Physiological Effects of Suspended Solids on Venerupis philippinarum and Argopecten irradians
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Experimental Organisms
2.2. Suspended Solids Exposure
2.3. Serum Analysis
2.4. Cortisol Analysis
2.5. Water Temperature Stress
2.6. Statistical Analysis
3. Results
3.1. Cumulative Survival During Suspended Solids
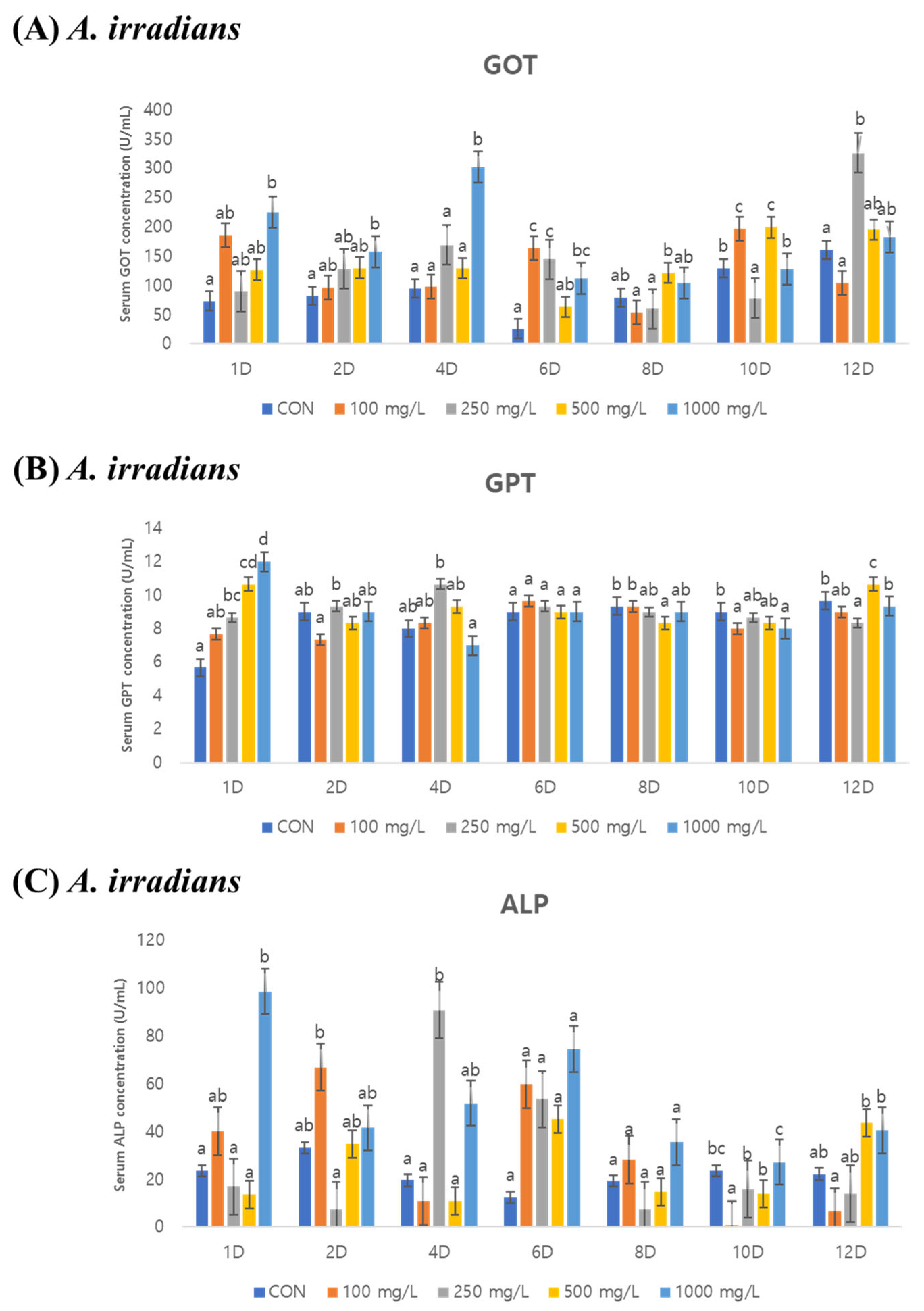
3.2. Serum Analysis
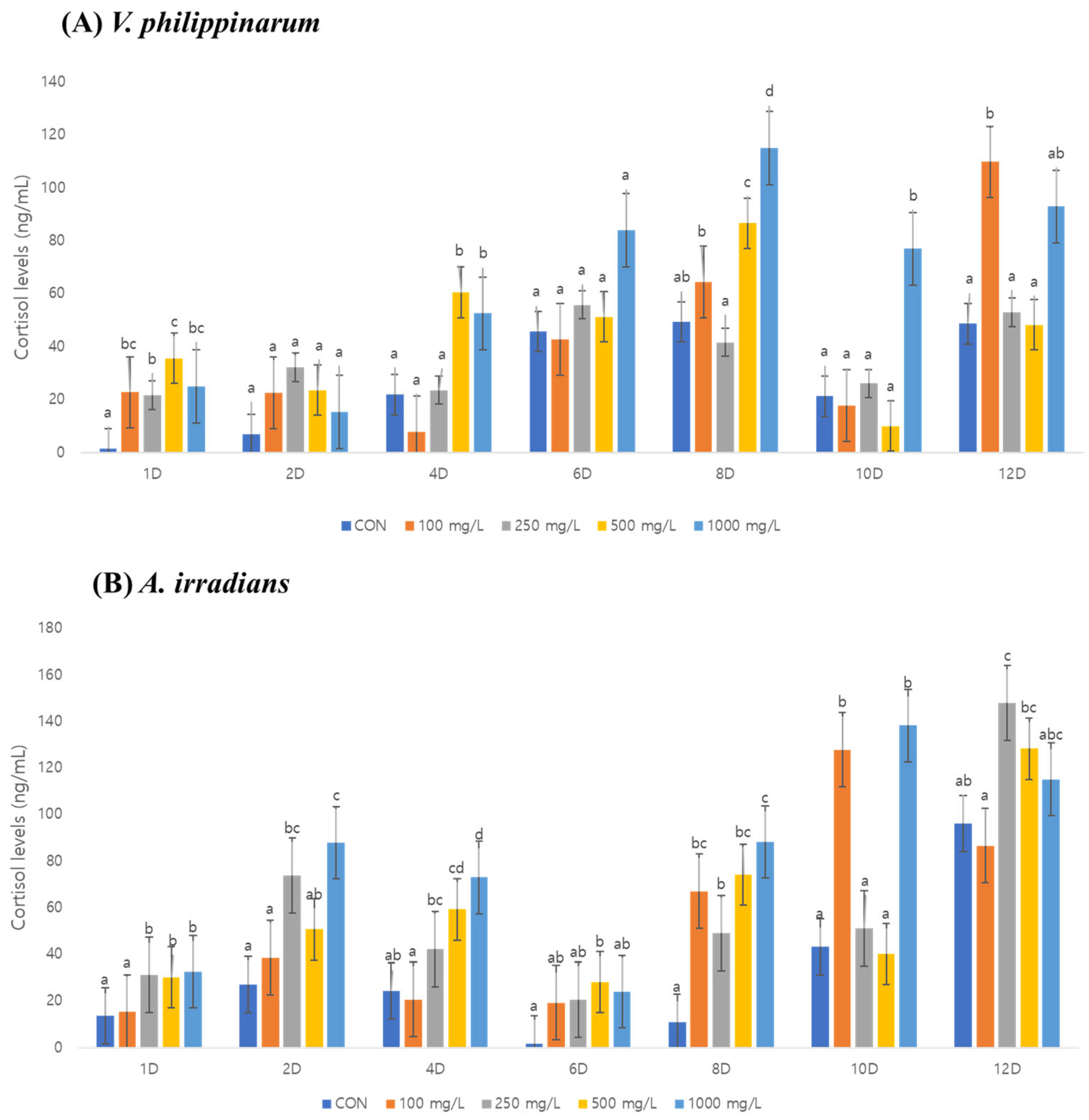
3.3. Cortisol Analysis
3.4. Cumulative Survival After Water Temperature Stress Stimulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, C.S.; Lim, H.S. Sediment dispersal and deposition due to sand mining in the coastal waters of Korea. Cont. Shelf Res. 2009, 29, 194–204. [Google Scholar] [CrossRef]
- Pollock, F.; Lamb, J.; Field, S.; Heron, S.; Schaffelke, B.; Shedrawi, G.; Bourne, D.; Willis, B. Sediment and turbidity associated with offshore dredging increase coral disease prevalence on nearby reefs. PLoS ONE 2014, 9, e102498. [Google Scholar] [CrossRef]
- Maeng, J.H.; Kim, S.D.; Park, C.W.; Sung, C.G. Evaluation of Marine Organisms Effects of Suspended Sediment through the Bioassay. Korean Soc. Environ. Health Toxicol. 2021, 24, 300–311. [Google Scholar]
- Zezulka, Š.; Maršálek, B.; Maršálková, E.; Odehnalová, K.; Pavlíková, M.; Lamaczová, A. Suspended particles in water and energetically sustainable solutions of their removal—A Review. Processes 2024, 12, 2627. [Google Scholar] [CrossRef]
- British Columbia Ministry of Environment. Water Quality Guidelines for Suspended and Benthic Sediments; British Columbia Ministry of Environment: Victoria, BC, Canada, 2014.
- Minnesota Pollution Control Agency (MPCA). Water Quality Standards for Class 2B Waters; Minnesota Pollution Control Agency: St. Paul, MN, USA, 2015.
- South African Department of Water Affairs and Forestry. South African Water Quality Guidelines: Marine & Coastal Water; South African Department of Water Affairs and Forestry: Pretoria, South Africa, 1995.
- European Commission. Directive 2000/60/EC Establishing a Framework for Community Action in the Field of Water Policy (Water Framework Directive); European Commission: Brussels, Belgium, 2000.
- Netherlands Ministry of Infrastructure and Environment. Water Quality Guidelines; Netherlands Ministry of Infrastructure and Environment: The Hague, The Netherlands, 2014.
- Ellis, M.M. Some Factors Affecting the Replacement of the Commercial Fresh-Water Mussel; U.S. Department of Commerce, Bureau of Fisheries: Washington, DC, USA, 1931; Volume 7, 10p.
- Moor, M. The Cutaneous sense organs of barbled minnows adapted to life in the muddy waters of the Great Plains Resion. Trans. Am. Micro. Soc. 1950, 69, 69–95. [Google Scholar] [CrossRef]
- Gammon, J.R. The Effect of Inorganic Sediment on Stream Biota; Environmental Protection Agency, Water Quality Office: Canberra, Australia, 1970; Volume 12, pp. 1–141.
- Park, S.Y.; Kwon, I.; Lee, J.; Yoon, S.J.; Lee, C.; Khim, J.S. Range of the Biological Effects and Threshold Concentrations on Marine Organisms by suspended Solids. J. Korean Soc. Mar. Environ. Energy 2022, 25, 29–40. [Google Scholar] [CrossRef]
- Quinn, J.M.; Davies-Colley, R.J.; Hichey, C.W.; Vickers, M.L.; Ryan, P.A. Effects of clay discharges on streams; 2. Benethic invertebrates. Hydrobiologia 1992, 248, 235–247. [Google Scholar] [CrossRef]
- Ellis, J.; Cummings, V.; Hewitt, J.; Thrush, S.; Norkko, A. Determining effects of suspended sediment on condition of a suspension feeding bivalve(Atrina zelandica): Results of a survey, a laboratory experiment and a field transplant experiment. J. Exp. Mar. Biol. Ecol. 2002, 267, 147–174. [Google Scholar] [CrossRef]
- Bilotta, G.S.; Brazier, R.E. Understanding the influence of suspended solids on water quality and aquatic biota. Water Res. 2008, 42, 2849–2861. [Google Scholar] [CrossRef]
- Newcombe, C.P.; Jensen, J.O.T. Channel suspended sediment and fisheries: A synthesis for quantitative assessment of risk and impact. N. Am. J. Fish. Manag. 1996, 16, 693–727. [Google Scholar] [CrossRef]
- Park, K.J.; Yoon, S.P.; Song, J.h.; Han, H.S. Improvement of Manila clam (Ruditapes philippinarum) habitat condition by adding crushed oyster (Crassostrea gigas) shells to the substratum. Korean J. Malacol. 2011, 27, 291–297. [Google Scholar] [CrossRef][Green Version]
- Choi, M.; Lee, I.S.; Kim, C.S.; Kim, H.C.; Hwang, D.W. Distributions of Organic Matter and Trace Metals in Surface Sediments around a Manila Clam Ruditapes phillippinarum Farming Area in Gomso Bay, Korea. J. Fish. Aquat. Sci. 2015, 48, 555–563. [Google Scholar][Green Version]
- Shumway, S.E.; Parsons, G.J. Scallops: Biology, Ecology and Aquaculture, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Lee, J.Y. The effects of suspended solids on the mortality and the glycogen content of abalone, Haliotis discus hannai. J. Korean Soc. Mar. Environ. Saf. 1994, 14, 183–187. [Google Scholar][Green Version]
- Shin, P.K.S.; Yau, F.N.; Chow, S.H.; Tai, K.K.; Cheung, S.G. Responses of the green-lipped mussel Perna viridis (L.) to suspended solids. Mar. Pollut. Bull. 2002, 45, 157–162. [Google Scholar] [CrossRef]
- Norkko, J.; Hewitt, J.E.; Thrus, S.F. Effects of increased sedimentation on the physiology of two estuarine soft-sediment bivalves, Austrovenus stutchburyi and Paphies australis. J. Exp. Mar. Biol. Ecol. 2006, 333, 12–26. [Google Scholar] [CrossRef]
- Casillas, E.; Sundquist, J.; Ames, W.E. Optimization of assay conditions for and the selected tissue distribution of alanine aminotransferase and aspartate aminotransferase of English sole. Parophrys vetulus Girard. J. Fish. Biol. 1982, 21, 197–204. [Google Scholar] [CrossRef]
- Myeong, J.I.; Pack, S.Y.; Chang, Y.J. Effects of water temperature and feeding rate on growth and feed efficiency of Korean rockfish, Sebastes schlegeli. J. Aquac. 1997, 10, 311–320. [Google Scholar]
- Eune, J.J.; Lee, E.S.; Rim, J.S.; Jang, H.S.; Woo, H.I. Changes of serum alkaline phosphatase after enucleation of systs in the jaws. J. Korean Assoc. Oral Maxillofac. Surg. 2005, 31, 417–421. [Google Scholar]
- Liang, S.; Luo, X.; You, W.; Luo, L.; Liu, C.K.; Zhang, J.F.; Shen, H.; Wang, X.R.; Wang, W.M. The role of hybridiztion in improving the immune response and thermal tolerance of abalone. Fish. Shellfish. Immunol. 2014, 39, 69–77. [Google Scholar] [CrossRef]
- Choi, Y.K. Molecular and Physiological Studies on the Effects of Waterborne Pollutants in Pacific Oyster, Crassostrea gigas. Master’s Thesis, Korea Maritime University, Busan, Republic of Korea, 2009. [Google Scholar]
- Min, E.Y.; Lee, J.S.; Kim, J.W.; Jeon, M.A.; Kang, J.C. Influence of Elevated Temperatures on the Physiological Response of Hemolymph from Two Species of the Abalone, Haliotis discus hannai and H. discus discus. Korean J. Malacol. 2015, 31, 1–8. [Google Scholar]
- Choi, S.K.; Min, E.Y.; Jee, J.H.; Kim, J.W.; Ahn, C.M.; Kang, J.C. Changes of inorganic matter and enzyme activity in the hemolymph of oyster, Crassostrea gigas exposed to TBTO. J. Fish. Pathol. 2001, 14, 65–70. [Google Scholar]
- Choi, Y.K.; Jo, P.G.; Choi, C.Y. Cadmium affects the expression of heat shock protein 90 and metallothionein mRNA in the Pacific oyster, Crassstrea gigas. Comp. Biochem. Physiol. 2008, 286, 29. [Google Scholar]
- Schreck, C.B.; Bradford, C.S.; Fitzpatrick, M.S.; Patino, R. Regulation of the interrenal of fishes: Non-classical control mechanism. Fish. Physiol. Biochem. 1989, 7, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.F.; Reid, S.D. β-adrenergic signal transduction in fish: Interactive effects of catecholamines and cortisol. Fish. Physiol. Biochem. 1993, 11, 195–203. [Google Scholar] [CrossRef]
- Chang, Y.J.; Hur, J.W. Physiological responses of grey mullet, Mugil cephalus and nile tilapia, Oreochromis niloticus by rapid changes in salinity of rearing water. J. Korean Fish Soc. 1999, 32, 310–316. [Google Scholar]
- Tomasso, J.R.; Davis, K.B.; Parker, N.C. Plasma corticosteroid and electrolyte dynamics of hybrid striped bass (white bass × strped bass) during netting and hauling stress. Proc. World Maric. Soc. 1980, 11, 303–310. [Google Scholar] [CrossRef]
- Eddy, F.B. Effects of Stress on Osmotic and Ionic Regulation in Fish; Academic Press: London, UK, 1981; pp. 77–102. [Google Scholar]
- Carmichael, G.J.; Tomasso, J.R.; Simco, B.S.; Davis, K.B. Characterization and alleviation of stress associated with hauling laremouth bass. Trans. Am. Fish. Soc. 1984, 113, 778–785. [Google Scholar] [CrossRef]
- McDonald, G.; Milligan, L. Ionic, Osmotic and Acidbase Regulation in Stress; University Press: Cambridge, UK, 1997; pp. 119–144. [Google Scholar]
- Lagos, L.; Herrera, M.; Sanchez-Lazo, C.; Martinez-Pita, I. Effect of larval stocking density on growth, survival and whole body cortisol of the Mediterranean mussel Mytilus galloprovincialis (Lamarck, 1819) larvae reared under laboratory conditions. Aquac. Res. 2013, 46, 1648–1656. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Liu, C.; Liu, R.; Yang, C.; Wang, L.; Song, L. Cortisol modulates glucose metabolism and oxidative response after acute high temperature stress in Pacific oyster Crassostrea gigas. Fish. Shellfish. Immunol. 2022, 126, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Chapple, J.P.; Smerdon, G.R.; Berry, R.J.; Hawkins, A.J.S. Seasonal changes in stress-70 protein levels reflect thermal tolerance in the marine bivalve Mytilus edulis L. J. Exp. Mar. Biol. Ecol. 1998, 229, 53–68. [Google Scholar] [CrossRef]
- Velasco, L.A.; Navarro, J.M. Feeding physiology of infaunal (Mulinia edulis) and epifaunal (Mytilus chilensis) bivalves under a wide range of concentrations and qualities of seston. Mar. Ecol. Prog. Ser. 2002, 240, 143–155. [Google Scholar] [CrossRef]


| Group | Suspended Solids (mg/L) | Nephelometric (NTU) |
|---|---|---|
| Control | 0 | 0 |
| G1 | 100 | 55.2 |
| G2 | 250 | 158 |
| G3 | 500 | 413 |
| G4 | 1000 | 718 |
| Survival Number/Total Number of Species | ||||||
|---|---|---|---|---|---|---|
| Species | Group | 1D (19 °C) | 2D (20 °C) | 3D (21 °C) | 4D (22 °C) | 5D (23 °C) |
| V. philippinarum | Control | 30/30 | 30/30 | 30/30 | 30/30 | 30/30 |
| 100 mg/L | 30/30 | 30/30 | 30/30 | 30/30 | 30/30 | |
| 250 mg/L | 29/30 | 27/30 | 26/30 | 26/30 | 26/30 | |
| 500 mg/L | 28/30 | 26/30 | 26/30 | 25/30 | 25/30 | |
| 1000 mg/L | 29/30 | 29/30 | 28/30 | 28/30 | 28/30 | |
| A. irradians | Control | 30/30 | 30/30 | 30/30 | 30/30 | 30/30 |
| 100 mg/L | 29/30 | 28/30 | 28/30 | 28/30 | 28/30 | |
| 250 mg/L | 30/30 | 30/30 | 30/30 | 30/30 | 30/30 | |
| 500 mg/L | 29/30 | 29/30 | 29/30 | 29/30 | 29/30 | |
| 1000 mg/L | 29/30 | 26/30 | 26/30 | 25/30 | 25/30 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, H.-J.; Kim, J.-W.; Sohn, M.-Y.; Ji, C.-y.; Jeong, S.B.; Kim, I.G.; Kang, J.-H.; Kong, H.J.; Park, C.-I.; Kang, G. Physiological Effects of Suspended Solids on Venerupis philippinarum and Argopecten irradians. Fishes 2025, 10, 432. https://doi.org/10.3390/fishes10090432
Son H-J, Kim J-W, Sohn M-Y, Ji C-y, Jeong SB, Kim IG, Kang J-H, Kong HJ, Park C-I, Kang G. Physiological Effects of Suspended Solids on Venerupis philippinarum and Argopecten irradians. Fishes. 2025; 10(9):432. https://doi.org/10.3390/fishes10090432
Chicago/Turabian StyleSon, Ha-Jeong, Ju-Won Kim, Min-Young Sohn, Chae-yeong Ji, Su Bhin Jeong, In Gu Kim, Jung-Ha Kang, Hee Jeong Kong, Chan-Il Park, and Gyoungsik Kang. 2025. "Physiological Effects of Suspended Solids on Venerupis philippinarum and Argopecten irradians" Fishes 10, no. 9: 432. https://doi.org/10.3390/fishes10090432
APA StyleSon, H.-J., Kim, J.-W., Sohn, M.-Y., Ji, C.-y., Jeong, S. B., Kim, I. G., Kang, J.-H., Kong, H. J., Park, C.-I., & Kang, G. (2025). Physiological Effects of Suspended Solids on Venerupis philippinarum and Argopecten irradians. Fishes, 10(9), 432. https://doi.org/10.3390/fishes10090432






