 26 |
| Steam fish meal 1 | 20 |
| Soybean meal 1 | 20 |
| Wheat flour 1 | 14 |
| Soybean protein concentrate 1 | 6 |
| Corn protein 1 | 5 |
| Soybean oil | 3 |
| Fish oil | 2 |
| Lecithin | 0.6 |
| Ca(H2PO4)2 | 1.8 |
| Choline chloride | 0.5 |
| Vitamin C | 0.1 |
| Mineral premix 2 | 0.6 |
| Vitamin premix 3 | 0.4 |
| Proximate composition (g/100 g dry matter basis) | |
| Crude protein | 47.4 |
| Crude fat | 10.4 |
| DRE0 | DRE1 | DRE2 | DRE3 | |
|---|---|---|---|---|
| FBW (g/fish) | 42.80 ± 0.90 | 41.66 ± 0.50 | 41.63 ± 0.34 | 41.65 ± 1.04 |
| WGR (%) | 684.79 ± 16.24 | 662.26 ± 8.70 | 662.69 ± 6.23 | 663.55 ± 18.97 |
| SGR (%/d) | 4.90 ± 0.05 | 4.84 ± 0.03 | 4.84 ± 0.02 | 4.84 ± 0.06 |
| FCR | 0.83 ± 0.02 | 0.80 ± 0.01 | 0.83 ± 0.01 | 0.81 ± 0.01 |
| CF (g/cm3) | 1.74 ± 0.04 | 1.73 ± 0.01 | 1.68 ± 0.01 | 1.73 ± 0.04 |
| HSI (%) | 2.01 ± 0.06 | 1.95 ± 0.07 | 1.96 ± 0.09 | 1.89 ± 0.03 |
| SR (%) | 100 | 100 | 100 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, P.-A.; Yang, H.-L.; Huang, J.; Li, F.; Huang, K.-Y.; Liu, Z.-X.; Cai, G.-H.; Sun, Y.-Z. Effect of Dandelion Root Extract on Growth, Biochemical Indices, Antioxidant Capacity, Intestinal Histomorphology and Microbiota in Micropterus salmoides. Fishes 2025, 10, 446. https://doi.org/10.3390/fishes10090446
Liu P-A, Yang H-L, Huang J, Li F, Huang K-Y, Liu Z-X, Cai G-H, Sun Y-Z. Effect of Dandelion Root Extract on Growth, Biochemical Indices, Antioxidant Capacity, Intestinal Histomorphology and Microbiota in Micropterus salmoides. Fishes. 2025; 10(9):446. https://doi.org/10.3390/fishes10090446
Chicago/Turabian StyleLiu, Peng-Ao, Hong-Ling Yang, Jian Huang, Fen Li, Ke-Yun Huang, Zi-Xin Liu, Guo-He Cai, and Yun-Zhang Sun. 2025. "Effect of Dandelion Root Extract on Growth, Biochemical Indices, Antioxidant Capacity, Intestinal Histomorphology and Microbiota in Micropterus salmoides" Fishes 10, no. 9: 446. https://doi.org/10.3390/fishes10090446
APA StyleLiu, P.-A., Yang, H.-L., Huang, J., Li, F., Huang, K.-Y., Liu, Z.-X., Cai, G.-H., & Sun, Y.-Z. (2025). Effect of Dandelion Root Extract on Growth, Biochemical Indices, Antioxidant Capacity, Intestinal Histomorphology and Microbiota in Micropterus salmoides. Fishes, 10(9), 446. https://doi.org/10.3390/fishes10090446







