Ecosystem Size and Functional Group Relative Abundance Drive Stream Community Body Size Structure †
Abstract
1. Introduction
2. Methods
2.1. Study Site Description
2.2. Sampling Methods and Watershed Factors
2.3. Macroinvertebrate Sampling and Laboratory Protocols
2.4. Fish Sampling and Laboratory Protocols
2.5. Data Analysis
2.6. Data and Code Availability
3. Results
3.1. Combined Fish and Macroinvertebrate Size Spectra
3.2. Macroinvertebrate Only Size Spectra
3.3. Fish Only Size Spectra
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Power, M.E.; Dietrich, W.E. Food webs in river networks. Ecol. Res. 2002, 17, 451–471. [Google Scholar] [CrossRef]
- Hardin, G. The competitive exclusion principle: An idea that took a century to be born has implications in ecology, economics, and genetics. Science 1960, 131, 1292–1297. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Kitchell, J.F. (Eds.) The Trophic Cascade in Lakes; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar]
- Beisel, J.N.; Usseglio-Polatera, P.; Thomas, S.; Moreteau, J.C. Stream community structure in relation to spatial variation: The influence of mesohabitat characteristics. Hydrobiologia 1998, 389, 73–88. [Google Scholar] [CrossRef]
- Sarremejane, R.; Truchy, A.; McKie, B.G.; Mykrä, H.; Johnson, R.K.; Huusko, A.; Sponseller, R.A.; Muotka, T. Stochastic processes and ecological connectivity drive stream invertebrate community responses to short-term drought. J. Anim. Ecol. 2021, 90, 886–898. [Google Scholar] [CrossRef]
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, C.E. The river continuum concept. Can. J. Fish. Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- Thorp, J.H.; Thoms, M.C.; Delong, M.D.; Maasri, A. The ecological nature of whole river macrosystems: New perspectives from the riverine ecosystem synthesis. Front. Ecol. Evol. 2023, 11, 1184433. [Google Scholar] [CrossRef]
- Poff, N.L.; Allan, J.D.; Bain, M.B.; Karr, J.R.; Prestegaard, K.L.; Richter, B.D.; Sparks, R.E.; Stromberg, J.C. The Natural Flow Regime: A paradigm for river conservation and restoration. Bioscience 1997, 47, 769–784. [Google Scholar] [CrossRef]
- Junk, W.J. The flood pulse concept of large rivers: Learning from the tropics. Arch. Hydrobiol. Suppl. 1999, 115, 261–280. [Google Scholar] [CrossRef]
- Marin, V.; Arranz, I.; Grenouillet, G.; Cucherousset, J. Fish size spectrum as a complementary biomonitoring approach of freshwater. Ecol. Indic. 2023, 146, 1–8. [Google Scholar] [CrossRef]
- Royer, T.V. Human-dominated rivers and river management in the Anthropocene. In Stream Ecosystems in a Changing Environment; Academic Press: Cambridge, MA, USA, 2016; pp. 491–524. [Google Scholar]
- Dudgeon, D. Multiple threats imperil freshwater biodiversity in the Anthropocene. Curr. Biol. 2019, 29, R960–R967. [Google Scholar] [CrossRef]
- King, R.S.; Walker, C.M.; Whigham, D.F.; Baird, S.J.; Back, J.A. Catchment topography and wetland geomorphology drive macroinvertebrate community structure and juvenile salmonid distributions in south-central Alaska headwater streams. Freshw. Sci. 2012, 31, 341–364. [Google Scholar] [CrossRef]
- Benejam, L.; Tobes, I.; Brucet, S.; Miranda, R. Size spectra and other size-related variables of river fish communities: Systematic changes along the altitudinal gradient on pristine Andean streams. Ecol. Indic. 2018, 90, 366–378. [Google Scholar] [CrossRef]
- Murry, B.A.; Olmeda, M.D.L.; Lilyestrom, C.; Adams, D.S.; Adase, K.; García-Bermudez, M. Community size-spectra applied to recreational freshwater fisheries in Puerto Rican reservoirs. Fish. Manag. Ecol. 2024, 31, e12671. [Google Scholar] [CrossRef]
- Liao, H.; Sarver, E.; Krometis, L.A.H. Interactive effects of water quality, physical habitat, and watershed anthropogenic activities on stream ecosystem health. Water Res. 2018, 130, 69–78. [Google Scholar] [CrossRef]
- Pomeranz, J.P.F.; Warburton, H.J.; Harding, J.S. Anthropogenic mining alters macroinvertebrate size spectra in streams. Freshw. Biol. 2019, 64, 81–92. [Google Scholar] [CrossRef]
- Clement, T.A.; Murry, B.A.; Uzarski, D.G. Fish community size structure of small lakes: The role of lake size, biodiversity and disturbance. J. Freshw. Ecol. 2015, 30, 557–568. [Google Scholar] [CrossRef]
- Greig, H.S.; McHugh, P.A.; Thompson, R.M.; Warburton, H.J.; McIntosh, A.R. Habitat size influences community stability. Ecology 2022, 103, e03545. [Google Scholar] [CrossRef]
- Tong, S.T.Y.; Chen, W. Modeling the relationship between land use and surface water quality. J. Environ. Manag. 2002, 66, 377–393. [Google Scholar] [CrossRef]
- Du, J.; Qian, L.; Rui, H.; Zuo, T.; Zheng, D.; Xu, Y.; Xu, C.Y. Assessing the effects of urbanization on annual runoff and flood events using an integrated hydrological modeling system for Qinhuai River basin, China. J. Hydrol. 2012, 464, 127–139. [Google Scholar] [CrossRef]
- Merriam, E.R.; Petty, J.T.; Merovich, G.T.; Fulton, J.B.; Strager, M.P. Additive effects of mining and residential development on stream conditions in a central Appalachian watershed. J. N. Am. Benthol. Soc. 2011, 30, 399–418. [Google Scholar] [CrossRef]
- Brose, U.; Blanchard, J.L.; Eklöf, A.; Galiana, N.; Hartvig, M.; Hirt, M.R.; Kalinkat, G.; Nordström, M.C.; O’Gorman, E.J.; Rall, B.C.; et al. Predicting the consequences of species loss using size-structured biodiversity approaches. Biol. Rev. 2017, 92, 684–697. [Google Scholar] [CrossRef]
- Brown, J.H.; Gillooly, J.F.; Allen, A.P.; Savage, V.M.; West, G.B. Toward a metabolic theory of ecology. Ecology 2004, 85, 1771–1789. [Google Scholar] [CrossRef]
- Woodward, G.; Brown, L.E.; Edwards, F.K.; Hudson, L.N.; Milner, A.M.; Reuman, D.C.; Ledger, M.E. Climate change impacts in multispecies systems: Drought alters food web size structure in a field experiment. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 2990–2997. [Google Scholar] [CrossRef]
- Schorr, M.S.; Backer, J.C. Localized effects of coal mine drainage on fish assemblages in a Cumberland Plateau stream in Tennessee. J. Freshw. Ecol. 2006, 21, 17–24. [Google Scholar] [CrossRef]
- Hilsenhoff, W.L. An improved biotic index of organic stream pollution. Great Lakes Entomol. 1987, 20, 7. [Google Scholar] [CrossRef]
- Bailey, R.C.; Norris, R.H.; Reynoldson, T.B. Taxonomic resolution of benthic macroinvertebrate communities in bioassessments. J. N. Am. Benthol. Soc. 2001, 20, 280–286. [Google Scholar] [CrossRef]
- Blocksom, K.A.; Johnson, B.R. Development of a regional macroinvertebrate index for large river bioassessment. Ecol. Indic. 2009, 9, 313–328. [Google Scholar] [CrossRef]
- Petchey, O.L.; Belgrano, A. Body-size distributions and size-spectra: Universal indicators of ecological status? Biol. Lett. 2010, 6, 434–437. [Google Scholar] [CrossRef]
- Novak, B.; Murry, B.A.; Wesner, J.S.; Gjoni, V.; Arantes, C.C.; Shepta, E.; Pomeranz, J.P.; Junker, J.R.; Zipfel, K.; Stump, A.; et al. Threshold responses of freshwater fish community size spectra to invasive species. Ecosphere 2024, 15, e70090. [Google Scholar] [CrossRef]
- Sheldon, R.W.; Prakash, A.; Sutcliffe, W., Jr. The size distribution of particles in the ocean. Limnol. Oceanogr. 1972, 17, 327–340. [Google Scholar]
- Kerr, S.R.; Dickie, L.M. The Biomass Spectrum; Columbia University Press: New York, NY, USA, 2001. [Google Scholar]
- McGarvey, D.J.; Kirk, A.J. Seasonal comparison of community-level size-spectra in southern coalfield streams of West Virginia (USA). Hydrobiologia 2018, 809, 65–77. [Google Scholar] [CrossRef]
- Dimech, M.; Camilleri, M.; Hiddink, J.G.; Kaiser, M.J.; Ragonese, S.; Schembri, P.J. Differences in demersal community structure and biomass size spectra within and outside the Maltese Fishery Management Zone (FMZ). Sci. Mar. 2008, 72, 669–682. [Google Scholar] [CrossRef]
- Blanchard, J.L.; Andersen, K.H.; Scott, F.; Hintzen, N.T.; Piet, G.; Jennings, S. Evaluating targets and trade-offs among fisheries and conservation objectives using a multispecies size spectrum model. J. Appl. Ecol. 2014, 51, 612–622. [Google Scholar] [CrossRef]
- Murry, B.A.; Farrell, J.M. Resistance of the size structure of the fish community to ecological perturbations in a large river ecosystem. Freshw. Biol. 2014, 59, 155–167. [Google Scholar] [CrossRef]
- White, E.P.; Ernest, S.K.M.; Kerkhoff, A.J.; Enquist, B.J. Relationships between body size and abundance in ecology. Trends Ecol. Evol. 2007, 22, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.M.; Robinson, J.P.W.; Plank, M.J.; Baum, J.K.; Blanchard, J.L. Testing and recommending methods for fitting size spectra to data. Methods Ecol. Evol. 2017, 8, 57–67. [Google Scholar] [CrossRef]
- Pomeranz, J.P.F.; Junker, J.R.; Gjoni, V.; Wesner, J.S. Maximum likelihood outperforms binning methods for detecting differences in abundance size spectra across environmental gradients. J. Anim. Ecol. 2024, 93, 267–280. [Google Scholar] [CrossRef]
- Trebilco, R.; Baum, J.K.; Salomon, A.K.; Dulvy, N.K. Ecosystem ecology: Size-based constraints on the pyramids of life. Trends Ecol. Evol. 2013, 28, 423–431. [Google Scholar] [CrossRef]
- Daan, N.; Gislason, H.; Pope, J.; Rice, J.C. Changes in the North Sea fish community: Evidence of indirect effects of fishing? ICES J. Mar. Sci. 2005, 62, 177–188. [Google Scholar] [CrossRef]
- Sweeting, C.J.; Badalamenti, F.; D’Anna, G.; Pipitone, C.; Polunin, N.V.C. Steeper biomass spectra of demersal fish communities after trawler exclusion in Sicily. ICES J. Mar. Sci. 2009, 66, 195–202. [Google Scholar] [CrossRef]
- Mehner, T.; Keeling, C.; Emmrich, M.; Holmgren, K.; Argillier, C.; Volta, P.; Winfield, I.J.; Brucet, S. Effects of fish predation on density and size spectra of prey fish communities in lakes. Can. J. Fish. Aquat. Sci. 2016, 73, 506–518. [Google Scholar] [CrossRef]
- Broadway, K.J.; Pyron, M.; Gammon, J.R.; Murry, B.A. Shift in a large river fish assemblage: Body-size and trophic structure dynamics. PLoS ONE 2015, 10, e0125178. [Google Scholar] [CrossRef] [PubMed]
- Law, R.; Plank, M.J.; Kolding, J. Balanced exploitation and coexistence of interacting, size-structured, fish species. Fish Fish. 2016, 17, 281–302. [Google Scholar] [CrossRef]
- Shin, Y.J.; Rochet, M.J.; Jennings, S.; Field, J.G.; Gislason, H. Using size-based indicators to evaluate the ecosystem effects of fishing. ICES J. Mar. Sci. 2005, 62, 384–396. [Google Scholar] [CrossRef]
- Collyer, G.; Perkins, D.M.; Petsch, D.K.; Siqueira, T.; Saito, V. Land-use intensification systematically alters the size structure of aquatic communities in the Neotropics. Glob. Change Biol. 2023, 29, 4094–4106. [Google Scholar] [CrossRef]
- Benejam, L.; Teixeira-de Mello, F.; Meerhoff, M.; Loureiro, M.; Jeppesen, E.; Brucet, S. Assessing effects of change in land use on size-related variables of fish in subtropical streams. Can. J. Fish. Aquat. Sci. 2016, 73, 547–556. [Google Scholar] [CrossRef]
- Arranz, I.; Hsieh, C.H.; Mehner, T.; Brucet, S. Systematic deviations from linear size spectra of lake fish communities are correlated with predator–prey interactions and lake-use intensity. Oikos 2019, 128, 33–44. [Google Scholar] [CrossRef]
- USGS. MRLC: NLCD 2019 Land Cover (CONUS). U.S. Geological Survey. 2023. Available online: https://www.mrlc.gov/data/nlcd-2019-land-cover-conus (accessed on 1 March 2023).
- USGS. National Hydrography: NHDPlus High Resolution. U.S. Geological Survey. 2023. Available online: https://www.usgs.gov/national-hydrography/nhdplus-high-resolution (accessed on 1 March 2023).
- National Aquatic Monitoring Center (NAMC). Protocol for the Collection of Aquatic Macroinvertebrate Samples. 2015. Available online: https://www.usu.edu/buglab/Content/NAMC_Macroinvertebrate_Protocol.pdf (accessed on 8 March 2022).
- Merritt, R.W.; Cummins, K.W.; Berg, M.B. An Introduction to the Aquatic Insects of North America, 5th ed.; Kendall Hunt Publishing Company: Dubuque, IA, USA, 2019. [Google Scholar]
- Benke, A.C.; Huryn, A.D.; Smock, L.A.; Wallace, J.B. Length-mass relationships for freshwater macroinvertebrates in North America with particular reference to the southeastern United States. J. N. Am. Benthol. Soc. 1999, 18, 308–343. [Google Scholar] [CrossRef]
- Smock, L.A. Relationships between body size and biomass of aquatic insects. Freshw. Biol. 1980, 10, 375–383. [Google Scholar] [CrossRef]
- Kelly, D.W.; Dick, J.T.; Montgomery, W.I. The functional role of Gammarus (Crustacea, Amphipoda): Shredders, predators, or both? Hydrobiologia 2002, 485, 199–203. [Google Scholar] [CrossRef]
- Reid, S.M.; Yunker, G.; Jones, N.E. Evaluation of single-pass backpack electric fishing for stream fish community monitoring. Fish. Manag. Ecol. 2009, 16, 1–9. [Google Scholar] [CrossRef]
- West Virginia Department of Environmental Protection, Watershed Assessment Branch (WVDEP-WAB). Chapter VI: Fish Collection Protocols—Wadeable Streams; 2011. Available online: https://dep.wv.gov/WWE/watershed/bio_fish/Documents/WBFish.pdf (accessed on 8 March 2022).
- Driehaus, E.R.; Landreth, J.; Adase, K.; Smith, D.; Wellman, D.; Arantes, C.C.; Murry, B.A. Length-weight relationships for 44 Central Appalachian fish species. J. Appl. Ichthyol. 2023, 39, e5573054. [Google Scholar] [CrossRef]
- Waters, T.F. Secondary production in inland waters. Adv. Ecol. Res. 1977, 10, 91–164. [Google Scholar]
- Poff, N.L.; Allan, J.D. Functional organization of stream fish assemblages in relation to hydrological variability. Ecology 1995, 76, 606–627. [Google Scholar] [CrossRef]
- Gido, K.B.; Franssen, N.R. Invasion of stream fishes into low trophic positions. Ecol. Freshw. Fish 2007, 16, 457–464. [Google Scholar] [CrossRef]
- Taylor, C.M.; Warren, M.L., Jr. Dynamics in species composition of stream fish assemblages: Environmental variability and nested subsets. Ecology 2001, 82, 2320–2330. [Google Scholar] [CrossRef]
- Wesner, J.S.; Pomeranz, J.P.F.; Junker, J.R.; Gjoni, V. Bayesian hierarchical modelling of size spectra. Methods Ecol. Evol. 2024, 15, 856–867. [Google Scholar] [CrossRef]
- Virkar, Y.; Clauset, A. Power-law distributions in binned empirical data. Ann. Appl. Stat. 2014, 8, 89–119. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 29 July 2024).
- Bürkner, P.-C. Bayesian item response modeling in R with brms and Stan. J. Stat. Softw. 2021, 100, 1–54. [Google Scholar] [CrossRef]
- Stan Development Team. RStan: The R Interface to Stan; 2024; Available online: https://mc-stan.org/ (accessed on 9 July 2024).
- Kay, M. Tidybayes: Tidy Data and Geoms for Bayesian Models. 2023. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; Available online: https://ggplot2.tidyverse.org (accessed on 29 July 2024).
- Wesner, J.S.; Pomeranz, J.P.F. Choosing priors in Bayesian ecological models by simulating from the prior predictive distribution. Ecosphere 2021, 12, e03739. [Google Scholar] [CrossRef]
- Pomeranz, J.P.F.; Junker, J.R.; Wesner, J.S. Individual size distributions across North American streams vary with local temperature. Glob. Change Biol. 2022, 28, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S. A widely applicable Bayesian information criterion. J. Mach. Learn. Res. 2013, 14, 867–897. [Google Scholar]
- Sprules, W.G.; Barth, L.E. Surfing the biomass size spectrum: Some remarks on history, theory, and application. Can. J. Fish. Aquat. Sci. 2016, 73, 477–495. [Google Scholar] [CrossRef]
- Platt, T.; Denman, K. The structure of pelagic marine ecosystems. Rapp. P. V. Réun. Cons. Int. Explor. Mer. 1978, 173, 60–65. [Google Scholar]
- Gaedke, U. The size distribution of plankton biomass in a large lake and its seasonal variability. Limnol. Oceanogr. 1992, 37, 1202–1220. [Google Scholar] [CrossRef]
- Benkendorf, D.J.; Whiteman, H.H. Omnivore density affects community structure through multiple trophic cascades. Oecologia 2021, 195, 397–407. [Google Scholar] [CrossRef]
- Baumgartner, M.T.; Faria, L.D.B. The sensitivity of complex dynamic food webs to the loss of top omnivores. J. Theor. Biol. 2022, 538, 111027. [Google Scholar] [CrossRef]
- Perkins, D.M.; Hatton, I.A.; Gauzens, B.; Barnes, A.D.; Ott, D.; Rosenbaum, B.; Vinagre, C.; Brose, U. Consistent predator-prey biomass scaling in complex food webs. Nat. Commun. 2022, 13, 4990. [Google Scholar] [CrossRef]
- Perkins, D.M.; Durance, I.; Edwards, F.K.; Grey, J.; Hildrew, A.G.; Jackson, M.; Jones, J.I.; Lauridsen, R.B.; Layer-Dobra, K.; Thompson, M.S.; et al. Bending the rules: Exploitation of allochthonous resources by a top-predator modifies size-abundance scaling in stream food webs. Ecol. Lett. 2018, 21, 1771–1780. [Google Scholar] [CrossRef] [PubMed]
- Zatkos, L.; Arismendi, I.; Johnson, S.L.; Penaluna, B.E. Geophysical templates modulate the structure of stream food webs dominated by omnivory. Ecosphere 2021, 12, e03444. [Google Scholar] [CrossRef]
- Jeppesen, E.; Jensen, J.P.; Søndergaard, M.; Lauridsen, T.; Landkildehus, F. Trophic structure, species richness and biodiversity in Danish lakes: Changes along a phosphorus gradient. Freshw. Biol. 2000, 45, 201–218. [Google Scholar] [CrossRef]
- Brucet, S.; Pédron, S.; Mehner, T.; Lauridsen, T.L.; Argillier, C.; Winfield, I.J.; Volta, P.; Emmrich, M.; Hesthagen, T.; Holmgren, K.; et al. Fish diversity in European lakes: Geographical factors dominate over anthropogenic pressures. Freshw. Biol. 2013, 58, 1779–1793. [Google Scholar] [CrossRef]
- Marin, V.; Cucherousset, J.; Grenouillet, G. Interactive effects of anthropogenic stressors on the temporal changes in the size spectrum of lake fish communities. Ecol. Freshw. Fish 2024, 34, e12826. [Google Scholar] [CrossRef]
- Elton, C.S. The nature and origin of soil-polygons in Spitsbergen. Q. J. Geol. Soc. Lond. 1927, 83, 163-NP. [Google Scholar] [CrossRef]
- Lindeman, R.L. The trophic-dynamic aspect of ecology. Ecology 1942, 23, 399–417. [Google Scholar] [CrossRef]
- Mehner, T.; Lischke, B.; Scharnweber, K.; Attermeyer, K.; Brothers, S.; Gaedke, U.; Hilt, S.; Brucet, S. Empirical correspondence between trophic transfer efficiency in freshwater food webs and the slope of their size spectra. Ecology 2018, 99, 1463–1472. [Google Scholar] [CrossRef]
- Thiebaux, M.L.; Dickie, L.M. Models of aquatic biomass size spectra and the common structure of their solutions. J. Theor. Biol. 1992, 159, 147–161. [Google Scholar] [CrossRef]
- Sprules, W.G.; Goyke, A.P. Size-based structure and production in the pelagia of Lakes Ontario and Michigan. Can. J. Fish. Aquat. Sci. 1994, 51, 2603–2611. [Google Scholar] [CrossRef]
- Blanchard, J.L.; Jennings, S.; Law, R.; Castle, M.D.; McCloghrie, P.; Rochet, M.J.; Benoît, E. How does abundance scale with body size in coupled size-structured food webs? J. Anim. Ecol. 2009, 78, 270–280. [Google Scholar] [CrossRef]
- Loreau, M.; Barbier, M.; Filotas, E.; Gravel, D.; Isbell, F.; Miller, S.J.; Montoya, J.M.; Wang, S.; Aussenac, R.; Germain, R.; et al. Biodiversity as insurance: From concept to measurement and application. Biol. Rev. 2021, 96, 2333–2354. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Isbell, F.; Deng, W.; Hong, P.; Dee, L.E.; Thompson, P.; Loreau, M. How complementarity and selection affect the relationship between ecosystem functioning and stability. Ecology 2021, 102, e03347. [Google Scholar] [CrossRef] [PubMed]


| Watershed Factor | CSS Slope (λ) | Literature Source |
|---|---|---|
| Drainage Area | Flatten | Vannote et al., 1980 [6] |
| Mean Drainage Slope | Steepen | King et al., 2012 [13] |
| Mean Drainage Slope STDEV | None | King et al., 2012 [13] |
| Mean Drainage Elevation | Flatten | Benejam et al., 2018 [14] |
| Drainage % Forested | Steepen | Collyer et al., 2023 [48] |
| Drainage % Agriculture | Flatten | Benejam et al., 2016, Arranz et al., 2019 [49,50] |
| Stream Specific Conductance | None | Pomeranz et al., 2019 [17] |
| % Predators | Steepen | Murry et al., 2024 [15] |
| % Omnivores | Flatten * | Broadway et al., 2015 [45] |
| % Herbivores | Flatten * | --- |
| Stream | Major Watershed | Mean Wetted Width (m) | Mean Stream Depth (cm) | Drainage Area (km2) | Mean Drainage Slope (%) | Mean Drainage Slope STDEV | Mean Drainage Elevation (m) | Drainage % Agriculture | Stream SpC (mS/cm) |
|---|---|---|---|---|---|---|---|---|---|
| Beaver | Cheat | 7.2 | 26.5 | 33.97 | 6.51 | 4.52 | 649.06 | 26.53 | 0.07 |
| Big Sandy | Cheat | 19.4 | 43.7 | 538.32 | 8.69 | 6.01 | 598.11 | 17.26 | 0.13 |
| Buffalo | Monongahela | 18.0 | 48.6 | 324.71 | 17.17 | 6.80 | 373.41 | 7.81 | 0.37 |
| Dry Fork | Cheat | 45.9 | 40.3 | 1269.06 | 15.08 | 8.65 | 987.51 | 18.66 | 0.10 |
| Dunkard | Monongahela | 16.4 | 20.8 | 602.84 | 16.01 | 6.44 | 370.39 | 10.72 | 0.61 |
| Elk | Monongahela | 13.4 | 34.8 | 312.62 | 13.76 | 7.47 | 384.97 | 20.36 | 1.12 |
| Fish | Ohio | 18.7 | 46.1 | 648.76 | 18.92 | 7.00 | 372.88 | 7.26 | 0.25 |
| Fishing | Ohio | 17.0 | 53.6 | 565.45 | 21.21 | 7.10 | 341.71 | 2.57 | 0.20 |
| Horseshoe | Cheat | 10.2 | 43.7 | 143.05 | 19.28 | 8.64 | 732.72 | 6.35 | 0.07 |
| Paw Paw | Monongahela | 13.7 | 33.7 | 108.48 | 16.07 | 6.50 | 370.96 | 13.55 | 0.45 |
| Simpson | Monongahela | 13.7 | 25.0 | 188.67 | 13.62 | 7.57 | 377.03 | 17.44 | 0.97 |
| Tenmile | Monongahela | 18.8 | 35.7 | 323.60 | 16.98 | 6.90 | 370.71 | 8.42 | 0.78 |
| Three Fork | Monongahela | 22.3 | 53.4 | 261.92 | 12.89 | 7.16 | 508.32 | 11.25 | 0.29 |
| Wheeling | Ohio | 15.3 | 33.6 | 770.94 | 14.70 | 6.67 | 364.15 | 16.38 | 0.54 |
| Whiteday | Monongahela | 8.7 | 19.4 | 85.26 | 13.60 | 6.20 | 444.98 | 36.91 | 0.15 |
| Mean | 1.9 | 17.1 | 37.0 | 411.85 | 14.97 | 6.91 | 483.13 | 12.19 | 0.41 |
| STDEV | 0.7 | 11.9 | 18.3 | 328.11 | 3.82 | 1.01 | 184.53 | 6.60 | 0.33 |
| Site Name | Site Coordinates | County | Watershed |
|---|---|---|---|
| Beaver Creek DS | N 39°37′36.4″, W 79°35′58.2″ | Preston | Cheat |
| Beaver Creek US | N 39°36.679′, W 79°31.031′ | Preston | Cheat |
| Big Sandy Creek DS | N 39°39.480′, W 79°38.339′ | Preston | Cheat |
| Big Sandy Creek US | N 39°43.142′, W 79°39.561′ | Preston | Cheat |
| Buffalo Creek DS | N 39°29.919′, W 80°11.066′ | Marion | Monongahela |
| Buffalo Creek US | N 39°30.690′, W 80°14.885′ | Marion | Monongahela |
| Dry Fork DS | N 39°00.791′, W 79°31.738′ | Tucker | Cheat |
| Dry Fork US | N 38°59.554′, W 79°31.826′ | Tucker | Cheat |
| Dunkard Creek DS | N 39°43.054′, W 80°07.126′ | Monongalia | Monongahela |
| Dunkard Creek US | N 39°42.932′, W 80°09.928 | Monongalia | Monongahela |
| Elk Creek DS | N 39°15.634′, W 80°19.086′ | Harrison | Monongahela |
| Elk Creek US | N 39°13.560′, W 80°18.371 | Harrison | Monongahela |
| Fish Creek DS | N 39°46′00.7”, W 80°42′33.4″ | Marshall | Ohio |
| Fish Creek US | N 39°46.040′, W 80°38.705′ | Marshall | Ohio |
| Fishing Creek DS | N 39°35.447′, W 80°48.750′ | Wetzel | Ohio |
| Fishing Creek US | N 39°33.794′, W 80°42.322′ | Wetzel | Ohio |
| Horseshoe Run DS | N 39°09.242′, W 79°39.677′ | Tucker | Cheat |
| Horseshoe Run US | N 39°10.844′, W 79°36.140′ | Tucker | Cheat |
| Paw Paw Creek DS | N 39°33.204′, W 80°10.050′ | Marion | Monongahela |
| Paw Paw Creek US | N 39°33.646′, W 80°11.218′ | Marion | Monongahela |
| Simpson Creek DS | N 39°18.528′, W 80°16.734′ | Harrison | Monongahela |
| Simpson Creek US | N 39°17.353′, W 80°15.935′ | Harrison | Monongahela |
| Tenmile Creek DS | N 39°22.408′, W 80°20.863′ | Harrison | Monongahela |
| Tenmile Creek US | N 39°20.703′, W 80°24.038′ | Harrison | Monongahela |
| Three Fork Creek DS | N 39°20.181′, W 79°59.473′ | Taylor | Monongahela |
| Three Fork Creek US | N 39°22.844′, W 79°54.825′ | Taylor | Monongahela |
| Wheeling Creek DS | N 39°58.542′, W 80°37.846′ | Marshall | Ohio |
| Wheeling Creek US | N 39°57.528′, W 80°32.953′ | Marshall | Ohio |
| Whiteday Creek DS | N 39°31.849′, W 80°02.652′ | Monongalia | Monongahela |
| Whiteday Creek US | N 39°29.256′, W 80°00.415′ | Monongalia | Monongahela |
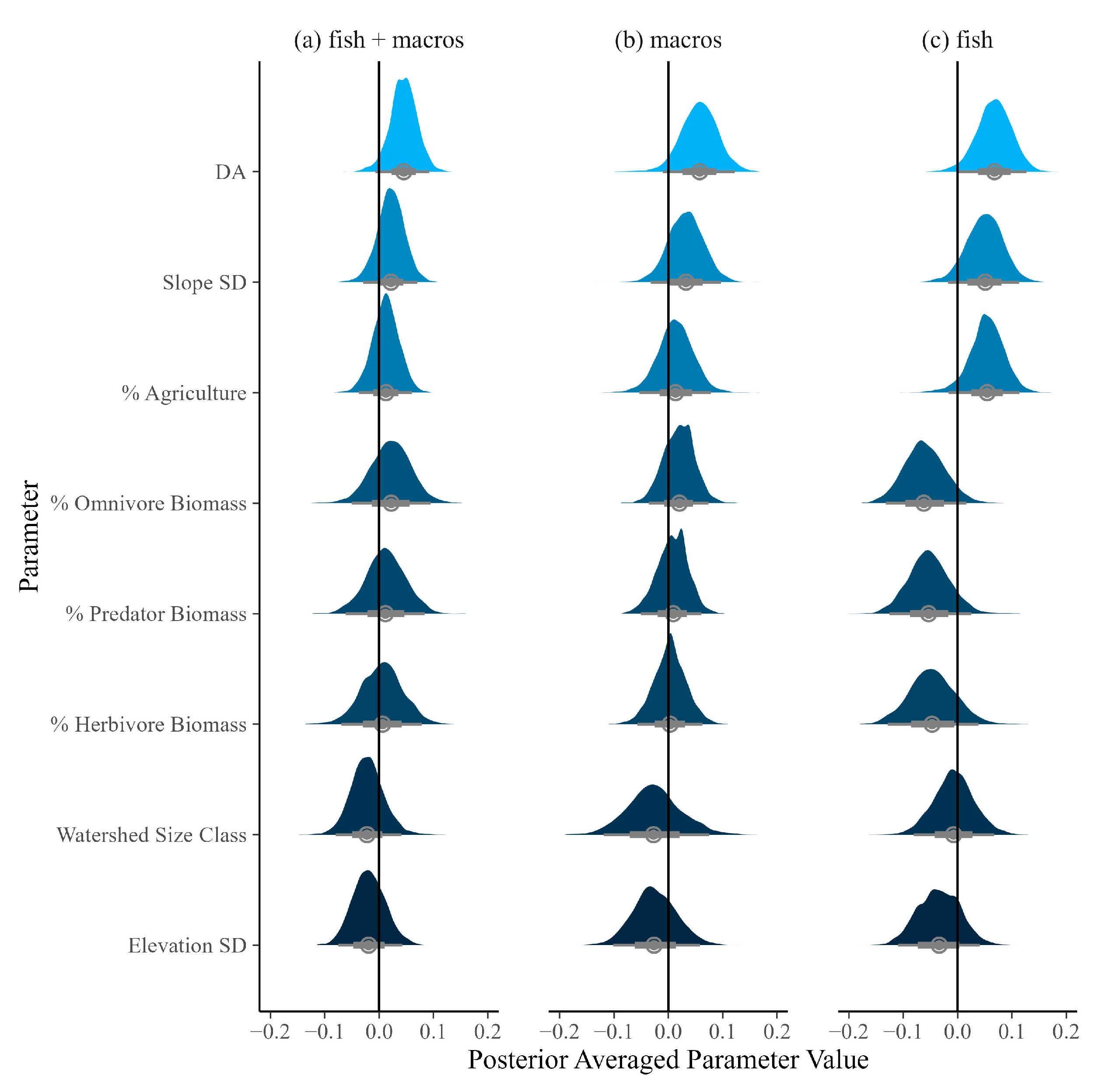
| Predictor | Fish + Macros | Macros | Fish |
|---|---|---|---|
| DA | 0.05 (−0.01, 0.09) | 0.06 (−0.01, 0.12) | 0.07 (0, 0.13) |
| % Agriculture | 0.01 (−0.04, 0.06) | 0.01 (−0.05, 0.08) | 0.05 (−0.02, 0.11) |
| Slope SD | 0.02 (−0.03, 0.07) | 0.03 (−0.03, 0.1) | 0.05 (−0.02, 0.11) |
| % Omnivore Biomass | 0.02 (−0.05, 0.09) | 0.02 (−0.04, 0.07) | −0.06 (−0.13, 0.02) |
| % Predator Biomass | 0.01 (−0.06, 0.08) | 0.01 (−0.05, 0.06) | −0.05 (−0.13, 0.02) |
| % Herbivore Biomass | 0.01 (−0.07, 0.08) | 0 (−0.06, 0.06) | −0.05 (−0.13, 0.04) |
| Watershed Size Class | −0.02 (−0.08, 0.04) | −0.03 (−0.12, 0.07) | −0.01 (−0.08, 0.07) |
| Elevation SD | −0.02 (−0.08, 0.04) | −0.03 (−0.1, 0.06) | −0.03 (−0.11, 0.04) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landreth, J.H.; Murry, B.A.; Adase, K.A.; Arantes, C.C.; Smith, D.M.; Wellman, D.I., Jr.; Junker, J.R.; Pomeranz, J.P.F.; Wesner, J.S. Ecosystem Size and Functional Group Relative Abundance Drive Stream Community Body Size Structure. Fishes 2025, 10, 419. https://doi.org/10.3390/fishes10080419
Landreth JH, Murry BA, Adase KA, Arantes CC, Smith DM, Wellman DI Jr., Junker JR, Pomeranz JPF, Wesner JS. Ecosystem Size and Functional Group Relative Abundance Drive Stream Community Body Size Structure. Fishes. 2025; 10(8):419. https://doi.org/10.3390/fishes10080419
Chicago/Turabian StyleLandreth, Jarrett H., Brent A. Murry, Katherine A. Adase, Caroline C. Arantes, Dustin M. Smith, David I. Wellman, Jr., James R. Junker, Justin P. F. Pomeranz, and Jeff S. Wesner. 2025. "Ecosystem Size and Functional Group Relative Abundance Drive Stream Community Body Size Structure" Fishes 10, no. 8: 419. https://doi.org/10.3390/fishes10080419
APA StyleLandreth, J. H., Murry, B. A., Adase, K. A., Arantes, C. C., Smith, D. M., Wellman, D. I., Jr., Junker, J. R., Pomeranz, J. P. F., & Wesner, J. S. (2025). Ecosystem Size and Functional Group Relative Abundance Drive Stream Community Body Size Structure. Fishes, 10(8), 419. https://doi.org/10.3390/fishes10080419






