Phosphorus in Salmonid Aquaculture: Sources, Requirements, and System-Level Implications
Abstract
1. Introduction
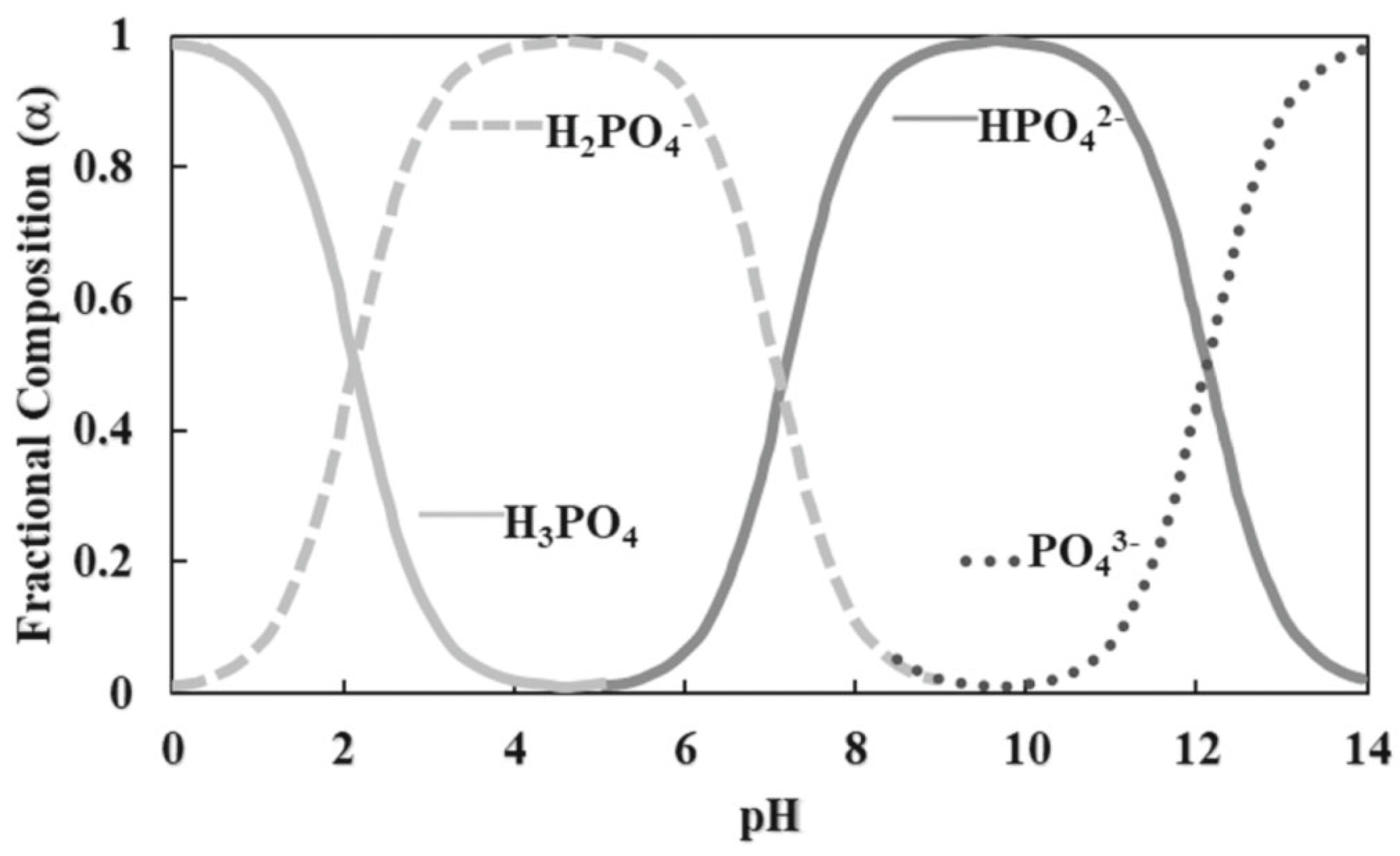
2. The Chemical Element P and Its Cycle
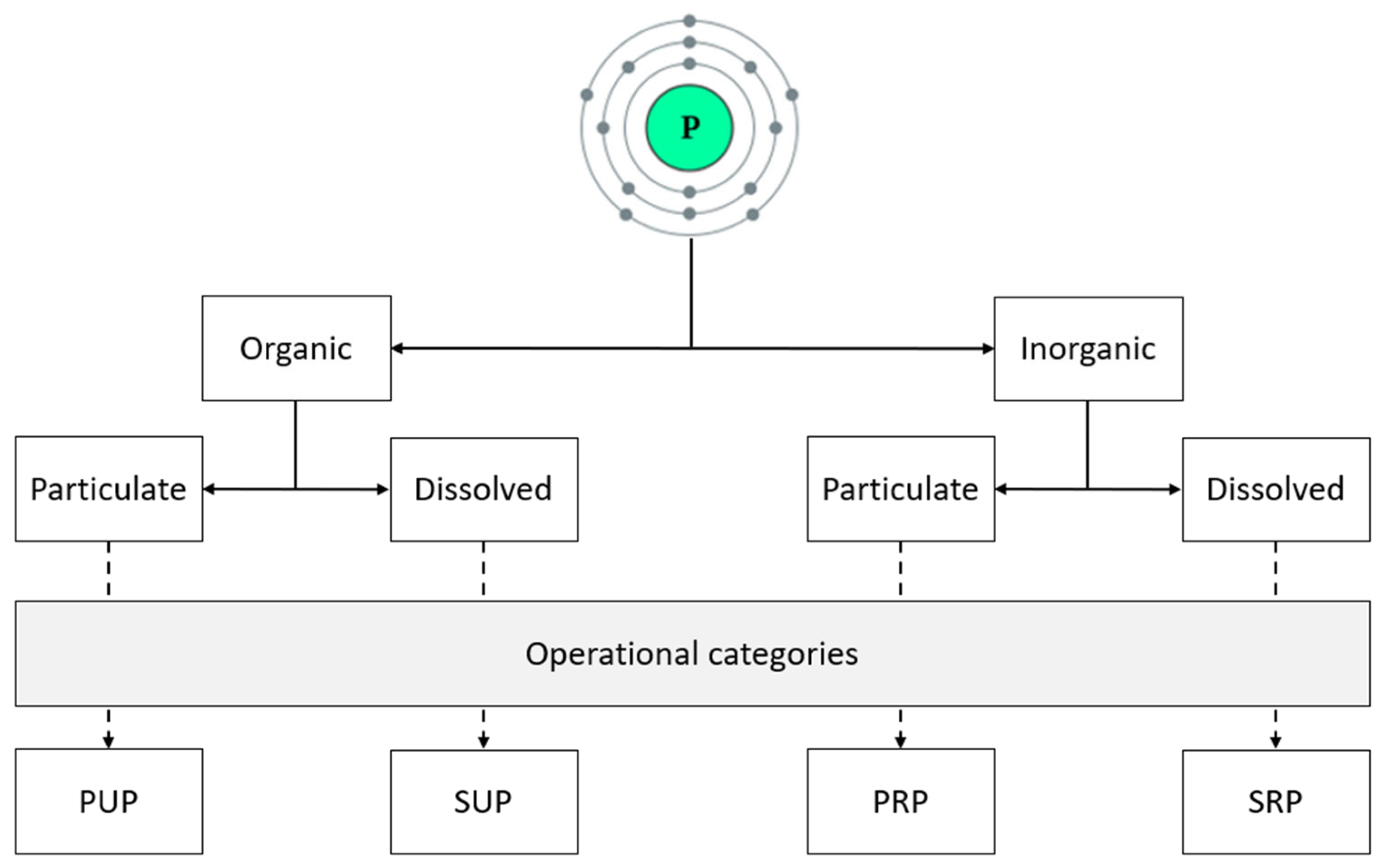
3. Phosphorus Terminology Used in Aquaculture
- Soluble Reactive Phosphorus (SRP)
- Definition: This is the fraction of P that is dissolved in water and readily available for biological uptake. It reacts with molybdate to form a detectable compound.
- Importance: SRP is considered immediately bioavailable and can be quickly utilized by aquatic plants and algae.
- 2.
- Soluble Unreactive Phosphorus (SUP)
- Definition: This includes dissolved phosphorus that does not react with molybdate. It is often Po, such as that found in DNA, RNA, and phospholipids.
- Importance: SUP is not immediately available for biological uptake but can become available over time through biological or chemical processes.
- 3.
- Particulate Reactive Phosphorus (PRP)
- Definition: This is P that is attached to particles in water and can react with molybdate. It includes P bound to sediments or organic matter.
- Importance: PRP can be a significant source of P in aquatic systems, especially when particles are resuspended into the water column.
- 4.
- Particulate Unreactive Phosphorus (PUP)
- Definition: This includes P that is attached to particles but does not react with molybdate. It is often in a form that is not readily available for biological uptake.
- Importance: PUP represents a more stable form of P that can be stored in sediments and may become available under certain conditions.
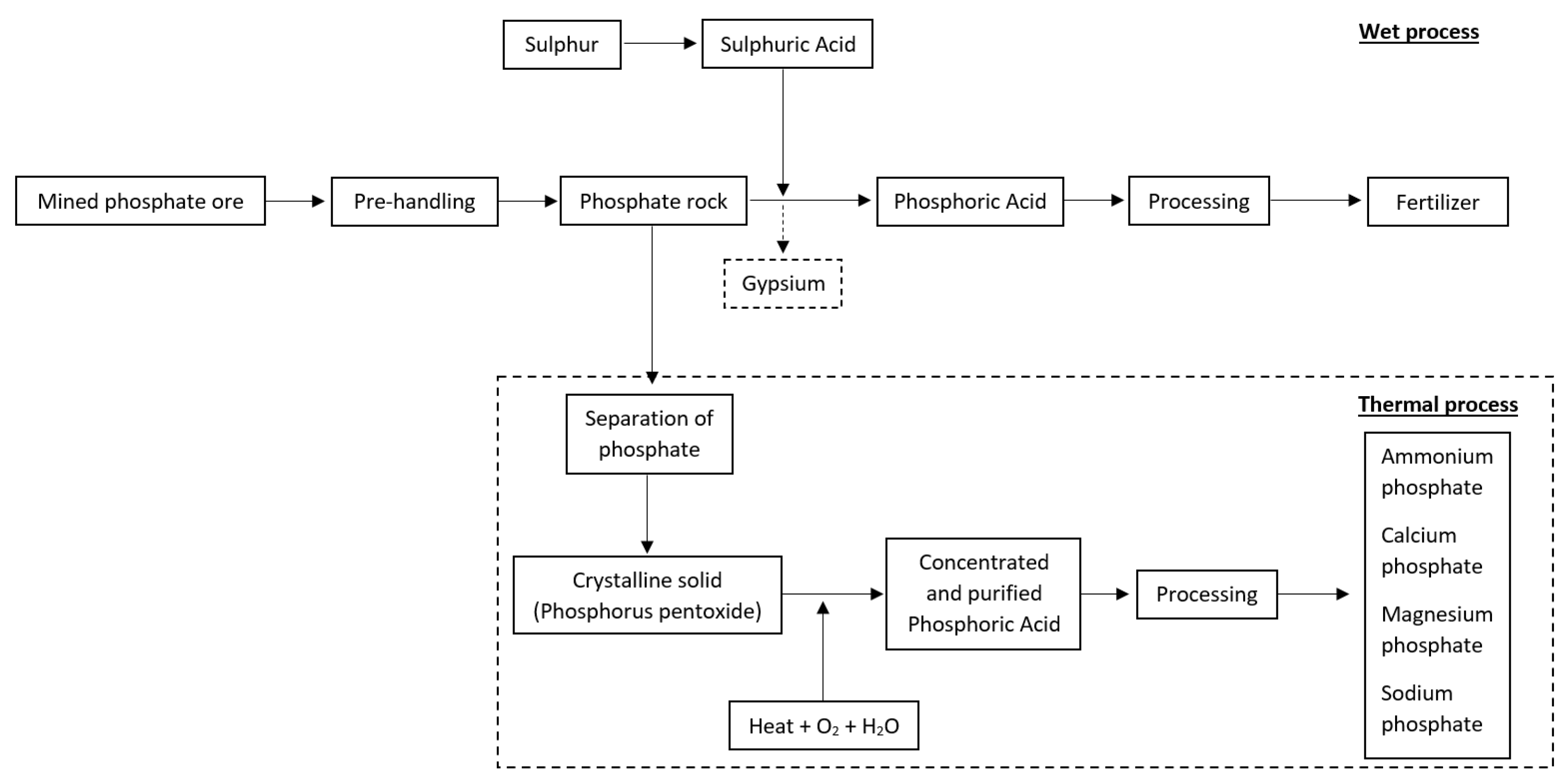
4. The Global Phosphorus Market and Processing Industry
5. Methods for Determining Phosphorus Requirements
5.1. Principles and Practices in Phosphorus Nutrition
5.1.1. Differentiating Between Digestible and Available P
5.1.2. Conducting Requirement Studies
5.2. Modeling Phosphorus Availability in Feed
5.3. Statistical Method
6. Dietary Requirements and Commercial Diet Composition
7. Sources of Phosphorus in Aquafeed
7.1. Marine and Animal Ingredients
7.2. Vegetable Ingredients
7.3. Inorganic Phosphorus Supplements
8. Phosphorus in Salmonids
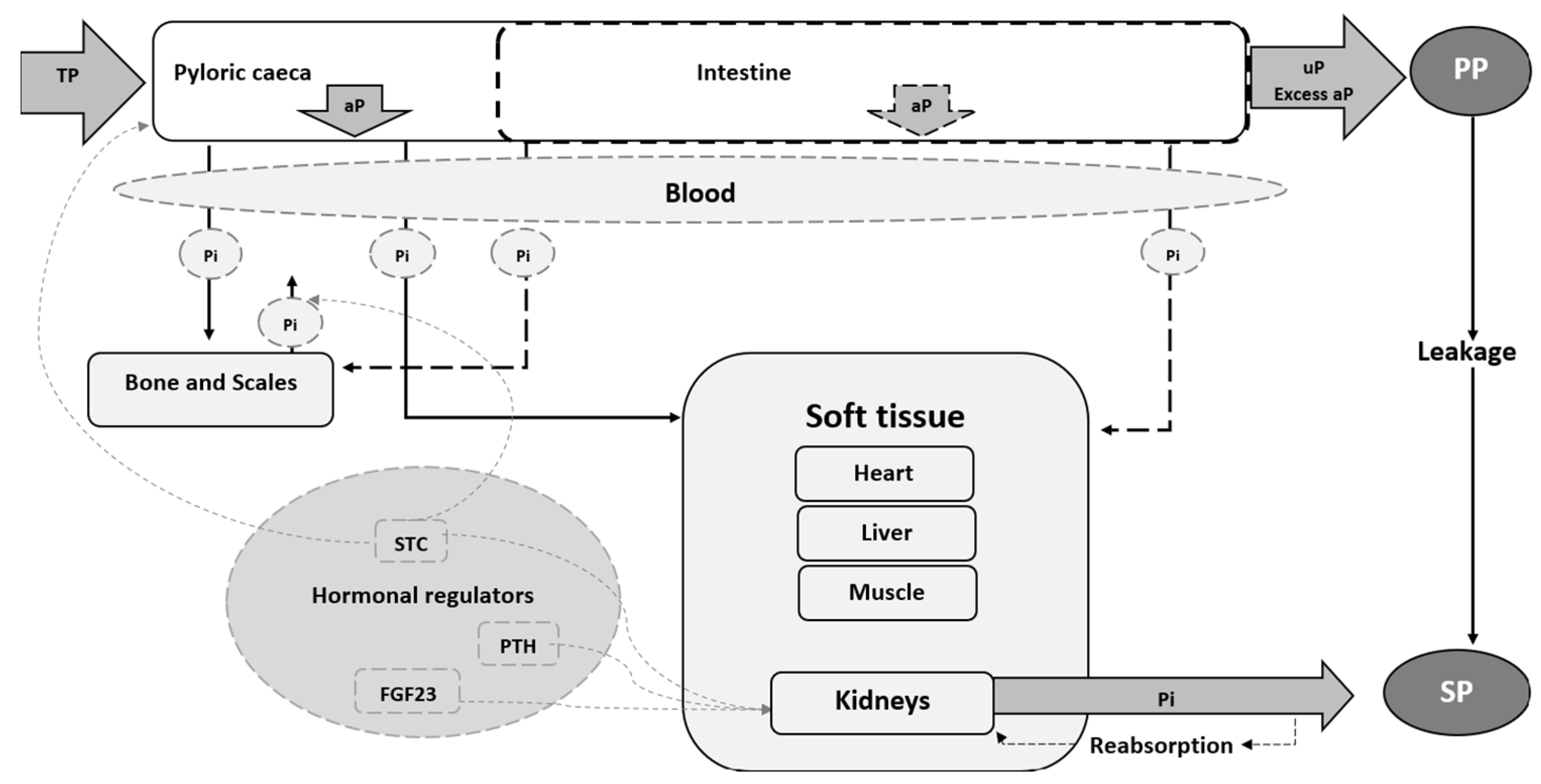
8.1. Absorption, Digestion, Storage, and Loss
- Bones and scales
- 2.
- Soft tissue (heart, liver, muscles, and kidneys)
8.2. Hormone, Vitamin, and Mineral Interactions
9. Relevance for Farming Practices
- Economic Considerations and Available Methods for Phosphorus Removal
10. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lall, S.P. The minerals. In Fish Nutrition, 4th ed.; Hardy, R.W., Kaushik, S.J., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2021; pp. 469–554. [Google Scholar]
- Berndt, T.; Kumar, R. Phosphatonins and the regulation of phosphate homeostasis. Annu. Rev. Physiol. 2007, 69, 341–359. [Google Scholar] [CrossRef]
- Holland, J. Kontali: Worldwide Salmon Supply in 2023 Grew on the Back of Wild Catch, Not Aquaculture. Available online: https://www.seafoodsource.com/news/supply-trade/kontali-worldwide-salmon-supply-growing-on-the-back-of-wild-catch-not-aquaculture (accessed on 6 December 2024).
- Iversen, A.; Asche, F.; Hermansen, Ø.; Nystøyl, R. Production cost and competitiveness in major salmon farming countries 2003–2018. Aquaculture 2020, 522, 735089. [Google Scholar] [CrossRef]
- Hamilton, H.A.; Brod, E.; Hanserud, O.S.; Gracey, E.O.; Vestrum, M.I.; Bøen, A.; Steinhoff, F.S.; Müller, D.B.; Brattebø, H. Investigating cross-sectoral synergies through integrated aquaculture, fisheries, and agriculture Phosphorus assessments: A case study of Norway. J. Ind. Ecol. 2016, 20, 867–881. [Google Scholar] [CrossRef]
- Rognstad, O. Små endringar i mengd gjødsel brukt i jordbruket. Available online: https://www.ssb.no/jord-skog-jakt-og-fiskeri/jordbruk/artikler/sma-endringar-i-mengd-gjodsel-brukt-i-jordbruket (accessed on 28 March 2023).
- akvafakta.no. Akvafakta Måned 2101. Available online: https://akvafakta.no/wp-content/uploads/Maned/2021/2101_Akvafakta.pdf (accessed on 6 December 2024).
- Aas, T.S.; Åsgård, T.; Ytrestøyl, T. Utilization of feed resources in the production of Atlantic salmon (Salmo salar) in Norway: An update for 2020. Aquaulture Rep. 2022, 26, 101316. [Google Scholar] [CrossRef]
- akvafakta.no. Akvafakta Måned 2201. Available online: https://akvafakta.no/wp-content/uploads/Maned/2022/2201_Akvafakta.pdf (accessed on 6 December 2024).
- akvafakta.no. Akvafakta Måned 2301. Available online: https://akvafakta.no/wp-content/uploads/Maned/2023/2301_Akvafakta.pdf (accessed on 6 December 2024).
- Sundnes, H.M.; Tande, T.; Tande, T. Matfisk på Land. Available online: https://norskfisk.no/2024/11/07/matfisk-pa-land-2/? (accessed on 6 December 2024).
- Correll, D.L. The role of phosphorus in the eutrophication of receiving waters: A review. J. Environ. Qual. 1998, 27, 261–266. [Google Scholar] [CrossRef]
- Daniel, T.C.; Sharpley, A.N.; Lemunyon, J.L. Agricultural phosphorus and eutrophication: A symposium overview. J. Environ. Qual. 1998, 27, 251–257. [Google Scholar] [CrossRef]
- Herath, S.S.; Satoh, S. Environmental impact of phosphorus and nitrogen from aquaculture. In Feed and Feeding Practices in Aquaculture; Elsevier: Amsterdam, The Netherlands, 2015; pp. 369–386. [Google Scholar]
- Boyd, C.E. Guidelines for aquaculture effluent management at the farm-level. Aquaculture 2003, 226, 101–112. [Google Scholar] [CrossRef]
- Bergheim, A.; Brinker, A. Effluent treatment for flow through systems and European environmental regulations. Aquac. Eng. 2003, 27, 61–77. [Google Scholar] [CrossRef]
- NEA. Land-Based Aquaculture: Results from the nationwide Inspection Campaign in 2024; 2025. Available online: https://www.miljodirektoratet.no/publikasjoner/2025/mai-2025/landbasert-akvakultur-resultater-fra-landsdekkende-tilsynsaksjon-i-2024/ (accessed on 14 May 2025).
- Reijnders, L. Phosphorus resources, their depletion and conservation, a review. Resour. Conserv. Recycl. 2014, 93, 32–49. [Google Scholar] [CrossRef]
- Boyd, C.E. (Ed.) Phosphorus. In Water Quality; Springer: Cham, Switzerland, 2015; pp. 243–261. [Google Scholar]
- Sugiura, S.H. Digestion and absorption of dietary phosphorus in fish. Fishes 2024, 9, 324. [Google Scholar] [CrossRef]
- Sugiura, S.H. Urinary phosphorus excretion in fish: Environmental and aquaculture implications. Aquat. Living Resour. 2025, 38, 7. [Google Scholar] [CrossRef]
- Nathanailides, C.; Kolygas, M.; Tsoumani, M.; Gouva, E.; Mavraganis, T.; Karayanni, H. Addressing phosphorus waste in open flow freshwater fish farms: Challenges and solutions. Fishes 2023, 8, 442. [Google Scholar] [CrossRef]
- Baumeister, R.F.; Leary, M.R. Writing narrative literature reviews. Rev. Gen. Psychol. 1997, 1, 311–320. [Google Scholar] [CrossRef]
- Rother, E.T. Systematic literature review X narrative review. Acta Paul. De Enferm. 2007, 20, v–vi. [Google Scholar] [CrossRef]
- Ruttenberg, K.C. The global phosphorus cycle. Treatise Geochem. 2003, 8, 682. [Google Scholar] [CrossRef]
- Kroiss, H.; Rechberger, H.; Egle, L. Phosphorus in water quality and waste management. In Integrated Waste Management-Volume II; Kumar, S., Ed.; InTech: London, UK, 2011; pp. 181–214. [Google Scholar]
- LibreTexts. REACTIVITY of Phosphorus Compounds. Available online: https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_%28Organic_Chemistry%29/Organo-phosphorus_Compounds/Reactivity_of_Phosphorus_Compounds (accessed on 13 December 2024).
- Durif, A. Crystal Chemistry of Condensed Phophates; Springer: New York, NY, USA, 1995. [Google Scholar]
- Griffith, E.J. The chemical and physical properties of condensed phosphates. In Proceedings of the Plenary Lectures Presented at the Second Symposium on Inorganic Phosphorus Compounds, Prague, Czech Republic, 9–13 September 1974; pp. 173–200. [Google Scholar]
- Islam, R.; Barman, D.K.; Kabir, M.; Sabur, M.A. Salinity-induced phosphate binding to soil particles: Effects of divalent cations. Water Air Soil Pollut. 2023, 234, 697. [Google Scholar] [CrossRef]
- Zeitoun, R.; Biswas, A. Potentiometric determination of phosphate using cobalt: A review. J. Electrochem. Soc. 2020, 167, 127507. [Google Scholar] [CrossRef]
- Noller, C.R.; Usselman, M.C.; Norman, R.O.C. Organic Compound. Available online: https://www.britannica.com/science/organic-compound (accessed on 14 February 2025).
- Whitford, D. Proteins: Structure and Function; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Turner, B.L.; Frossard, E.; Baldwin, D.S. Organic Phosphorus in the Environment; CABI Publishing: Oxfordshire, UK, 2005. [Google Scholar]
- Zheng, L.; Ren, M.; Xie, E.; Ding, A.; Liu, Y.; Deng, S.; Zhang, D. Roles of phosphorus sources in microbial community assembly for the removal of organic matters and ammonia in activated sludge. Front. Microbiol. 2019, 10, 1023. [Google Scholar] [CrossRef]
- Wetzel, R.G. 13-The phosphorus cycle. In Limnology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2001; pp. 239–288. [Google Scholar] [CrossRef]
- USEPA. VMS56. Available online: https://archive.epa.gov/water/archive/web/html/vms56.html (accessed on 15 December 2024).
- Kirchman, D.L. Phytoplankton death in the sea. Nature 1999, 398, 293–294. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Microbial transformations in the phosphorus cycle. In Advances in Microbial Ecology; Alexander, M., Ed.; Springer: Boston, MA, USA, 1977; Volume 1. [Google Scholar]
- Van Cappellen, P.; Ingall, E.D. Redox stabilization of the atmosphere and oceans by phosphorus-limited marine productivity. Science 1996, 271, 493–496. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality: Incorporating the First and Second Addenda; World Health Organization: Geneva, Switzerland, 2022.
- Lekang, O.-I. Aquaculture Engineering; Wiley Online Library: Hoboken, NJ, USA, 2013. [Google Scholar]
- Karl, D.M.; Björkman, K.M. Dynamics of dissolved organic phosphorus. In Biogeochemistry of Marine Dissolved Organic Matter; Elsevier: Amsterdam, The Netherlands, 2015; pp. 233–334. [Google Scholar]
- Benitez-Nelson, C.R.; O’Neill, L.; Kolowith, L.C.; Pellechia, P.; Thunell, R. Phosphonates and particulate organic phosphorus cycling in an anoxic marine basin. Limnol. Oceanogr. 2004, 49, 1593–1604. [Google Scholar] [CrossRef]
- Uglem, I.; Järnegren, J.; Bloecher, N. Effekter av Organisk Utslipp fra Havbruk i Norge–En Kunnskapsoppsummering; Norwegian Institute for Nature Research: Trondheim, Norway, 2020. [Google Scholar]
- Olsen, Y.; Olsen, L.M. Environmental impact of aquaculture on coastal planktonic ecosystems. In Fisheries for Global Welfare and Environment. Memorial Book of the 5th World Fisheries Congress 2008; Terrapub: Tokyo, Japan, 2008. [Google Scholar]
- Ulrich, A.E.; Frossard, E. On the history of a reoccurring concept: Phosphorus scarcity. Sci. Total Environ. 2014, 490, 694–707. [Google Scholar] [CrossRef]
- Sharpley, A.; Tunney, H. Phosphorus research strategies to meet agricultural and environmental challenges of the 21st century. J. Environ. Qual. 2000, 29, 176–181. [Google Scholar] [CrossRef]
- Van Kauwenbergh, S.J. World Phosphate Rock Reserves and Resources; International Fertilizer Development Center: Muscle Shoals, AL, USA, 2010. [Google Scholar]
- Jasinski, S.M. U.S. Geological Survey. Mineral Commodity Summaries. Phosphate Rock Statistics and Information 1999–2024. Available online: https://www.usgs.gov/centers/national-minerals-information-center/phosphate-rock-statistics-and-information (accessed on 12 December 2024).
- Jasinski, S.M. Mineral Commodity Summaries: Phosphate Rock; United States Geological Survey: Reston, VA, USA, 2021.
- GVR. Phosphate rock market size, share & trends analysis report by application (fertilizers, food & feed additives, industrial), by region (North America, Europe, APAC, South America, MEA), and segment forecasts, 2022–2030 (Rep. ID: GVR-3-68038-281-5). 2021. Available online: https://www.grandviewresearch.com/industry-analysis/phosphate-rock-market (accessed on 12 November 2024).
- Li, B.; Bicknell, K.B.; Renwick, A. Peak phosphorus, demand trends and implications for the sustainable management of phosphorus in China. Resour. Conserv. Recycl. 2019, 146, 316–328. [Google Scholar] [CrossRef]
- Gilmour, R. Phosphoric Acid. Purification, Uses, Technology and Econimics; Routledge: London, UK, 2013. [Google Scholar]
- USEPA. Phosphoric Acid and Phosphatic Fertilizers: A Profile. Available online: https://www.epa.gov/sites/production/files/2020-07/documents/phosphoric-acid-phosphatic-fertilizers_ip_07-1993.pdf (accessed on 16 December 2024).
- Europe, F. Production of Phosphoric Acid. Available online: https://fertilizerseurope.com/wp-content/uploads/2019/08/Booklet_4_final.pdf (accessed on 16 December 2024).
- The Essential Chemical Industry-Online. Phosphoric Acid. Available online: https://www.essentialchemicalindustry.org/chemicals/phosphoric-acid.html (accessed on 3 June 2022).
- Hawkesford, M.J.; Cakmak, I.; Coskun, D.; De Kok, L.J.; Lambers, H.; Schjoerring, J.K.; White, P.J. Functions of macronutrients. In Marschner’s Mineral Nutrition of Plants; Elsevier: Amsterdam, The Netherlands, 2023; pp. 201–281. [Google Scholar]
- Germeau, A.; Heptia, B. Method and device for producing polyphosphoric acid. WO2010108991A1, 30 September 2010. [Google Scholar]
- Hervé, E. IFP-Inorganic Feed Phosphates. Available online: https://specialty-chemicals.eu/ifp/#:~:text=Most%20inorganic%20feed%20phosphates%20are,magnesium%2C%20ammonium%20and%20sodium%20phosphates (accessed on 3 June 2022).
- Epps, E.A. Photometric determination of available phosphorus pentoxide in fertilizers. Anal. Chem. 1950, 22, 1062–1063. [Google Scholar] [CrossRef]
- Hua, K.; Bureau, D.P. Modelling digestible phosphorus content of salmonid fish feeds. Aquaculture 2006, 254, 455–465. [Google Scholar] [CrossRef]
- Boyd, C.E.; McNevin, A. Aquaculture, Resource Use, and the Environment; John Eiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Kim, E.; Yoo, S.; Ro, H.Y.; Han, H.J.; Baek, Y.W.; Eom, I.C.; Kim, H.M.; Kim, P.; Choi, K. Aquatic toxicity assessment of phosphate compounds. Env. Health Toxicol. 2013, 28, e2013002. [Google Scholar] [CrossRef]
- Goodman, B.E. Insights into digestion and absorption of major nutrients in humans. Adv. Physiol. Educ. 2010, 34, 44–53. [Google Scholar] [CrossRef]
- Traylor, S.L.; Cromwell, G.L.; Lindemann, M.D.; Knabe, D.A. Effects of level of supplemental phytase on ileal digestibility of amino acids, calcium, and phosphorus in dehulled soybean meal for growing pigs. J. Anim. Sci. 2001, 79, 2634–2642. [Google Scholar] [CrossRef]
- Austreng, E. Digestibility determination in fish using chromic oxide marking and analysis of contents from different segments of the gastrointestinal tract. Aquaculture 1978, 13, 265–272. [Google Scholar] [CrossRef]
- Austreng, E.; Storebakken, T.; Thomassen, M.S.; Refstie, S.; Thomassen, Y. Evaluation of selected trivalent metal oxides as inert markers used to estimate apparent digestibility in salmonids. Aquaculture 2000, 188, 65–78. [Google Scholar] [CrossRef]
- Albrektsen, S.; Hope, B.; Aksnes, A. Phosphorous (P) deficiency due to low P availability in fishmeal produced from blue whiting (Micromesistius poutassou) in feed for under-yearling Atlantic salmon (Salmo salar) smolt. Aquaculture 2009, 296, 318–328. [Google Scholar] [CrossRef]
- Storebakken, T.; Shearer, K.D.; Roem, A.J. Growth, uptake and retention of nitrogen and phosphorus, and absorbtion of other minerals in Atlantic salmon Salmo salar fed diets with fish meal and soy-protein concentrate as the main source of protein. Aquac. Nutr. 2000, 6, 103–108. [Google Scholar] [CrossRef]
- Hansen, J.Ø.; Sharma, S.; Horn, S.J.; Eijsink, V.G.H.; Øverland, M.; Mydland, L.T. Fecal excretion and whole-body retention of macro and micro minerals in Atlantic Salmon fed torula yeast grown on sugar kelp hydrolysate. Animals 2021, 11, 2409. [Google Scholar] [CrossRef]
- Agboola, J.O.; Lapeña, D.; Øverland, M.; Arntzen, M.Ø.; Mydland, L.T.; Hansen, J.Ø. Yeast as a novel protein source-Effect of species and autolysis on protein and amino acid digestibility in Atlantic salmon (Salmo salar). Aquaculture 2022, 546, 737312. [Google Scholar] [CrossRef]
- Storebakken, T.; Shearer, K.D.; Roem, A.J. Availability of protein, phosphorus and other elements in fish meal, soy-protein concentrate and phytase-treated soy-protein-concentrate-based diets to Atlantic salmon. Salmo salar. Aquaculture 1998, 161, 365–379. [Google Scholar] [CrossRef]
- Weththasinghe, P.; Hansen, J.Ø.; Nøkland, D.; Lagos, L.; Rawski, M.; Øverland, M. Full-fat black soldier fly larvae (Hermetia illucens) meal and paste in extruded diets for Atlantic salmon (Salmo salar): Effect on physical pellet quality, nutrient digestibility, nutrient utilization and growth performances. Aquaculture 2021, 530, 735785. [Google Scholar] [CrossRef]
- Kraugerud, O.F.; Penn, M.; Storebakken, T.; Refstie, S.; Krogdahl, Å.; Svihus, B. Nutrient digestibilities and gut function in Atlantic salmon (Salmo salar) fed diets with cellulose or non-starch polysaccharides from soy. Aquaculture 2007, 273, 96–107. [Google Scholar] [CrossRef]
- Riche, M.; Brown, P.B. Availability of phosphorus from feedstuffs fed to rainbow trout, Oncorhynchus mykiss. Aquaculture 1996, 142, 269–282. [Google Scholar] [CrossRef]
- Parada, J.; Aguilera, J.M. Food microstructure affects the bioavailability of several nutrients. J. Food Sci. 2007, 72, R21–R32. [Google Scholar] [CrossRef]
- Lall, S.P.; Kaushik, S.J. Nutrition and metabolism of minerals in fish. Animals 2021, 11, 2711. [Google Scholar] [CrossRef] [PubMed]
- Shastak, Y.; Rodehutscord, M. Determination and estimation of phosphorus availability in growing poultry and their historical development. World’s Poult. Sci. J. 2019, 69, 569–586. [Google Scholar] [CrossRef]
- Åsgård, T.; Shearer, K.D. Dietary phosphorus requirement of juvenile Atlantic salmon. Salmo salar L. Aquac. Nutr. 1997, 3, 17–23. [Google Scholar] [CrossRef]
- Nordrum, S.; Åsgård, T.; Shearer, K.D.; Arnessen, P. Availability of phosphorus in fish bone meal and inorganic salts to Atlantic salmon (Salmo salar) as determined by retention. Aquaculture 1997, 157, 51–61. [Google Scholar] [CrossRef]
- Baeverfjord, G.; Åsgård, T.; Shearer, K.D. Development and detection of phosphorus deficiency in Atlantic Salmon, Salmo salar L., parr and post-smolts. Aquac. Nutr. 1998, 4, 51–61. [Google Scholar] [CrossRef]
- Greiling, A.M.; Tschesche, C.; Baardsen, G.; Kröckel, S.; Koppe, W.; Rodehutscord, M. Effects of phosphate and phytase supplementation on phytate degradation in rainbow trout (Oncorhynchus mykiss W.) and Atlantic salmon (Salmo salar L.). Aquaculture 2019, 503, 467–474. [Google Scholar] [CrossRef]
- Shearer, K.D. Experimental design, statistical analysis and modelling of dietary nutrient requirement studies for fish: A critical review. Aquac. Nutr. 2000, 6, 91–102. [Google Scholar] [CrossRef]
- Ketola, G.H. Requirement of Atlantic salmon for dietary phosphorus. Trans. Am. Fish. Soc. 1975, 104, 548–551. [Google Scholar] [CrossRef]
- Pelech, S.L.; Vance, D.E. Regulation of phosphatidylcholine biosynthesis. Biochim. Biophys. Acta 1984, 779, 217–251. [Google Scholar] [CrossRef]
- Hovde, G. Validation of a Method for Analysis of Soluble Phosphorus by Use of Alkaline Extraction and Spectrophotometric Determination; Nofima: Tromsø, Norway, 2013. [Google Scholar]
- Pfeffer, E.; Pieper, A. Application of the factorial approach for deriving nutrient requirements of growing fish. In Proceedings of the World Symposium on Finfish Nutrition and Fishfeed Technology, Hamburg: Heeneman Verlagsgell-schaft, 1979; pp. 545–553. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/19801497196 (accessed on 15 March 2024).
- Shearer, K.D. Determination of the Dietary Requirements for Essential Elements in Fish; University of Bergen, Institute of Fisheries and Marine Biology: Bergen, Norway, 1991. [Google Scholar]
- Shearer, K.D. Factors affecting the proximate composition of cultured fishes with emphasis on salmonids. Aquaculture 1994, 119, 63–88. [Google Scholar] [CrossRef]
- Dersjant-Li, Y.; Awati, A.; Schulze, H.; Partridge, G. Phytase in non-ruminant animal nutrition: A critical review on phytase activities in the gastrointestinal tract and influencing factors. J. Sci. Food Agric. 2015, 95, 878–896. [Google Scholar] [CrossRef] [PubMed]
- Shearer, K.D. The use of factorial modeling to determine the dietary requirements for essential elements in fishes. Aquaculture 1995, 133, 57–72. [Google Scholar] [CrossRef]
- Baeverfjord, G.; Prabhu, A.J.; Fjelldal, P.G.; Albrektsen, S.; Hatlen, B.; Denstadli, V.; Ytteborg, E.; Takle, H.; Lock, E.-J.; Berntssen, M.H.G.; et al. Mineral nutrition and bone health in salmonids. Rev. Aquac. 2018, 11, 740–765. [Google Scholar] [CrossRef]
- Huxley, J. Problems of relative growth. Found. Nat. Hist. 1936, 276, 221. [Google Scholar]
- Laird, A.K.; Barton, A.D.; Tyler, S.A. Growth and time: An interpretation of allometry. Growth 1968, 32, 347–354. [Google Scholar]
- Papoutsoglou, S.E.; Papaparaskeva-Papoutsoglou, E.G. Comparative studies on body composition of rainbow trout (Salmo gairdneri R.) in relation to type of diet and growth rate. Aquaculture 1978, 13, 235–243. [Google Scholar] [CrossRef]
- Shearer, K.D. Changes in elemental composition of hatchery-reared rainbow trout, Salmo gairdneri, associated with growth and reproduction. Can. J. Fish. Aquat. Sci. 1984, 41, 1592–1600. [Google Scholar] [CrossRef]
- Shearer, K.D.; Åsgård, T.; Andorsdottir, G.; Aas, G.H. Whole body elemental and proximate composition of Atlantic salmon (Salmo salar) during the life cycle. J. Fish Biol. 1994, 44, 785–797. [Google Scholar] [CrossRef]
- Aas, T.S.; Ytrestøyl, T.; Åsgård, T. Utilization of feed resources in the production of Atlantic salmon (Salmo salar) in Norway: An update for 2016. Aquac. Rep. 2019, 15, 100216. [Google Scholar] [CrossRef]
- Talbot, C.; Preston, T.; East, B.W. Body composition of Atlantic salmon (Salmo salar L.) studied by neutron activation analysis. Comp. Biochem. Physiol. A Comp. Physiol. 1986, 85, 445–450. [Google Scholar] [CrossRef]
- Sambraus, F.; Hansen, T.; Daae, B.S.; Thorsen, A.; Sandvik, R.; Stien, L.H.; Fraser, T.W.K.; Fjelldal, P.G. Triploid Atlantic salmon (Salmo salar) have a higher dietary phosphorus requirement for bone mineralization during early development. J. Fish Biol. 2020, 97, 137–147. [Google Scholar] [CrossRef]
- Godoy, S.; Chicco, C.; Meschy, F.; Requena, F. Phytic phosphorus and phytase activity of animal feed ingredients. Interciencia 2005, 30, 24–28. [Google Scholar]
- Kumar, V.; Sinha, A.K.; Makkar, H.P.; De Boeck, G.; Becker, K. Phytate and phytase in fish nutrition. J. Anim. Physiol. Anim. Nutr. 2011, 96, 335–364. [Google Scholar] [CrossRef]
- Selle, P.H.; Walker, A.R.; Bryden, W.L. Total and phytate-phosphorus contents and phytase activity of Australian-sourced feed ingredients for pigs and poultry. Aust. J. Exp. Agric. 2003, 43, 475–479. [Google Scholar] [CrossRef]
- Lall, S.P. Digestibility, metabolism and excretion of dietary phosphorus in fish. In Nutritional Strategies and Aquaculture Waste; Cowey, C.B., Cho, C.Y., Eds.; University of Guelph, Department of Nutritional Sciences: Guelph, ON, Canada, 1991; pp. 21–36. [Google Scholar]
- Miles, R.D.; Chapman, F.A. The benefits of fish meal in aquaculture diets: FA122/FA122, 5/2006. Edis 2006, 2006. Available online: https://journals.flvc.org/edis/article/view/115917 (accessed on 7 January 2025).
- Lall, S.P.; Bishop, F.J. Studies on Mineral and Protein Utilization by Atlantic Salmon Grown in Sea Water; Disease and Nutrition Section, Department of Fisheries and Environment: Ottawa, ON, Canada, 1977.
- Ogino, C.; Takeda, H. Requirements of rainbow trout for dietary calcium and phosphorus. Bull. Jpn. Soc. Sci. Fish 1978, 44, 1019–1022. [Google Scholar] [CrossRef]
- Ketola, G.H.; Richmond, M.E. Requirement of rainbow trout for dietary phosphorus and its relationship to the amount discharged in hatchery effluents. Trans. Am. Fish. Soc. 1994, 123, 587–594. [Google Scholar] [CrossRef]
- Rodehutscord, M. Response of rainbow trout (Oncorhynchus mykiss) growing from 50 to 200 g to supplements of dibasic sodium phosphate in a semipurified diet. J. Nutr. 1996, 126, 324–331. [Google Scholar] [CrossRef]
- Chatvijitkul, S.; Boyd, C.E.; Davis, D.A. Nitrogen, phosphorus, and carbon concentrations in some common aquaculture feeds. J. World Aquac. Soc. 2018, 49, 477–483. [Google Scholar] [CrossRef]
- Flo, V.Ø.; Cavrois-Rogacki, T.; Hansen, J.Ø.; Vigen, J.; Gitlesen, T.; Lekang, O.-I. RAS-designed diets result in lower accumulation of nitrogen, phosphorus, and zinc in recirculating aquaculture system compared with traditional flow-through designed diets. Fishes 2024, 9, 300. [Google Scholar] [CrossRef]
- Witten, P.E.; Fjelldal, P.G.; Huysseune, A.; McGurk, C.; Obach, A.; Owen, M.A.G. Bone without minerals and its secondary mineralization in Atlantic salmon (Salmo salar): The recovery from phosphorus deficiency. J. Exp. Biol. 2019, 222, jeb188763. [Google Scholar] [CrossRef]
- Fjelldal, P.G.; Hansen, T.; Breck, O.; Sandvik, R.; Waagbø, R.; Berg, A.; Ørnsrud, R. Supplementation of dietary minerals during the early seawater phase increase vertebral strength and reduce the prevalence of vertebral deformities in fast-growing under-yearling Atlantic salmon (Salmo salar L). smolt. Aquac. Nutr. 2009, 15, 366–378. [Google Scholar] [CrossRef]
- Sugiura, S.H.; Hardy, R.W.; Roberts, R.J. The pathology of phosphorus deficiency in fish–a review. J. Fish Dis. 2004, 27, 255–265. [Google Scholar] [CrossRef]
- Fjelldal, P.G.; Hansen, T.; Albrektsen, S. Inadequate phosphorus nutrition in juvenile Atlantic salmon has a negative effect on long-term bone health. Aquaculture 2012, 334, 117–123. [Google Scholar] [CrossRef]
- Fraser, T.W.K.; Witten, P.E.; Albrektsen, S.; Breck, O.; Fontanillas, R.; Nankervis, L.; Thomsen, T.H.; Koppe, W.; Sambraus, F.; Fjelldal, P.G. Phosphorus nutrition in farmed Atlantic salmon (Salmo salar): Life stage and temperature effects on bone pathologies. Aquaculture 2019, 511, 734246. [Google Scholar] [CrossRef]
- Drábiková, L.; Fjelldal, P.G.; De Clercq, A.; Yousaf, M.N.; Morken, T.; McGurk, C.; Witten, P.E. What will happen to my smolt at harvest? Individually tagged Atlantic salmon help to understand possible progression and regression of vertebral deformities. Aquaculture 2022, 559, 738430. [Google Scholar] [CrossRef]
- Fjelldal, P.G.; Hansen, T.J.; Lock, E.-J.; Wargelius, A.; Fraser, T.W.K.; Sambraus, F.; El-Mowafi, A.; Albrektsen, S.; Waagbø, R.; Ørnsrud, R. Increased dietary phosphorous prevents vertebral deformities in triploid Atlantic salmon (Salmo salar L). Aquac. Nutr. 2016, 22, 72–90. [Google Scholar] [CrossRef]
- Berge, G.M.; Witten, P.E.; Baeverfjord, G.; Vegusdal, A.; Wadsworth, S.; Ruyter, B. Diets with different n− 6/n− 3 fatty acid ratio in diets for juvenile Atlantic salmon, effects on growth, body composition, bone development and eicosanoid production. Aquaculture 2009, 296, 299–308. [Google Scholar] [CrossRef]
- Flik, G.; Van Der Velden, J.A.; Dechering, K.J.; Verbost, P.M.; Schoenmakers, T.J.M.; Kolar, Z.I.; Bonga, S.E.W. Ca2+ and Mg2+ transport in gills and gut of tilapia, Oreochromis mossambicus: A review. J. Exp. Zool. 1993, 265, 356–365. [Google Scholar] [CrossRef]
- Skonberg, D.I.; Yogev, L.; Hardy, R.W.; Dong, F.M. Metabolic response to dietary phosphorus intake in rainbow trout (Oncorhynchus mykiss). Aquaculture 1997, 157, 11–24. [Google Scholar] [CrossRef]
- Vielma, J.; Lall, S.P. Phosphorus utilization by Atlantic salmon (Salmo salar) reared in freshwater is not influenced by higher dietary calcium intake. Aquaculture 1998, 160, 117–128. [Google Scholar] [CrossRef]
- Metz, J.R.; Leeuwis, R.H.J.; Zethof, J.; Flik, G. Zebrafish (D anio rerio) in calcium-poor water mobilise calcium and phosphorus from scales. J. Appl. Ichthyol. 2014, 30, 671–677. [Google Scholar] [CrossRef]
- Persson, P.; Sundell, K.; Björnsson, B.T.; Lundqvist, H. Calcium metabolism and osmoregulation during sexual maturation of river running Atlantic salmon. J. Fish Biol. 1998, 52, 334–349. [Google Scholar] [CrossRef]
- Jobling, M. National research council (NRC): Nutrient requirements of fish and shrimp. Aquac. Int. 2011, 20, 601–602. [Google Scholar] [CrossRef]
- Lazzari, R.; Baldisserotto, B. Nitrogen and phosphorus waste in fish farming. Bol. Do Inst. De Pesca 2008, 34, 591–600. [Google Scholar]
- Albrektsen, S.; Lock, E.-J.; Baeverfjord, G.; Pedersen, M.; Krasnov, A.; Takle, H.; Veiseth-Kent, E.; Ørnsrud, R.; Waagbø, R.; Ytteborg, E. Utilization of H2SO4-hydrolysed phosphorus from herring bone by-products in feed for Atlantic salmon (Salmo salar) 0+postsmolt. Aquac. Nutr. 2018, 24, 348–365. [Google Scholar] [CrossRef]
- Egerton, S.; Wan, A.; Murphy, K.; Collins, F.; Ahern, G.; Sugrue, I.; Busca, K.; Egan, F.; Muller, N.; Whooley, J.; et al. Replacing fishmeal with plant protein in Atlantic salmon (Salmo salar) diets by supplementation with fish protein hydrolysate. Sci. Rep. 2020, 10, 4194. [Google Scholar] [CrossRef]
- Kangsen, M.; Xue, M.; He, G.; Xie, S.Q.; Kaushik, S. Protein and amino acids. In Fish Nutrition, 4th ed.; Hardy, R.W., Kaushik, S.J., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2021; pp. 181–302. [Google Scholar]
- Shepherd, C.J.; Jackson, A.J. Global fishmeal and fish-oil supply: Inputs, outputs and marketsa. J. Fish Biol. 2013, 83, 1046–1066. [Google Scholar] [CrossRef]
- Albrektsen, S. Soluble phosphorus in salmon feed: Suitable as a measure of available phosphorus? Int. Aquafeed 2015, 18, 14–15. [Google Scholar]
- NRC. Nutrient Requirements of Fish; National Academies Press: Washington, DC, USA, 1993. [Google Scholar]
- Ytteborg, E.; Baeverfjord, G.; Lock, E.-J.; Pedersen, M.; Takle, H.; Ørnsrud, R.; Waagbø, R.; Albrektsen, S. Utilization of acid hydrolysed phosphorous from herring bone by-products in feed for Atlantic salmon (Salmo salar) start-feeding fry. Aquaculture 2016, 459, 173–184. [Google Scholar] [CrossRef]
- Skoglund, E.; Carlsson, N.-G.; Sandberg, A.-S. Phytate. In Analysis of Bioactive Components in Small Grain Cereals; Shewry, P.R., Ward, J.L., Eds.; Woodhead Publishing and AACC International Press: St. Paul, MN, USA, 2009; pp. 129–139. [Google Scholar]
- Reddy, N.R. Occurrence, distribution, content, and dietary intake of phytate. In Food Phytates; CRC Press: Boca Raton, FL, USA, 2001; pp. 41–68. [Google Scholar]
- Nolan, K.B.; Duffin, P.A.; McWeeny, D.J. Effects of phytate on mineral bioavailability. In vitro studies on Mg2+, Ca2+, Fe3+, Cu2+ and Zn2+ (also Cd2+) solubilities in the presence of phytate. J. Sci. Food Agric. 1987, 40, 79–85. [Google Scholar] [CrossRef]
- Thompson, L.U.; Yoon, J.H. Starch digestibility as affected by polyphenols and phytic acid. J. Food Sci. 1984, 49, 1228–1229. [Google Scholar] [CrossRef]
- Dendougui, F.; Schwedt, G. In vitro analysis of binding capacities of calcium to phytic acid in different food samples. Eur. Food Res. Technol. 2004, 219, 409–415. [Google Scholar] [CrossRef]
- Denstadli, V.; Skrede, A.; Krogdahl, Å.; Sahlstrøm, S.; Storebakken, T. Feed intake, growth, feed conversion, digestibility, enzyme activities and intestinal structure in Atlantic salmon (Salmo salar L.) fed graded levels of phytic acid. Aquaculture 2006, 256, 365–376. [Google Scholar] [CrossRef]
- Humer, E.; Schwarz, C.; Schedle, K. Phytate in pig and poultry nutrition. J. Anim. Physiol. Anim. Nutr. 2015, 99, 605–625. [Google Scholar] [CrossRef]
- Konietzny, U.; Greiner, R. Phytic acid/Properties and determination. In Encyclopedia of Food Science and Nutrition; Elsevier: Amsterdam, The Netherlands, 2003; pp. 4546–4555. [Google Scholar]
- Igbasan, F.A.; Männer, K.; Miksch, G.; Borriss, R.; Farouk, A.; Simon, O. Comparative studies on the in vitro properties of phytases from various microbial origins. Arch. Anim. Nutr. 2000, 53, 353–373. [Google Scholar] [CrossRef]
- Deak, N.A.; Johnson, L.A. Fate of phytic acid in producing soy protein ingredients. J. Am. Oil Chem. Soc. 2007, 84, 369–376. [Google Scholar] [CrossRef]
- Kim, J.; Kaushik, S.; Breque, J. Nitrogen and phosphorus utilisation in rainbow trout fed diets with or without fish meal. Aquat. Living Resour. 1998, 11, 261–264. [Google Scholar] [CrossRef]
- Denstadli, V.; Vestre, R.; Svihus, B.; Skrede, A.; Storebakken, T. Phytate degradation in a mixture of ground wheat and ground defatted soybeans during feed processing: Effects of temperature, moisture level, and retention time in small-and medium-scale incubation systems. J. Agric. Food Chem. 2006, 54, 5887–5893. [Google Scholar] [CrossRef]
- Sørensen, M.; Stjepanovic, N.; Romarheim, O.H.; Krekling, T.; Storebakken, T. Soybean meal improves the physical quality of extruded fish feed. Anim. Feed Sci. Technol. 2009, 149, 149–161. [Google Scholar] [CrossRef]
- Barrows, F.T.; Stone, D.A.J.; Hardy, R.W. The effects of extrusion conditions on the nutritional value of soybean meal for rainbow trout (Oncorhynchus mykiss). Aquaculture 2007, 265, 244–252. [Google Scholar] [CrossRef]
- Morken, T.; Kraugerud, O.F.; Barrows, F.T.; Sørensen, M.; Storebakken, T.; Øverland, M. Sodium diformate and extrusion temperature affect nutrient digestibility and physical quality of diets with fish meal and barley protein concentrate for rainbow trout (Oncorhynchus mykiss). Aquaculture 2011, 317, 138–145. [Google Scholar] [CrossRef]
- Cain, K.D.; Garling, D.L. Pretreatment of soybean meal with phytase for salmonid diets to reduce phosphorus concentrations in hatchery effluents. Progress. Fish-Cult. 1995, 57, 114–119. [Google Scholar] [CrossRef]
- Denstadli, V.; Storebakken, T.; Svihus, B.; Skrede, A. A comparison of online phytase pre-treatment of vegetable feed ingredients and phytase coating in diets for Atlantic salmon (Salmo salar L.) reared in cold water. Aquaculture 2007, 269, 414–426. [Google Scholar] [CrossRef]
- Rodehutscord, M.; Pfeffer, E. Effects of supplemental microbial phytase on phosphorus digestibility and utilization in rainbow trout (oncorhynchus mykiss). Water Sci. Technol. 1995, 31, 143–147. [Google Scholar] [CrossRef]
- Dalsgaard, J.; Ekmann, K.S.; Pedersen, P.B.; Verlhac, V. Effect of supplemented fungal phytase on performance and phosphorus availability by phosphorus-depleted juvenile rainbow trout (Oncorhynchus mykiss), and on the magnitude and composition of phosphorus waste output. Aquaculture 2009, 286, 105–112. [Google Scholar] [CrossRef]
- DS. Water Quality: Determination of Phosphorus—Ammonium Molybdate Spectrometric Method; DS/EN ISO: Copenhagen, Denmark, 2004. [Google Scholar]
- Milian-Sorribes, M.C.; Tomas-Vidal, A.; Penaranda, D.S.; Carpintero, L.; Mesa, J.S.; Dupuy, J.; Donadeu, A.; Macias-Vidal, J.; Martinez-Llorens, S. Estimation of phosphorus and nitrogen waste in rainbow trout (Oncorhynchus mykiss, Walbaum, 1792) diets including different inorganic phosphorus sources. Animals 2021, 11, 1700. [Google Scholar] [CrossRef]
- Morales, G.A.; Azcuy, R.L.; Casaretto, M.E.; Márquez, L.; Hernández, A.J.; Gómez, F.; Koppe, W.; Mereu, A. Effect of different inorganic phosphorus sources on growth performance, digestibility, retention efficiency and discharge of nutrients in rainbow trout (Oncorhynchus mykiss). Aquaculture 2018, 495, 568–574. [Google Scholar] [CrossRef]
- Bakke, A.M.; Glover, C.; Krogdahl, A. Feeding, digestion and absorption of nutrients. Fish Physiol. 2010, 30, 57–110. [Google Scholar] [CrossRef]
- Kryvi, H.; Poppe, T. Fiskeanatomi; Fagbokforlaget: Bergen, Norway, 2021; Volume 2, p. 256. [Google Scholar]
- Wood, R.J.; Serfaty-Lacrosniere, C. Gastric acidity, atrophic gastritis, and calcium absorption. Nutr. Rev. 1992, 50, 33–40. [Google Scholar] [CrossRef]
- Sugiura, S.H.; Ferraris, R.P. Contributions of different NaPi cotransporter isoforms to dietary regulation of P transport in the pyloric caeca and intestine of rainbow trout. J. Exp. Biol. 2004, 207, 2055–2064. [Google Scholar] [CrossRef]
- Marks, J.; Debnam, E.S.; Unwin, R.J. The role of the gastrointestinal tract in phosphate homeostasis in health and chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2013, 22, 481–487. [Google Scholar] [CrossRef]
- Sugiura, S.H.; McDaniel, N.K.; Ferraris, R.P. In vivo fractional P(i) absorption and NaPi-II mRNA expression in rainbow trout are upregulated by dietary P restriction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 285, R770–R781. [Google Scholar] [CrossRef]
- Weber, R.E.; Jensen, F.B. Functional adaptations in hemoglobins from ectothermic vertebrates. Annu. Rev. Physiol. 1988, 50, 161–179. [Google Scholar] [CrossRef] [PubMed]
- Schönbörner, A.A.; Boivin, G.; Baud, C.A. The mineralization processes in teleost fish scales. Cell Tissue Res. 1979, 202, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Verri, T.; Werner, A. Type II Na(+)-phosphate cotransporters and phosphate balance in teleost fish. Pflügers Arch.-Eur. J. Physiol. 2019, 471, 193–212. [Google Scholar] [CrossRef]
- McDaniel, N.K.; Sugiura, S.H.; Kehler, T.; Fletcher, J.W.; Coloso, R.M.; Weis, P.; Ferraris, R.P. Dissolved oxygen and dietary phosphorus modulate utilization and effluent partitioning of phosphorus in rainbow trout (Oncorhynchus mykiss) aquaculture. Env. Pollut. 2005, 138, 350–357. [Google Scholar] [CrossRef]
- Piper, R.G. Fish Hatchery Management; American Fisheries Society: Bethesda, MD, USA, 1982. [Google Scholar]
- Ketola, G.H.; Harland, B.F. Influence of phosphorus in rainbow trout diets on phosphorus discharges in effluent water. Trans. Am. Fish. Soc. 1993, 122, 1120–1126. [Google Scholar] [CrossRef]
- Coloso, R.M.; Basantes, S.P.; King, K.; Hendrix, M.A.; Fletcher, J.W.; Weis, P.; Ferraris, R.P. Effect of dietary phosphorus and vitamin D3 on phosphorus levels in effluent from the experimental culture of rainbow trout (Oncorhynchus mykiss). Aquaculture 2001, 202, 145–161. [Google Scholar] [CrossRef]
- Vielma, J.; Lall, S.P. Control of phosphorus homeostasis of Atlantic salmon (Salmo salar) in fresh water. Fish Physiol. Biochem. 1998, 19, 83–93. [Google Scholar] [CrossRef]
- Clark, I. Effects of magnesium ions on calcium and phosphorus metabolism. Am. J. Physiol. 1968, 214, 348–356. [Google Scholar] [CrossRef]
- Pahuja, D.N.; DeLuca, H.F. Stimulation of intestinal calcium transport and bone calcium mobilization by prolactin in vitamin D-deficient rats. Science 1981, 214, 1038–1039. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.F.; Jaworski, E.M.; Haddad, M. Stanniocalcin in the seawater salmon: Structure, function, and regulation. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1998, 274, R1177–R1185. [Google Scholar] [CrossRef] [PubMed]
- Radman, D.P.; McCudden, C.; James, K.; Nemeth, E.M.; Wagner, G.F. Evidence for calcium-sensing receptor mediated stanniocalcin secretion in fish. Mol. Cell. Endocrinol. 2002, 186, 111–119. [Google Scholar] [CrossRef]
- Suarez-Bregua, P.; Cal, L.; Canestro, C.; Rotllant, J. PTH reloaded: A new evolutionary perspective. Front. Physiol. 2017, 8, 776. [Google Scholar] [CrossRef]
- Abbink, W.; Flik, G. Parathyroid hormone-related protein in teleost fish. Gen. Comp. Endocrinol. 2007, 152, 243–251. [Google Scholar] [CrossRef][Green Version]
- Danks, J.A.; Devlin, A.J.; Ho, P.M.W.; Diefenbach-Jagger, H.; Power, D.M.; Canario, A.; Martin, T.J.; Ingleton, P.M. Parathyroid hormone-related protein is a factor in normal fish pituitary. Gen. Comp. Endocrinol. 1993, 92, 201–212. [Google Scholar] [CrossRef]
- Nguyen-Yamamoto, L.; Karaplis, A.C.; St–Arnaud, R.; Goltzman, D. Fibroblast growth factor 23 regulation by systemic and local osteoblast-synthesized 1,25-dihydroxyvitamin D. J. Am. Soc. Nephrol. 2017, 28, 586–597. [Google Scholar] [CrossRef]
- Henry, H.L. The 25 (OH) D3/1α, 25 (OH) 2D3-24R-hydroxylase: A catabolic or biosynthetic enzyme? Steroids 2001, 66, 391–398. [Google Scholar] [CrossRef]
- Lopez, E.; Peignoux-Deville, J.; Lallier, F.; Colston, K.; MacIntyre, I. Responses of bone metabolism in the eel (Anguilla anguilla) to injections of 1, 25-dihydroxyvitamin D 3. Calcif. Tissue Res. 1976, 22, 19–23. [Google Scholar] [CrossRef]
- Swarup, K.; Das, V.; Norman, A. Dose-dependent vitamin D3 and 1, 25-dihydrxyvitamin D3 induced hypercalcemia and hyperphosphatemia in male cyprinoid Cyprinus carpio. Comp. Biochem. Physiol. A 1991, 100, 445–447. [Google Scholar] [CrossRef]
- Vielma, J.; Lall, S.P.; Koskela, J.; Mattila, P. Influence of low dietary cholecalciferol intake on phosphorus and trace element metabolism by rainbow trout (Oncorhynchus mykiss, Walbaum). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 1999, 122, 117–125. [Google Scholar] [CrossRef]
- Vielma, J.; Lall, S.P.; Koskela, J.; Schöner, F.-J.; Mattila, P. Effects of dietary phytase and cholecalciferol on phosphorus bioavailability in rainbow trout (Oncorhynchus mykiss). Aquaculture 1998, 163, 309–323. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D insufficiency/deficiency in gastrointestinal disorders. J. Bone Miner. Res. 2007, 22, V50–V54. [Google Scholar] [CrossRef]
- Grant, W.B. Epidemiology of disease risks in relation to vitamin D insufficiency. Prog. Biophys. Mol. Biol. 2006, 92, 65–79. [Google Scholar] [CrossRef]
- Bonewald, L.F.; Wacker, M.J. FGF23 production by osteocytes. Pediatr. Nephrol. 2013, 28, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Ben-Dov, I.Z.; Galitzer, H.; Lavi-Moshayoff, V.; Goetz, R.; Kuro-o, M.; Mohammadi, M.; Sirkis, R.; Naveh-Many, T.; Silver, J. The parathyroid is a target organ for FGF23 in rats. J. Clin. Investig. 2007, 117, 4003–4008. [Google Scholar] [CrossRef] [PubMed]
- Grefsrud, E.S.; Andersen, L.B.; Grøsvik, B.E.; Hansen, P.K.; Husa, V.; Karlsen, Ø.; Madhun, A.S.; Samuelsen, O.; Sandlund, N.; Solberg, M.F.; et al. Risikorapport Norsk Fiskeoppdrett 2024; Havforskningsinstituttet: Bergen, Norway, 2024. [Google Scholar]
- Jensen, D.; Sønvisen, S.A.; Johnsen, J.P. A governability lesson for the Blue Economy: The collapse of mobile salmon aquaculture in the Norwegian coastal zone. Marit. Stud. 2025, 24, 2. [Google Scholar] [CrossRef]
- Ellis, T.; Turnbull, J.F.; Knowles, T.G.; Lines, J.A.; Auchterlonie, N.A. Trends during development of Scottish salmon farming: An example of sustainable intensification? Aquaculture 2016, 458, 82–99. [Google Scholar] [CrossRef]
- Quiñones, R.A.; Fuentes, M.; Montes, R.M.; Soto, D.; León-Muñoz, J. Environmental issues in Chilean salmon farming: A review. Rev. Aquac. 2019, 11, 375–402. [Google Scholar] [CrossRef]
- Ytrestøyl, T.; Aas, T.S.; Åsgård, T. Utilisation of feed resources in production of Atlantic salmon (Salmo salar) in Norway. Aquaculture 2015, 448, 365–374. [Google Scholar] [CrossRef]
- Kronvang, B.; Andersen, H.E.; Børgesen, C.; Dalgaard, T.; Larsen, S.E.; Bøgestrand, J.; Blicher-Mathiasen, G. Effects of policy measures implemented in Denmark on nitrogen pollution of the aquatic environment. Environ. Sci. Policy 2008, 11, 144–152. [Google Scholar] [CrossRef]
- NEA. TEOTIL-SUPPLY of Nitrogen and Phosphorus to Costal Areas. Available online: https://www.miljodirektoratet.no/ansvarsomrader/overvaking-arealplanlegging/miljoovervaking/overvakingsprogrammer/forurensning-og-klimagasser/teotil/ (accessed on 29 December 2024).
- Liltved, H.; Hansen, B.R. Screening as a method for removal of parasites from inlet water to fish farms. Aquac. Eng. 1990, 9, 209–215. [Google Scholar] [CrossRef]
- Naylor, R.L.; Goldburg, R.J.; Primavera, J.H.; Kautsky, N.; Beveridge, M.C.M.; Clay, J.; Folke, C.; Lubchenco, J.; Mooney, H.; Troell, M. Effect of aquaculture on world fish supplies. Nature 2000, 405, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Couturier, M.; Trofimencoff, T.; Buil, J.U.; Conroy, J. Solids removal at a recirculating salmon-smolt farm. Aquac. Eng. 2009, 41, 71–77. [Google Scholar] [CrossRef]
- Ciao, R.; Wei, Y.; An, D.; Li, D.; Ta, X.; Wu, Y.; Ren, Q. A review on the research status and development trend of equipment in water treatment processes of recirculating aquaculture systems. Rev. Aquac. 2019, 11, 863–895. [Google Scholar]
- Liao, P.B.; Mayo, R.D. Salmonid hatchery water reuse systems. Aquaculture 1972, 1, 317–335. [Google Scholar] [CrossRef]
- Summerfelt, S.T.; Davidson, J.W.; Waldrop, T.B.; Tsukuda, S.M.; Bebak-Williams, J. A partial-reuse system for coldwater aquaculture. Aquac. Eng. 2004, 31, 157–181. [Google Scholar] [CrossRef]
- Colt, J.; Lamoureux, J.; Patterson, R.; Rogers, G. Reporting standards for biofilter performance studies. Aquac. Eng. 2006, 34, 377–388. [Google Scholar] [CrossRef]
- Jokumsen, A.; Svendsen, L.M. Farming of Freshwater Rainbow Trout in Denmark; DTU Aqua: Copenhagen, Denmark, 2010. [Google Scholar]
- Martins, C.I.M.; Eding, E.H.; Verdegem, M.C.J.; Heinsbroek, L.T.N.; Schneider, O.; Blancheton, J.-P.; d’Orbcastel, E.R.; Verreth, J.A.J. New developments in recirculating aquaculture systems in Europe: A perspective on environmental sustainability. Aquac. Eng. 2010, 43, 83–93. [Google Scholar] [CrossRef]
- Fernandes, P. Interactions Between Micro-Particles and the Rearing Environment in Recirculating Aquaculture Systems; DTU Aqua: Copenhagen, Denmark, 2015. [Google Scholar]
- Bureau, D.P.; Hua, K. Towards effective nutritional management of waste outputs in aquaculture, with particular reference to salmonid aquaculture operations. Aquac. Res. 2010, 41, 777–792. [Google Scholar] [CrossRef]
- Dalsgaard, J.; Pedersen, P.B. Solid and suspended/dissolved waste (N, P, O) from rainbow trout (Oncorynchus mykiss). Aquaculture 2011, 313, 92–99. [Google Scholar] [CrossRef]
- Dalsgaard, J.; Larsen, B.K.; Pedersen, P.B. Nitrogen waste from rainbow trout (Oncorhynchus mykiss) with particular focus on urea. Aquac. Eng. 2015, 65, 2–9. [Google Scholar] [CrossRef]
- Hrubec, T.C.; Smith, S.A.; Robertson, J.L. Nitrate toxicity: A potential problem of recirculating systems. Successes Fail. Commer. Recirc. Aquac. 1996, 1, 41–48. [Google Scholar]
- Martins, C.I.M.; Eding, E.H.; Verreth, J.A.J. The effect of recirculating aquaculture systems on the concentrations of heavy metals in culture water and tissues of Nile tilapia Oreochromis niloticus. Food Chem. 2011, 126, 1001–1005. [Google Scholar] [CrossRef]
- Davidson, J.; Good, C.; Welsh, C.; Brazil, B.; Summerfelt, S. Heavy metal and waste metabolite accumulation and their potential effect on rainbow trout performance in a replicated water reuse system operated at low or high system flushing rates. Aquac. Eng. 2009, 41, 136–145. [Google Scholar] [CrossRef]
- Davidson, J.; Good, C.; Welsh, C.; Summerfelt, S. The effects of ozone and water exchange rates on water quality and rainbow trout oncorhynchus mykiss performance in replicated water recirculating systems. Aquac. Eng. 2011, 44, 80–96. [Google Scholar] [CrossRef]
- van Bussel, C.G.J.; Schroeder, J.P.; Mahlmann, L.; Schulz, C. Aquatic accumulation of dietary metals (Fe, Zn, Cu, Co, Mn) in recirculating aquaculture systems (RAS) changes body composition but not performance and health of juvenile turbot (Psetta maxima). Aquac. Eng. 2014, 61, 35–42. [Google Scholar] [CrossRef]
- Eding, E.H.; Kamstra, A.; Verreth, J.A.J.; Huisman, E.A.; Klapwijk, A. Design and operation of nitrifying trickling filters in recirculating aquaculture: A review. Aquac. Eng. 2006, 34, 234–260. [Google Scholar] [CrossRef]
- Szwerinski, H.; Arvin, E.; Harremoës, P. pH-decrease in nitrifying biofilms. Water Res. 1986, 20, 971–976. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, S. The impact of temperature on nitrification rate in fixed film biofilters. Aquac. Eng. 2002, 26, 221–237. [Google Scholar] [CrossRef]
- Heinen, J.M. In Water Quality Criteria, Uptake, Bioaccumulation, and Public Health Considerations for Chemicals of Possible Concern in West Virginia Mine Waters Used for Culture of Rainbow Trout; Jenkins, M.R., Wade, E.M., Eds.; Conservation Fund’s Freshwater Institute: Sheperdstown, PA, USA, 1996. [Google Scholar]
- Meade, J.W. Aquaculture Management; Van Nostrand Reinhold: New York, NY, USA, 1989; p. 9. [Google Scholar]
- Wedemeyer, G.A. Physiology of Fish in Intensive Culture System; Chapman and Hall: New York, NY, USA, 1996. [Google Scholar]
- USEPA. Quality Criteria for Water 1986; Publication EPA 440/5-86-001; USEPA: Washington, DC, USA, 1986.
- USEPA. Ambient Water Quality Criteria for Selenium-1987; Publication EPA 440/5-87-006; USEPA: Washington, DC, USA, 1987.
- USEPA. 1995 Updates: Water Quality Criteria for the Protection of Aquatic Life in Ambient Water; Publication EPA-820-B-96-001; USEPA: Washington, DC, USA, 1996.
- USEPA. National Recommended Water Quality Criteria; USEPA: Washington, DC, USA, 2002.
- USEPA. Aquatic Life Ambient Fresh Quality Criteria: Copper (2007 Revision); Publication EPA-822-R-07-001; USEPA: Washington, DC, USA, 2007.
- Boyd, C.E. Trace metals toxic at high concentration. Global aquaculture. Advocate (July/August) 2009, 24–26. Available online: https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=boyd+Trace+metals+toxic+at+high+concentration.+Global+aquaculture&btnG= (accessed on 3 April 2025).
- Usher, M.L.; Talbot, C.; Eddy, F.B. Drinking in Atlantic salmon smolts transferred to seawater and the relationship between drinking and feeding. Aquaculture 1988, 73, 237–246. [Google Scholar] [CrossRef]
- Fuentes, J.; Eddy, F.B. Drinking in Atlantic salmon presmolts and smolts in response to growth hormone and salinity. Comp. Biochem. Physiol. Part A Physiol. 1997, 117, 487–491. [Google Scholar] [CrossRef]
- Cripps, S.J.; Bergheim, A. Solids management and removal for intensive land-based aquaculture production systems. Aquac. Eng. 2000, 22, 33–56. [Google Scholar] [CrossRef]
- Wang, X.; Andresen, K.; Handå, A.; Jensen, B.; Reitan, K.I.; Olsen, Y. Chemical composition and release rate of waste discharge from an Atlantic salmon farm with an evaluation of IMTA feasibility. Aquac. Environ. Interact. 2013, 4, 147–162. [Google Scholar] [CrossRef]
- Boyd, C.E. Practical aspects of chemistry in pond aquaculture. N. Am. J. Aquac. 1997, 59, 85–93. [Google Scholar] [CrossRef]
- Adler, P.R.; Harper, J.K.; Takeda, F.; Wade, E.M.; Summerfelt, S.T. Economic evaluation of hydroponics and other treatment options for phosphorus removal in aquaculture effluent. HortScience 2000, 35, 993–999. [Google Scholar] [CrossRef]
- Sickander, O.; Filgueira, R. Factors affecting IMTA (integrated multi-trophic aquaculture) implementation on Atlantic Salmon (Salmo salar) farms. Aquaculture 2022, 561, 738716. [Google Scholar] [CrossRef]
- Carras, M.A.; Knowler, D.; Pearce, C.M.; Hamer, A.; Chopin, T.; Weaire, T. A discounted cash-flow analysis of salmon monoculture and integrated multi-trophic aquaculture in eastern Canada. Aquac. Econ. Manag. 2020, 24, 43–63. [Google Scholar] [CrossRef]
| Mine Production | |||
|---|---|---|---|
| Country | 2022 | 2023 e | Reserves |
| United States | e 19,800 | 20,000 | 1,000,000 |
| Algeria | e 1800 | 1800 | 2,200,000 |
| Australia | e 2500 | 2500 | 1 1,100,000 |
| Brazil | e 6200 | 5300 | 1,600,000 |
| China 2 | e 93,000 | 90,000 | 3,800,000 |
| Egypt | e 5000 | 4800 | 2,800,000 |
| Finland | 923 | 950 | 1,000,000 |
| India | e 1740 | 1500 | 31,000 |
| Israel | 2170 | 2500 | 60,000 |
| Jordan | 11,300 | 12,000 | 1,000,000 |
| Kazakhstan | e 1500 | 2000 | 260,000 |
| Mexico | 442 | 500 | 30,000 |
| Morocco | 39,000 | 35,000 | 50,000,000 |
| Peru | 4200 | 4200 | 210,000 |
| Russia | e 14,000 | 14,000 | 2,400,000 |
| Saudi Arabia | e 9000 | 8500 | 1,400,000 |
| Senegal | e 2600 | 2500 | 50,000 |
| South Africa | 1990 | 1600 | 1,500,000 |
| Syria | e 1100 | 800 | 250,000 |
| Togo | e 1500 | 1500 | 30,000 |
| Tunisia | 3560 | 3600 | 2,500,000 |
| Turkey | e 900 | 800 | 71,000 |
| Uzbekistan | e 900 | 900 | 100,000 |
| Vietnam | e 2000 | 2000 | 30,000 |
| Other countries | 750 | 800 | 800,000 |
| World total (rounded) | 228,000 | 220,000 | 74,000,000 |
| Requirement (g/kg) of Diet | Species | Fish Weight (g) | Method of Dose–Response Analysis | Reference |
|---|---|---|---|---|
| 10–11 | Atlantic salmon | 1.4 | Regression | [80] |
| 6 | Atlantic salmon | 6.5 | ANOVA | [85] |
| 6 | Atlantic salmon | 57 | ANOVA | [107] |
| 7–8 | Rainbow trout | 1.2 | Broken line | [108] |
| 3.4–5.4 | Rainbow trout | 35 | ANOVA | [109] |
| 2.4–5.9 | Rainbow trout | 50 | Regression | [110] |
| Commercial salmonid diets | ||||
| 16 | Salmonids | Starter | [111] | |
| 14 | Salmonids | Fingerling | [111] | |
| 13–14 | Salmonids | Grower | [111,112] | |
| Ingredients | aP Without Pre-Treatment (g kg−1) | aP Without Pre-Treatment (%) |
|---|---|---|
| Maize | 0.35 | 15 |
| Maize gluten | 0.80 | 16 |
| Corn | 0.80 | 32 |
| Gross defatted corn germ and bran | 2.40 | 36 |
| Fine defatted corn germ and bran | 4.30 | 36 |
| Hominy meal | 0.70 | 10 |
| Rice bran | 1.68 | 10 |
| Rice | 0.40 | 33 |
| Rice broken | 0.45 | 53 |
| Rice polishing | 4.40 | 28 |
| Wheat bran | 2.60 | 24 |
| Wheat by-products | 1.02 | 13 |
| Wheat | 0.88 | 29 |
| Sorghum | 0.51 | 17 |
| Barley | 0.87 | 32 |
| Oats | 0.33 | 14 |
| Oats, dehulled | 1.10 | 49 |
| Groundnut meal | 1.40 | 23 |
| Palm oil meal | 2.20 | 43 |
| Soybeans, whole | 2.47 | 45 |
| Soybean meal | 2.13 | 32 |
| Coconut meal | 1.90 | 44 |
| Cotton seed, whole | 1.80 | 30 |
| Cotton seed meal | 2.25 | 20 |
| Sunflower meal | 1.57 | 17 |
| Rapeseed meal | 4.80 | 41 |
| Canola meal | 2.07 | 24 |
| Peas | 1.78 | 52 |
| Faba beans | 1.58 | 39 |
| L. albus, whole | 1.98 | 44 |
| L. angustifolius, whole | 1.50 | 48 |
| L. angustifolius, dehulled | 1.91 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flo, V.Ø.; Åsgård, T.; Lekang, O.-I. Phosphorus in Salmonid Aquaculture: Sources, Requirements, and System-Level Implications. Fishes 2025, 10, 388. https://doi.org/10.3390/fishes10080388
Flo VØ, Åsgård T, Lekang O-I. Phosphorus in Salmonid Aquaculture: Sources, Requirements, and System-Level Implications. Fishes. 2025; 10(8):388. https://doi.org/10.3390/fishes10080388
Chicago/Turabian StyleFlo, Vegard Øvstetun, Torbjørn Åsgård, and Odd-Ivar Lekang. 2025. "Phosphorus in Salmonid Aquaculture: Sources, Requirements, and System-Level Implications" Fishes 10, no. 8: 388. https://doi.org/10.3390/fishes10080388
APA StyleFlo, V. Ø., Åsgård, T., & Lekang, O.-I. (2025). Phosphorus in Salmonid Aquaculture: Sources, Requirements, and System-Level Implications. Fishes, 10(8), 388. https://doi.org/10.3390/fishes10080388






