Histochemical Study of Enzyme Activity in the Digestive Tract of the Small-Spotted Catshark (Scyliorhinus canicula) and the Smooth-Hound (Mustelus mustelus)
Abstract
1. Introduction
2. Materials and Methods
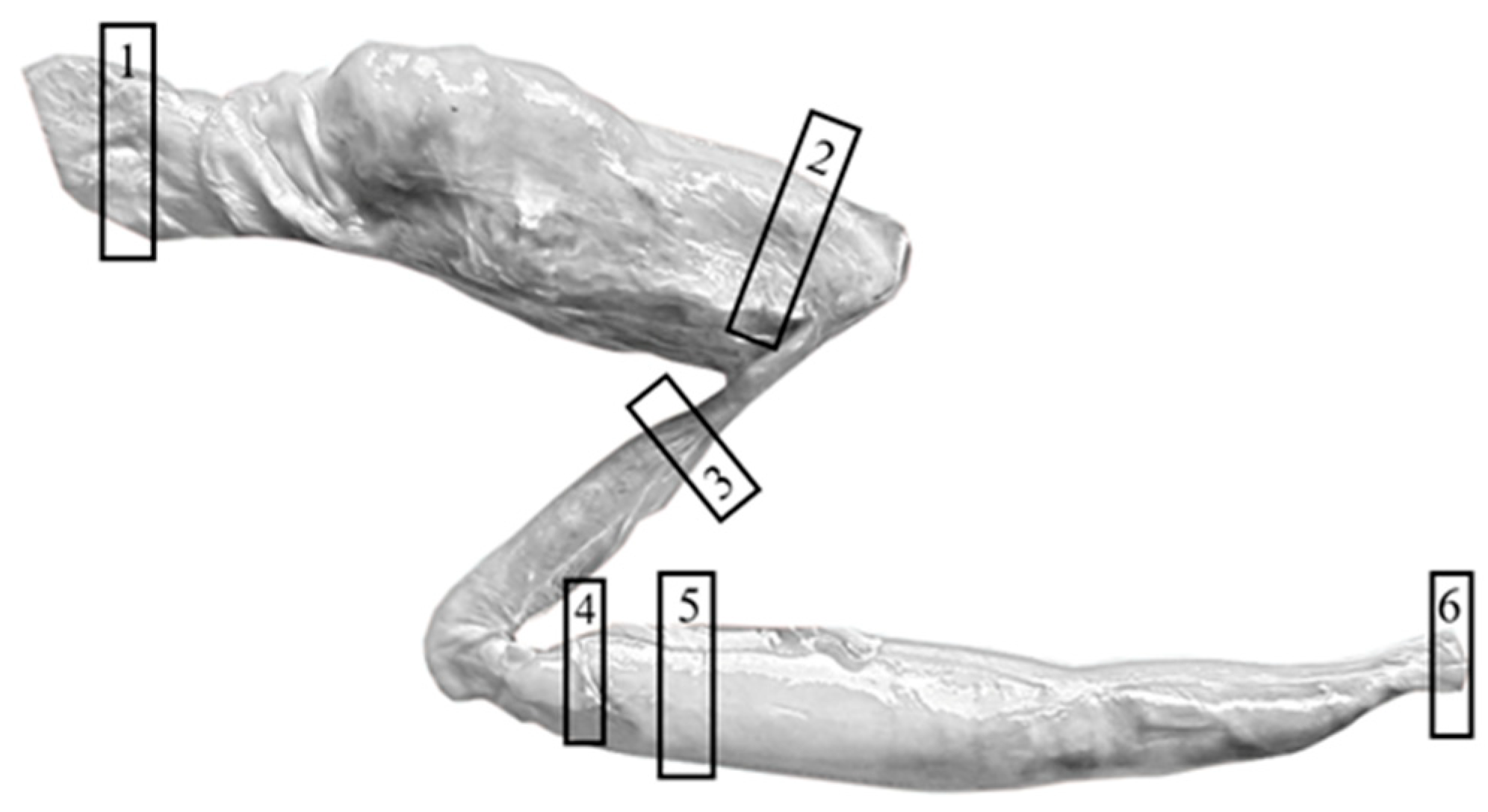
2.1. Sampling
2.2. Microscopic Observation and Statistical Analysis
3. Results
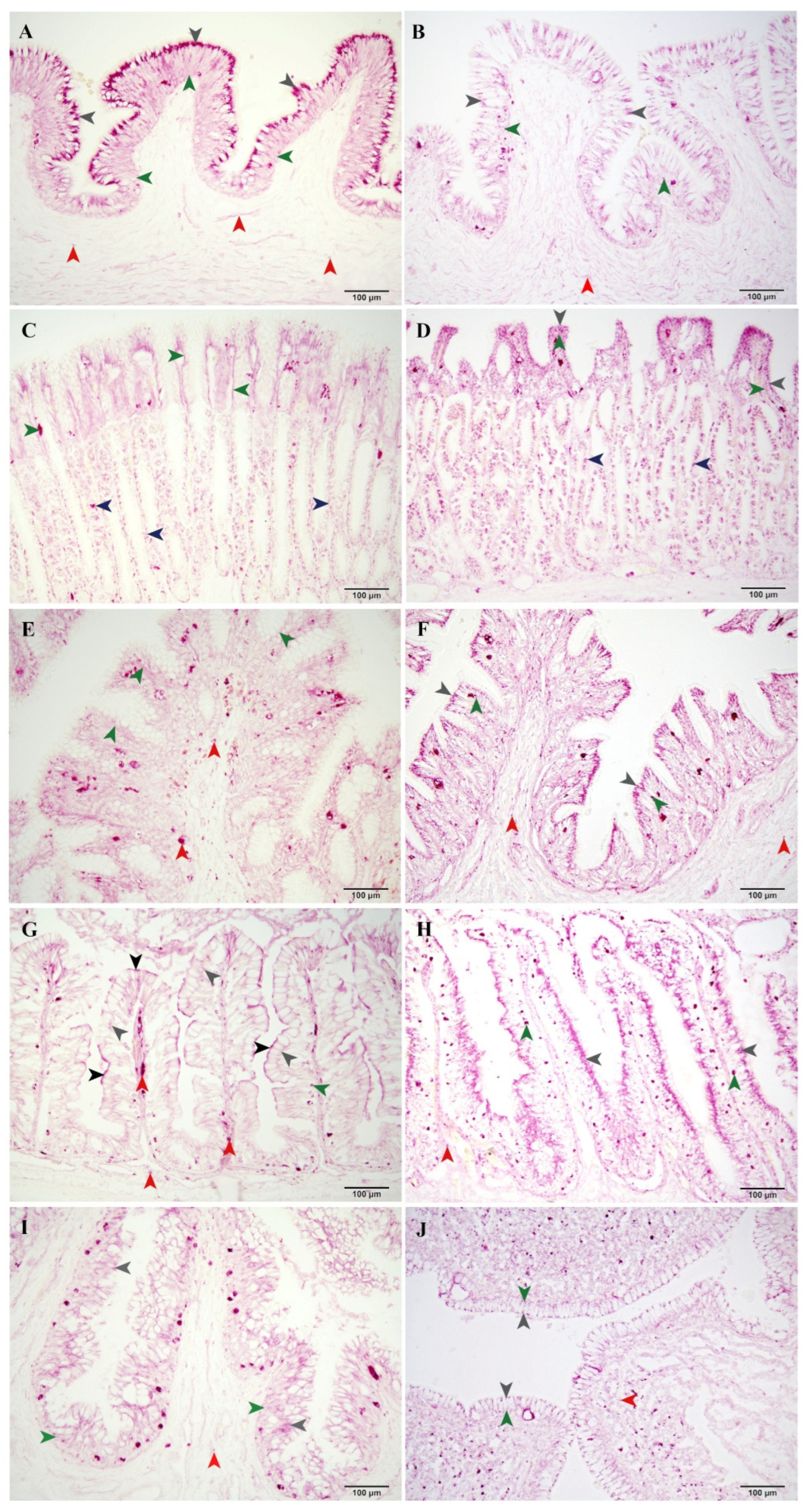
3.1. Enzyme Localization Among Digestive Tract
3.1.1. Esophagus
3.1.2. Stomach
3.1.3. Spiral Intestine
3.1.4. Rectum
3.2. Enzyme Activities in the Digestive Tract
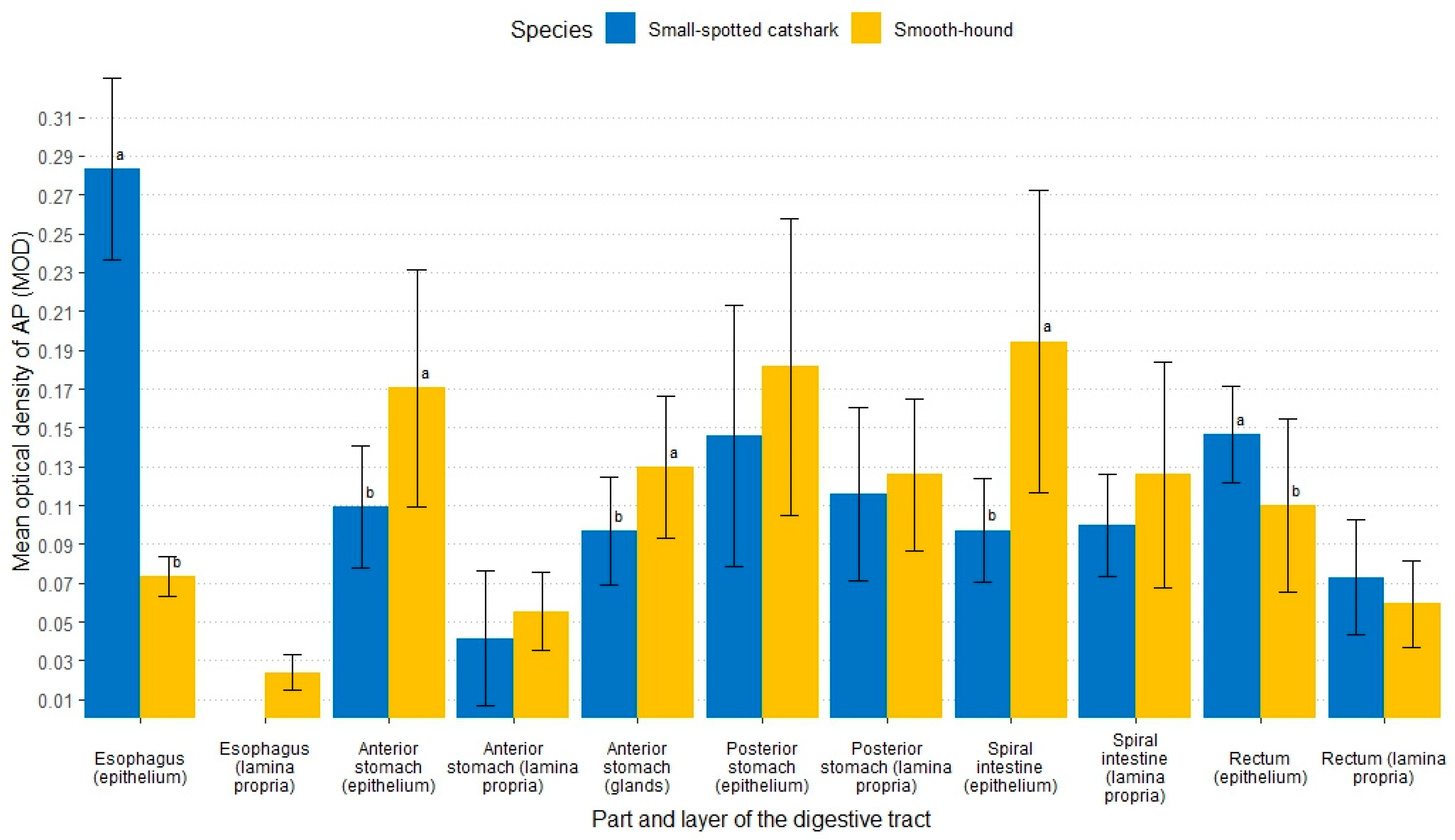
3.2.1. Alkaline Phosphatase
3.2.2. Acid Phosphatase
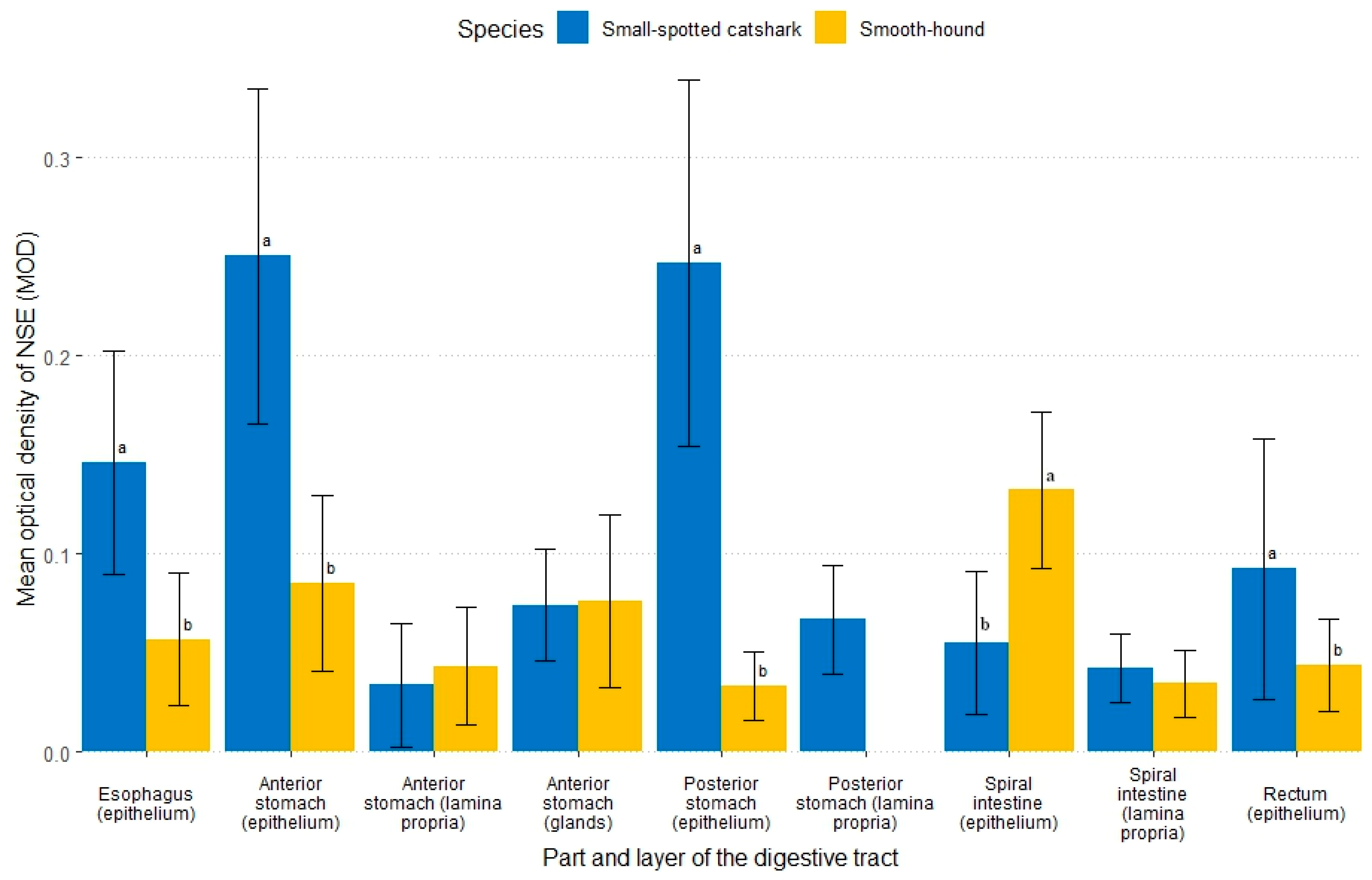
3.2.3. Non-Specific Esterase
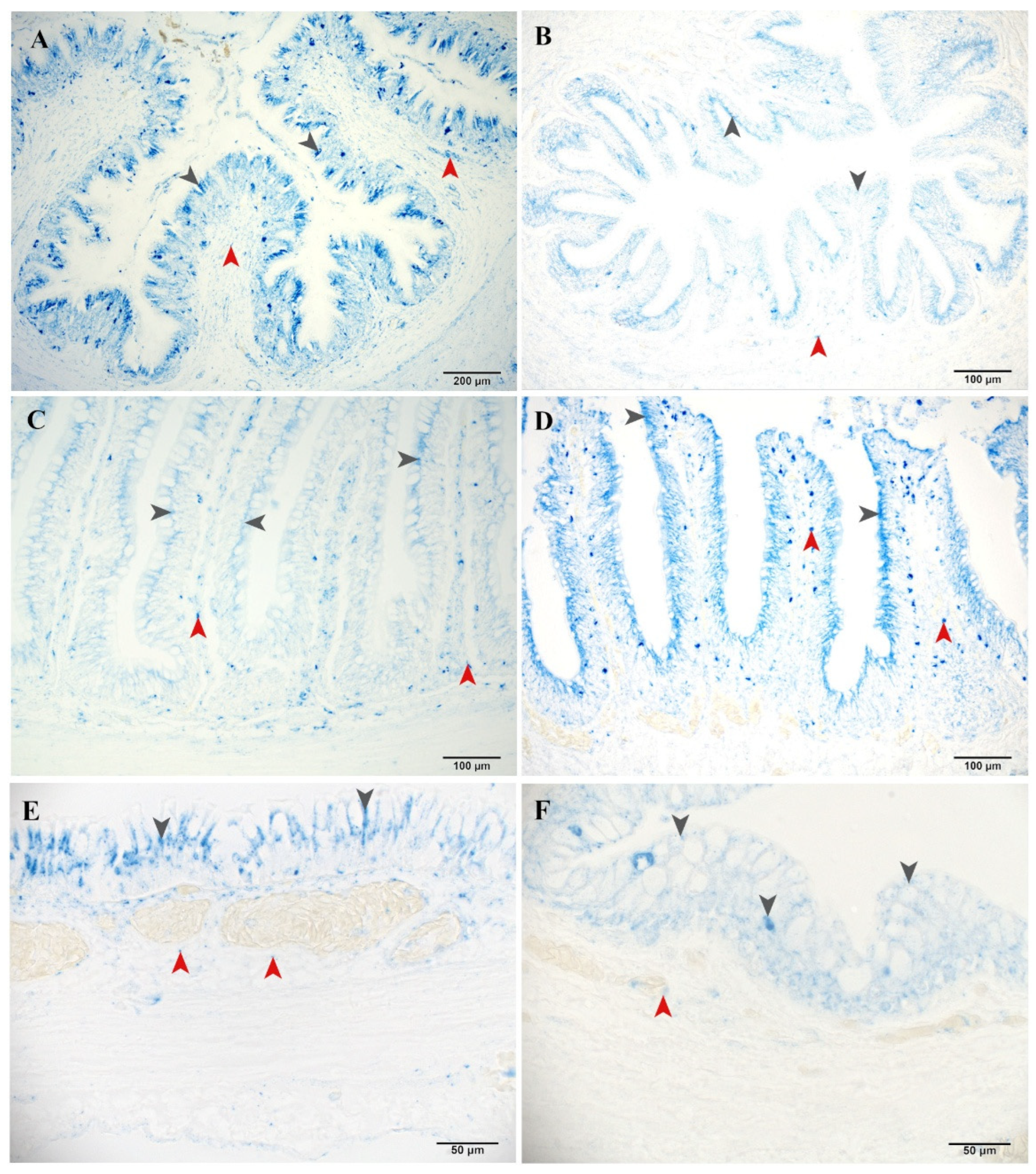
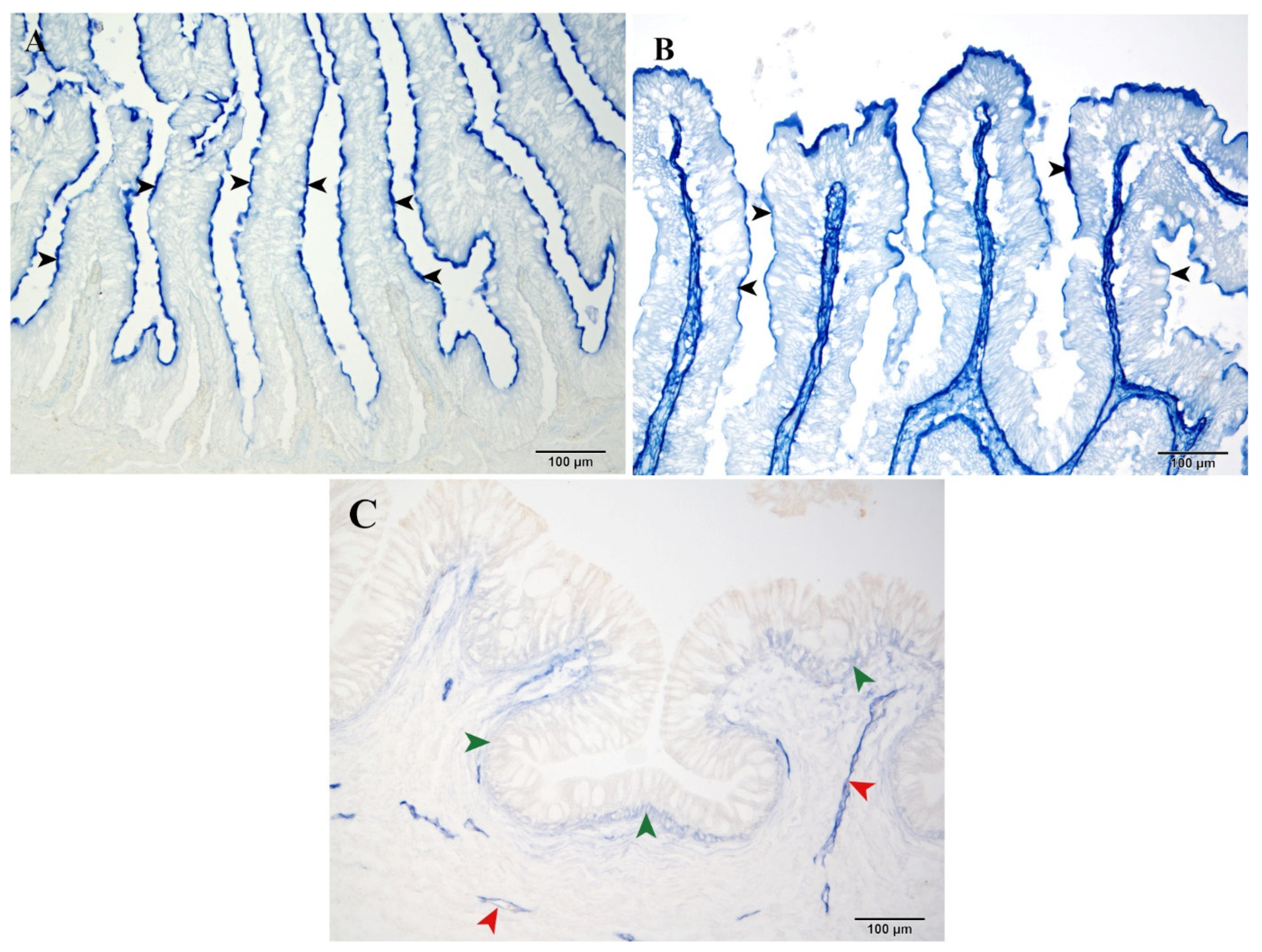
3.2.4. Aminopeptidase

4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ray, A.K.; Ringø, E. The gastrointestinal tract of fish. In Aquacult Nutr: Gut Health, Probiotics and Prebiotics; Merrifield, D., Ringø, E., Eds.; John Wiley & Sons: Chichester, UK, 2014; pp. 1–10. [Google Scholar]
- Duque-Correa, M.J.; Clements, K.D.; Meloro, C.; Ronco, F.; Boila, A.; Indermaur, A.; Salzburger, W.; Clauss, M. Diet and habitat as determinants of intestine length in fishes. Rev. Fish Biol. Fish. 2024, 34, 1017–1034. [Google Scholar] [CrossRef]
- German, D.P.; Nagle, B.C.; Villeda, J.M.; Ruiz, A.M.; Thomson, A.W.; Contreras Balderas, S.; Evans, D.H. Evolution of herbivory in a carnivorous clade of minnows (Teleostei: Cyprinidae): Effects on gut size and digestive physiology. Physiol. Biochem. Zool. 2010, 83, 1–18. [Google Scholar] [CrossRef]
- Horn, M.H.; Gawlicka, A.; German, D.P.; Logothetis, E.A.; Cavanagh, J.W.; Boyle, K.S. Structure and function of the stomachless digestive system in three related species of New World silverside fishes (Atherinopsidae) representing herbivory, omnivory, and carnivory. Mar. Biol. 2006, 149, 1237–1245. [Google Scholar] [CrossRef]
- Denstadli, V.; Vegusdal, A.; Krogdahl, Å.; Bakke-McKellep, A.M.; Berge, G.M.; Holm, H.; Hillestad, M.; Ruyter, B. Lipid absorption in different segments of the gastrointestinal tract of Atlantic salmon (Salmo salar L.). Aquaculture 2004, 240, 385–398. [Google Scholar] [CrossRef]
- Kozarić, Z.; Kužir, S.; Nejedli, S.; Petrinec, Z.; Srebočan, E. Histochemical distribution of digestive enzymes in hake, Merluccius merluccius L. 1758. Vet. Arh. 2004, 74, 299–308. [Google Scholar]
- Refstie, S.; Bakke-McKellep, A.M.; Penn, M.H.; Sundby, A.; Shearer, K.D.; Krogdahl, A. Capacity for digestive hydrolysis and amino acid absorption in Atlantic salmon (Salmo salar) fed diets with soybean meal or inulin with or without addition of antibiotics. Aquaculture 2006, 261, 392–406. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, Y.; Zhou, X.Q.; Zeng, X.Y.; Feng, L.; Liu, Y.; Jiang, W.D.; Li, S.H.; Li, D.B.; Wu, X.Q.; et al. Effects of dietary glutamate supplementation on growth performance, digestive enzyme activities and antioxidant capacity in intestine of grass carp (Ctenopharyngodon idella). Aquacult. Nutr. 2015, 21, 935–941. [Google Scholar] [CrossRef]
- Cohen, S.; Diaz, M.V.; Díaz, A.O. Development of the digestive system of Argentine hake, Merluccius hubbsi, larvae. J. Morphol. 2020, 281, 578–590. [Google Scholar] [CrossRef] [PubMed]
- Ballantyne, J.S. Membranes and metabolism. In The Physiology of Fishes, 4th ed.; Evans, D.H., Claiborne, J.B., Currie, S., Eds.; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2014; pp. 81–149. [Google Scholar]
- Cho, J.-H.; Park, J.W.; Ryu, Y.-W.; Kim, K.-W.; Hur, S.-W. Morphology, Histology, and Histochemistry of the Digestive Tract of the Marbled Flounder Pseudopleuronectes yokohamae. Animals 2023, 13, 936. [Google Scholar] [CrossRef]
- Ugolev, A.M. Membrane (contact) digestion. Physiol. Rev. 1965, 45, 555–595. [Google Scholar] [CrossRef]
- Kozarić, Z.; Petrinec, Z.; Kužir, S.; Gjurčević, E.; Baždarić, B. Histochemical analyses of digestive enzymes in the intestine of adult large-scaled gurnard (Lepidotrigla cavillone, Lacepède, 1801). Anat. Histol. Embryol. 2011, 40, 314–320. [Google Scholar] [CrossRef]
- Kužir, S.; Gjurčević, E.; Nejedli, S.; Baždarić, B.; Kozarić, Z. Morphological and histochemical study of intestine in wild and reared European eel (Anguilla anguilla L.). Fish Physiol. Biochem. 2012, 38, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Weinrauch, A.M.; Schaefer, C.M.; Goss, G.G. Activity and post-prandial regulation of digestive enzyme activity along the Pacific hagfish (Eptatretus stoutii) alimentary canal. PLoS ONE 2019, 14, e0215027. [Google Scholar] [CrossRef] [PubMed]
- Kolkovski, S. Digestive enzymes in fish larvae and juveniles—Implications and applications to formulated diets. Aquaculture 2001, 200, 181–201. [Google Scholar] [CrossRef]
- Gawlicka, A.; Herold, M.A.; Barrows, F.T.; De La Noüe, J.; Hung, S.S.O. Effects of dietary lipids on growth, fatty acid composition, intestinal absorption and hepatic storage in white sturgeon (Acipenser transmontanus R.) larvae. J. Appl. Ichthyol. 2002, 18, 673–681. [Google Scholar] [CrossRef]
- Solovyev, M.M.; Kashinskaya, E.N.; Izvekova, G.I.; Glupov, V.V. pH Values and Activity of Digestive Enzymes in the Gastrointestinal Tract of Fish in Lake Chany (West Siberia). J. Ichthyol. 2015, 55, 251–258. [Google Scholar] [CrossRef]
- Solovyev, M.; Gisbert, E. Influence of time, storage temperature and freeze/thaw cycles on the activity of digestive enzymes from gilthead sea bream (Sparus aurata). Fish Physiol. Biochem. 2016, 42, 1383–1394. [Google Scholar] [CrossRef]
- Faccioli, C.K.; Chedid, R.A.; Mori, R.H.; Amaral, A.C.; Belmont, R.A.F.; Vicentini, I.B.F.; Vicentini, C.A. Organogenesis of the digestive system in Neotropical carnivorous freshwater catfish Hemisorubim platyrhynchos (Siluriformes: Pimelodidae). Aquaculture 2016, 451, 205–212. [Google Scholar] [CrossRef]
- Hani, Y.M.I.; Marchand, A.; Turiès, C.; Kerambrun, E.; Palluel, O.; Bado-Nilles, A.; Beaudouin, R.; Porcher, J.; Geffard, A.; Dedourge-Geffard, O. Digestive enzymes and gut morphometric parameters of threespine stickleback (Gasterosteus aculeatus): Influence of body size and temperature. PLoS ONE 2018, 13, e0194932. [Google Scholar] [CrossRef]
- Abdulla, A. Status and Conservation of Sharks in the Mediterranean Sea; IUCN Technical Paper, 2004; p. 7. Available online: https://www.researchgate.net/publication/237278296_Status_and_Conservation_of_Sharks_in_the_Mediterranean_Sea (accessed on 28 May 2025).
- Simpfendorfer, C.A.; Dulvy, N.K. Bright spots of sustainable shark fishing. Curr. Biol. 2017, 27, 97–98. [Google Scholar] [CrossRef]
- Nuez, I.; Giovos, I.; Tiralongo, F.; Penadés-Suay, J.; Cetkovic, I.; Di Lorenzo, M.; Kleitou, P.; Bakiu, R.; Bradai, M.N.; Almabruk, S.A.A.; et al. Assessing the current status of Hexanchus griseus in the Mediterranean Sea using local ecological knowledge. Mar. Policy 2023, 147, 105378. [Google Scholar] [CrossRef]
- Ebert, D.A.; Stehmann, M.F.W. Sharks, Batoids, and Chimaeras of the North Atlantic; FAO Species Catalogue for Fishery Purposes, No. 7; FAO: Rome, Italy, 2013; pp. 188–219. [Google Scholar]
- Jardas, I. Jadranska Ihtiofauna; Školska Knjiga: Zagreb, Croatia, 1996. [Google Scholar]
- Jardas, I.; Anti, M.; Nerlović, V.; Pallaoro, A. Diet of the smooth-hound, Mustelus mustelus (Chondrichthyes: Triakidae), in the eastern Adriatic Sea. Cybium 2007, 31, 459–464. [Google Scholar]
- Šantić, M.; Rađa, B.; Pallaoro, A. Feeding habits of small-spotted catshark (Scyliorhinus canicula Linnaeus, 1758) from the eastern central Adriatic Sea. Mar. Biol. Res. 2012, 8, 1003–1011. [Google Scholar] [CrossRef]
- Koutseni, V.; Karachle, P.K.; Megalofonou, P. Diet of the small-spotted catshark Scyliorhinus canicula in the Aegean Sea (eastern Mediterranean). Mar. Biol. Res. 2017, 13, 161–173. [Google Scholar] [CrossRef]
- Dulčić, J.; Kovačić, M. Ihtiofauna Jadranskoga Mora; Golden marketing—Tehnička Knjiga: Zagreb, Croatia, 2020. [Google Scholar]
- Suvarna, S.K.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques; Elsevier: Scotland, UK, 2019. [Google Scholar]
- Lojda, Z.; Gossrau, R.; Schiebler, T.H. Enzyme Histochemistry. A Laboratory Manual; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1979. [Google Scholar]
- Pearse, A.G.E. Histochemistry, Theoretical and Applied, 3rd ed.; J. & A. Churchill Ltd.: London, UK, 1968. [Google Scholar]
- Sheehan, D.C.; Hrapchak, B.B. Theory and Practice of Histotechnology; Battelle Press: Columbus, OH, USA, 1980. [Google Scholar]
- Crane, R.K.; Boge, G.; Rigal, A. Isolation of brush border membranes in vesicular form from the intestinal spiral valve of the small dogfish (Scyliorhinus canicula). Biochim. Biophys. Acta 1979, 554, 264–267. [Google Scholar] [CrossRef]
- Jhaveri, P.; Papastamatiou, Y.P.; German, D.P. Digestive enzyme activities in the guts of bonnethead sharks (Sphyrna tiburo) provide insight into their digestive strategy and evidence for microbial digestion in their hindguts. Comp. Biochem. Physiol. A 2015, 189, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Z.; Sun, J.F.; Lv, A.J.; Zhang, S.L.; Sung, Y.Y.; Shi, H.Y.; Hu, X.C.; Chen, S.J.; Xing, K.Z. Histochemical distribution of four types of enzymes and mucous cells in the gastrointestinal tract of reared half-smooth tongue sole Cynoglossus semilaevis. J. Fish Biol. 2018, 92, 3–16. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Y.; Lv, A.; Xian, J.-A.; Wang, Q.; Zhang, S.; Guo, Y.; Xing, K. Histochemical distribution of four types of enzymes and mucous cells in the intestine of koi carp (Cyprinus carpio var. koi). Fish Physiol. Biochem. 2019, 45, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Bastiančić, L.; Vlahek, I.; Benko, V.; Lovrić, M.; Valić, D.; Kužir, S. Histochemical research of enzymes involved in cellular digestion in the digestive tract of tub gurnard, Chelidonichthys lucerna. Fish Physiol. Biochem. 2023, 50, 157–170. [Google Scholar] [CrossRef]
- Xiao, W.; Jiang, W.; Feng, L.; Liu, Y.; Wu, P.; Jiang, J.; Zhang, Y.; Zhou, X. Supplementation of enzyme-treated soy protein saves dietary protein and promotes digestive and absorptive ability referring to TOR signaling in juvenile fish. Fish Physiol. Biochem. 2017, 43, 1657–1675. [Google Scholar] [CrossRef]
- Wei, S.P.; Jiang, W.D.; Wu, P.; Liu, Y.; Zeng, Y.Y.; Jiang, J.; Kuang, S.Y.; Tang, L.; Zhang, Y.A.; Zhou, X.Q.; et al. Dietary magnesium deficiency impaired intestinal structural integrity in grass carp (Ctenopharyngodon idella). Sci. Rep. 2018, 8, 12705. [Google Scholar] [CrossRef]
- Pasquariello, R.; Pavlovic, R.; Chacon, M.A.; Camin, F.; Verdile, N.; Løkka, G.; Panseri, S.; Faustini, M.; Tandler, A.; Peggs, D.; et al. Development of a Rainbow Trout (Oncorhynchus mykiss) Intestinal In Vitro Platform for Profiling Amino Acid Digestion and Absorption of a Complete Diet. Animals 2023, 13, 2278. [Google Scholar] [CrossRef]
- Tlak Gajger, I.; Nejedli, S.; Kozarić, Z.; Vlainić, J. Histochemical analysis and distribution of digestive enzymes in the gastrointestinal system of the European barracuda Sphyraena sphyraena (Linnaeus, 1758). Animals 2024, 14, 2798. [Google Scholar] [CrossRef]
- Lallès, J.P. Intestinal alkaline phosphatase in the gastrointestinal tract of fish: Biology, ontogeny, and environmental and nutritional modulation. Rev. Aquacult. 2019, 12, 555–581. [Google Scholar] [CrossRef]
- Devčić, L.; Valić, D.; Lovrić, M.; Vlahek, I.; Benko, V.; Kužir, S. Comprehensive study of the digestive tract in the European hake (Merluccius merluccius). Slov. Vet. Res. 2024; in press. [Google Scholar] [CrossRef]
- Lallès, J.P. Intestinal alkaline phosphatase, inflammation and nutrition: Recent advances. Nutr. Rev. 2019, 77, 710–724. [Google Scholar] [CrossRef]
- Lallès, J.P. Intestinal alkaline phosphatase: Novel functions and protective effects. Nutr. Rev. 2014, 72, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Rader, B.A. Alkaline phosphatase, an unconventional immune protein. Front. Immunol. 2017, 8, 897. [Google Scholar] [CrossRef]
- Ostaszewska, T.; Kamaszewski, M. Digestive system. In The Histology of Fishes; Kirschbaum, F., Formicki, K., Eds.; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2019; pp. 88–100. [Google Scholar]
- Hirji, K.N.; Courtney, W.A.M. Leucine aminopeptidase activity in the digestive tract of perch, Perca fluviatilis L. J. Fish Biol. 1982, 21, 615–622. [Google Scholar] [CrossRef]
- Tengjaroenkul, B.; Smith, B.J.; Caceci, T.; Smith, S.A. Distribution of intestinal enzyme activities along the intestinal tract of cultured Nile tilapia, Oreochromis niloticus L. Aquaculture 2000, 182, 317–327. [Google Scholar] [CrossRef]
| Fish Species | Digestive Tract | Epithelium (MOD) | Connective Tissue (MOD) | Gastric Glands (MOD) | |
|---|---|---|---|---|---|
| Brush Border | Cytoplasm | ||||
| Small-spotted catshark | Esophagus | / | 0.283 a ± 0.047 | / | / |
| Stomach | |||||
| Anterior part | / | 0.109 bA ± 0.032 | 0.042 cB ± 0.034 | 0.097 A ± 0.028 | |
| Posterior part | / | 0.146 b ± 0.067 | 0.116 a ± 0.045 | / | |
| Spiral intestine | 0.227 A ± 0.117 | 0.097 bB ± 0.027 | 0.100 abB ± 0.026 | / | |
| Rectum | / | 0.146 bA ± 0.025 | 0.073 bcB ± 0.030 | / | |
| Smooth-hound | Esophagus | / | 0.074 c ± 0.010 | 0.024 ± 0.009 | / |
| Stomach | |||||
| Anterior part | / | 0.170 abA ± 0.061 | 0.056 B ± 0.020 | 0.130 A ± 0.037 | |
| Posterior part | / | 0.181 ab ± 0.076 | 0.126 ± 0.039 | / | |
| Spiral intestine | / | 0.194 a ± 0.078 | 0.126 ± 0.058 | / | |
| Rectum | / | 0.110 bc ± 0.044 | 0.059 ± 0.022 | / | |
| Fish Species | Digestive Tract | Epithelium (MOD) | Connective Tissue (MOD) | Gastric Glands (MOD) |
|---|---|---|---|---|
| Small-spotted catshark | Esophagus | 0.146 b ± 0.056 | / | / |
| Stomach | ||||
| Anterior part | 0.250 aA ± 0.085 | 0.034 bB ± 0.031 | 0.074 B ± 0.028 | |
| Posterior part | 0.247 a ± 0.092 | 0.067 a ± 0.027 | / | |
| Spiral intestine | 0.055 c ± 0.036 | 0.042 ab ± 0.017 | / | |
| Rectum | 0.093 bc ± 0.066 | / | / | |
| Smooth-hound | Esophagus | 0.057 bc ± 0.033 | / | / |
| Stomach | ||||
| Anterior part | 0.086 b ± 0.044 | 0.043 ± 0.029 | 0.076 ± 0.044 | |
| Posterior part | 0.033 c ± 0.017 | / | / | |
| Spiral intestine | 0.132 aA ± 0.039 | 0.034 B ± 0.017 | / | |
| Rectum | 0.044 bc ± 0.023 | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devčić, L.; Vlahek, I.; Palić, M.; Benko, V.; Faraguna, S.; Lovrić, M.; Valić, D.; Kužir, S. Histochemical Study of Enzyme Activity in the Digestive Tract of the Small-Spotted Catshark (Scyliorhinus canicula) and the Smooth-Hound (Mustelus mustelus). Fishes 2025, 10, 386. https://doi.org/10.3390/fishes10080386
Devčić L, Vlahek I, Palić M, Benko V, Faraguna S, Lovrić M, Valić D, Kužir S. Histochemical Study of Enzyme Activity in the Digestive Tract of the Small-Spotted Catshark (Scyliorhinus canicula) and the Smooth-Hound (Mustelus mustelus). Fishes. 2025; 10(8):386. https://doi.org/10.3390/fishes10080386
Chicago/Turabian StyleDevčić, Lucija, Ivan Vlahek, Magdalena Palić, Valerija Benko, Siniša Faraguna, Marin Lovrić, Damir Valić, and Snježana Kužir. 2025. "Histochemical Study of Enzyme Activity in the Digestive Tract of the Small-Spotted Catshark (Scyliorhinus canicula) and the Smooth-Hound (Mustelus mustelus)" Fishes 10, no. 8: 386. https://doi.org/10.3390/fishes10080386
APA StyleDevčić, L., Vlahek, I., Palić, M., Benko, V., Faraguna, S., Lovrić, M., Valić, D., & Kužir, S. (2025). Histochemical Study of Enzyme Activity in the Digestive Tract of the Small-Spotted Catshark (Scyliorhinus canicula) and the Smooth-Hound (Mustelus mustelus). Fishes, 10(8), 386. https://doi.org/10.3390/fishes10080386







