Effects of Probiotic-Fermented Chinese Herb on Immune Response and Growth Performance in Common Carp (Cyprinus carpio)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials, Reagents, and Instruments
2.1.1. Experimental Strains
2.1.2. Chinese Herbal Medicines
2.1.3. Fermentation Process
2.1.4. Experimental Feed
2.1.5. Reagents
2.1.6. Instruments
2.2. Experimental Fish and Feeding Management
2.3. Sample Collection
2.4. Peripheral Blood Leukocyte Separation
2.5. Peripheral Blood Leukocyte Respiratory Burst Assay
2.6. Peripheral Blood Leukocyte Phagocytosis Assay
2.7. Physiological and Biochemical Index Determination
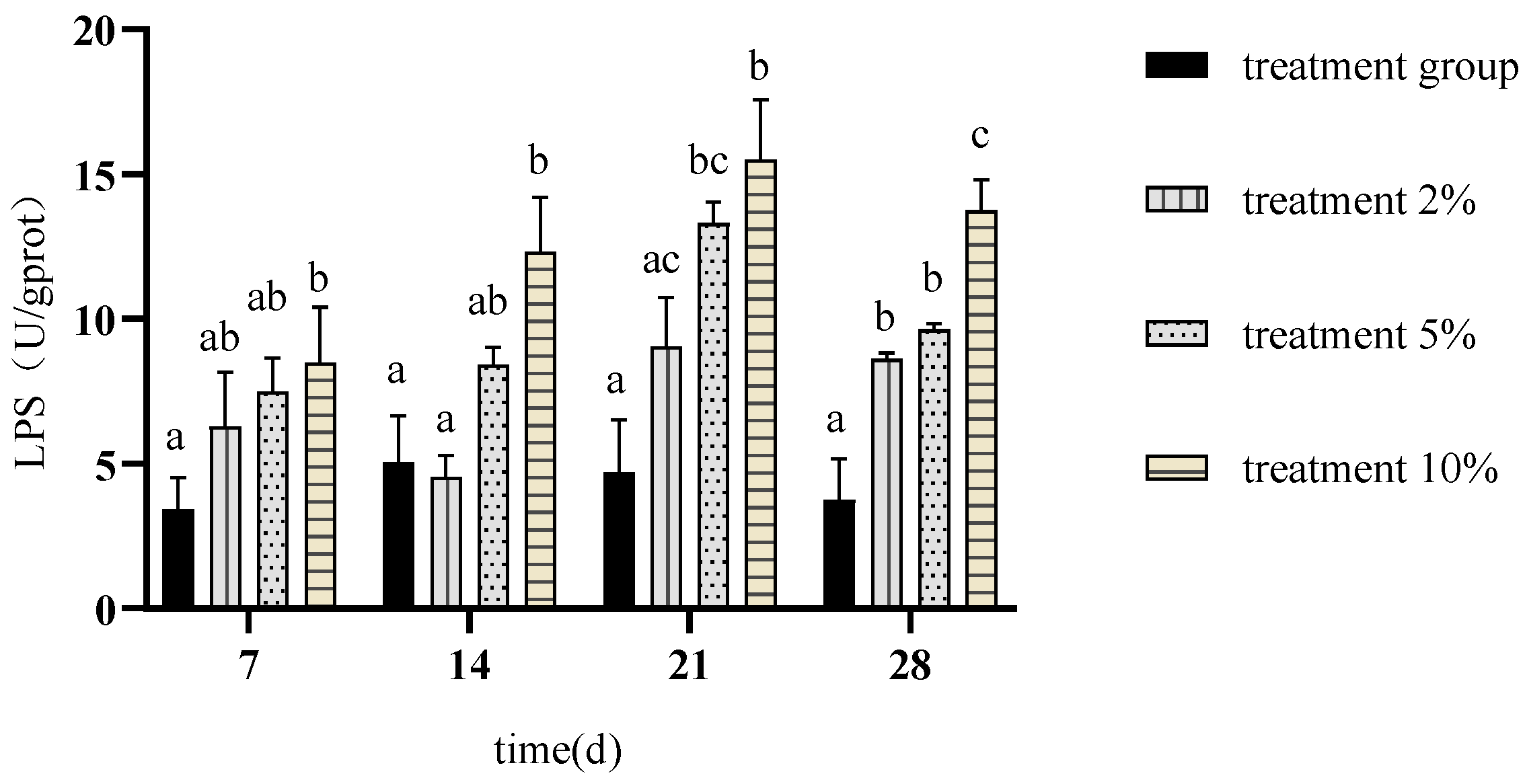
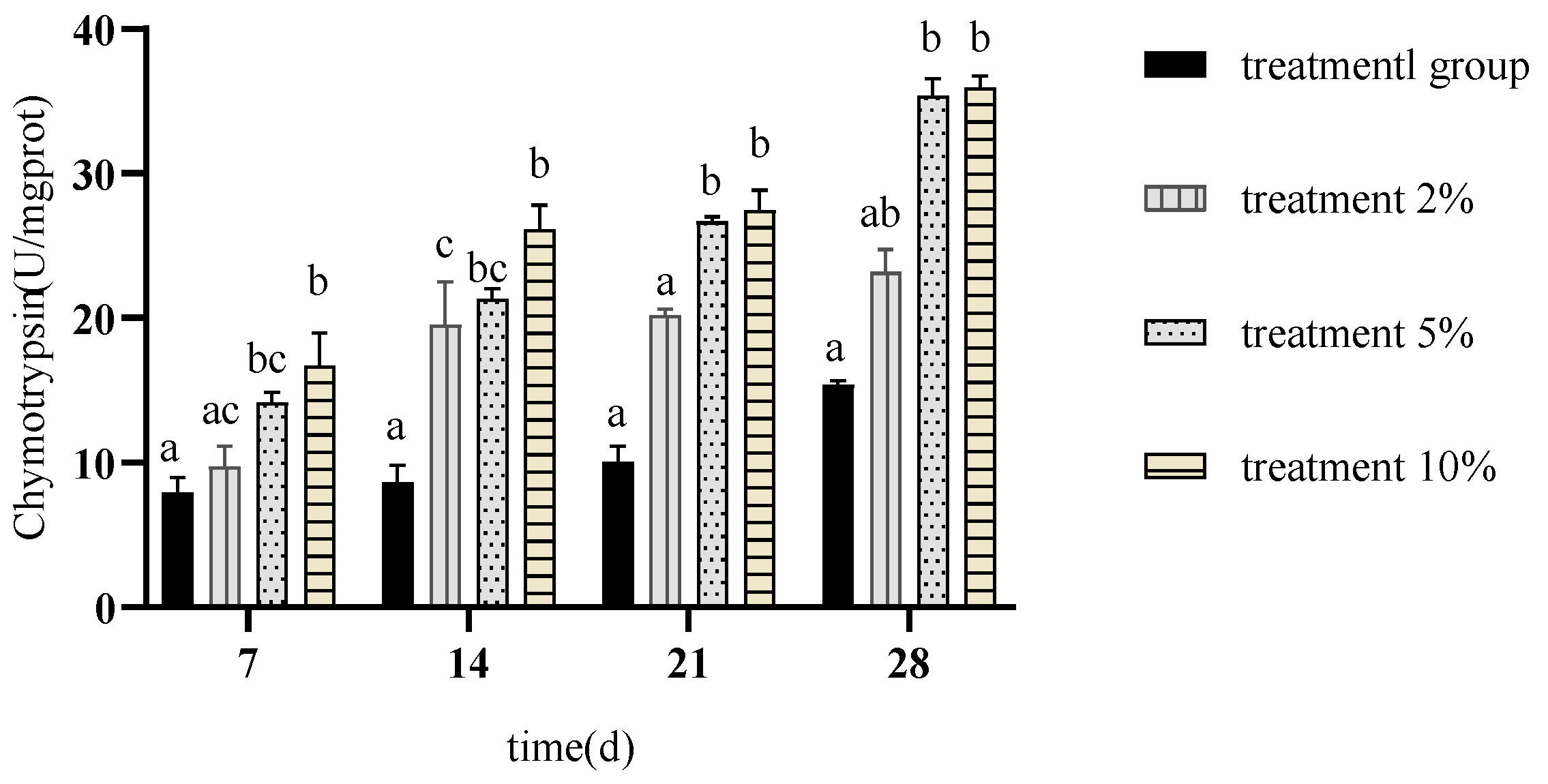
2.7.1. Digestive Enzyme Assay
2.7.2. Biochemical Indices
2.7.3. Antioxidant Indices
2.8. Calculation Formulas
feeding days × 100%
2.9. Statistical Analysis
3. Results and Analysis
3.1. Effects of FCH on Carp Leukocyte Activity
3.1.1. Optimization of Peripheral Blood Leukocyte Separation Solution Ratio
3.1.2. Effects of FCH on Carp Leukocyte Respiratory Burst Activity
3.1.3. Effects of FCH on Carp Leukocyte Phagocytic Activity
3.2. Effects of FCH on Carp Growth Performance
3.3. Effects of FCH on Carp Digestive Enzymes
3.4. Effects of FCH on Carp Liver Transaminase Activity
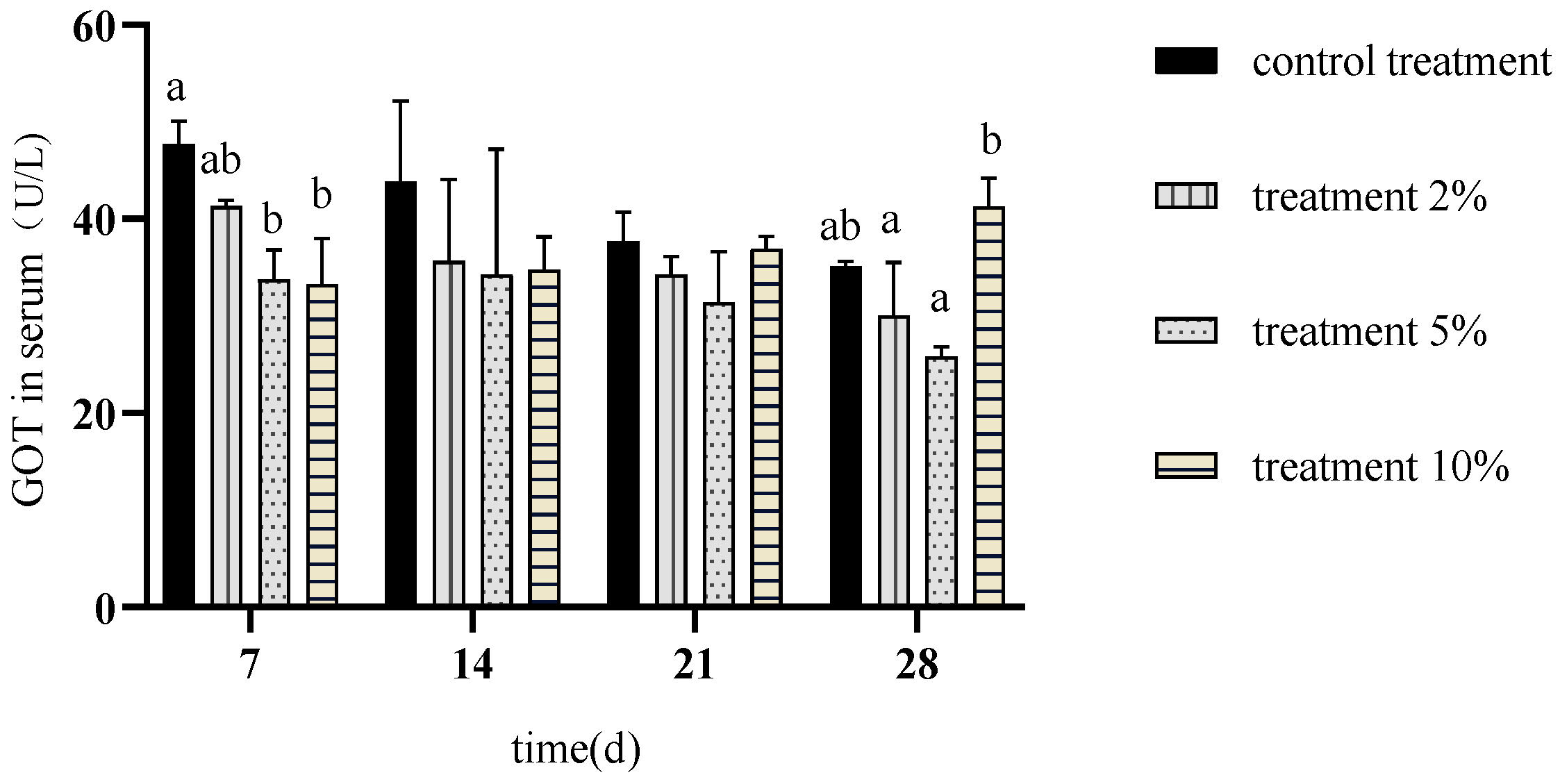
3.5. Effects of FCH on Carp Serum Transaminase Activity
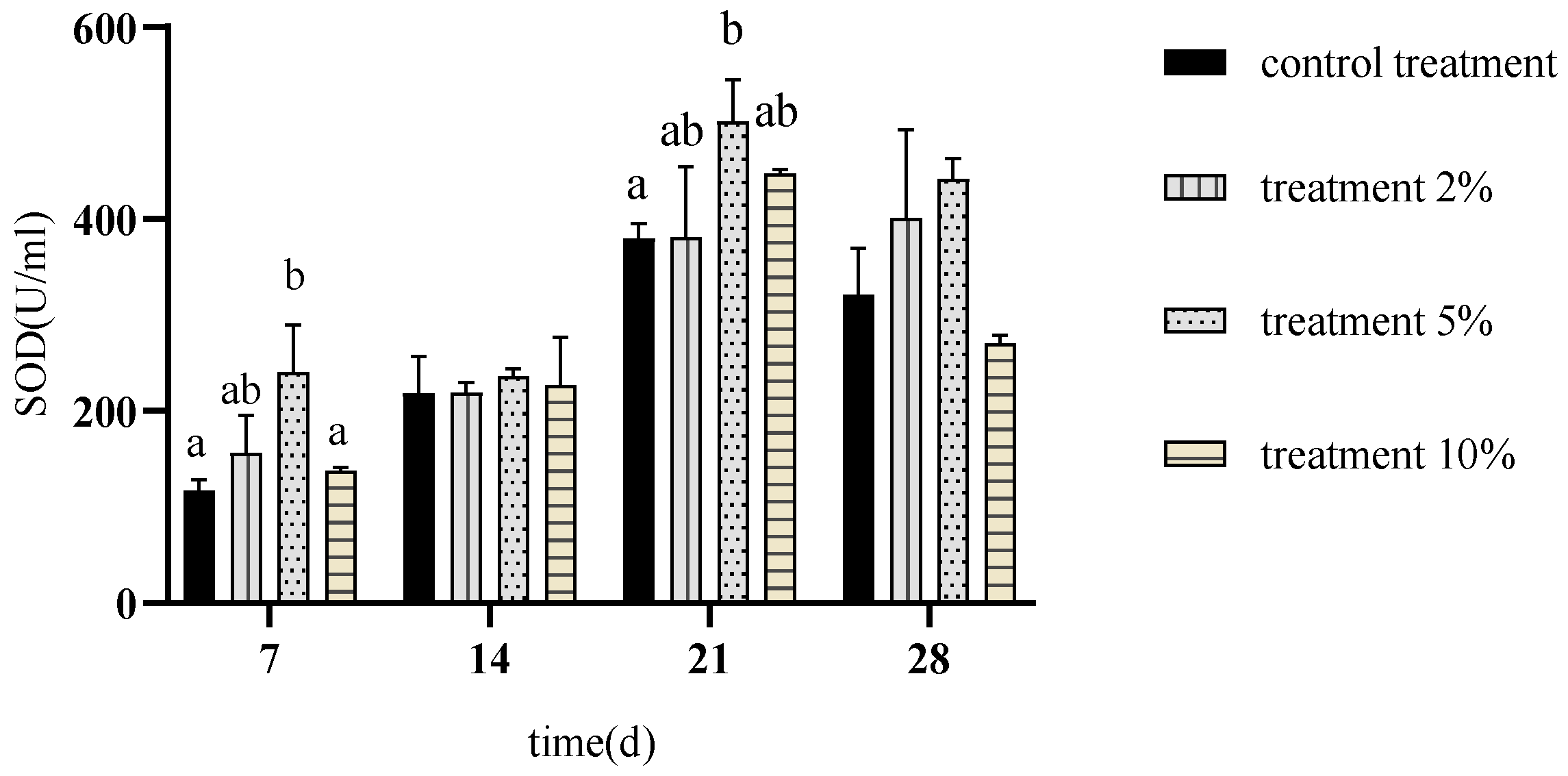
3.6. Effects of FCH on Carp Serum Superoxide Dismutase Activity
4. Discussion
4.1. Effects of FCH on Carp Peripheral Leukocytes
4.2. Growth-Promoting Effects of FCH on Carp
4.3. Effects of FCH on Carp Transaminase Activity
4.4. Effects of FCH on Carp Antioxidant Capacity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Balkrishna, A.; Sharma, N.; Srivastava, D.; Kukreti, A.; Srivastava, S.; Arya, V. Exploring the Safety, Efficacy, and Bioactivity of Herbal Medicines: Bridging Traditional Wisdom and Modern Science in Healthcare. Future Integr. Med. 2024, 3, 35–49. [Google Scholar] [CrossRef]
- Rātu; Bhavan, P.S.; Seenivasan, C.; Muralisankar, T.; Shanthi, R. Effects of native medicinal herbs (Alternanthera sessilis, Eclipta alba and Cissus quadrangularis) on growth performance, digestive enzymes and biochemical constituents of the monsoon river prawn Macrobrachium malcolmsonii. Aquac. Nutr. 2015, 21, 496–506. [Google Scholar]
- Hussain, A.; Bose, S.; Wang, J.-H.; Yadav, M.K.; Mahajan, G.B.; Kim, H. Fermentation, a feasible strategy for enhancing bioactivity of herbal medicines. Food Res. Int. 2016, 81, 1–16. [Google Scholar] [CrossRef]
- Choi, W.M.; Mak, N.K.; Bian, Z.X.; Nie, X.P.; Wong, M.H. Effects of traditional Chinese medicines (TCM) on the immune response of grass carp (Ctenopharyngodon idellus). Aquac. Int. 2014, 22, 361–377. [Google Scholar] [CrossRef]
- Zhang, X.; Miao, Q.; Pan, C.; Yin, J.; Wang, L.; Qu, L.; Yin, Y.; Wei, Y. Research advances in probiotic fermentation of Chinese herbal medicines. iMeta 2023, 2, e93. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Cai, H.; Li, H.; You, F.; Jiang, A.; Hu, W.; Li, K.; Zhang, S.; Zhang, Y.; Chang, X.; et al. Clostridium butyricum-FCH enhances the immunity by modulating the intestinal microflora of largemouth bass (Micropterus salmoides). Aquaculture 2023, 562, 738768. [Google Scholar] [CrossRef]
- Moslehi, F.; Sattari, M.; Masouleh, A.S. Effects of Pediococcus pentosaceus as a probiotic on intestinal microbiota and body composition of Siberian sturgeon, Acipenser baerii Brandt, 1869. Int. J. Aquat. Biol. 2016, 4, 11–16. [Google Scholar]
- Sun, Y.; Wang, X.; Zhou, H.; Mai, K.; He, G. Dietary Astragalus polysaccharides ameliorates the growth performance, antioxidant capacity and immune responses in turbot (Scophthalmus maximus L.). Fish Shellfish. Immunol. 2020, 99, 603–608. [Google Scholar] [CrossRef]
- Chen, G.; Xu, J.; Yuan, J.; Li, H.; Gou, D.; Wu, M.; Yang, Q.; Jiang, J. The protective effect of dietary extract of Astragalus membranaceus on the high stocking density, copper and trichlorfon in Jian carp (Cyprinus carpio var. Jian). Aquac. Rep. 2024, 37, 102226. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, X. Comprehensive Application of Pericarpium Citri Reticulatae in the Healthy Aquaculture of Aquatic Animals. Beijing Fish. 2007, 4, 26–28. [Google Scholar]
- Liu, H.; Liu, G.; Li, G.; Jiang, Z.Q. Effects of compound traditional Chinese medicine additive on the growth and digestive enzyme activities of juvenile Lateolabrax japonicus. Feed Ind. 2008, 6, 4–7. [Google Scholar]
- Du, J.; Cao, L.P.; Jia, R.; Gu, Z.Y.; He, Q.; Xu, P.; Yin, G.J.; Ma, Y.Z. Alleviative effects of total flavones of Glycyrrhiza uralensis Fisch on oxidative stress and lipid metabolism disorder induced by high-fat diet in intestines of Tilapia (Oreochromis niloticus). 3 Biotech 2021, 11, 348. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.I.; Shen, J.; Yan, Z.; Xiang, X.; Mu, R.; Zhu, P.; Yao, Y.; Zhu, F.; Chen, K.; Chi, S.; et al. Dietary Glycyrrhiza uralensis extracts supplementation elevated growth performance, immune responses and disease resistance against Flavobacterium columnare in yellow catfish (Pelteobagrus fulvidraco). Fish Shellfish Immunol. 2020, 97, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.S.; Kim, S.G.; Jun, J.W.; Chi, C.; Saha, S.; Sukumaran, V.; Park, S.C. Jamun fruit extract enhances growth performance, mucosal immunity, disease resistance, and immune- and antioxidant-related gene expression of Cyprinus carpio juveniles. Aquac. Rep. 2024, 36, 102123. [Google Scholar] [CrossRef]
- Fouad, A.M.; Abo-Al-Ela, H.G.; Moneeb, R.H.; Alfons, M.S.; Salah, A.S.; Yusuf, S. Impact of Bambusa vulgaris-supplemented diet on Nile tilapia challenged with Pseudomonas putida: Hematological, immune, and oxidative responses. Fish Shellfish Immunol. 2025, 157, 110102. [Google Scholar] [CrossRef]
- Windyaswari, A.S.; Nugraha, M.F.I.; Hartati, R.; Elfahmi. Isolation and antimicrobial activity of secondary metabolites of pothos tener wall. Nat. Prod. Res. 2024, 5, 1–9. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, J.; Ma, Y.; Li, J.; Chen, X. The effective components of herbal medicines used for prevention and control of fish diseases. Fish Shellfish Immunol. 2022, 126, 73–83. [Google Scholar] [CrossRef]
- Zhu, F. A review on the application of herbal medicines in the disease control of aquatic animals. Aquaculture 2020, 526, 735422. [Google Scholar] [CrossRef]
- Gholipour-Kanani, H.; Sahandi, J.; Taheri, A. Influence of Garlic (Allium sativum) and Mother worth (Matricaria chamomilla) Extract on Ichthyophtirius multifilus Parasite Treatment in Sail Fin Molly (Poecilia latipinna) Ornamental Fish. APCBEE Procedia 2012, 4, 6–11. [Google Scholar] [CrossRef]
- de Andrade, J.I.A.; Jerônimo, G.T.; Brasil, E.M.; Nunez, C.V.; Gonçalves, E.L.T.; Ruiz, M.L.; Martins, M.L. Efficacy of seed extract of Bixa orellana against monogenean gill parasites and physiological aspects of Colossoma macropomum after bath treatment. Aquaculture 2016, 462, 40–46. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Y.; Li, G.; Huang, X.; Zheng, L.; Peng, M.; Cao, Y.; Wang, X. Effect of dietary supplementation Ampelopsis grossedentata extract on growth performance and muscle nutrition of Megalobrama hoffmanni by gut bacterial mediation. Heliyon 2024, 10, e29008. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Yang, Z.; Huang, Y.; Jian, J.; Tang, J. Effects of Chinese herbal medicines on growth performance, intestinal flora, immunity and serum metabolites of hybrid grouper (Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂). Fish Shellfish. Immunol 2023, 140, 108946. [Google Scholar] [CrossRef]
- Wang, X.; Qu, P.; Huangfu, Y.; Liu, D.; Wu, Y.; Chen, P.; Mai, K.; Zhang, W. A compound of herbs improves the growth performance, intestinal and liver histology, antioxidative capacity and immunity of juvenile large yellow croaker Larimichthys crocea. Aquac. Rep. 2024, 36, 102086. [Google Scholar] [CrossRef]
- Hajek, G.J.; Kłyszejko, B.; Dziaman, R. The anaesthetic effects of clove oil on common carp, Cyprinus carpio L. Acta Ichthyol. Et Piscat. 2006, 36, 93–97. [Google Scholar] [CrossRef]
- Vera-Jimenez, N.I.; Pietretti, D.; Wiegertjes, G.F.; Nielsen, M.E. Comparative study of β-glucan induced respiratory burst measured by nitroblue tetrazolium assay and real-time luminol-enhanced chemiluminescence assay in common carp (Cyprinus carpio L.). Fish Shellfish Immunol. 2013, 34, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Li, Z.Q.; Zhai, S.W.; Lai, X.J.; Yang, Q.H.; Jiang, X.L. In vitro effect of alcohol extracts of 5 traditional Chinese medicines on leukocyte immune activity in peripheral blood of Nile tilapia. Fish. Sci. 2020, 39, 160–167. [Google Scholar]
- Li, K.F.; Xu, Q.Y. Effects of resveratrol on growth performance, intestinal digestive enzyme activities, liver antioxidant indices and serum biochemical indices of Songpu mirror carp. Chin. J. Anim. Nutr. 2019, 31, 1833–1841. [Google Scholar]
- Zhu, L.; Zhao, T.; Wang, X.; Yang, S.; Hou, L.; Li, C.; Jiang, X.; Zhang, J.; Zhao, X.; Pei, C.; et al. Separation and phagocytosis analysis of peripheral blood leukocytes from Qihe crucian carp Carassius auratus. Aquaculture 2022, 552, 737992. [Google Scholar] [CrossRef]
- Shi, H.T.; Zhao, S.Z.; Wang, K.L.; Fan, M.X.; Han, Y.Q.; Wang, H.L. Effects of dietary Astragalus Membranaceus supplementation on growth performance, and intestinal morphology, microbiota and metabolism in common carp (Cyprinus carpio). Aquac. Rep. 2022, 22, 100955. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, Y.C.; Gao, X.N.; Guo, T.T.; Wang, B.; Gu, W. Effect of fermented Chinese medicine preparation by on growth performance, biochemical indices, antioxidant parameters and ability of anti-infection of common carp. China Anim. Husb. Vet. Med. 2017, 44, 724–731. [Google Scholar]
- Zhao, Q.; Chen, Y.C.; Gao, X.N.; Guo, T.T.; Gu, W. Effects of different probiotics fermented Chinese herb on growth performance and anti-infection ability in common carp. China Feed 2017, 2, 35–39. [Google Scholar]
- Jin, W.; Jiang, L.; Hu, S.; Zhu, A. Effects of Lactobacillus plantarum and Bacillus subtilis on growth, immunity and intestinal flora of largemouth bass(Micropterus salmoides). Aquaculture 2024, 583, 740581. [Google Scholar] [CrossRef]
- Wangkahart, E.; Wachiraamonloed, S.; Lee, P.T.; Subramani, P.A.; Qi, Z.; Wang, B. Impacts of Aegle marmelos fruit extract as a medicinal herb on growth performance, antioxidant and immune responses, digestive enzymes, and disease resistance against Streptococcus agalactiae in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2022, 120, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.J.; Du, Q.Y.; Yang, Y.; Wu, X.H. The Studies on the acute toxicity and physiology toxicity of powerful kitchen cleaner on Paramisgurnus dabryanus. Hubei Agric. Sci. 2011, 50, 557–559. [Google Scholar]
- Meng, Z.N.; Chen, Y.C.; Guan, X.T.; Zhang, H.; Liu, M. Effect of Chinese herb compounds on activities of transaminase in-serum and antioxidase in erythrocyte of Cyprinus carpio L. J. Northeast Agric. Univ. 2010, 41, 75–80. [Google Scholar]
- Tang, J.F.; Huang, Y.; Cai, J.; Lu, Y.S.; Wu, J.H.; Jian, J.C. Effects of a probiotics combined with Chinese herbal medicine on growth performance, water quality and resistance to diseases for Litopenaeus vannamei. J. Guangdong Ocean Univ. 2015, 35, 47–52. [Google Scholar]
- Ma, Y.-H.; Sheng, Y.-D.; Zhang, D.; Liu, J.-T.; Tian, Y.; Li, H.; Li, X.-F.; Li, N.; Sun, P.; Siddiqui, S.A.; et al. Acanthopanax senticosus cultures fermented by Lactobacillus rhamnosus enhanced immune response through improvement of antioxidant activity and inflammation in crucian carp (Carassius auratus). Microb. Pathog. 2024, 190, 106614. [Google Scholar] [CrossRef]
- Gou, G.; Jiang, M.; Wen, H.; Wu, F.; Liu, W.; Tian, J.; Yang, C.G. Effects of silymarin addition in feed on the growth performance, hepatic lipid metabolic enzymes, and antioxidant capacity of GIFT tilapia. J. Fish. 2016, 40, 1309–1320. [Google Scholar]
- Zhang, X.; Sun, Z.; Cai, J.; Wang, J.; Wang, G.; Zhu, Z.; Cao, F. Effects of dietary fish meal replacement by fermented moringa (Moringa oleifera Lam.) leaves on growth performance, nonspecific immunity and disease resistance against Aeromonas hydrophila in juvenile gibel carp (Carassius auratus gibelio var. CAS III). Fish Shellfish Immunol. 2020, 102, 430–439. [Google Scholar] [CrossRef]






| Items | Content (%) | Items | Content (%) |
|---|---|---|---|
| Shandong fish meal | 2.5 | Soybean lecithin | 0.5 |
| Soybean meal | 34.5 | CaH2PO4 | 1.5 |
| Rapeseed meal | 20 | Vitamin premix | 0.4 |
| Flour | 21.9 | Mineral premix | 0.6 |
| DDGS | 7.5 | Fungicide | 0.05 |
| Rice bran | 8.0 | Antioxidant | 0.05 |
| Fish oil | 2.5 | Total | 100 |
| Control Group | Experimental Group | Blank Group | |
|---|---|---|---|
| Leukocyte suspension (μL) | 100 | 100 | 100 |
| 5% DMSO solution (μL) | 100 | ||
| FCH (μL) | 100 | 100 | |
| PBS (μL) | 100 | 100 | 100 |
| Incubate at room temperature for 2 h, aspirate 100 μL of the supernatant, and wash three times with 100 μL of L-15 cell culture medium. | |||
| L-15 cell culture medium (μL) | 50 | 50 | |
| 0.2% NBT solution (μL) | 50 | ||
| PBS (μL) | 50 | 100 | |
| Incubate at room temperature for 1 h, aspirate 100 μL of the supernatant, wash three times with 100 μL of a 70% methanol solution, blow out the liquid from each well, and air-dry at room temperature. | |||
| 100 μL of 2 mol/L KOH solution and 200 μL of 100% dimethyl sulfoxide. | |||
| Absorbance reading at 630 nm using a microplate reader. | |||
| Control Group | Experimental Group | |
|---|---|---|
| Leukocyte suspension (μL) | 200 | 200 |
| FCH (μL) | 100 | |
| 5% DMSO solution (μL) | 100 | |
| Incubated at room temperature for 2 h. | ||
| Microsphere suspension (μL) | 50 | 50 |
| Mix thoroughly, room temperature, protect from light, and react for 1 h. | ||
| 70% methanol solution (μL) | 250 | 250 |
| Flow cytometry analysis. | ||
| Percoll (%) Percoll Concentration | 20% | 30% | 40% | 50% | 60% | 70% |
|---|---|---|---|---|---|---|
| (g/mL) Separation medium density | 1.031 | 1.043 | 1.056 | 1.067 | 1.077 | 1.090 |
| (105/mL) Leukocyte concentration | 2.37 | 2.42 | 3.60 | 6.19 | 8.79 | 14.25 |
| Items | Addition Amount of FCH | |||
|---|---|---|---|---|
| Control Treatment | Treatment 2% | Treatment 5% | Treatment 10% | |
| IBW/g | 44.61 ± 3.45 | 40.41 ± 1.08 | 42.85 ± 0.58 | 42.38 ± 3.78 |
| FBW/g | 48.64 ± 1.67 | 44.66 ± 1.29 | 47.42 ± 1.15 | 46.76 ± 1. 6 |
| WGR/% | 9.02 a ± 3.25 | 10.51 b ± 3.23 | 10.67 b ± 7.0 | 10.34 b ± 6.44 |
| SGR/% | 0.30 a ± 0.12 | 0.35 b ± 0.13 | 0.36 b ± 0.21 | 0.35 b ± 0.20 |
| FCR/% | 1.85 | 1.75 | 1.63 | 1.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, W.; Huang, X.; Han, F.; Li, Z. Effects of Probiotic-Fermented Chinese Herb on Immune Response and Growth Performance in Common Carp (Cyprinus carpio). Fishes 2025, 10, 196. https://doi.org/10.3390/fishes10050196
Zou W, Huang X, Han F, Li Z. Effects of Probiotic-Fermented Chinese Herb on Immune Response and Growth Performance in Common Carp (Cyprinus carpio). Fishes. 2025; 10(5):196. https://doi.org/10.3390/fishes10050196
Chicago/Turabian StyleZou, Wenzheng, Xuanxuan Huang, Fang Han, and Zhongqin Li. 2025. "Effects of Probiotic-Fermented Chinese Herb on Immune Response and Growth Performance in Common Carp (Cyprinus carpio)" Fishes 10, no. 5: 196. https://doi.org/10.3390/fishes10050196
APA StyleZou, W., Huang, X., Han, F., & Li, Z. (2025). Effects of Probiotic-Fermented Chinese Herb on Immune Response and Growth Performance in Common Carp (Cyprinus carpio). Fishes, 10(5), 196. https://doi.org/10.3390/fishes10050196





