Mechanisms of Laurel (Laurus nobilis) Essential Oil on Oxidative Stress and Apoptosis in Hybrid Grouper (Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂) During Keep Live Transport
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish and Laurel Essential Oil
2.2. Experimental Design
2.3. Methods
2.3.1. Analysis of Relevant Enzyme Activities and Oxidizing Substances
2.3.2. Serum Biochemical Analysis
2.3.3. Analysis of Enzymes Related to the Internal Environment
2.3.4. Histological Analysis
2.3.5. Transmission Electron Microscopy (TEM)
2.3.6. Real-Time Quantitative PCR (qPCR)
2.3.7. Nontargeted Metabolomics Analysis in Hybrid Grouper Under Transport Stress
2.4. Statistical Analysis
3. Results
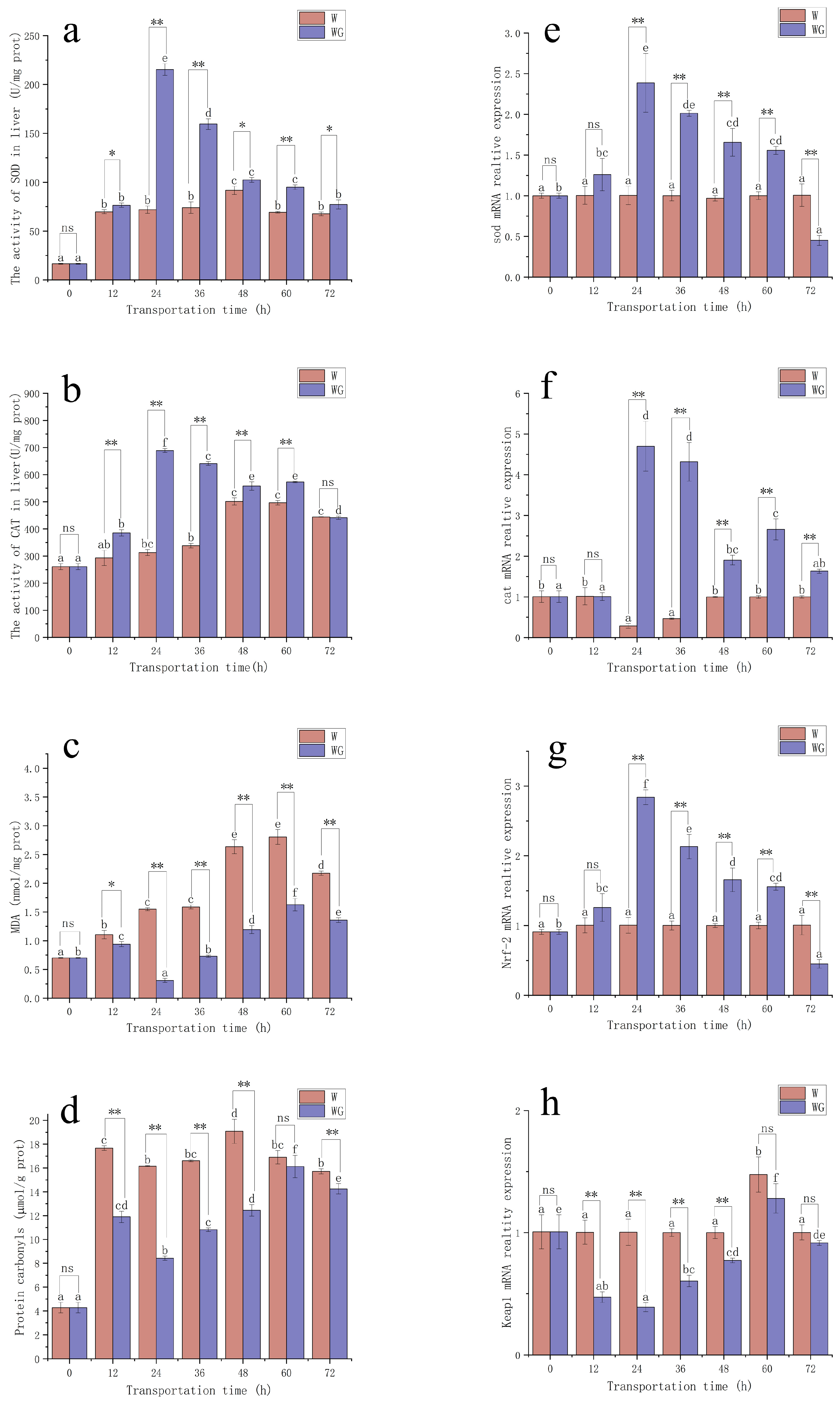
3.1. Oxidative Stress
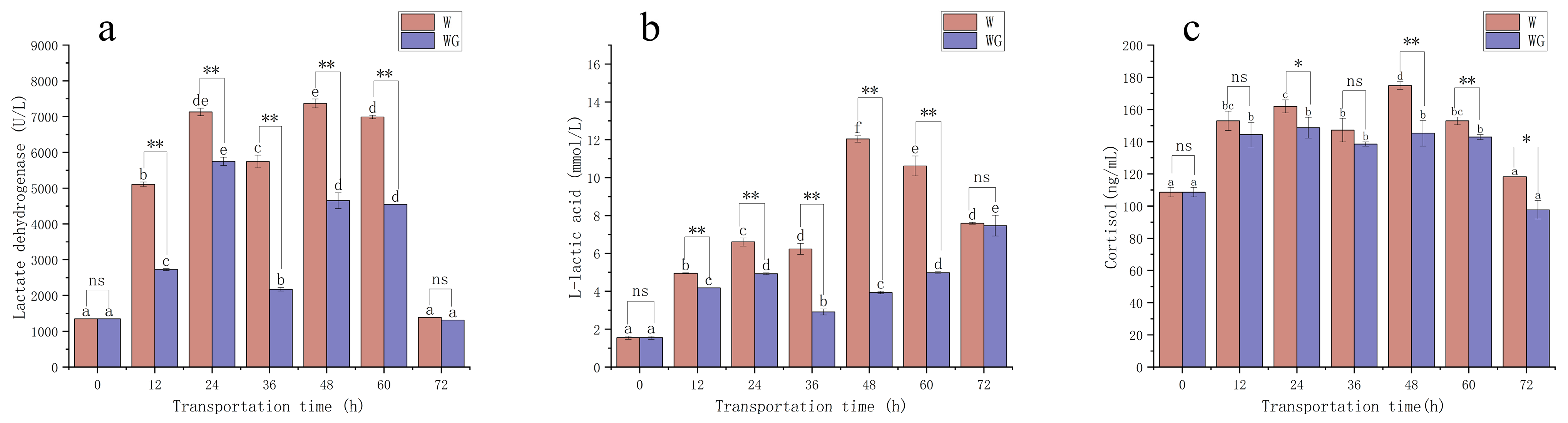
3.2. Serum Biochemical Indicators
3.3. Homeostasis of the Internal Environment
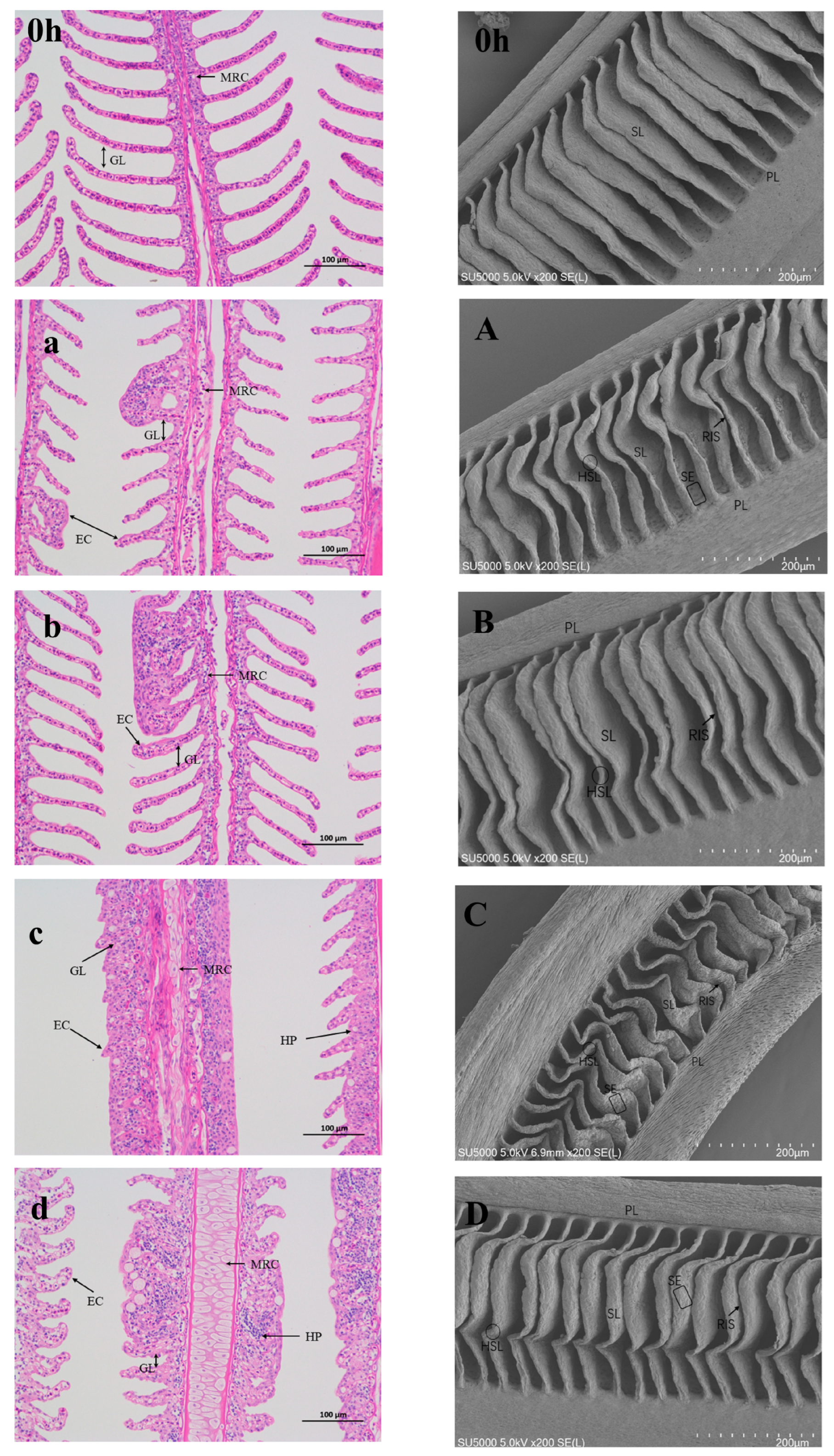
3.4. Stress in Gill Filaments
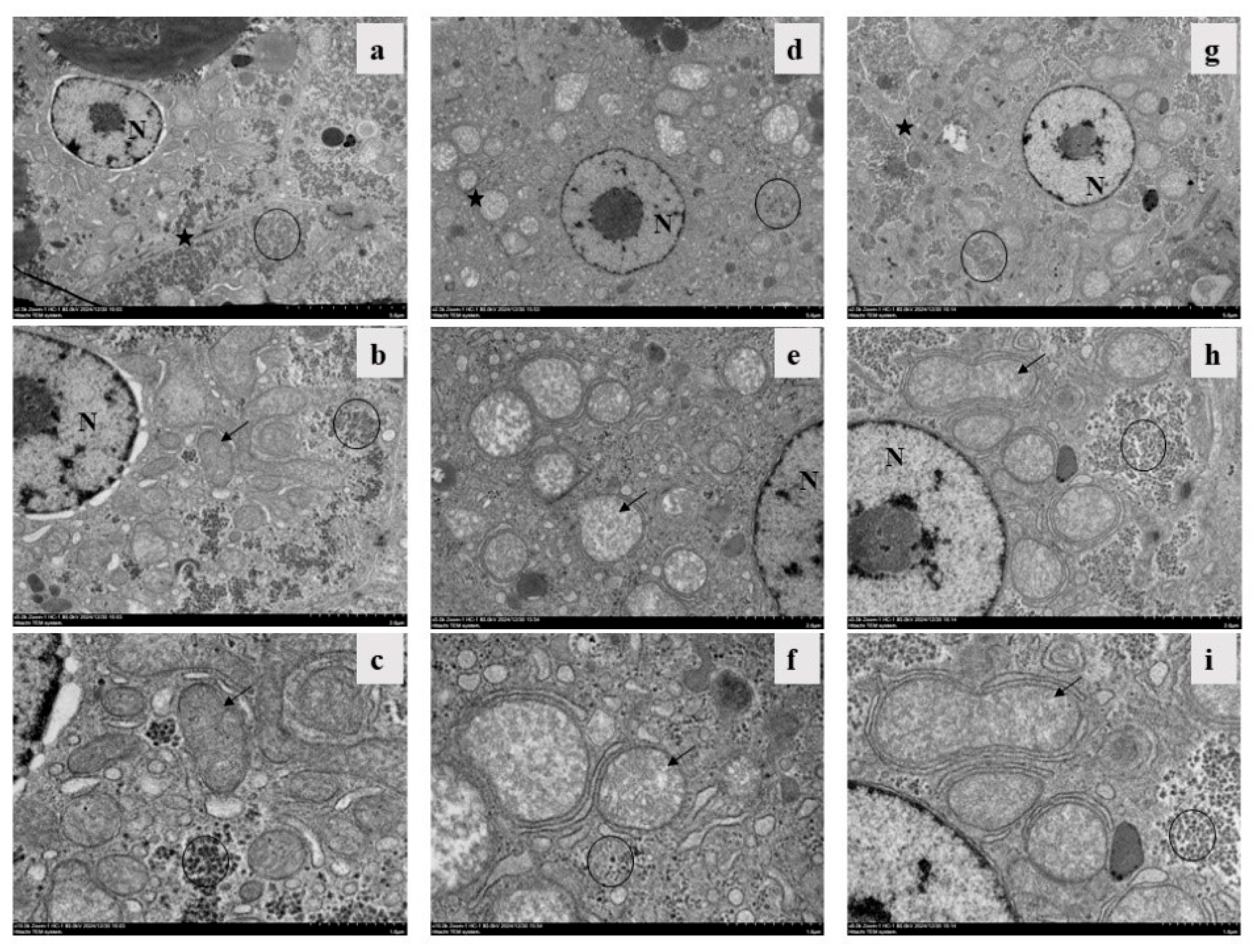
3.5. Electron Micrographs of Livers
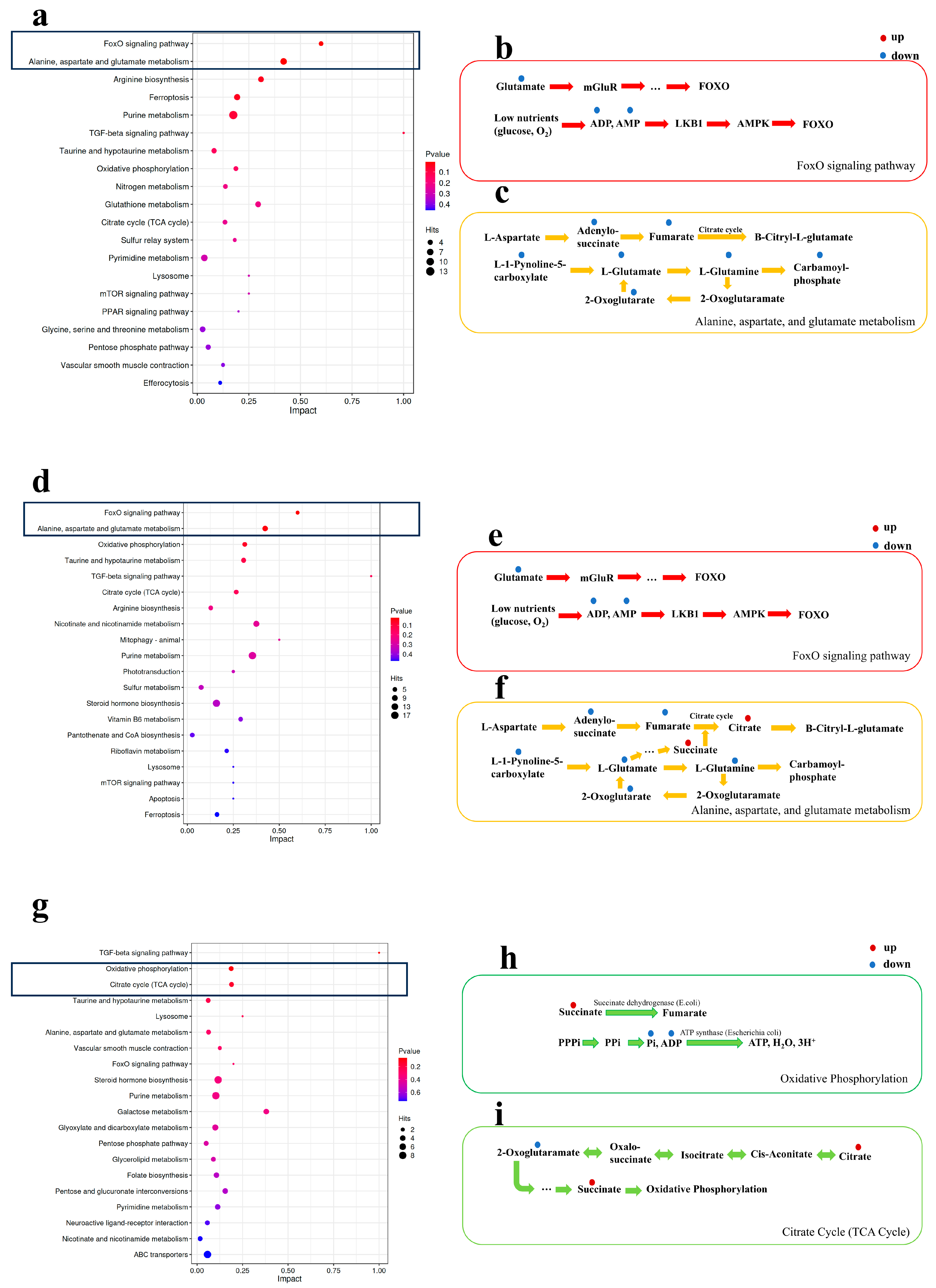
3.6. Analysis of Metabolic Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, L.; Meng, X.; Wang, X.; Fan, J.; Tan, B.; Deng, J. Comparative effects of different dietary pectin types on growth performance and intestinal health in pearl gentian grouper (Epinephelus fuscoguttatus♀ × E. lanceolatus♂). Aquacult. Rep. 2025, 40, 102547. [Google Scholar] [CrossRef]
- Harmon, T.S. Methods for reducing stressors and maintaining water quality associated with live fish transport in tanks: A review of the basics. Rev. Aquacult. 2009, 1, 58–66. [Google Scholar] [CrossRef]
- Purbosari, N.; Warsiki, E.; Syamsu, K.; Santoso, J. Natural versus synthetic anesthetic for transport of live fish: A review. Aquacult. Fish. 2019, 4, 129–133. [Google Scholar] [CrossRef]
- Beman, J.M.; Vargas, S.M.; Wilson, J.M.; Perez-Coronel, E.; Karolewski, J.S.; Vazquez, S.; Yu, A.; Cairo, A.E.; White, M.E.; Koester, I.; et al. Substantial oxygen consumption by aerobic nitrite oxidation in oceanic oxygen minimum zones. Nat. Commun. 2021, 12, 7043. [Google Scholar] [CrossRef]
- Fang, D.; Mei, J.; Xie, J.; Qiu, W. The Effects of Transport Stress (Temperature and Vibration) on Blood Biochemical Parameters, Oxidative Stress, and Gill Histomorphology of Pearl Gentian Groupers. Fishes 2023, 8, 218. [Google Scholar] [CrossRef]
- Kim, J.-H.; Cho, J.-H.; Kim, S.-R.; Hur, Y.B. Toxic effects of waterborne ammonia exposure on hematological parameters, oxidative stress and stress indicators of juvenile hybrid grouper, Epinephelus lanceolatus ♂ × Epinephelus fuscoguttatus ♀. Environ. Toxicol. Pharmacol. 2020, 80, 103453. [Google Scholar] [CrossRef]
- Vinagre, C.; Madeira, D.; Narciso, L.; Cabral, H.N.; Diniz, M. Effect of temperature on oxidative stress in fish: Lipid peroxidation and catalase activity in the muscle of juvenile seabass, Dicentrarchus labrax. Ecol. Indic. 2012, 23, 274–279. [Google Scholar] [CrossRef]
- Skår, M.W.; Haugland, G.T.; Powell, M.D.; Wergeland, H.I.; Samuelsen, O.B. Development of anaesthetic protocols for lumpfish (Cyclopterus lumpus L.): Effect of anaesthetic concentrations, sea water temperature and body weight. PLoS ONE 2017, 12, e0179344. [Google Scholar] [CrossRef]
- Aydin, B.; Barbas, L.A.L. Sedative and anesthetic properties of essential oils and their active compounds in fish: A review. Aquaculture 2020, 520, 734999. [Google Scholar] [CrossRef]
- Wang, Q.; Mei, J.; Xie, J. The Effects of Lemon Balm (Melissa officinalis L.) Essential Oil on the Stress Response, Anti-Oxidative Ability, and Kidney Metabolism of Sea Bass during Live Transport. Animals 2022, 12, 339. [Google Scholar] [CrossRef] [PubMed]
- Boaventura, T.P.; Souza, C.F.; Ferreira, A.L.; Favero, G.C.; Baldissera, M.D.; Heinzmann, B.M.; Baldisserotto, B.; Luz, R.K. The use of Ocimum gratissimum L. essential oil during the transport of Lophiosilurus alexandri: Water quality, hematology, blood biochemistry and oxidative stress. Aquaculture 2021, 531, 735964. [Google Scholar] [CrossRef]
- Cao, J.; Guo, M.J.; Wang, J.F.; Mei, J.; Xie, J. Efficiency of Cinnamomum camphora var. linaloofera Fujita essential oil, MS-222, and linalool on anesthesia and transportation of spotted seabass (Lateolabrax maculatus). Fish Physiol. Biochem. 2025, 51, 103. [Google Scholar] [CrossRef]
- Fang, D.; Zhang, C.; Mei, J.; Qiu, W.; Xie, J. Effects of Ocimum basilicum essential oil and ginger extract on serum biochemistry, oxidative stress and gill tissue damage of pearl gentian grouper during simulated live transport. Vet. Res. Commun. 2024, 48, 139–152. [Google Scholar] [CrossRef]
- Ferreira, A.L.; dos Santos, F.A.C.; Souza, A.D.; Favero, G.C.; Pinheiro, C.G.; Heinzmann, B.M.; Baldisserotto, B.; Luz, R.K. Anesthetic and sedative efficacy of essential oil of Hesperozygis ringens and the physiological responses of Oreochromis niloticus after biometric handling and simulated transport. Fish Physiol. Biochem. 2022, 48, 1155–1166. [Google Scholar] [CrossRef]
- Hoch, C.C.; Petry, J.; Griesbaum, L.; Weiser, T.; Werner, K.; Ploch, M.; Verschoor, A.; Multhoff, G.; Dezfouli, A.B.; Wollenberg, B. 1,8-cineole (eucalyptol): A versatile phytochemical with therapeutic applications across multiple diseases. Biomed. Pharmacother. 2023, 167, 115467. [Google Scholar] [CrossRef]
- Yigit, N.O.; Metin, S.; Didinen, B.I.; Didinen, H.; Ozmen, O. Effect of lavander (Lavandula angustifolia) and laurel (Laurus nobilis) essential oils as anesthesics in rainbow trout (Oncorhynchus mykiss). Aquaculture 2022, 557, 738328. [Google Scholar] [CrossRef]
- Jung, J.-W.; Wang, F.; Turk, A.; Park, J.-S.; Ma, H.; Ma, Y.; Noh, H.-R.; Sui, G.; Shin, D.-S.; Lee, M.-K.; et al. Zaluzanin C Alleviates Inflammation and Lipid Accumulation in Kupffer Cells and Hepatocytes by Regulating Mitochondrial ROS. Molecules 2023, 28, 7484. [Google Scholar] [CrossRef]
- Li, S.; Wang, A.; Li, Z.; Zhang, J.; Sang, C.; Chen, N. Antioxidant defenses and non-specific immunity at enzymatic and transcriptional levels in response to dietary carbohydrate in a typical carnivorous fish, hybrid grouper (Epinephelus fuscoguttatus ♀ × E. lanceolatus ♂). Fish Shellfish Immunol. 2020, 100, 109–116. [Google Scholar] [CrossRef]
- Wang, Y.X.; Xu, S.F.; Han, C.Z.; Wang, L.Q.; Zheng, Q.; Wang, S.W.; Huang, Y.H.; Wei, S.A.; Qin, Q.W. Curcumin inhibits Singapore grouper iridovirus infection through multiple antiviral mechanisms. Aquaculture 2023, 562, 738870. [Google Scholar] [CrossRef]
- Ahmed, I.; Ahmad, I.; Malla, B.A. Effects of dietary tryptophan levels on growth performance, plasma profile, intestinal antioxidant capacity and growth related genes in rainbow trout (Oncorhynchus mykiss) fingerlings. Aquaculture 2024, 585, 740710. [Google Scholar] [CrossRef]
- Wang, Q.; Mei, J.; Cao, J.; Xie, J. Effects of Melissa officinalis L. Essential Oil in Comparison with Anaesthetics on Gill Tissue Damage, Liver Metabolism and Immune Parameters in Sea Bass (Lateolabrax maculatus) during Simulated Live Transport. Biology 2022, 11, 11. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, F.; Chen, K.; Guo, Y.; Liang, Y.; Zhao, H.; Chen, S. Exposure of zebrafish to a cold environment triggered cellular autophagy in zebrafish liver. J. Fish Dis. 2022, 45, 991–1000. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Cao, J.; Fang, D.; Qiu, W.Q.; Xie, J. Effects of Exogenous Tryptophan in Alleviating Transport Stress in Pearl Gentian Grouper (Epinephelus fuscoguttatus ♀ × E. lanceolatus ♂). Animals 2024, 14, 3583. [Google Scholar] [CrossRef]
- Cao, J.; Mei, J.; Xie, J. Combined effects of hypoxia and ammonia-N exposure on the immune response, oxidative stress, tissue injury and apoptosis of hybrid grouper (Epinephelus fuscoguttatus ♀ × E. lanceolatus ♂). Environ. Sci. Pollut. Res. 2024, 31, 1050–1063. [Google Scholar] [CrossRef]
- Chu, Y.; Mei, J.; Xie, J. Integrated volatile compounds and non-targeted metabolomics analysis reveal the characteristic flavor formation of proteins in grouper (Epinephelus coioides) during cold storage. Food Res. Int. 2023, 172, 113145. [Google Scholar] [CrossRef]
- Hong, J.; Chen, X.; Liu, S.; Fu, Z.; Han, M.; Wang, Y.; Gu, Z.; Ma, Z. Impact of fish density on water quality and physiological response of golden pompano (Trachinotus ovatus) flingerlings during transportation. Aquaculture 2019, 507, 260–265. [Google Scholar] [CrossRef]
- Wang, J.; Lu, Q.; Cai, J.; Wang, Y.; Lai, X.; Qiu, Y.; Huang, Y.; Ke, Q.; Zhang, Y.; Guan, Y.; et al. Nestin regulates cellular redox homeostasis in lung cancer through the Keap1–Nrf2 feedback loop. Nat. Commun. 2019, 10, 5043. [Google Scholar] [CrossRef]
- Zhang, D.D. The Nrf2-Keap1-ARE Signaling Pathway: The Regulation and Dual Function of Nrf2 in Cancer. Antioxid. Redox Signal. 2010, 13, 1623–1626. [Google Scholar] [CrossRef]
- McCarroll, M.N.; Gendelev, L.; Kinser, R.; Taylor, J.; Bruni, G.; Myers-Turnbull, D.; Helsell, C.; Carbajal, A.; Rinaldi, C.; Kang, H.J.; et al. Zebrafish behavioural profiling identifies GABA and serotonin receptor ligands related to sedation and paradoxical excitation. Nat. Commun. 2019, 10, 4078. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Brooks, G.A. The Science and Translation of Lactate Shuttle Theory. Cell Metab. 2018, 27, 757–785. [Google Scholar] [CrossRef]
- Magistretti, P.J.; Allaman, I. Lactate in the brain: From metabolic end-product to signalling molecule. Nat. Rev. Neurosci. 2018, 19, 235–249. [Google Scholar] [CrossRef]
- Wu, S.M.; Chen, J.-R.; Chang, C.-Y.; Tseng, Y.-J.; Pan, B.S. Potential benefit of I-Tiao-Gung (Glycine tomentella) extract to enhance ornamental fish welfare during live transport. Aquaculture 2021, 534, 736304. [Google Scholar] [CrossRef]
- Biswal, A.; Srivastava, P.P.; Krishna, G.; Paul, T.; Pal, P.; Gupta, S.; Varghese, T.; Jayant, M. An Integrated biomarker approach for explaining the potency of exogenous glucose on transportation induced stress in Labeo rohita fingerlings. Sci. Rep. 2021, 11, 5713. [Google Scholar] [CrossRef]
- Zheng, T.; Song, Z.; Tao, Y.; Qiang, J.; Ma, J.; Lu, S.; Xu, P. Transport stress induces innate immunity responses through TLR and NLR signaling pathways and increases mucus cell number in gills of hybrid yellow catfish (Tachysurus fulvidraco ♀ × Pseudobagrus vachellii ♂). Fish Shellfis. Immunol. 2022, 127, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Nwosu, L.C.; Adedire, C.O.; Ogunwolu, E.O.; Ashamo, M.O. Toxicological and histopathological effects of Dennettia tripetala seed used as grain protectant, food, and medicine. Food Qual. Saf. 2017, 1, 211–219. [Google Scholar] [CrossRef]
- Chen, V.L.; Du, X.M.; Chen, Y.H.; Kuppa, A.; Handelman, S.K.; Vohnoutka, R.B.; Peyser, P.A.; Palmer, N.D.; Bielak, L.F.; Halligan, B.; et al. Genome-wide association study of serum liver enzymes implicates diverse metabolic and liver pathology. Nat. Commun. 2021, 12, 816. [Google Scholar] [CrossRef] [PubMed]
- Tikhe, C.V.; Issiaka, S.; Dong, Y.; Kefi, M.; Tavadia, M.; Bilgo, E.; Corder, R.M.; Marshall, J.; Diabate, A.; Dimopoulos, G. Chromobacterium biopesticide overcomes insecticide resistance in malaria vector mosquitoes. Sci. Adv. 2024, 10, eads3658. [Google Scholar] [CrossRef]
- Zeng, X.; Dong, H.; Yang, Y.; Li, T.; Li, C.; Zhang, J. Effects of essential oil of Magnolia denudata on spotted seabass (Lateolabrax maculatus) during simulated live transportation. Aquaculture 2023, 567, 739258. [Google Scholar] [CrossRef]
- Chin, A.C.; Gao, Z.; Riley, A.M.; Furkert, D.; Wittwer, C.; Dutta, A.; Rojas, T.; Semenza, E.R.; Felder, R.A.; Pluznick, J.L.; et al. The inositol pyrophosphate 5-InsP7 drives sodium-potassium pump degradation by relieving an autoinhibitory domain of PI3K p85α. Sci. Adv. 2020, 6, eabb8542. [Google Scholar] [CrossRef] [PubMed]
- Gatto, C.; McLoud, S.M.; Kaplan, J.H. Heterologous expression of Na+-K+-ATPase in insect cells: Intracellular distribution of pump subunits. Am. J. Physiol. Cell Physiol. 2001, 281, C982–C992. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.H.; Zheng, P.L.; Guo, H.Y.; Jiang, S.G.; Qin, J.G.; Zhang, D.C.; Liu, X.L. Salinity regulates antioxidant enzyme and Na+K+-ATPase activities of juvenile golden pompano Trachinotus ovatus (Linnaeus 1758). Aquacult. Res. 2016, 47, 1481–1487. [Google Scholar] [CrossRef]
- Liu, B.-L.; Jia, R.; Huang, B.; Lei, J.-L. Interactive effect of ammonia and crowding stress on ion-regulation and expression of immune-related genes in juvenile turbot (Scophthalmus maximus). Mar. Freshwater Behav. Physiol. 2017, 50, 179–194. [Google Scholar] [CrossRef]
- Popgeorgiev, N.; Sa, J.D.; Jabbour, L.; Banjara, S.; Nguyen, T.T.M.; Akhavan-E-Sabet, A.; Gadet, R.; Ralchev, N.; Manon, S.; Hinds, M.G.; et al. Ancient and conserved functional interplay between Bcl-2 family proteins in the mitochondrial pathway of apoptosis. Sci. Adv. 2020, 6, eabc4149. [Google Scholar] [CrossRef]
- Tait, S.W.; Oberst, A.; Quarato, G.; Milasta, S.; Haller, M.; Wang, R.; Karvela, M.; Ichim, G.; Yatim, N.; Albert, M.L.; et al. Widespread mitochondrial depletion via mitophagy does not compromise necroptosis. Cell Rep. 2013, 5, 878–885. [Google Scholar] [CrossRef]
- Wang, Y.T.; Meng, S.T.; Li, D.L.; Liu, S.Y.; Li, L.; Wu, L.F. Dietary chlorogenic acid supplementation protects against lipopolysaccharide-induced oxidative stress, inflammation and apoptosis in intestine of amur ide (Leuciscus waleckii). Aquat. Toxicol. 2025, 279, 107223. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Fu, Z.Y.; Liu, X.C.; Ma, Z.H. The Impact of Acute Ammonia Nitrogen Stress on the Gill Tissue Structure and Antioxidant Ability of Gills and Red and White Muscle in Juvenile Yellowfin Tuna (Thunnus albacares). Antioxidants 2024, 13, 1357. [Google Scholar] [CrossRef]
- Yao, Y.; Lin, J.; Yang, P.; Chen, Q.; Chu, X.; Gao, C.; Hu, J. Fine structure, enzyme histochemistry, and immunohistochemistry of liver in zebrafish. Anat. Rec. 2012, 295, 567–576. [Google Scholar] [CrossRef]
- Aghaallaei, N.; Bajoghli, B.; Schwarz, H.; Schorpp, M.; Boehm, T. Characterization of mononuclear phagocytic cells in medaka fish transgenic for a cxcr3a:gfp reporter. Proc. Natl. Acad. Sci. USA 2010, 107, 18079–18084. [Google Scholar] [CrossRef] [PubMed]
- Samanta, P.; Pal, S.; Senapati, T.; Mukherjee, A.K.; Ghosh, A.R. Assessment of adverse outcome of Excel Mera 71 in Anabas testudineus by histological and ultrastructural alterations. Aquat. Toxicol. 2018, 205, 19–24. [Google Scholar] [CrossRef]
- Clifton, L.A.; Wacklin-Knecht, H.P.; Ådén, J.; Mushtaq, A.U.; Sparrman, T.; Gröbner, G. Creation of distinctive Bax-lipid complexes at mitochondrial membrane surfaces drives pore formation to initiate apoptosis. Sci. Adv. 2023, 9, eadg7940. [Google Scholar] [CrossRef]
- Hwang, S.; Song, S.; Hong, Y.K.; Choi, G.; Suh, Y.S.; Han, S.Y.; Lee, M.; Park, S.H.; Lee, J.H.; Lee, S.; et al. Drosophila DJ-1 decreases neural sensitivity to stress by negatively regulating Daxx-like protein through dFOXO. PLoS Genet. 2013, 9, e1003412. [Google Scholar] [CrossRef]
- Ma, F.; Zhao, L.; Ma, R.L.; Wang, J.; Du, L.Q. FoxO signaling and mitochondria-related apoptosis pathways mediate tsinling lenok trout (Brachymystax lenok tsinlingensis) liver injury under high temperature stress. Int. J. Biol. Macromol. 2023, 251, 126404. [Google Scholar] [CrossRef]
- Anupama, N.; Sindhu, G.; Raghu, K.G. Significance of mitochondria on cardiometabolic syndromes. Fundam. Clin. Pharmacol. 2018, 32, 346–356. [Google Scholar] [CrossRef]
- Rainey, N.E.; Moustapha, A.; Parker, R.; Petit, P.X. Curcumin a Multifaceted Compound with Hormetic Behaviour that Mediates an Intricate Crosstalk between Mitochondrial Biogenesis, Mitophagy, Mitophagic Death and Apoptosis. Preprints 2017, 25687225. [Google Scholar]
- Qu, L.L.; Yu, B.; Li, Z.; Jiang, W.X.; Jiang, J.D.; Kong, W.J. Gastrodin Ameliorates Oxidative Stress and Proinflammatory Response in Nonalcoholic Fatty Liver Disease through the AMPK/Nrf2 Pathway. Phytother. Res. 2016, 30, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.L.; Liang, J.P.; Yang, W.W.; Wang, S.X.; Yu, J.; Jia, P.; Du, Y.P.; Wang, M.; Li, Y.; Zheng, X.H. Ultra-Performance Liquid Chromatography-Q-Exactive Orbitrap-Mass Spectrometry Analysis for Metabolic Communication between Heart and Kidney in Adriamycin-Induced Nephropathy Rats. Kidney Blood Pressure Res. 2021, 47, 31–42. [Google Scholar] [CrossRef]

| Group | Treatment |
|---|---|
| W | Transport; no additives |
| WG | Transport + 10 mg/L laurel oil |
| Gene | Forward Sequence | Reverse Sequence | GenBank Accession No. |
|---|---|---|---|
| gst | GGGTCTCCCCTCAAACACATCC | GGACCTGAATGGCTCACTGGAA | XM_033624859.1 |
| gadph | CATCACTGCCACCCAGAAGA | GACAGCTTTAGCAGCACCAGTAGA | [24] |
| sod | GAGACCAGTGGGACCGTGTATTT | GCATCTTGTCCGTGATGTCTATCTT | AY735008.1 |
| cat | CACATCACCGTCGTCAGGAACTA | CTATCATCCGTACTGATTCCTTGTT | KT884509.1 |
| Nrf2 | GTGGCAAGAACAAGGTAGC | GTATTCGGAGGGGGAGTAG | [18] |
| Keap1 | TACGCTGTTTGGACTGCTCT | GCTGGACTCGGTGTTGTTTT | [18] |
| Bcl-2 | CTCCCATCCTCTTTGGCTCTG | ATCGTAGGGCTTTTCGCTTTC | |
| Bax | CGTCCTGAAGAAATCCAAACA | ACTGGGGAAGAATCATCGTG | KP335157.1 |
| Caspase 8 | TGCTTCTTGTGTCGTGATGTTG | GCGTCGGTCTCTTCTGGTTG | [25] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, M.; Wang, J.; Mei, J.; Xie, J. Mechanisms of Laurel (Laurus nobilis) Essential Oil on Oxidative Stress and Apoptosis in Hybrid Grouper (Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂) During Keep Live Transport. Fishes 2025, 10, 436. https://doi.org/10.3390/fishes10090436
Yuan M, Wang J, Mei J, Xie J. Mechanisms of Laurel (Laurus nobilis) Essential Oil on Oxidative Stress and Apoptosis in Hybrid Grouper (Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂) During Keep Live Transport. Fishes. 2025; 10(9):436. https://doi.org/10.3390/fishes10090436
Chicago/Turabian StyleYuan, Ming, Jingjing Wang, Jun Mei, and Jing Xie. 2025. "Mechanisms of Laurel (Laurus nobilis) Essential Oil on Oxidative Stress and Apoptosis in Hybrid Grouper (Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂) During Keep Live Transport" Fishes 10, no. 9: 436. https://doi.org/10.3390/fishes10090436
APA StyleYuan, M., Wang, J., Mei, J., & Xie, J. (2025). Mechanisms of Laurel (Laurus nobilis) Essential Oil on Oxidative Stress and Apoptosis in Hybrid Grouper (Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂) During Keep Live Transport. Fishes, 10(9), 436. https://doi.org/10.3390/fishes10090436










