An Assessment of the Population Structure and Stock Dynamics of Megalobrama skolkovii During the Early Phase of the Fishing Ban in the Poyang Lake Basin
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Design and Methodology
2.2. Data Analysis
2.2.1. Length–Weight Relationship Estimation
2.2.2. Growth Model
2.2.3. Mortality Coefficients and Exploitation Rate
2.2.4. Length-Based Indicators (LBIs)
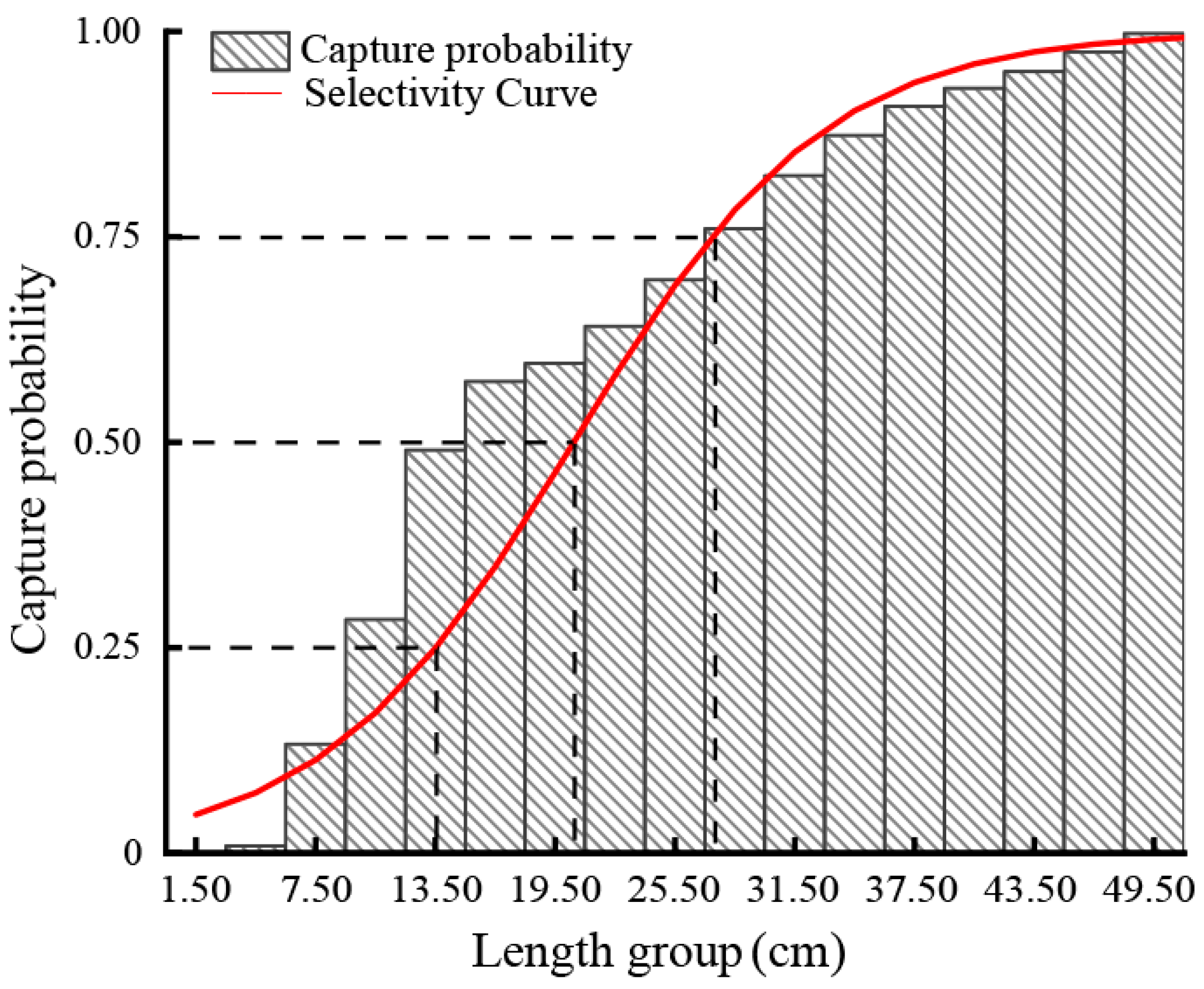
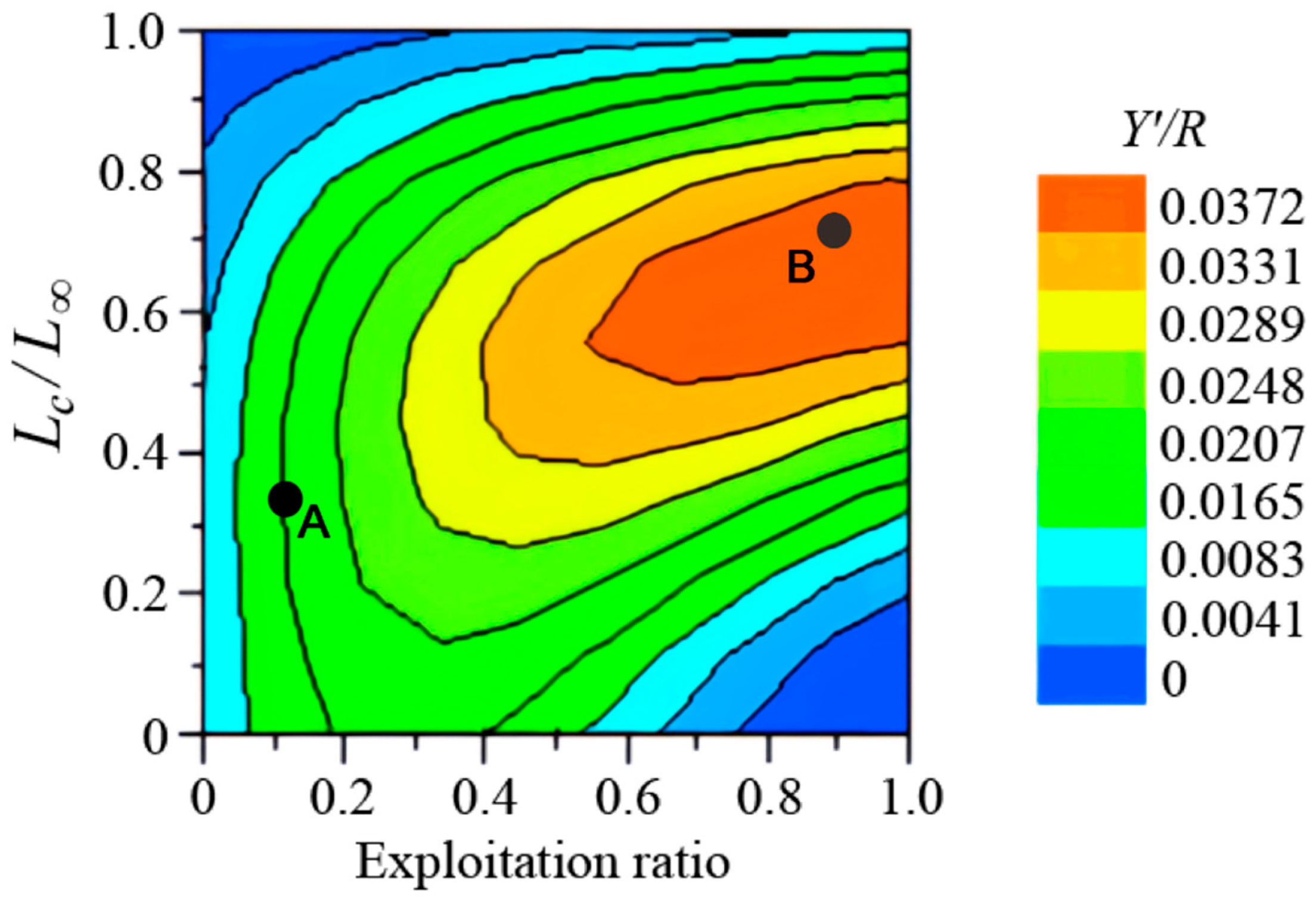
2.2.5. Selectivity and Yield-per-Recruit Analysis
2.3. Terminology and Definitions
3. Results
3.1. Population Structure
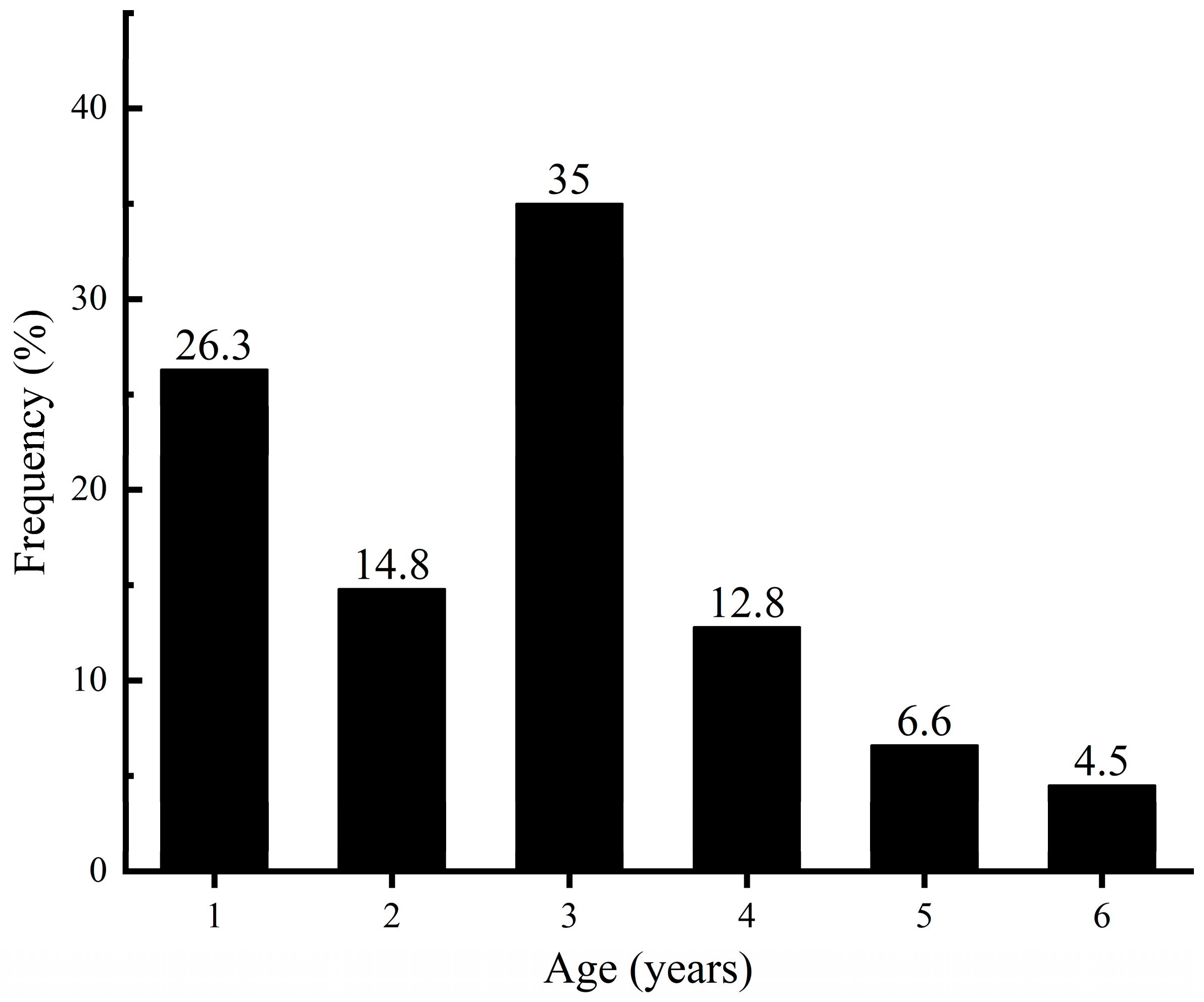
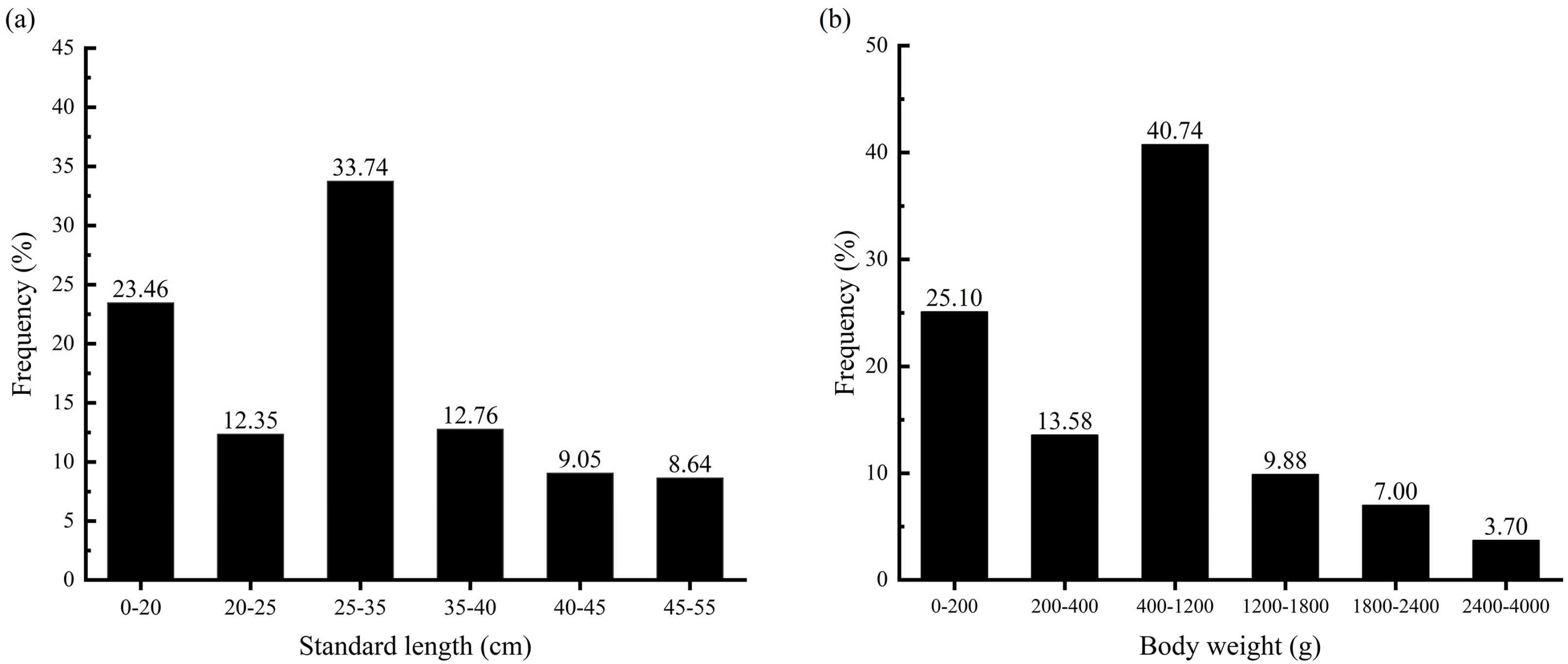
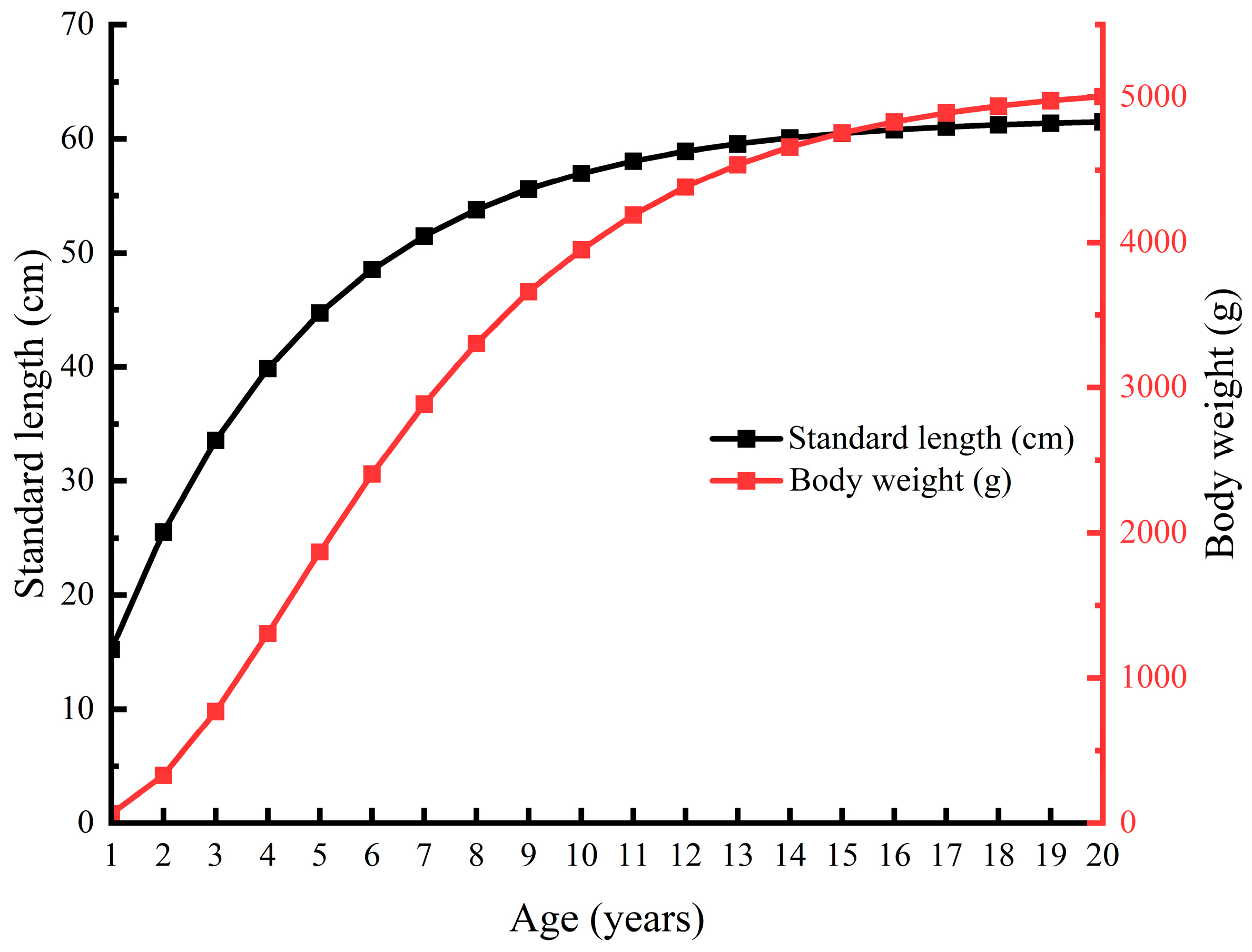
3.1.1. Standard Length, Body Weight, and Age
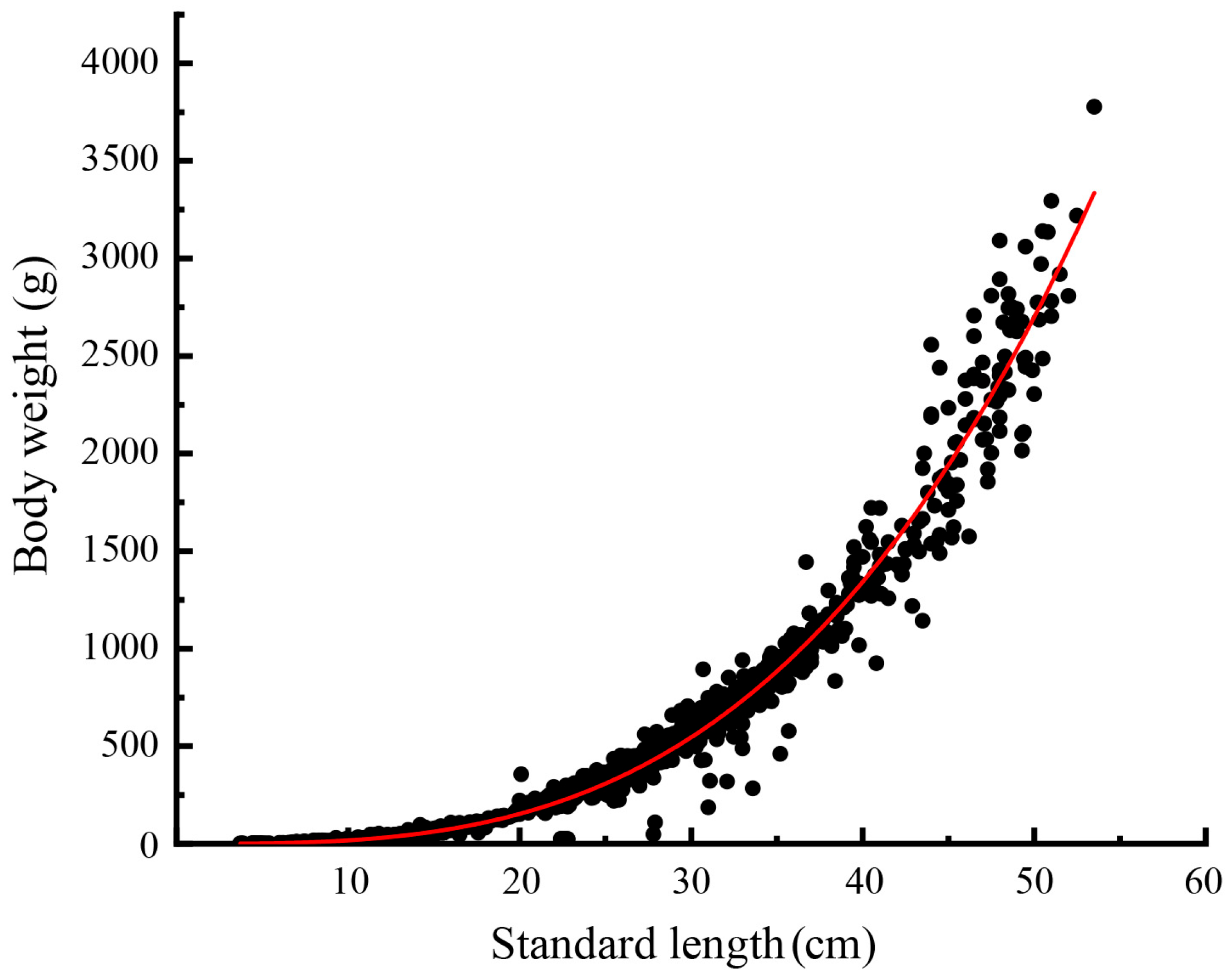
3.1.2. Length–Weight Relationship
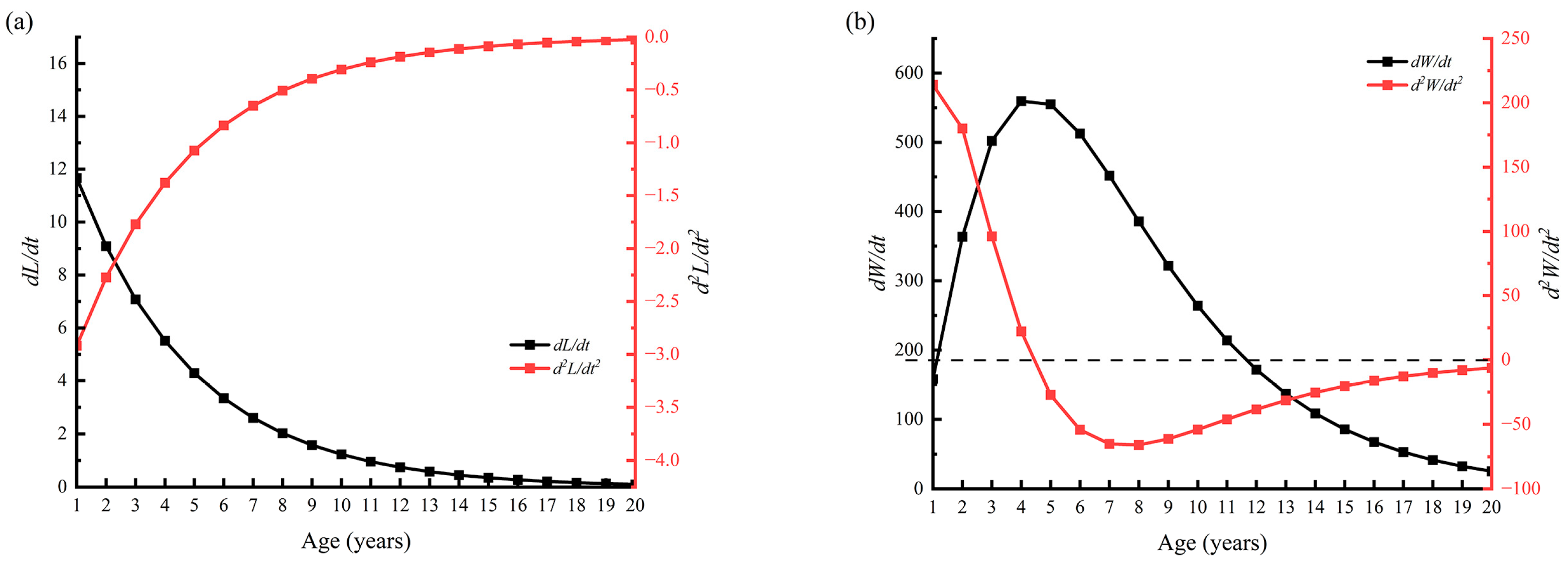
3.1.3. Growth Parameters and Model Results
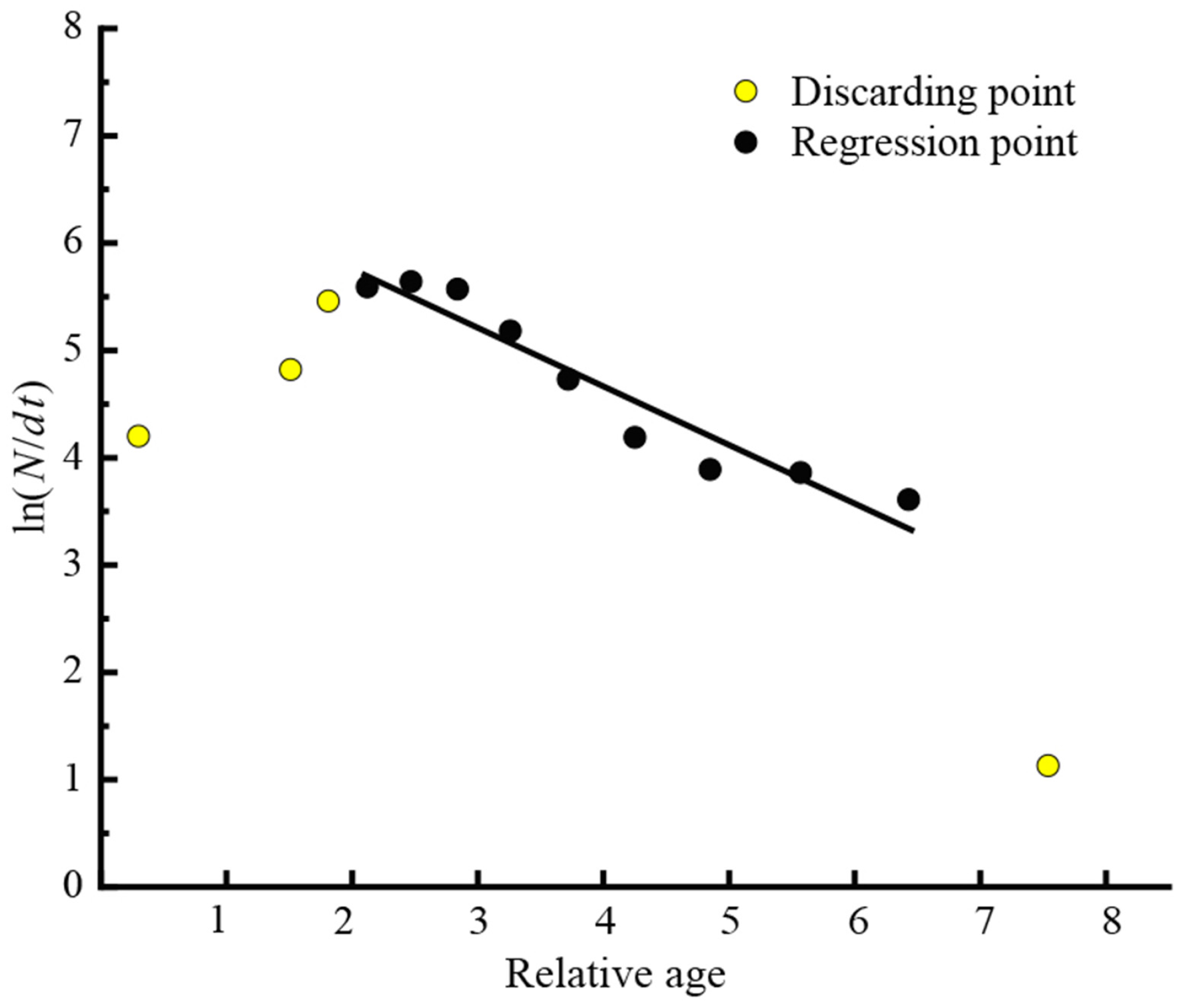
3.2. Mortality Parameters
3.3. Population Assessment Based on Length-Based Indicators (LBIs)
3.4. Stock Dynamics Modeling
4. Discussion
4.1. Appropriate Population Structure and Assessment Methods
4.2. Age Structure and Growth Characteristics of M. skolkovii
| Species | Sampling Site | a | k (Year−1) | ti (Year) | L∞ (cm) | W∞ (g) | Inflection Length (cm) | Inflection Weight (g) | Apparent Growth Index |
|---|---|---|---|---|---|---|---|---|---|
| M. skolkovii | Poyang Lake | 1.46 × 10−2 | 0.25 | 4.40 | 61.89 | 5106.07 | 41.80 | 1515.00 | 2.98 |
| M. skolkovii | Nanshui Reservoir [57] | 1.14 × 10−2 | 0.21 | 5.40 | 49.29 | 2875.63 | 33.81 | 902.15 | 2.71 |
| M. skolkovii | Beijiang River [58] | 4.00 × 10−5 | 0. 23 | 4.38 | 48.05 | 3052.92 | 31.76 | 900.22 | 2.73 |
| M. skolkovii | Xujiahe Reservoir [9] | 2.23 × 10−5 | 0.18 | 5.90 | 45.83 | 2197.30 | 30.47 | 665.68 | 2.58 |
| M. terminalis | Pearl River [59] | 1.55 × 10−2 | 0.18 | 5.91 | 47.20 | 2388.12 | 31.70 | 712.21 | 2.60 |
| M. terminalis | Xijiang River [60] | 7.00 × 10−5 | 0.22 | 4.70 | 42.01 | 1602.76 | 28.03 | 476.16 | 2.59 |
4.3. Stock Dynamics and Resource Status of M. skolkovii
4.4. Management Strategies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fu, C.; Wu, J.H.; Chen, J.K.; Wu, Q.H.; Lei, G.C. Freshwater Fish Biodiversity in the Yangtze River Basin of China: Patterns, Threats and Conservation. Biodivers. Conserv. 2003, 12, 1649–1685. [Google Scholar] [CrossRef]
- Xie, P. Three-Gorges Dam: Risk to Ancient Fish. Science 2003, 302, 1149–1151. [Google Scholar] [CrossRef]
- Jiangxi Provincial Local Chronicles Compilation Committee. Physical Geography of Jiangxi Province; Local Gazetteers Press: Beijing, China, 2003; ISBN 978-7-80122-914-4. [Google Scholar]
- Feng, L.; Hu, C.; Chen, X.; Cai, X.; Tian, L.; Gan, W. Assessment of Inundation Changes of Poyang Lake Using MODIS Observations between 2000 and 2010. Remote Sens. Environ. 2012, 121, 80–92. [Google Scholar] [CrossRef]
- Zhang, T.L.; Li, Z.J. Fish Resources and Fishery Utilization of Lake Poyang. J. Lake Sci. 2007, 19, 434–444. [Google Scholar] [CrossRef][Green Version]
- Froese, R.; Pauly, D. FishBase. Available online: https://www.fishbase.se (accessed on 22 May 2025).
- Chen, J.; Liu, H.; Gooneratne, R.; Wang, Y.; Wang, W. Population Genomics of Megalobrama Provides Insights into Evolutionary History and Dietary Adaptation. Biology 2022, 11, 186. [Google Scholar] [CrossRef]
- Huang, D.M.; Lin, Y.T.; Wan, C.Y.; Liu, Y.J. Reproductive Biology of the Fish, Megalobrama skolkovii, in Fuqiaohe Reservoir. Acta Hydrobiol. Sin. 1997, 21, 15–23. [Google Scholar] [CrossRef]
- Xu, W.; Xiong, B.X.; Cheng, Z.X.; Wang, D.W.; Ying, C.G. Growth and Fecundity of Megalobrama skolkovii in the Xujiahe Reservoir of Hubei, China. Chin. J. Appl. Environ. Biol. 2010, 16, 91–95. [Google Scholar] [CrossRef]
- Kong, C.; Luo, Y.; Xu, Q.; Zhang, B.; Gao, X.; Wang, X.; Luo, Z.; Luo, Z.; Li, L.; Gong, X. Post-Fishing Ban Period: The Fish Diversity and Community Structure in the Poyang Lake Basin, Jiangxi Province, China. Animals 2025, 15, 433. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jarić, I.; Roberts, D.L.; He, Y.; Du, H.; Wu, J.; Wang, C.; Wei, Q. Extinction of One of the World’s Largest Freshwater Fishes: Lessons for Conserving the Endangered Yangtze Fauna. Sci. Total Environ. 2020, 710, 136242. [Google Scholar] [CrossRef] [PubMed]
- Turvey, S.T.; Pitman, R.L.; Taylor, B.L.; Barlow, J.; Akamatsu, T.; Barrett, L.A.; Zhao, X.; Reeves, R.R.; Stewart, B.S.; Wang, K.; et al. First Human-Caused Extinction of a Cetacean Species? Biol. Lett. 2007, 3, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Tian, H.; Xiang, Z.; Zhao, K.; Yu, L.; Duan, X.; Chen, D.; Xu, J.; Liu, M. Impact of the Fishing Ban on Fish Diversity and Population Structure in the Middle Reaches of the Yangtze River, China. Front. Environ. Sci. 2025, 12, 1530716. [Google Scholar] [CrossRef]
- Chen, T.; Wang, Y.; Gardner, C.; Wu, F. Threats and Protection Policies of the Aquatic Biodiversity in the Yangtze River. J. Nat. Conserv. 2020, 58, 125931. [Google Scholar] [CrossRef]
- Wang, H.; Chen, J.; Wang, P.; Jeppesen, E.; Xie, P. How to Manage Fish within and after the 10-Year Fishing Ban. Innovation 2024, 5, 100694. [Google Scholar] [CrossRef]
- Han, Y.Q.; He, A.Y.; Shi, J.; Wang, D.P.; Wu, W.J.; Lei, J.J.; Li, Y.S. Effect Evaluation of Three-Year Fishing Ban in Pearl River Waters (Guangxi Section). Fish. Sci. Technol. Inf. 2015, 42, 135–139. [Google Scholar] [CrossRef]
- Xia, Z.J.; Liu, F.; Yu, F.D.; Tang, R.; Wang, J.W. Species, Functional and Taxonomic Diversity of Fish in the Chishui River Basin. J. Hydroecol. 2022, 43, 89–98. [Google Scholar] [CrossRef]
- Beamish, R.J.; McFarlane, G.A. The Forgotten Requirement for Age Validation in Fisheries Biology. Trans. Am. Fish. Soc. 1983, 112, 735–743. [Google Scholar] [CrossRef]
- Froese, R. Cube Law, Condition Factor and Weight-Length Relationships: History, Meta-Analysis and Recommendations. J. Appl. Ichthyol. 2006, 22, 241–253. [Google Scholar] [CrossRef]
- Munro, J.L.; Pauly, D. A Simple Method for Comparing the Growth of Fishes and Invertebrates. Fishbyte 1983, 1, 5–6. [Google Scholar]
- Branstetter, S. Age and Growth Estimates for Blacktip, Carcharhinus Limbatus, and Spinner, C. Brevipinna, Sharks from the Northwestern Gulf of Mexico. Copeia 1987, 1987, 964. [Google Scholar] [CrossRef]
- Pauly, D.; Munro, J.L. Once More on the Comparison of Growth in Fishes and Invertebrates. Fishbyte 1984, 2, 21. [Google Scholar]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 8th ed.; Iowa State University Press: Ames, IA, USA, 1996; ISBN 978-0-8138-1561-9. [Google Scholar]
- Sturges, H.A. The Choice of a Class Interval. J. Am. Stat. Assoc. 1926, 21, 65–66. [Google Scholar] [CrossRef]
- Gayanilo, F.C.; Sparre, P.; Pauly, D. FAO-ICLARM Stock Assessment Tools II: User’s Guide; FAO Computerized Information Series Fisheries; Rev. Version; Worldfish Center: Rome, Italy, 2005; ISBN 978-92-5-105300-3. [Google Scholar]
- Pauly, D. On the Interrelationships between Natural Mortality, Growth Parameters, and Mean Environmental Temperature in 175 Fish Stocks. ICES J. Mar. Sci. 1980, 39, 175–192. [Google Scholar] [CrossRef]
- Wu, B.; Fang, C.L.; Zhang, Y.P.; Fu, P.F.; Chen, W.J.; Xiong, X.Y.; Zhou, H.M.; He, G.; Wang, S.; Wang, Q.P. The Assessment of Biological Parameters and Stock Biomass of Siniperca Chuatsi in the Poyang Lake. Prog. Fish. Sci. 2015, 36, 21–26. [Google Scholar]
- Chen, M.; Zeng, S.; Jiang, B.; Wen, Z.; Wu, J.; Xia, J. The Comprehensive Evaluation of How Water Level Fluctuation and Temperature Change Affect Vegetation Cover Variations at a Lake of Ecological Importance (Poyang Lake), China. Ecol. Indic. 2023, 148, 110041. [Google Scholar] [CrossRef]
- Tian, M.; Mao, J.; Wang, K.; Xu, D. Spatio-Temporal Heterogeneity of Ecological Water Level in Poyang Lake, China. Ecol. Inform. 2024, 82, 102694. [Google Scholar] [CrossRef]
- Peng, Y.; Chen, G.; Chao, N.; Wang, Z.; Wu, T.; Luo, X. Detection of Extreme Hydrological Droughts in the Poyang Lake Basin during 2021–2022 Using GNSS-Derived Daily Terrestrial Water Storage Anomalies. Sci. Total Environ. 2024, 919, 170875. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Zhong, J.; You, Q.; Fang, C.; Hu, Q.; Liang, J.; He, J.; Yang, W. Land Use Modeling and Habitat Quality Assessment under Climate Scenarios: A Case Study of the Poyang Lake Basin. Ecol. Indic. 2025, 172, 113292. [Google Scholar] [CrossRef]
- Gulland, J.A. Fish Stock Assessment: A Manual of Basic Methods; FAO/Wiley Series on Food and Agriculture; Wiley: Chichester, NY, USA, 1983; ISBN 978-0-471-90027-6. [Google Scholar]
- Beverton, R.J.H. Patterns of Reproductive Strategy Parameters in Some Marine Teleost Fishes. J. Fish Biol. 1992, 41, 137–160. [Google Scholar] [CrossRef]
- Froese, R. Keep It Simple: Three Indicators to Deal with Overfishing. Fish Fish. 2004, 5, 86–91. [Google Scholar] [CrossRef]
- Herrón, P.; Mildenberger, T.K.; Díaz, J.M.; Wolff, M. Assessment of the Stock Status of Small-Scale and Multi-Gear Fisheries Resources in the Tropical Eastern Pacific Region. Reg. Stud. Mar. Sci. 2018, 24, 311–323. [Google Scholar] [CrossRef]
- Cope, J.M.; Punt, A.E. Length-Based Reference Points for Data-Limited Situations: Applications and Restrictions. Mar. Coast. Fish. 2009, 1, 169–186. [Google Scholar] [CrossRef]
- Beverton, R.J.H.; Holt, S.J. On the Dynamics of Exploited Fish Populations; Springer: Dordrecht, The Netherlands, 1993; ISBN 978-94-010-4934-4. [Google Scholar]
- Gheshlaghi, P.; Vahabnezhad, A.; Taghavi Motlagh, S.A. Growth Parameters, Mortality Rates, Yield per Recruit, Biomass, and MSY of Rutilus Frisii Kutum, Using Length Frequency Analysis in the Southern Parts of the Caspian Sea. Iran. J. Fish. Sci. 2012, 11, 48–62. [Google Scholar]
- Ohlberger, J.; Langangen, Ø.; Stige, L.C. Age Structure Affects Population Productivity in an Exploited Fish Species. Ecol. Appl. 2022, 32, e2614. [Google Scholar] [CrossRef] [PubMed]
- Hixon, M.A.; Johnson, D.W.; Sogard, S.M. BOFFFFs: On the Importance of Conserving Old-Growth Age Structure in Fishery Populations. ICES J. Mar. Sci. 2014, 71, 2171–2185. [Google Scholar] [CrossRef]
- Pauly, D.; Christensen, V.; Guénette, S.; Pitcher, T.J.; Sumaila, U.R.; Walters, C.J.; Watson, R.; Zeller, D. Towards Sustainability in World Fisheries. Nature 2002, 418, 689–695. [Google Scholar] [CrossRef]
- Abecasis, D.; Ogden, R.; Winkler, A.C.; Gandra, M.; Khallahi, B.; Diallo, M.; Cabrera-Castro, R.; Weiller, Y.; Erzini, K.; Afonso, P.; et al. Multidisciplinary Estimates of Connectivity and Population Structure Suggest the Use of Multiple Units for the Conservation and Management of Meagre, Argyrosomus regius. Sci. Rep. 2024, 14, 873. [Google Scholar] [CrossRef] [PubMed]
- Pikitch, E.K.; Santora, C.; Babcock, E.A.; Bakun, A.; Bonfil, R.; Conover, D.O.; Dayton, P.; Doukakis, P.; Fluharty, D.; Heneman, B.; et al. Ecosystem-Based Fishery Management. Science 2004, 305, 346–347. [Google Scholar] [CrossRef]
- Miranda, L.E. Fish Size Structure Analysis via Ordination: A Visualization Aid. N. Am. J. Fish. Manag. 2024, 44, 763–775. [Google Scholar] [CrossRef]
- Barua, S.; Liu, Q.; Rabby, A.F.; Al-Mamun, M.A.; Chen, X.; Sultana, R.; Baloch, A. Stock Appraisal for Atlantic Tripletail (Lobotes surinamensis; Bloch, 1790) in the Bay of Bengal, Bangladesh. J. Ocean. Limnol. 2024, 42, 1683–1694. [Google Scholar] [CrossRef]
- Barua, S.; Liu, Q.; Alam, M.S.; Kanak, M.K.; Ali, M.M. Population Dynamics and Stock Assessment of Red Snapper (Lutjanus johnii) in the Bay of Bengal Bangladesh Waters. Reg. Stud. Mar. Sci. 2023, 63, 102983. [Google Scholar] [CrossRef]
- Hard, J.J.; Gross, M.R.; Heino, M.; Hilborn, R.; Kope, R.G.; Law, R.; Reynolds, J.D. Evolutionary Consequences of Fishing and Their Implications for Salmon. Evol. Appl. 2008, 1, 388–408. [Google Scholar] [CrossRef]
- Hutchings, J.A. Life History Consequences of Overexploitation to Population Recovery in Northwest Atlantic Cod (Gadus morhua). Can. J. Fish. Aquat. Sci. 2005, 62, 824–832. [Google Scholar] [CrossRef]
- Clark, W.G.; Hare, S.R. Assessment and Management of Pacific Halibut: Data, Methods, and Policy; International Pacific Halibut Commission: Seattle, WA, USA, 2006. [Google Scholar]
- Kuparinen, A.; Merilä, J. Detecting and Managing Fisheries-Induced Evolution. Trends Ecol. Evol. 2007, 22, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.W.; Lu, W.K.; Li, M.Z.; Liu, H.Z. Evaluation of Fishing Ban Effect in the Poyang Lake Based on Fish Body Length Structure Analysis. Acta Hydrobiol. Sin. 2025, 49, 052502. [Google Scholar] [CrossRef]
- Quince, C.; Abrams, P.A.; Shuter, B.J.; Lester, N.P. Biphasic Growth in Fish I: Theoretical Foundations. J. Theor. Biol. 2008, 254, 197–206. [Google Scholar] [CrossRef]
- Feng, K.; Deng, W.; Li, H.; Guo, Q.; Tao, K.; Yuan, J.; Liu, J.; Li, Z.; Lek, S.; Hugueny, B.; et al. Direct and Indirect Effects of a Fishing Ban on Lacustrine Fish Community Do Not Result in a Full Recovery. J. Appl. Ecol. 2023, 60, 2210–2222. [Google Scholar] [CrossRef]
- Lester, S.; Halpern, B.; Grorud-Colvert, K.; Lubchenco, J.; Ruttenberg, B.; Gaines, S.; Airamé, S.; Warner, R. Biological Effects within No-Take Marine Reserves: A Global Synthesis. Mar. Ecol. Prog. Ser. 2009, 384, 33–46. [Google Scholar] [CrossRef]
- Pauly, D.; Christensen, V.; Dalsgaard, J.; Froese, R.; Torres, F. Fishing Down Marine Food Webs. Science 1998, 279, 860–863. [Google Scholar] [CrossRef]
- Sass, G.G.; Kitchell, J.F.; Carpenter, S.R.; Hrabik, T.R.; Marburg, A.E.; Turner, M.G. Fish Community and Food Web Responses to a Whole-Lake Removal of Coarse Woody Habitat. Fisheries 2006, 31, 321–330. [Google Scholar] [CrossRef]
- Cao, L.W.; Chen, X.L.; Zhao, J. Studies on Age and Growth of Megalobrama skolkovii in the Nanshui Reservoir. Fish. Sci. 2001, 20, 11–14. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Song, W.; Zhu, D.M.; Ren, L.; Wang, W.M. Age and Growth Characteristic of Megalobrama skolkovii in Beijiang River. Freshw. Fish. 2013, 43, 35–39. [Google Scholar] [CrossRef]
- Zhu, S.L.; Li, X.H.; Li, Y.F.; Liu, Y.Q.; Wu, Z.; Xia, Y.G.; Li, J. Population Structure and Growth Differences of Megalobrama terminalis from Different Water Areas in Middle and Lower Reaches of Pearl River. J. Huazhong Agric. Univ. 2023, 42, 75–81. [Google Scholar] [CrossRef]
- He, M.F.; Li, X.H.; Tan, X.C.; Li, J.; Wang, C.; Luo, J.R.; Lin, J.Z. Age Determination and the Growth of Megalobrama hoffmanni in Xijiang River. Freshw. Fish. 2007, 37, 54–58. [Google Scholar] [CrossRef]
- Shao, X.Y. Research on the Legal System for Sustainable Development of Aquatic Species in the Yangtze River under the Background of the Yangtze River Protection Law. OJLS 2024, 12, 1359–1367. [Google Scholar] [CrossRef]
- Amezcua, F.; Madrid-Vera, J.; Aguirre, H. Incidental Capture of Juvenile Fish from an Artisanal Fishery in a Coastal Lagoon in the Gulf of California. N. Am. J. Fish. Manag. 2009, 29, 245–255. [Google Scholar] [CrossRef]
- Shalehin, M.S.; Parvez, M.T.; Lucas, M.C.; Galib, S.M. A Case Study of Illegal Fishing Causes during Seasonal Fishery Closure in Kaptai Lake, Bangladesh. Fish. Manag. Ecol. 2022, 29, 542–551. [Google Scholar] [CrossRef]
- Martins, G.M.; Jenkins, S.R.; Hawkins, S.J.; Neto, A.I.; Medeiros, A.R.; Thompson, R.C. Illegal Harvesting Affects the Success of Fishing Closure Areas. J. Mar. Biol. Assoc. UK 2011, 91, 929–937. [Google Scholar] [CrossRef]
- Hilborn, R.; Walters, C.J. Quantitative Fisheries Stock Assessment; Springer: Boston, MA, USA, 1992; ISBN 978-1-4020-1845-9. [Google Scholar]
- Agnew, D.J.; Pearce, J.; Pramod, G.; Peatman, T.; Watson, R.; Beddington, J.R.; Pitcher, T.J. Estimating the Worldwide Extent of Illegal Fishing. PLoS ONE 2009, 4, e4570. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.S.; Liu, Q.; Nabi, M.R.-U.; Chowdhury, M.Z.R.; Duc-Hieu, N.T. Length-Based Indicators for the Sustainability of Small-Scale Fisheries in the Northern Bay of Bengal Coast, Bangladesh. Reg. Stud. Mar. Sci. 2022, 51, 102177. [Google Scholar] [CrossRef]
- Kell, L.T.; Minto, C.; Gerritsen, H.D. Evaluation of the Skill of Length-Based Indicators to Identify Stock Status and Trends. ICES J. Mar. Sci. 2022, 79, 1202–1216. [Google Scholar] [CrossRef]
- Vasilakopoulos, P.; O’Neill, F.G.; Marshall, C.T. Misspent Youth: Does Catching Immature Fish Affect Fisheries Sustainability? ICES J. Mar. Sci. 2011, 68, 1525–1534. [Google Scholar] [CrossRef]
- Taylor, J.J.; Rytwinski, T.; Bennett, J.R.; Smokorowski, K.E.; Lapointe, N.W.R.; Janusz, R.; Clarke, K.; Tonn, B.; Walsh, J.C.; Cooke, S.J. The Effectiveness of Spawning Habitat Creation or Enhancement for Substrate-Spawning Temperate Fish: A Systematic Review. Environ. Evid. 2019, 8, 19. [Google Scholar] [CrossRef]
- Pomeroy, R.S.; Berkes, F. Two to Tango: The Role of Government in Fisheries Co-Management. Mar. Policy 1997, 21, 465–480. [Google Scholar] [CrossRef]
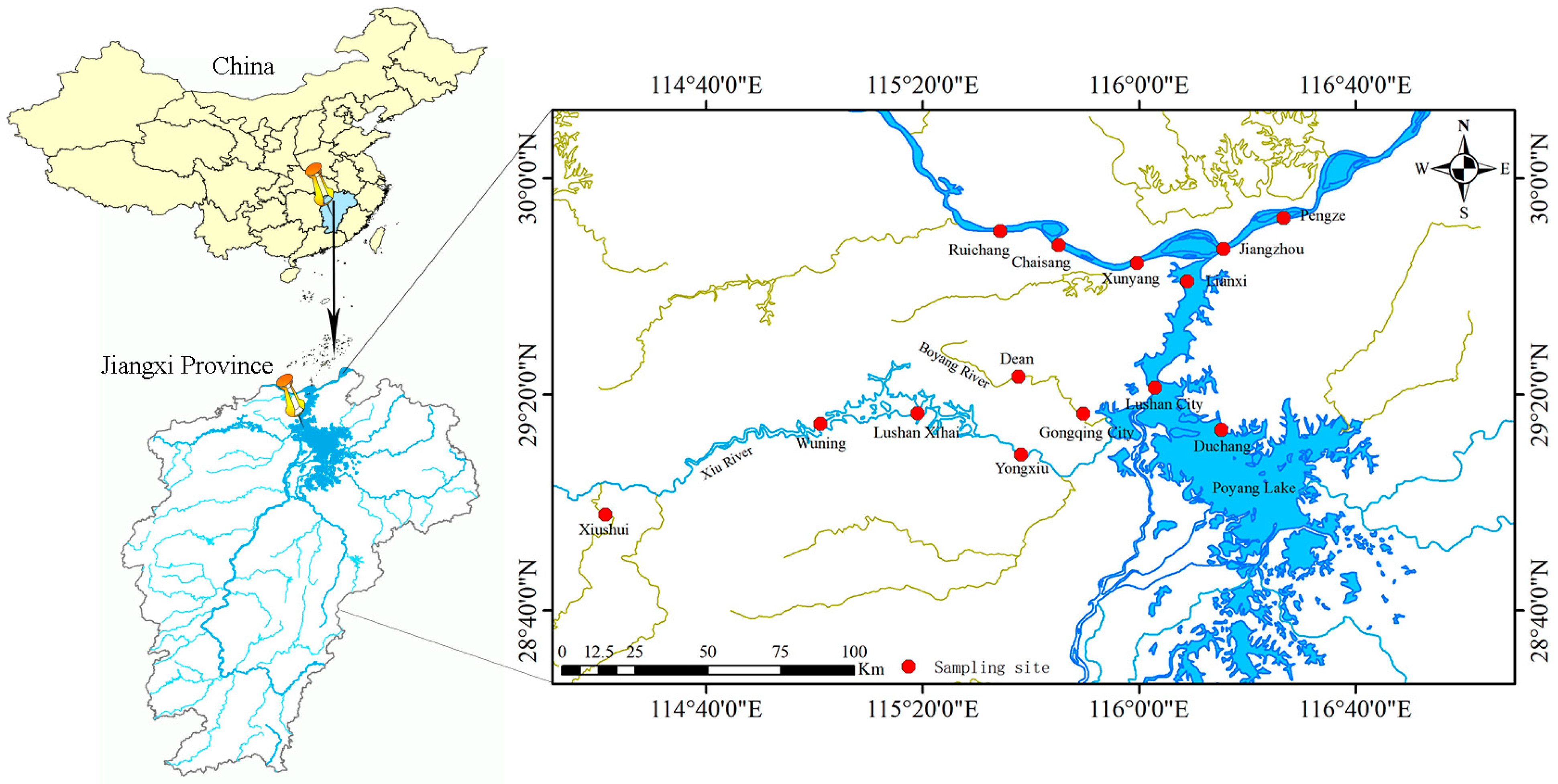

| Category | Subcategory | Sample Size (n) |
|---|---|---|
| Season | Spring | 312 |
| Summer | 496 | |
| Autumn | 789 | |
| Region | Jiangxi section of the Yangtze River mainstem | 699 |
| Northern Poyang Lake | 706 | |
| Xiu River | 79 | |
| Boyang River | 113 | |
| Total | \ | 1597 |
| Lm (cm) | Lopt (cm) | Pmat | Popt | Pmega | Pobj | Stock Condition | Probability of BeingSB < RP |
|---|---|---|---|---|---|---|---|
| 25.53 | 37.78 | 46.05% | 9.51% | 6.88% | 62.44% | SB > RP | 4% for TRP and 2% for LRP |
| Year | Standard Length Range (cm) | Mean Standard Length ± SD (cm) |
|---|---|---|
| 2017–2019 | 4.1–78.9 | 17.8 ± 10.9 |
| 2021–2022 | 5.8–56.3 | 20.0 ± 6.3 |
| 2024 | 3.7–53.5 | 20.6 ± 11.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Xu, Q.; Zhang, B.; Kong, C.; Fang, L.; Gao, X.; Sun, L.; Li, L.; Gong, X. An Assessment of the Population Structure and Stock Dynamics of Megalobrama skolkovii During the Early Phase of the Fishing Ban in the Poyang Lake Basin. Fishes 2025, 10, 378. https://doi.org/10.3390/fishes10080378
Huang X, Xu Q, Zhang B, Kong C, Fang L, Gao X, Sun L, Li L, Gong X. An Assessment of the Population Structure and Stock Dynamics of Megalobrama skolkovii During the Early Phase of the Fishing Ban in the Poyang Lake Basin. Fishes. 2025; 10(8):378. https://doi.org/10.3390/fishes10080378
Chicago/Turabian StyleHuang, Xinwen, Qun Xu, Bao Zhang, Chiping Kong, Lei Fang, Xiaoping Gao, Leyi Sun, Lekang Li, and Xiaoling Gong. 2025. "An Assessment of the Population Structure and Stock Dynamics of Megalobrama skolkovii During the Early Phase of the Fishing Ban in the Poyang Lake Basin" Fishes 10, no. 8: 378. https://doi.org/10.3390/fishes10080378
APA StyleHuang, X., Xu, Q., Zhang, B., Kong, C., Fang, L., Gao, X., Sun, L., Li, L., & Gong, X. (2025). An Assessment of the Population Structure and Stock Dynamics of Megalobrama skolkovii During the Early Phase of the Fishing Ban in the Poyang Lake Basin. Fishes, 10(8), 378. https://doi.org/10.3390/fishes10080378






