Integrated Transcriptomic and Metabolomic Analysis Reveals the Molecular Mechanisms Involved in the Adaptations of Mandarin Fish (Siniperca chuatsi) to Compound Feed
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Fish Culture
2.2. Preparation of Liver Tissue Sections and Hematoxylin–Eosin (H&E) Staining
2.3. Total RNA Extraction, cDNA Library Construction, and Transcriptome Sequencing
2.4. Sequencing Data Quality Control, Screening, and Functional Annotation of the DEGs
2.5. Validation Using Quantitative PCR (qPCR)
2.6. Metabolite Extraction and Liquid Chromatography–Mass Spectrometry (LC-MS) Analysis
2.7. DM Screening and Analysis
2.8. Correlation Analysis of the DEGs and DMs
3. Results
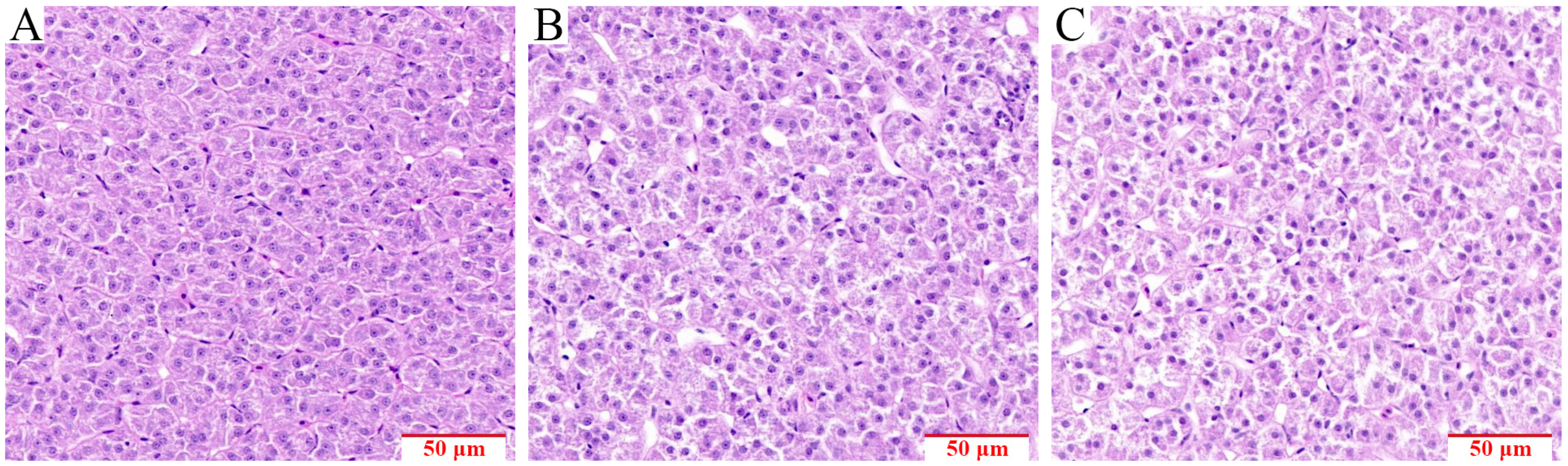
3.1. Effect of Compound Feed Ingestion on the Tissue Microstructure of S. chuatsi Liver
3.2. Transcriptome Sequencing Data Analysis
3.3. PCA of the Transcriptome Data
3.4. DEG Selection
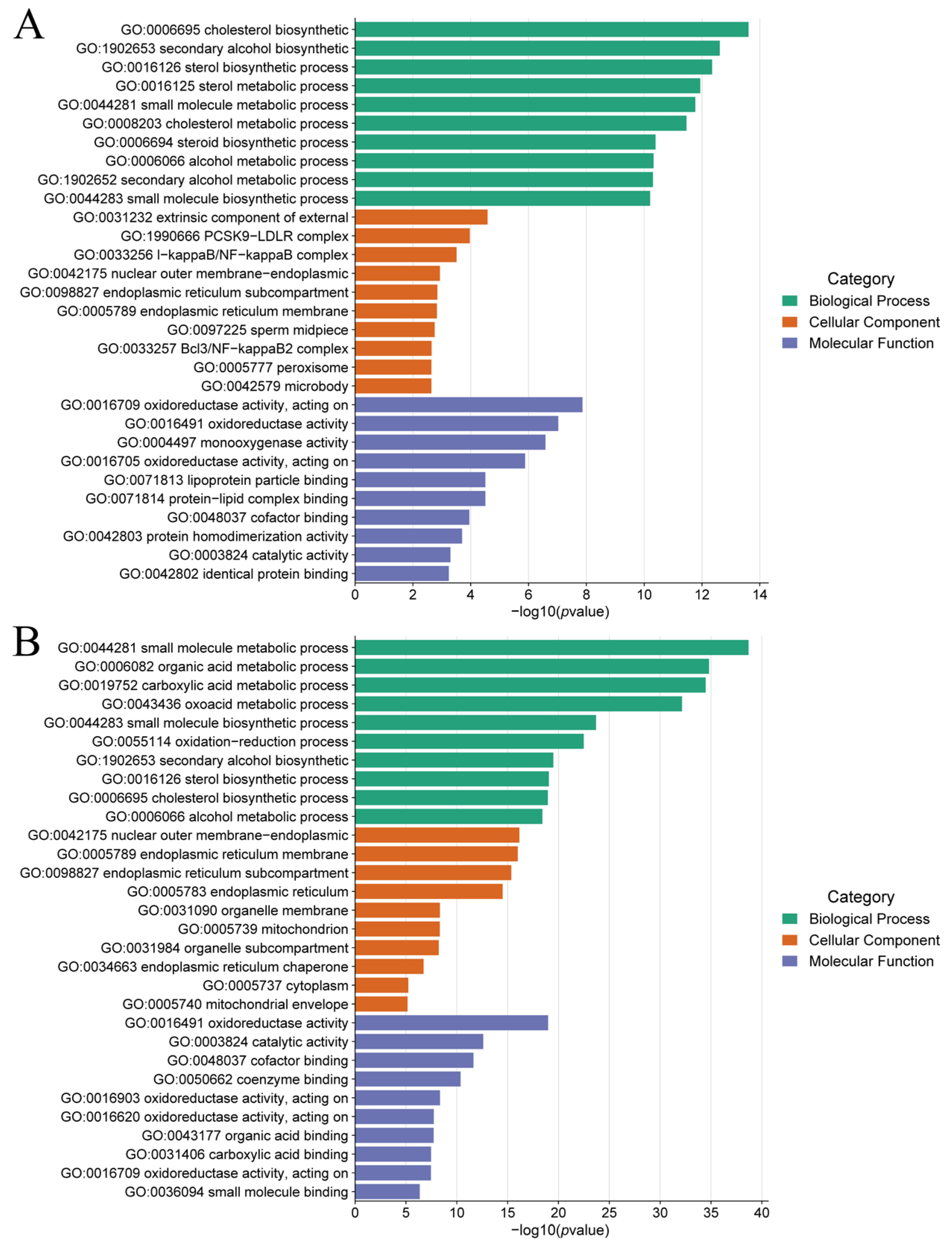
3.5. GO and KEGG Enrichment Analysis of the DEGs
3.6. qPCR Validation
3.7. Statistical Analysis of the Multivariate Variables Between Metabolic Groups
3.7.1. PCA of the Metabolome Data
3.7.2. OPLS-DA Analysis
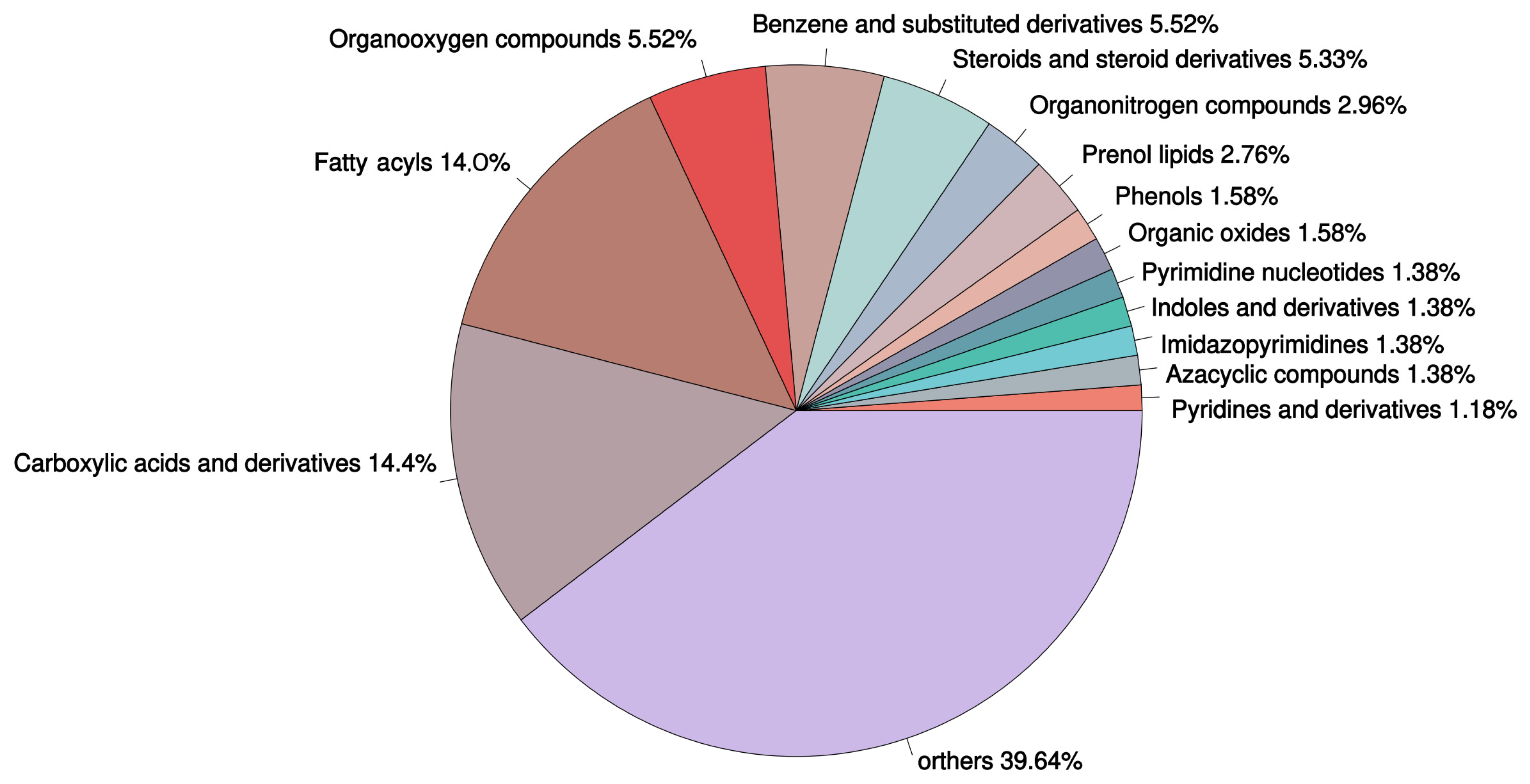
3.8. DM Selection
3.9. KEGG Enrichment Analysis of the DMs
3.10. Integrated Analysis of the Transcriptomic and Metabolomic Data
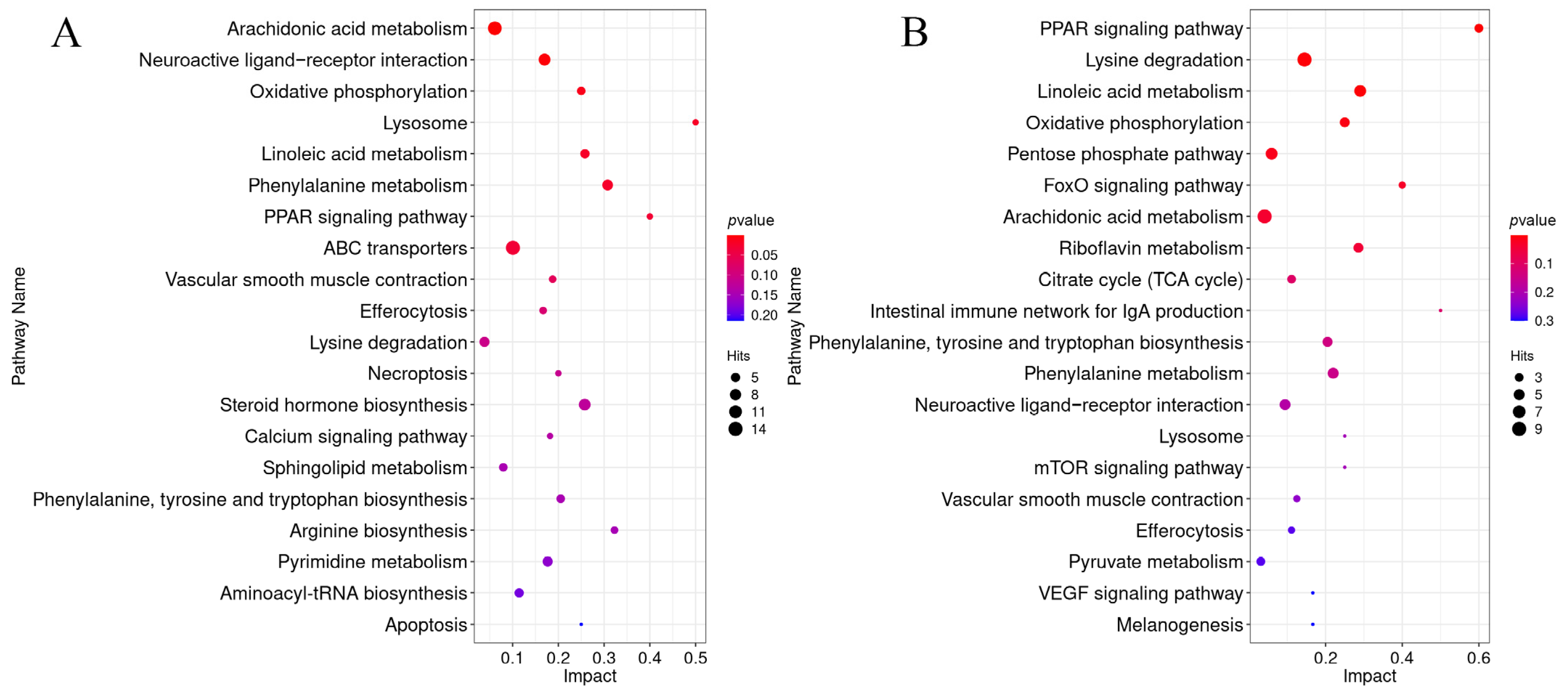
3.10.1. Common Pathway Enrichment Analysis
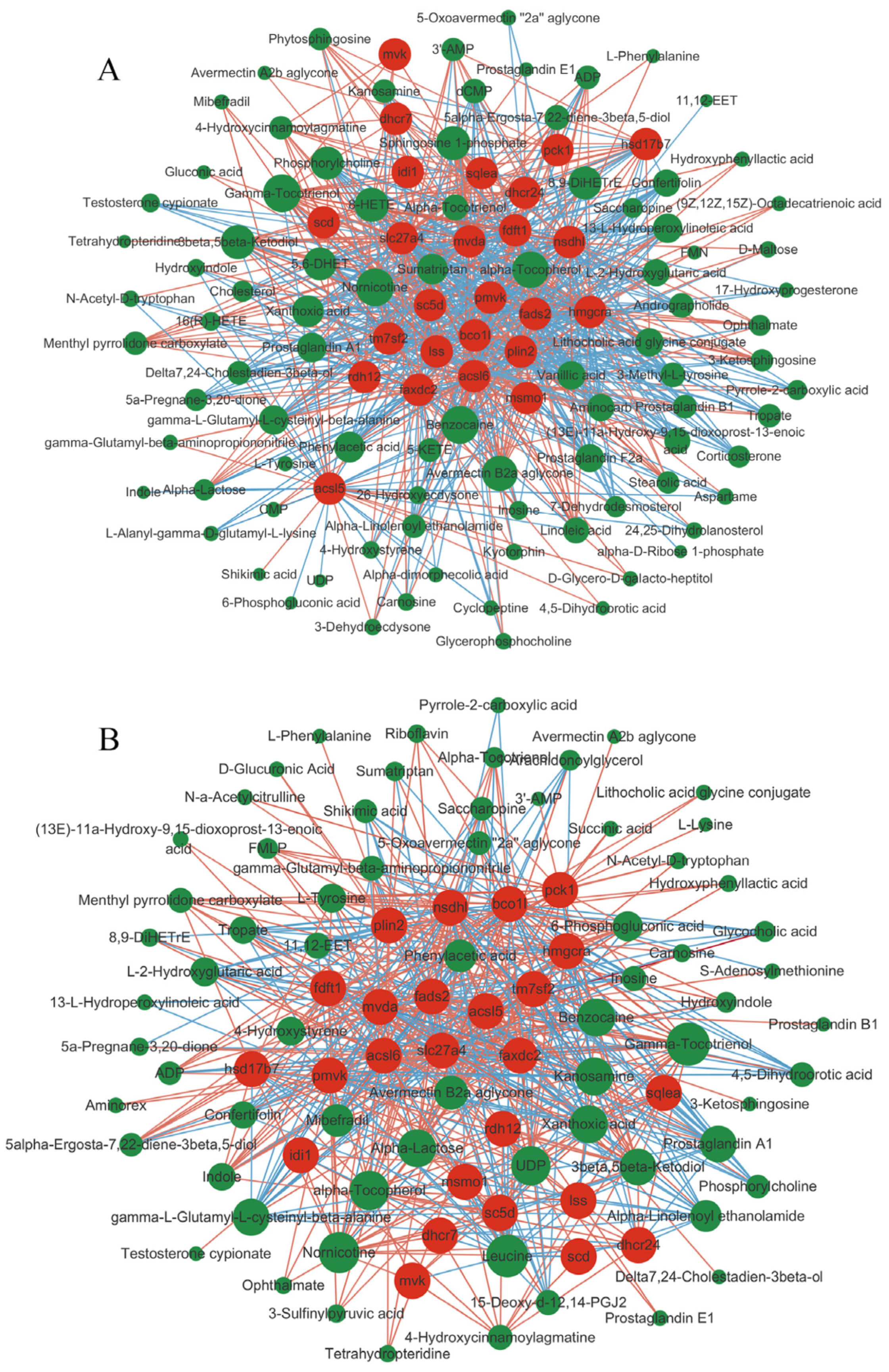
3.10.2. Correlation Analysis of the DEGs and DMs
4. Discussion
4.1. Key Genes and Metabolic Pathways Based on Transcriptome Analysis
4.2. Key Metabolites and Metabolic Pathways Based on Metabolome Analysis
4.3. Combined Multi-Omics Analysis of Pathways
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ministry of Agriculture and Rural Affairs. China Fishery Statistics Yearbook; China Agriculture Press: Beijing, China, 2024; p. 25. [Google Scholar]
- Liang, X.F.; Liu, J.K.; Huang, B.Y. The role of sense organs in the feeding behaviour of Chinese perch. J. Fish Biol. 1998, 52, 1058–1067. [Google Scholar] [CrossRef]
- He, S.; Li, L.; Lv, L.Y.; Cai, W.J.; Dou, Y.Q.; Li, J.; Tang, S.L.; Chen, X.; Zhang, Z.; Xu, J.; et al. Mandarin fish (Sinipercidae) genomes provide insights into innate predatory feeding. Commun. Biol. 2020, 3, 361. [Google Scholar] [CrossRef]
- Liang, X.F.; Lin, X.T.; Li, S.Q.; Liu, J.K. Impact of environmental and innate factors on the food habit of Chinese perch Siniperca chuatsi (Basilewsky) (Percichthyidae). Aquac. Res. 2008, 39, 150–157. [Google Scholar] [CrossRef]
- Shen, Y.W.; Li, H.Y.; Zhao, J.L.; Tang, S.J.; Zhao, Y.; Bi, Y.H.; Chen, X.W. The digestive system of mandarin fish (Siniperca chuatsi) can adapt to domestication by feeding with artificial diet. Aquaculture 2021, 538, 736546. [Google Scholar] [CrossRef]
- Dou, Y.Q.; He, S.; Liang, X.F.; Cai, W.J.; Wang, J.; Shi, L.J.; Li, J. Memory Function in Feeding Habit Transformation of Mandarin Fish (Siniperca chuatsi). Int. J. Mol. Sci. 2018, 19, 1254. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Leng, X.J.; Yun, B.; Wang, L.; Qian, X.Q. Artificial diets affect glucose and lipid metabolism, antioxidant capacity, and inflammatory response in the muscle of mandarin fish (Siniperca chuatsi). Front. Mar. Sci. 2024, 11, 1445902. [Google Scholar] [CrossRef]
- Li, Y.; Shi, J.H.; Shi, S.C.; Liu, Z.J.; Li, Z.; Li, J.Z. Effect of Live, Frozen and Artificial Feeds on Digestive Enzymes, Aminotransferase, Histology of Liver and Intestine in Mandarin Fish Hybrid (Siniperca chuatsi ♀ x Siniperca scherzeri ♂). Isr. J. Aquac.-Bamidgeh 2015, 67, 1185. [Google Scholar] [CrossRef]
- Cui, J.; Jiang, Z.; Wang, Z.R.; Shao, J.Q.; Dong, C.J.; Wang, L.; Li, X.J.; Du, J.X.; Li, S.J.; Qiao, Z.G.; et al. Eight single nucleotide polymorphisms and their association with food habit domestication traits and growth traits in largemouth bass fry (Micropterus salmoides) based on PCR-RFLP method. PeerJ 2023, 11, e14588. [Google Scholar] [CrossRef]
- He, S.; Liang, X.F.; Li, L.; Sun, J.; Wen, Z.Y.; Cheng, X.Y.; Li, A.X.; Cai, W.J.; He, Y.H.; Wang, Y.P.; et al. Transcriptome analysis of food habit transition from carnivory to herbivory in a typical vertebrate herbivore, grass carp Ctenopharyngodon idella. BMC Genom. 2015, 16, 15. [Google Scholar] [CrossRef]
- Li, L.; He, S.; Lin, M.H.; Zhang, Y.P.; Kuhl, H.; Liang, X.F. Whole-genome resequencing and bisulfite sequencing provide new insights into the feeding habit domestication in mandarin fish (Siniperca chuatsi). Front. Genet. 2023, 13, 1088081. [Google Scholar] [CrossRef]
- Shen, Y.W.; Song, L.Y.; Chen, T.T.; Jiang, H.W.; Yang, G.K.; Zhang, Y.M.; Zhang, X.D.; Lim, K.K.; Meng, X.L.; Zhao, J.L.; et al. Identification of hub genes in digestive system of mandarin fish (Siniperca chuatsi) fed with artificial diet by weighted gene co-expression network analysis. Comp. Biochem. Physiol. D-Genom. Proteom. 2023, 47, 101112. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tang, S.L.; He, S.; Liang, X.F. Transcriptome Analysis Provides an Overview of Genes Involved in the Peculiar Food Preference at First-Feeding Stage in Mandarin Fish (Siniperca chuatsi). Fishes 2023, 8, 17. [Google Scholar] [CrossRef]
- Yi, T.L.; Sun, J.; Liang, X.F.; He, S.; Li, L.; Wen, Z.Y.; Shen, D. Effects of Polymorphisms in Pepsinogen (PEP), Amylase (AMY) and Trypsin (TRY) Genes on Food Habit Domestication Traits in Mandarin Fish. Int. J. Mol. Sci. 2013, 14, 21504–21512. [Google Scholar] [CrossRef] [PubMed]
- He, S.; You, J.J.; Liang, X.F.; Zhang, Z.L.; Zhang, Y.P. Transcriptome sequencing and metabolome analysis of food habits domestication from live prey fish to artificial diets in mandarin fish (Siniperca chuatsi). BMC Genom. 2021, 22, 129. [Google Scholar] [CrossRef]
- Li, H.Q.; Li, W.H.; Su, J.S.; Zhou, Z.X.; Miao, Y.; Tian, X.L.; Tao, M.; Zhang, C.; Zhou, Y.; Qin, Q.B.; et al. Integration of transcriptome and metabolome reveals molecular mechanisms responsive to cold stress in gynogenetic mrigal carp (Cirrhinus mrigala). Aquaculture 2024, 579, 740200. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S.P.; Zhang, J.W.; Zhu, C.H.; Chen, S.L.; Zhou, Q. Integrated transcriptomic and metabolomic analysis provides insights into the responses to Vibrio infection in Plectropomus leopardus. Aquaculture 2024, 587, 740854. [Google Scholar] [CrossRef]
- Wang, J.A.; Hou, X.; Chen, X.W.; Zhang, K.J.; Wang, J.; Wang, C.H. Comprehensive analysis of metabolomics and transcriptomics provides insights into growth difference of juvenile Eriocheir sinensis during the molting cycle. Aquaculture 2021, 539, 736661. [Google Scholar] [CrossRef]
- Mitra, V.; Metcalf, J. Metabolic functions of the liver. Anaesth. Intensiv. Care Med. 2012, 13, 54–55. [Google Scholar] [CrossRef]
- Schiffer, L.; Barnard, L.; Baranowski, E.S.; Gilligan, L.C.; Taylor, A.E.; Arlt, W.; Shackleton, C.H.L.; Storbeck, K.H. Human steroid biosynthesis, metabolism and excretion are differentially reflected by serum and urine steroid metabolomes: A comprehensive review. J. Steroid Biochem. Mol. Biol. 2019, 194, 105439. [Google Scholar] [CrossRef]
- Si, Y.F.; Wen, H.S.; Li, Y.; He, F.; Li, J.F.; Li, S.P.; He, H.W. Liver transcriptome analysis reveals extensive transcriptional plasticity during acclimation to low salinity in Cynoglossus semilaevis. BMC Genom. 2018, 19, 464. [Google Scholar] [CrossRef]
- Shi, K.P.; Dong, S.L.; Zhou, Y.G.; Li, Y.; Gao, Q.F.; Sun, D.J. RNA-seq reveals temporal differences in the transcriptome response to acute heat stress in the Atlantic salmon (Salmo salar). Comp. Biochem. Physiol. D-Genom. Proteom. 2019, 30, 169–178. [Google Scholar] [CrossRef]
- Zhou, T.J.; Jia, C.F.; Meng, Q.; Xu, D.F.; Zhang, Z.W.; Zhu, F.; Zhao, Y.L.; Sun, R.J.; Yang, Y.X.; Chen, S.Y. Transcriptome-based analysis of the liver response mechanism of black porgy (Acanthopagrus schlegelii) to stocking density. Fishes 2023, 8, 356. [Google Scholar] [CrossRef]
- Wei, L.; Li, Y.; Ye, H.Z.; Xiao, J.; Hogstrand, C.; Green, I.; Guo, Z.Q.; Han, D. Dietary Trivalent Chromium Exposure Up-Regulates Lipid Metabolism in Coral Trout: The Evidence From Transcriptome Analysis. Front. Physiol. 2021, 12, 640898. [Google Scholar] [CrossRef] [PubMed]
- Kuhre, R.E.; Wewer Albrechtsen, N.J.; Larsen, O.; Jepsen, S.L.; Balk-Møller, E.; Andersen, D.B.; Deacon, C.F.; Schoonjans, K.; Reimann, F.; Gribble, F.M.; et al. Bile acids are important direct and indirect regulators of the secretion of appetite- and metabolism-regulating hormones from the gut and pancreas. Mol. Metab. 2018, 11, 84–95. [Google Scholar] [CrossRef]
- Wang, Y.J.; Rogers, P.M.; Su, C.; Varga, G.; Stayrook, K.R.; Burris, T.P. Regulation of cholesterologenesis by the oxysterol receptor, LXRα. J. Biol. Chem. 2008, 283, 26332–26339. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, A.V.; Luu, W.; Sharpe, L.J.; Brown, A.J. Phosphorylation regulates activity of 7-dehydrocholesterol reductase (DHCR7), a terminal enzyme of cholesterol synthesis. J. Steroid Biochem. Mol. Biol. 2017, 165, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Luu, W.; Zerenturk, E.J.; Kristiana, I.; Bucknall, M.P.; Sharpe, L.J.; Brown, A.J. Signaling regulates activity of DHCR24, the final enzyme in cholesterol synthesis. J. Lipid Res. 2013, 55, 410–420. [Google Scholar] [CrossRef]
- Lluch, M.A.; Masferrer, A.; Arró, M.; Boronat, A.; Ferrer, A. Molecular cloning and expression analysis of the mevalonate kinase gene from Arabidopsis thaliana. Plant Mol. Biol. 2000, 42, 365–376. [Google Scholar] [CrossRef]
- Zhao, L.L.; Liao, L.; Tang, X.H.; Liang, J.; Liu, Q.; Luo, W.; Adam, A.A.; Luo, J.; Li, Z.Q.; Yang, S.; et al. High-carbohydrate diet altered conversion of metabolites, and deteriorated health in juvenile largemouth bass. Aquaculture 2022, 549, 737816. [Google Scholar] [CrossRef]
- Li, H.Y.; Niu, S.H.; Pan, H.J.; Wang, G.J.; Xie, J.; Tian, J.J.; Zhang, K.; Xia, Y.; Li, Z.F.; Yu, E.M.; et al. Integrated miRNA-mRNA analysis reveals the molecular mechanism in mandarin fish (Siniperca chuatsi) in response to fresh baits and artificial diets feeding. Aquacult. Rep. 2023, 30, 101554. [Google Scholar] [CrossRef]
- Ngoh, S.Y.; Tan, D.; Shen, X.Y.; Kathiresan, P.; Jiang, J.H.; Liew, W.C.; Thevasagayam, N.M.; Kwan, H.Y.; Saju, J.M.; Prakki, S.R.S.; et al. Nutrigenomic and Nutritional Analyses Reveal the Effects of Pelleted Feeds on Asian Seabass (Lates calcarifer). PLoS ONE 2015, 10, e0145456. [Google Scholar] [CrossRef]
- Du, J.X.; Shao, J.Q.; Li, S.J.; Zhu, T.; Song, H.M.; Lei, C.X.; Zhang, M.; Cen, Y.K. Integrated transcriptomic and proteomic analyses reveal the mechanism of easy acceptance of artificial pelleted diets during food habit domestication in Largemouth bass (Micropterus salmoides). Sci. Rep. 2023, 13, 18461. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.Q.; Hao, Y.Y.; Song, Y.D.; Zhao, J.L. Predation behavior of mandarin fish (Siniperca chuatsi) regulated by visual and lateral line sensory. J. Fish. Sci. China 2022, 27, 1136–1144. (In Chinese) [Google Scholar]
- Lhor, M.; Salesse, C. Retinol dehydrogenases: Membrane-bound enzymes for the visual function. Biochem. Cell Biol. 2014, 92, 510–523. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Thompson, D.A.; Koutalos, Y. Reduction of all-trans-retinal in vertebrate rod photoreceptors requires the combined action of RDH8 and RDH12. J. Biol. Chem. 2012, 287, 24662–24670. [Google Scholar] [CrossRef]
- Sarkar, H.; Moosajee, M. Retinol dehydrogenase 12 (RDH12): Role in vision, retinal disease and future perspectives. Exp. Eye Res. 2019, 188, 107793. [Google Scholar] [CrossRef]
- He, S.; Liang, X.F.; Sun, J.; Li, L.; Yu, Y.; Huang, W.; Qu, C.M.; Cao, L.; Bai, X.L.; Tao, Y.X. Insights into food preference in hybrid F1 of Siniperca chuatsi (♀) x Siniperca scherzeri (♂) mandarin fish through transcriptome analysis. BMC Genom. 2013, 14, 601. [Google Scholar] [CrossRef]
- House, R.L.; Cassady, J.P.; Eisen, E.J.; McIntosh, M.K.; Odle, J. Conjugated linoleic acid evokes de-lipidation through the regulation of genes controlling lipid metabolism in adipose and liver tissue. Obes. Rev. 2005, 6, 247–258. [Google Scholar] [CrossRef]
- Xu, H.G.; Meng, X.X.; Wei, Y.L.; Ma, Q.; Liang, M.Q.; Turchini, G.M. Arachidonic acid matters. Rev. Aquac. 2022, 14, 1912–1944. [Google Scholar] [CrossRef]
- Bell, J.G.; Sargent, J.R. Arachidonic acid in aquaculture feeds: Current status and future opportunities. Aquaculture 2003, 218, 491–499. [Google Scholar] [CrossRef]
- Wang, B.; Wu, L.J.; Chen, J.; Dong, L.L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Signal Transduct. Target. Ther. 2021, 6, 94. [Google Scholar]
- Fleming, I. Cytochrome p450 enzymes and vascular homeostasis. Circ. Res. 2001, 89, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Oltman, C.L.; Weintraub, N.L.; VanRollins, M.; Dellsperger, K.C. Epoxyeicosatrienoic Acids and Dihydroxyeicosatrienoic Acids Are Potent Vasodilators in the Canine Coronary Microcirculation. Circ. Res. 1998, 83, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.S.; Wu, P.H.; Cao, J.L.; Luo, Y.J.; Chen, J.J.; Wang, G.D.; Guo, W.J.; Wang, T.Y.; He, X.J. The PFOS disturbed immunomodulatory functions via nuclear Factor-κB signaling in liver of zebrafish (Danio rerio). Fish Shellfish Immunol. 2019, 91, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.G.; Wang, C.Q.; Zhang, Y.Q.; Wei, Y.L.; Liang, M.Q. Moderate levels of dietary arachidonic acid reduced lipid accumulation and tended to inhibit cell cycle progression in the liver of Japanese seabass Lateolabrax japonicus. Sci. Rep. 2018, 8, 10682. [Google Scholar] [CrossRef]
- Huang, Y.S.; Lin, Z.D.; Rong, H.; Hao, M.L.; Zhu, D.S.; Li, S.K.; Wen, X.B. Effects of conjugated linoleic acid on growth, body composition, antioxidant status, lipid metabolism and immunity parameters of juvenile Chu’s croaker, Nibea coibor. Aquac. Res. 2018, 49, 546–556. [Google Scholar] [CrossRef]
- Li, L.; Liang, X.F.; Cai, W.J.; Li, J.; Zhang, Y.P.; Wang, J.; Huang, K.; Liu, Y.; Zhu, Q.S.; Alam, M.S.; et al. Dietary with proper ratio of alpha-linolenic acid to linoleic acid enhanced the unsaturated fatty acids deposition of Chinese perch (Siniperca Chuatsi). Aquac. Nutr. 2021, 27, 73–85. [Google Scholar] [CrossRef]
- Zuo, R.T.; Ai, Q.H.; Mai, K.S.; Xu, W. Effects of conjugated linoleic acid on growth, non-specific immunity, antioxidant capacity, lipid deposition and related gene expression in juvenile large yellow croaker (Larmichthys crocea) fed soyabean oil-based diets. Br. J. Nutr. 2013, 110, 1220–1232. [Google Scholar] [CrossRef]
- Zhao, H.H.; Chong, J.; Tang, R.; Li, L.; Xia, J.G.; Li, D.P. Metabolomics investigation of dietary effects on flesh quality in grass carp (Ctenopharyngodon idellus). GigaScience 2018, 7, giy111. [Google Scholar] [CrossRef]
- Tian, J.J.; Ji, H.; Oku, H.; Zhou, J.S. Effects of dietary arachidonic acid (ARA) on lipid metabolism and health status of juvenile grass carp, Ctenopharyngodon idellus. Aquaculture 2014, 430, 57–65. [Google Scholar] [CrossRef]
- Xu, H.G.; Ai, Q.H.; Mai, K.S.; Xu, W.; Wang, J.; Ma, H.M.; Zhang, W.B.; Wang, X.J.; Liufu, Z.G. Effects of dietary arachidonic acid on growth performance, survival, immune response and tissue fatty acid composition of juvenile Japanese seabass, Lateolabrax japonicus. Aquaculture 2010, 307, 75–82. [Google Scholar] [CrossRef]
- Wang, Y.K.; Pan, Y.; Hou, M.R.; Luo, R.S.Q.; He, J.W.; Lin, F.; Xia, X.F.; Li, P.; He, C.X.; He, P.; et al. Danggui Shaoyao San ameliorates the lipid metabolism via the PPAR signaling pathway in a Danio rerio (zebrafish) model of hyperlipidemia. Biomed. Pharmacother. 2023, 168, 115736. [Google Scholar] [CrossRef]
- Zhong, H.; Hu, J.; Zhou, Y. Transcriptomic evidence of luteinizing hormone-releasing hormone agonist (LHRH-A) regulation on lipid metabolism in grass carp (Ctenopharyngodon idella). Genomics 2021, 113, 1265–1271. [Google Scholar] [CrossRef]
- Luo, J.X.; Gao, X.T.; Rong, Z.; Zhang, L.H.; Sun, Y.F.; Qi, Z.L.; Yu, Q.; Waiho, K.; Zhao, W.X.; Xu, Y.H.; et al. Transcriptome Sequencing Reveals Effects of Artificial Feed Domestication on Intestinal Performance and Gene Expression of Carnivorous Mandarin Fish (Siniperca chuatsi) and Related Mechanisms. Mar. Biotechnol. 2025, 27, 41. [Google Scholar] [CrossRef]
- Najt, C.P.; Lwande, J.S.; McIntosh, A.L.; Senthivinayagam, S.; Gupta, S.; Kuhn, L.A.; Atshaves, B.P. Structural and functional assessment of perilipin 2 lipid binding domain(s). Biochemistry 2014, 53, 7051–7066. [Google Scholar] [CrossRef]
- Takahashi, Y.; Shinoda, A.; Kamada, H.; Shimizu, M.; Inoue, J.; Sato, R. Perilipin2 plays a positive role in adipocytes during lipolysis by escaping proteasomal degradation. Sci. Rep. 2016, 6, 20975. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.J.; Liang, X.F.; Cai, W.J.; Zhuang, W.Y. Bioinformatics and expression analysis of plin2 in Chinese perch (Siniperca Chuatsi) and its role in liver fat accumulation. Acta Hydrobiol. Sin. 2022, 46, 273–281. (In Chinese) [Google Scholar]
- Yu, S.; Meng, S.M.; Xiang, M.X.; Ma, H. Phosphoenolpyruvate carboxykinase in cell metabolism: Roles and mechanisms beyond gluconeogenesis. Mol. Metab. 2021, 53, 101257. [Google Scholar] [CrossRef]
- Ye, Q.; Liu, Y.; Zhang, G.J.; Deng, H.J.; Wang, X.J.; Tuo, L.; Chen, C.; Pan, X.M.; Wu, K.; Fan, J.G.; et al. Deficiency of gluconeogenic enzyme PCK1 promotes metabolic-associated fatty liver disease through PI3K/AKT/PDGF axis activation in male mice. Nat. Commun. 2023, 14, 1402. [Google Scholar] [CrossRef]
- You, J.J.; Ren, P.; He, S.; Liang, X.F.; Xiao, Q.Q.; Zhang, Y.P. Histone Methylation of H3K4 Involved in the Anorexia of Carnivorous Mandarin Fish (Siniperca chuatsi) After Feeding on a Carbohydrate-Rich Diet. Front. Endocrinol. 2020, 11, 323. [Google Scholar] [CrossRef]
- Liu, T.; Liang, X.F.; Zhuang, W.Y.; Cai, W.J.; Zhang, Y.P. Association of DNA methylation of pck1 and t1r1 with the food habits of Chinese perch, Siniperca chuatsi. J. Fish. Sci. China 2022, 29, 1064–1072. (In Chinese) [Google Scholar]
- Ma, X.Y.; Qiang, J.; He, J.; Gabriel, N.N.; Xu, P. Changes in the physiological parameters, fatty acid metabolism, and SCD activity and expression in juvenile GIFT tilapia (Oreochromis niloticus) reared at three different temperatures. Fish Physiol. Biochem. 2015, 41, 937–950. [Google Scholar] [CrossRef]
- Qiang, J.; Tao, Y.F.; Bao, J.W.; Chen, D.J.; Li, H.X.; He, J.; Xu, P. High Fat Diet-Induced miR-122 Regulates Lipid Metabolism and Fat Deposition in Genetically Improved Farmed Tilapia (GIFT, Oreochromis niloticus) Liver. Front. Physiol. 2018, 9, 1422. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Zhang, L.H.; Gao, X.W.; Sun, Y.F.; Zhao, C.L.; Gao, X.T.; Wu, C.B. Molecular Cloning of the scd1 Gene and Its Expression in Response to Feeding Artificial Diets to Mandarin Fish (Siniperca chuatsi). Genes 2024, 15, 1211. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.Z.; Ye, J.L.; Lu, M.S.; Wang, S.Q.; You, C.H.; Li, Y.Y. Comparsion of Activities of Fatty Acyl Desaturases and Elongases Among Six Teleosts With Different Feeding and Ecological Habits. Front. Mar. Sci. 2020, 7, 117. [Google Scholar] [CrossRef]
- Turkmen, S.; Perera, E.; Zamorano, M.J.; Simó-Mirabet, P.; Xu, H.L.; Pérez-Sánchez, J.; Izquierdo, M. Effects of Dietary Lipid Composition and Fatty Acid Desaturase 2 Expression in Broodstock Gilthead Sea Bream on Lipid Metabolism-Related Genes and Methylation of the fads2 Gene Promoter in Their Offspring. Int. J. Mol. Sci. 2019, 20, 6250. [Google Scholar] [CrossRef]
- Luo, Q.; Das, A.; Oldoni, F.; Wu, P.Y.; Wang, J.G.; Luo, F.; Fang, Z.F. Role of ACSL5 in fatty acid metabolism. Heliyon 2023, 9, e13316. [Google Scholar] [CrossRef]
- Bu, S.Y.; Mashek, D.G. Hepatic long-chain acyl-CoA synthetase 5 mediates fatty acid channeling between anabolic and catabolic pathways. J. Lipid Res. 2010, 51, 3270–3280. [Google Scholar] [CrossRef]
- Griffin, J.D.; Zhu, Y.; Reeves, A.; Buhman, K.K.; Greenberg, A.S. Intestinal Acyl-CoA synthetase 5 (ACSL5) deficiency potentiates postprandial GLP-1 & PYY secretion, reduces food intake, and protects against diet-induced obesity. Mol. Metab. 2024, 83, 101918. [Google Scholar]
- Teodoro, B.G.; Sampaio, I.H.; Bomfim, L.H.M.; Queiroz, A.L.; Silveira, L.R.; Souza, A.O.; Fernandes, A.M.A.P.; Eberlin, M.N.; Huang, T.Y.; Zheng, D.H.; et al. Long-chain acyl-CoA synthetase 6 regulates lipid synthesis and mitochondrial oxidative capacity in human and rat skeletal muscle. J. Physiol.-London 2017, 595, 677–693. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, A.R.; Jung, Y.H.; Bu, S.Y. Dissociation of Systemic Glucose Homeostasis from Triacylglyceride Accumulation by Reduced Acsl6 Expression in Skeletal Muscle. Biotechnol. Bioprocess Eng. 2018, 23, 465–472. [Google Scholar] [CrossRef]



| Items | Crude Protein | Crude Fat | Crude Ash | Moisture |
|---|---|---|---|---|
| Compound feed | 47.62 | 12.59 | 14.13 | 12.00 |
| Live food | 18.35 | 5.51 | 3.26 | 71.24 |
| Sample | Raw Reads | Raw Bases (bp) | Clean Reads | Clean Bases (bp) | Q20 (%) | Q30 (%) |
|---|---|---|---|---|---|---|
| C-1 | 56,182,872 | 8,483,613,672 | 55,466,800 | 8,354,240,007 | 98.87 | 96.64 |
| C-2 | 47,405,212 | 7,158,187,012 | 46,677,004 | 7,027,077,884 | 98.66 | 96.08 |
| C-3 | 54,969,954 | 8,300,463,054 | 54,136,846 | 8,143,475,626 | 98.70 | 96.20 |
| L-1 | 51,909,826 | 7,838,383,726 | 51,147,994 | 7,710,117,957 | 98.67 | 96.07 |
| L-2 | 53,048,796 | 8,010,368,196 | 52,287,502 | 7,878,504,720 | 98.69 | 96.14 |
| L-3 | 54,529,298 | 8,233,923,998 | 53,771,160 | 8,107,282,767 | 98.74 | 96.28 |
| M-1 | 56,344,264 | 8,507,983,864 | 55,546,234 | 8,378,330,464 | 98.81 | 96.46 |
| M-2 | 51,801,834 | 7,822,076,934 | 50,985,892 | 7,689,201,541 | 98.67 | 96.12 |
| M-3 | 50,750,324 | 7,663,298,924 | 49,956,536 | 7,533,954,136 | 98.69 | 96.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.; Zhang, Y.; Dong, J.; Wang, F.; Zhang, H.; Gao, F.; Ye, X.; Wu, C.; Sun, C. Integrated Transcriptomic and Metabolomic Analysis Reveals the Molecular Mechanisms Involved in the Adaptations of Mandarin Fish (Siniperca chuatsi) to Compound Feed. Fishes 2025, 10, 379. https://doi.org/10.3390/fishes10080379
Yan Y, Zhang Y, Dong J, Wang F, Zhang H, Gao F, Ye X, Wu C, Sun C. Integrated Transcriptomic and Metabolomic Analysis Reveals the Molecular Mechanisms Involved in the Adaptations of Mandarin Fish (Siniperca chuatsi) to Compound Feed. Fishes. 2025; 10(8):379. https://doi.org/10.3390/fishes10080379
Chicago/Turabian StyleYan, Yunyun, Yuan Zhang, Junjian Dong, Fubao Wang, Hetong Zhang, Fengying Gao, Xing Ye, Chengbin Wu, and Chengfei Sun. 2025. "Integrated Transcriptomic and Metabolomic Analysis Reveals the Molecular Mechanisms Involved in the Adaptations of Mandarin Fish (Siniperca chuatsi) to Compound Feed" Fishes 10, no. 8: 379. https://doi.org/10.3390/fishes10080379
APA StyleYan, Y., Zhang, Y., Dong, J., Wang, F., Zhang, H., Gao, F., Ye, X., Wu, C., & Sun, C. (2025). Integrated Transcriptomic and Metabolomic Analysis Reveals the Molecular Mechanisms Involved in the Adaptations of Mandarin Fish (Siniperca chuatsi) to Compound Feed. Fishes, 10(8), 379. https://doi.org/10.3390/fishes10080379






