1. Introduction
With the intensive development of aquaculture worldwide, the consumption of aquaculture products is increasing. To meet the needs of the population, more intensive aquaculture production technologies are being developed, growing products at high planting densities, which in turn leads to a deterioration of production ecology and the occurrence of hydrobiont diseases. Previously, antibiotics were intensively used to treat hydrobionts, leading to resistance of pathogenic bacteria. At the present stage, various preparations with probiotic properties have started to be used in aquaculture [
1,
2,
3].
The European Union has strict regulations prohibiting or minimizing the use of antibiotics in aquaculture. One of the modern approaches to organizing sustainable and biologically safe aquaculture is the use of probiotics as an alternative to antibiotics, which can be used to treat and prevent infectious diseases, improve microbial balance in the aquatic environment, stimulate growth, strengthen the immune system, and balance microorganisms in the gut while displacing pathogens.
Long-term experience of modern scientific research on the use of probiotics in aquaculture indicates the possibility of selecting probiotic cultures that not only suppress pathogenic microorganisms but also raise the overall resistance of the organism or hydrobiont, increase growth and the survival rate, and improve the quality of the obtained products.
The use of probiotic immunostimulating preparations, new effective probiotics, prebiotics, metabiotics, and phytobiotics is one of the promising directions in aquaculture feed production to increase productivity or improve the health of farmed hydrobionts. Probiotic preparations including phytocomponents are of particular interest; they are characterized by increased biological activity when combining the probiotic effect with the presence of metabolites, as well as the action of biologically active phytobiotic components [
2].
Large studies on the use of new effective probiotics and phytobiotics as a promising direction for the provision of feed additives to aquaculture enterprises have been conducted by Russian scientists on various species of fish [
2,
4].
The ProStor and HerbaStor preparations are characterized by the presence of phytoparticles of medicinal plants and represent a new class of biologics in the market of probiotics for aquaculture [
5].
At present, probiotic preparations obtained using less energy-intensive technological methods are entering the modern market.
Long-term experience of scientific research has shown that products with high titers of probiotic microorganisms can be obtained using less energy-intensive technological solutions. The use of microbial growth in biofilms is a key biotechnological technique that determines the possibility of abandoning energy- and material-intensive elements of technology, switching to the use of available and cheap raw materials, as well as achieving a high economic performance at small production scales and high technological flexibility. These advantages are determined by the fact that biofilms are a natural form of existence of microorganisms [
6].
Our earlier studies on the use of probiotic supplement B-1895, obtained by the method of two-phase fermentation on biofilms in feeds for juvenile trout, revealed its positive effect in increasing the growth, survival rate, and body weight of fish. The use of a probiotic provided the normal physiological conditions of young trout when reared under industrial conditions [
7].
Currently, there is a need for additional research to determine the effectiveness of introducing new probiotic biopreparations into feeds for steelhead salmon (Salmo gairdneri), testing novel probiotic cultures with different enzymatic and antioxidant actions, in order to develop environmentally friendly products for sustainable aquaculture.
2. Materials and Methods
2.1. Experimental Conditions
Juvenile steelhead salmon (Salmo gairdneri, Richardson, 1836) with an average weight of 1.04–1.13 g were used for the experiments. The juveniles were seeded in pools of 4 m3 volume into 3 groups of 1000 fish each. Two variants of feed with different probiotic additives were tested using two multistrain preparations developed during previous research work. Both preparations were obtained by solid-phase fermentation of soybean. In the course of the experiment, the main physicochemical parameters of the aquatic environment were controlled: temperature, oxygen saturation, pH, CO2, and nitrite and nitrate nitrogen. During the rearing of juveniles, the hydrochemical parameters were within normal limits: water temperature—11.26–11.29 °C, oxygen content—10.06–10.14 mg/L, and pH—7.44–7.75. Water hardness was 5–7 °H, nitrites were less than 0.3 mg/L, carbon dioxide was no more than 6 mg/L, ammonium nitrogen was 0–0.25 mg/L, and there were no nitrates.
2.2. Preparation of Probiotics and Experimental Feeds
The compound feed was produced on a laboratory feed mill. The protein content was 47%, and the fat content was 24%. The mixed fodder contained the following components: wheat, pork meat meal, shrimp meal, fish meal, chicken meat-bone meal, blood meal, corn gluten, wheat gluten, protein concentrate “Malt Pro”, pea protein, soy protein concentrate, premix (fry), probiotic preparation, refined rapeseed oil, and fish oil.
All the experimental probiotics that formed the basis of the biopreparations used to feed the experimental groups were isolated from the sediments of the lower reaches of the Don River (Rostov Region, Russia). Consequently, all of them were inherently adapted to an aquatic environment and low oxygen levels, and they had also been in contact with fish and fish microbiota.
Experimental group 1 received a biopreparation based on the probiotic bacteria B. velezensis MT14 and B. velezensis MT42. Both strains were selected for their high exoenzymatic activity, namely proteolytic, amylolytic, and cellulolytic.
Experimental group 2 received a biopreparation based on the probiotic bacteria B. subtilis MT48 and B. subtilis MT74. Both strains, in addition to their high exoenzymatic activity (proteolytic and amylolytic), also possessed high antioxidant activity due to the production of pulcherriminic acid.
The preparations were produced by solid-state fermentation of soybeans, followed by drying. The final bacillus content in the feed for both experimental groups was 4.0 × 106 CFU/g.
The control feed contained industrial probiotic Clostat (Toekomstlaan 42 2200 Herentals, Belgium), and the final bacterial content in the feed was 1.0–107 CFU/g.
Feeding and daily growth rates were calculated based on water temperatures and fish weight.
2.3. Analysis of Growth and Survival Rates
Standard methods [
8] and equipment were used to estimate size-mass biological and functional parameters of juvenile fish. The functional state of fish was analyzed after stable adaptation of juvenile fish to the growing conditions.
Growth, survival rate, feed consumption per unit of growth, and biochemical parameters reflecting the functional state of the organism were determined in the studied objects.
Changes in the weight of the objects were determined according to the data of control weighing. The sample size was 50 specimens in each variant.
Absolute growth was calculated according to the following formula:
where
P—absolute growth, g;
Wf—fish weight at the end of the experiment, g; and
Ws—fish weight at the beginning of the experiment, g.
Average daily gain was calculated by the following formula:
where
P average daily—average daily growth rate; g;
Wf—weight of fish at the end of the experiment, g;
Ws—weight of fish at the beginning of the experiment, g; and
t—duration of the experiment, days.
Average daily growth rate was determined by the following formula:
where A—average daily growth rate, %;
Wf—weight of fish at the end of the experiment, g;
Ws—weight of fish at the beginning of the experiment, g; and
t—duration of the experiment, days.
Specific growth rate was calculated by the following formula:
where
Cw—specific growth rate, %;
Wf—fish mass at the end of the experiment, g;
Ws—fish mass at the beginning of the experiment, g; and
t—duration of the experiment, days.
The mass accumulation coefficient was determined by Formula (5):
where
Km—total production coefficient of growth rate;
Wf—weight of fish at the end of the experiment, g;
Ws—weight of fish at the beginning of the experiment, g; and
t—duration of the experiment, days.
Feed inputs per unit of growth (Zk):
where Ck—the amount of feed spent on growing for the period of the experiment, mg (g);
Mk—fish weight at the end of the experiment, g;
Mn—fish weight at the beginning of the experiment, g.
The survival rate was calculated by counting dead juveniles daily.
2.4. Biochemical Analysis
Sampling and biochemical analysis (protein, fat, ash) of the fish bodies were carried out according to generally accepted methods (GOST ISO 5983-2-2016, Part 2; GOST 13496.15-2016 p. 10; GOST 26226-95 p. 1; GOST 32045, method A) [
9,
10,
11,
12]. Analysis of the chemical compositions of the bodies of the studied fish was carried out in a specialized laboratory: fat content—by the extraction method in a Soxhlet apparatus, protein content—by the Kjeldahl method, and ash—by burning in a muffle furnace.
2.5. Juvenile Fish Testing
To assess the behavioral reactions of fish in a minimally structured environment, the “open field” technique was used. The test is designed to characterize the reactivity of the central nervous system of fish. Young fish were evaluated by their reaction to external stimuli. The magnitude of motor activity was determined by the average number of fish crossing the lines of the coordinate grid drawn on the bottom of the experimental setup. In this study, we used a tank with a diameter of 55 cm, a water layer of 20 cm, and a size of grid squares—10 cm × 10 cm [
13].
The average number of crossings of coordinate lines after placing fish in a new environment characterizes their orientation activity (OA, units/min). Adaptation of juvenile sturgeon fish to a new environment takes no more than 3 min, after which their motor activity reaches a relative plateau. Accordingly, the average number of crossings of coordinate lines of each fish during the period from 4 to 7 min of the experiment was considered the background motor activity (FA, units/min). In 7 min after the beginning of the experiment, the stimulus imitating a low-frequency sound signal (impact on the vessel wall), the average frequencies of which lie in the range of 20–150 Hz, which is perceived by the lateral line of fish, was applied. The number of crossings of the grid lines per individual for a period of 30 s was recorded. Afterwards, the reactivity of fish to this stimulus was determined (P1, units/min). At the 9th minute, a light with a power of 250–300 lux was switched on above the setup, recording the reaction of fish to the short-term light stimulus (P2, units/min). After 10 min, a light with a power of 250–300 lux was switched on above the experimental chamber and the reaction of fish to a constant light stimulus was evaluated (P3, units/min). Illumination was measured using a luxmeter. The last stimulus has an effect on the visual lobes of the brain. Ten individuals each from the experimental and control groups were tested at stages after the experiments were completed.
To determine the viability of young fish, we used the method of functional loads [
14], testing using thermoresistance (25 °C) and salt tolerance (18‰). To investigate the thermoresistance of juvenile steelhead salmon fry, a 25 L volume tank filled with water was used and a batch of 10 fry was deposited.
After that, we measured the initial water temperature, performed active reaction of the medium, and determined the oxygen concentration using an oximeter; i.e., the main physicochemical parameters of the medium were controlled. To avoid various kinds of artifacts, fry before the experiment were kept in water, which would be used to fill the fish tank. The fry were not fed for 18–24 h before the experiment. After planting in the fish tank, the fry were incubated for 1–2 h, after which the water heater was turned on. The rate of temperature increase to a sublethal 25 °C was kept within 1 °C/h.
To study the salt tolerance of salmonids at early stages of their development, an artificially prepared salt solution with a salinity of 18‰ was used. A fish breeding tank with a volume of 25 L was used as a vessel.
Compressor units with air atomizers were used to maintain a normal oxygen saturation.
When juveniles were kept at a high temperature and high salinity concentration, their behavior was observed, and the time and number of deaths were recorded.
2.6. Determination of Probiotic Strains in Fish Intestinal Contents
The intestines of the fish were extracted and transported on ice to the laboratory. Then, the intestinal contents were extracted under aseptic conditions with sterile scissors and spatulas and placed in sterile microtubes. An average sample was prepared: equal amounts of intestinal contents from five fish were thoroughly mixed with a sterile glass rod or plastic microbiological loop, then mixed with a sterile physiological solution at a rate of 1:10. Then, a series of serial decimal dilutions were prepared. Seeding was performed on solid nutrient medium LB (Luria Bertani) twice, before and after pasteurization. The suspension was pasteurized at 95 °C for 5 min. The dishes were incubated at 25 °C for 48 h and then counted.
Colonies of probiotic strains were identified by morphological traits based on the fact that colony morphology is diverse, often individualized and genetically determined [
15,
16,
17,
18].
Laboratory dishes with the respective probiotic strains in the form of single colonies were used in this study. On cups seeded from the suspension before pasteurization, the total number of bacilli in the intestinal contents was counted. Only colonies in spore form were counted on the dishes sown from the pasteurized suspension. According to the difference between the numbers obtained, the number of bacilli in vegetative form was obtained.
2.7. Statistical Analysis
The results obtained during this study were subjected to statistical processing using Statistica and Excel software. If the data met the criteria of a normal distribution, the parametric Student’s test was used to evaluate differences between mean values. If the distribution was not normal, the nonparametric Mann–Whitney test was used, which ensured the correctness and reliability of statistical analysis.
3. Results and Discussion
The use of probiotic supplements in aquaculture plays a key role in maintaining the health and optimal physiological condition of fish. These supplements, containing live bacteria and their metabolites, are introduced directly into the feed, which allows for a multifaceted positive effect on the microflora of the gastrointestinal tract of fish and their metabolic processes. This approach contributes to the normalization of intestinal microbiocenosis, which is an important factor for maintaining fish health.
According to recent studies, Russia has already developed considerable experience in the use of probiotic supplements in the process of growing various species of fish. This experience shows positive results, which confirms the effectiveness of probiotics in aquaculture. Probiotics not only improve digestion and help to reduce the level of pathogenic bacteria in the aquatic environment but also increase the overall biological value of fish. This makes fish more resilient and better adapted to their environment, which in turn increases their survival rate [
2].
Hydrochemical parameters during the juvenile rearing period were in line with normative values. The water temperature averaged 11.26–11.29 °C, oxygen content was 10.06–10.14 mg/L, and pH was 7.44–7.75. Hydrochemical indicators of water corresponded to normative ones: water hardness 5–7 H0, nitrite less than 0.3 mg/L, carbon dioxide content no more than 6 mg/L, ammonium nitrogen 0–0.25 mg/L, and nitrates absent.
The results of this study of probiotics’ influence revealed that the experimental groups consuming feed with probiotic supplements had a better growth performance.
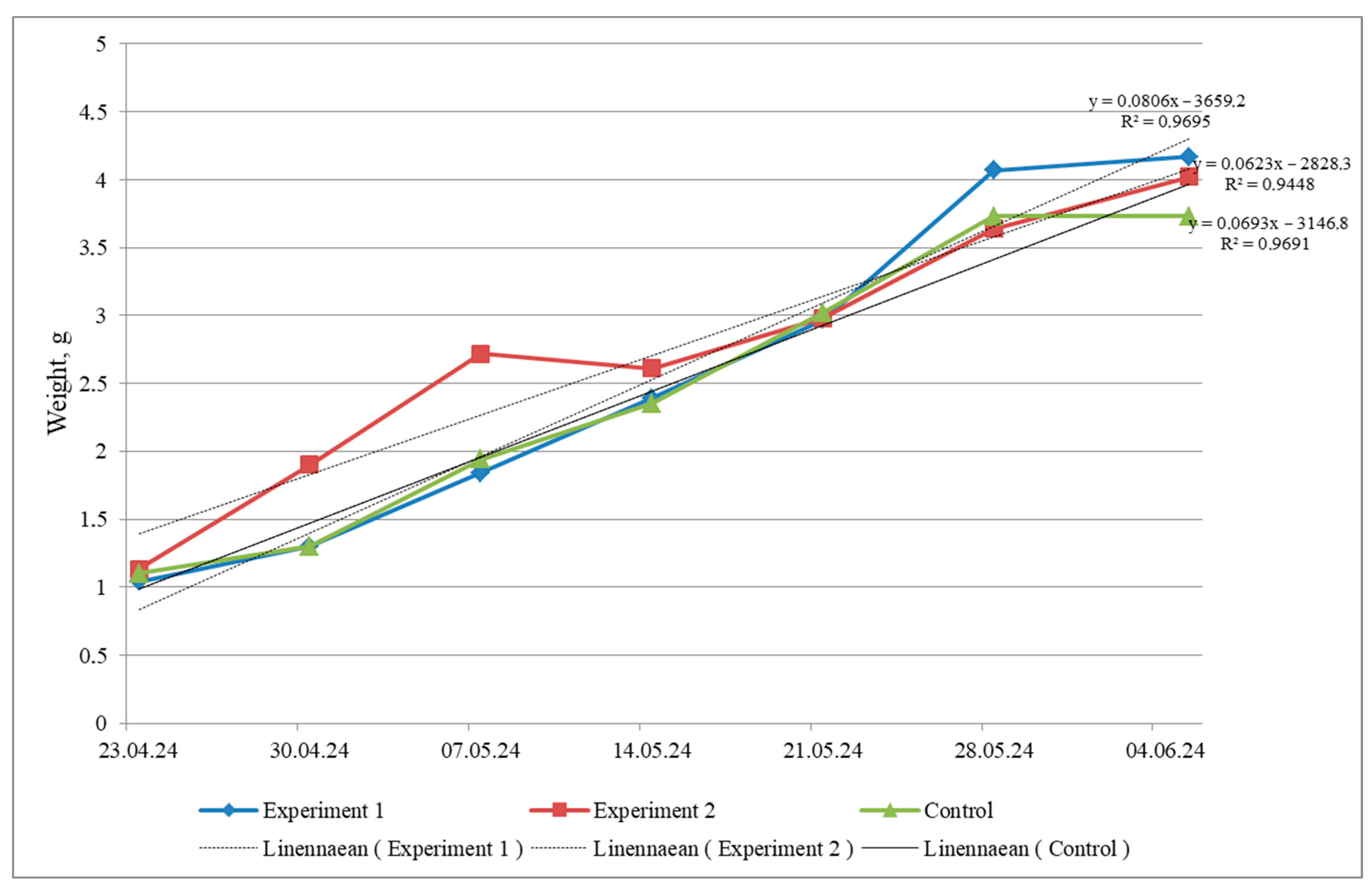
According to the studies, a statistically significant difference (
p < 0.01) in fish weight was found between the control group and Experiment 1. The weight of fish in variant 1 was significantly higher by 10.55% and amounted to 4.17 g. In the second variant, the weight of fish at the end of the rearing period was higher by 7.21% compared to the control group and amounted to 4.02 g (
p < 0.05) (
Table 1).
Absolute and average daily growth rates were higher by 9–16%, and mass accumulation ratios by 10.25% and 12.5% in the experimental variants, respectively, with lower feed inputs of 1.2–1.3 units.
Fish during the experiment grew unevenly in all variants (
Figure 1). At the end of rearing, juveniles in the experimental variants showed greater growth, whereas in the control, the weight did not change.
Figure 1 shows that the approximation reliability coefficient, which reflects the degree of correspondence of the trend model with the initial data in the control and experimental variants, approached the value of 1, which indicates that the model accurately describes the available data.
The probiotics included in the biopreparations for the experimental groups exhibit high exoenzymatic activity: proteolytic, amylolytic, and cellulolytic. The enzymes they produce aid in digestion and facilitate the assimilation of feed components. Due to increased access to nutrients, the fish’s body is able to grow and develop faster, which is evident from the presented data.
Along with studies of growth and mass accumulation indices, biochemical characteristics of fry were analyzed (
Table 2).
Analysis of data from
Table 2 shows that juvenile fish from the control variant differed from the experimental variant by a lower body fat content of 1.73% and protein content of 7.13–5.47%, indicating a better energy status and potential growth ability. All groups had similar ash contents, indicating the stability of the mineral composition with different probiotic additives.
The growing conditions of hydrobionts play a key role in the formation of their physiological state and adaptive abilities. The importance of these conditions is especially manifested during the transition of juveniles from artificial to natural water bodies, where they face completely different ecological characteristics. For this reason, attention is focused on conditioned-reflex techniques that allow us to assess how well fish are able to acquire and retain behavioral skills necessary for successful adaptation to habitat conditions.
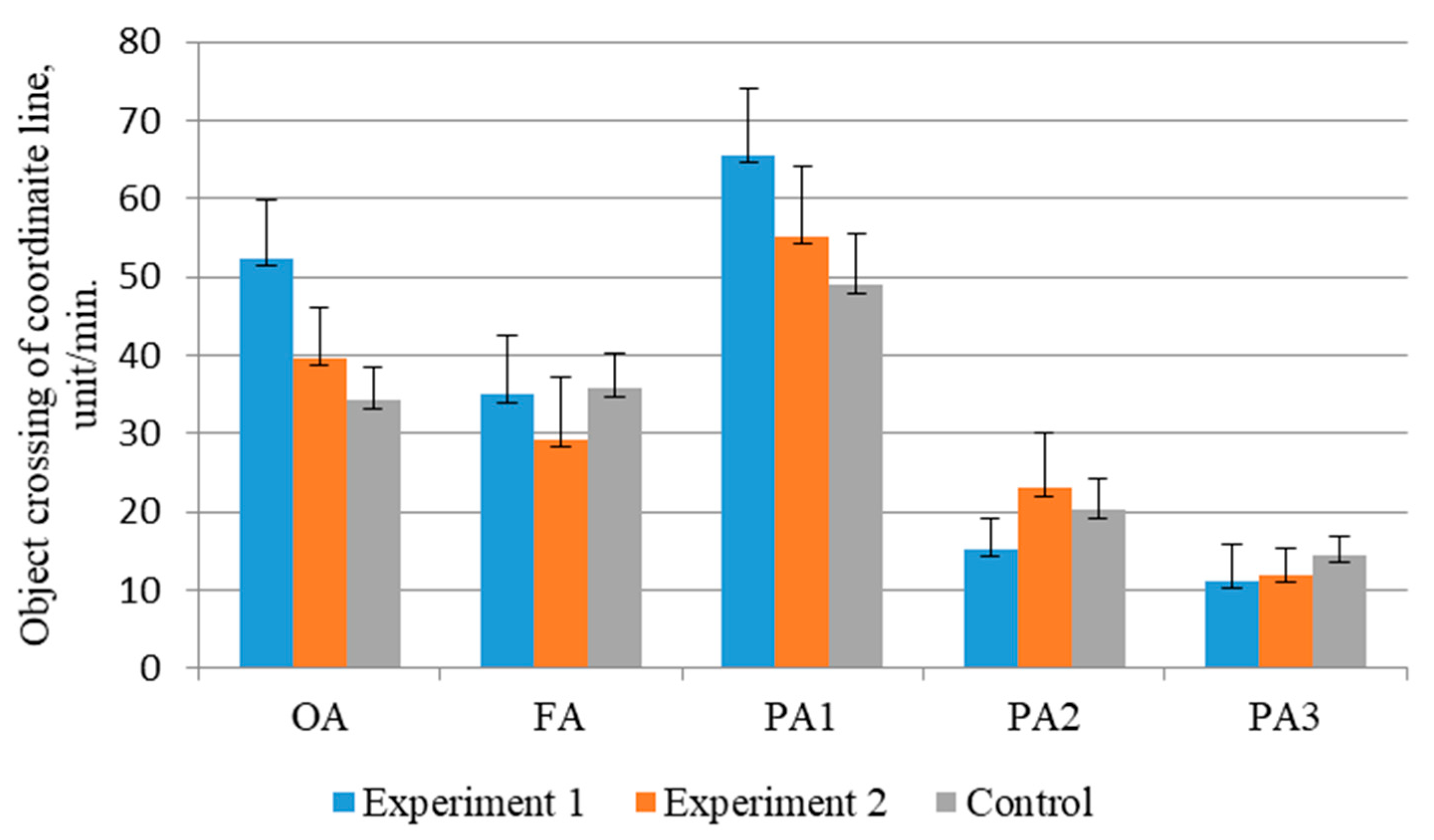
In the “open field” test in juvenile steelhead salmon from the first variant, it was found that the orienting activity was significantly (
p ≤ 0.05) higher than in the control group, by 34.68%, and also 24.43% higher than in the second variant of rearing, amounting to 54.2 units/min (
Figure 2). Fish from the experimental groups further showed a decrease in activity by 33.3% and 26.06%, and the background level was 29–35 units/min. In the control group of juvenile fish, this index was highest, and instead of stabilizing, it increased by 4.29%.
When exposed to low-frequency stimulus, motor activity increased compared to the background level in all groups, but the reaction to a nonspecific vibroacoustic stimulus was the most pronounced (more than 46%) in the experimental variants. In control fish, the reaction was more inhibited and increased by 26.99% in relation to the background activity. The reaction to a short-term light stimulus in juvenile fish was less intense compared to the first exposure, i.e., it had the character of inhibition. The activity of young fish decreased 4.3 times in the first variant and 2.4 times in the second and control groups. Under prolonged exposure to the light stimulus, individuals from the experimental groups showed less activity. Clearly, juveniles in variants 1 and 2 adapted to the new environment more easily.
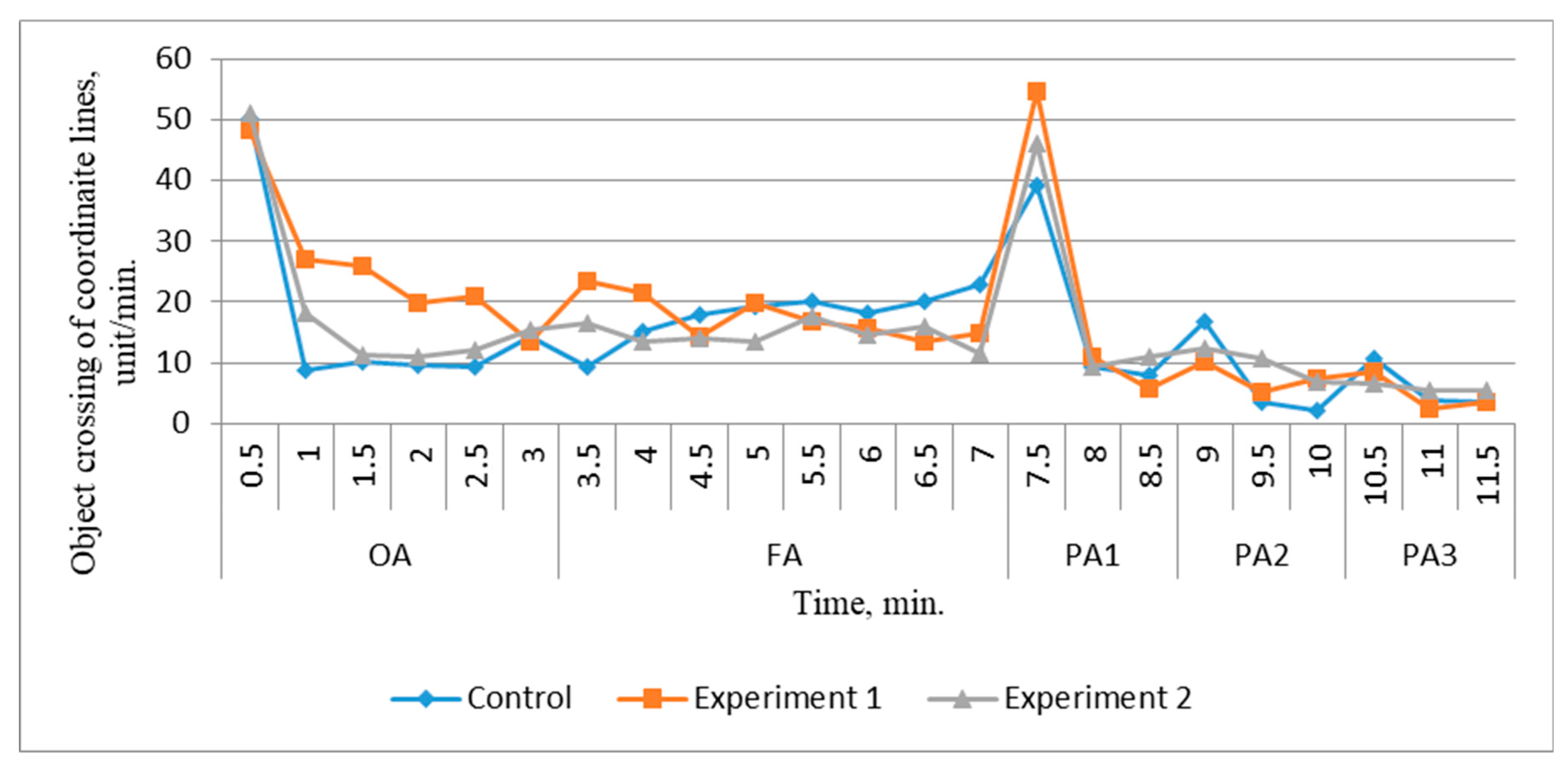
In the course of analyzing the dynamics of reactions to stimuli (
Figure 3), it was found that juveniles of the control group, initially showing a similar motor reaction to the experimental groups, after 30 s in the new environment, sharply decreased their activity by 5.7 times. At the same time, juveniles of the experimental groups, placed in similar conditions, showed a gradual normalization of motor activity, indicating the process of adaptation to new conditions.
By the end of the third minute, all groups showed a stabilization of the condition, and the level of motor activity was 13–15 units/min. In the next time interval (4–7 min), juveniles of the experimental group showed slight fluctuations in activity, indicating their habituation to the experimental setup, while juveniles of the control group showed an increase in motor activity.
After a significant response to the sound stimulus, all groups of juveniles showed a marked decrease in motility to 5–8 units/min. The juveniles of the experimental groups did not show a pronounced reaction to light stimuli, which indicates their ability to adapt more quickly to the changed conditions. The reaction of juveniles of the control group to visual stimuli was more pronounced.
The results of the “open field” test showed differences in the reactivity of the central nervous system between the experimental and control groups. Juveniles from the experimental groups showed more intensive motor activity in response to the first stimulus, and with further influence on the CNS showed stress resistance, which may be important for their adaptation to artificial housing conditions. These observations emphasize the potential of probiotic additives in feed to improve the adaptive abilities of juvenile salmonids, which in turn may contribute to their more successful survival and development.
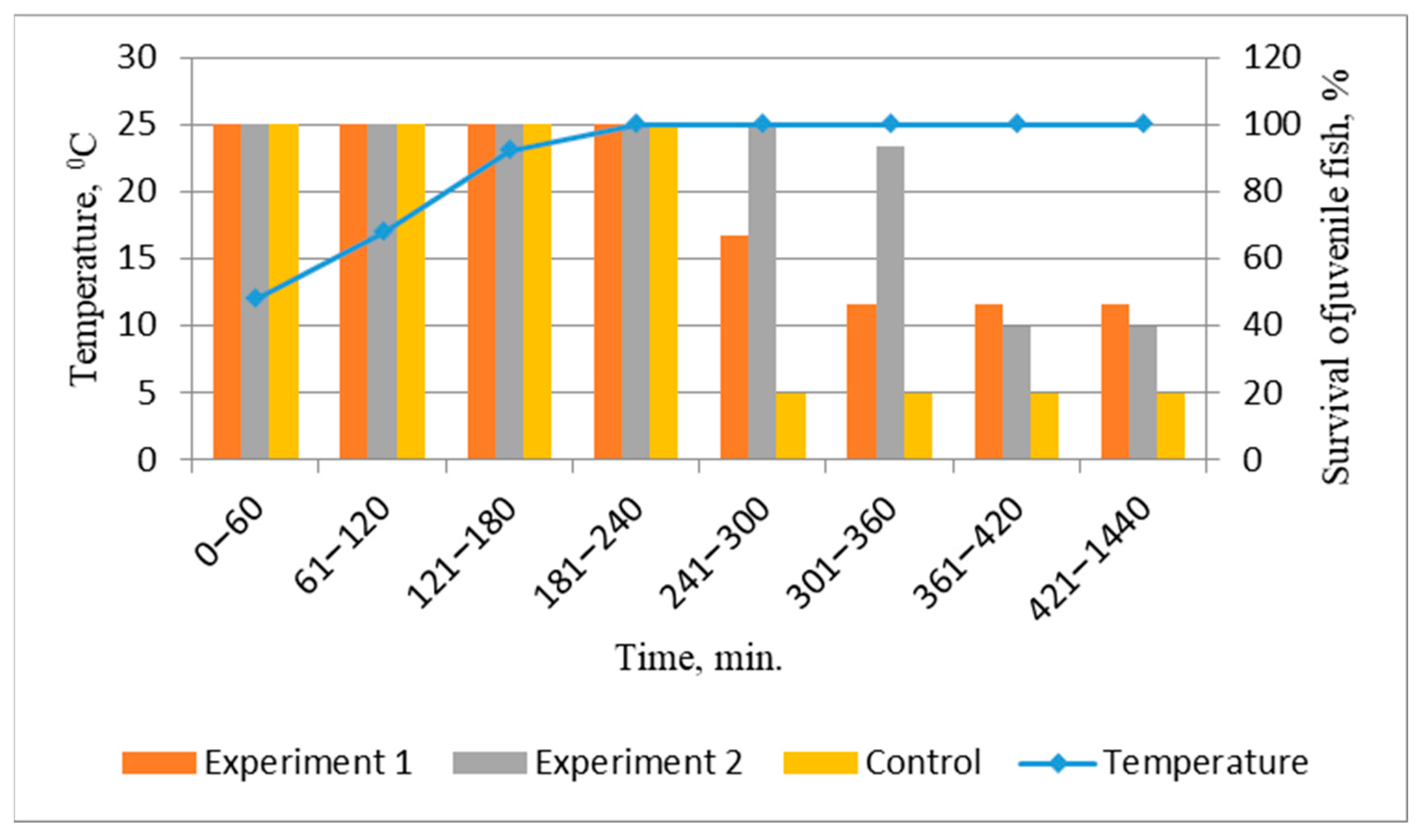
Studies conducted on the functional state of juvenile salmonids reared using probiotic supplemented feeds have shown their increased resistance to extreme aquatic environmental conditions. Special attention should be paid to the analysis of fish’s ability to survive at high water salinity reaching 18‰ and at sublethal temperatures of 25 °C. This test is important for determining the viability of steelhead salmon juveniles for artificial reproduction purposes when releasing juveniles into a natural habitat with a salinity of 18‰.
The thermal resistance of juvenile salmonids in the experimental variants was higher, so the average time of death of juveniles at a temperature of 25 °C was higher (variant 1—280.50 + 5.44 min, variant 2—373.22 + 7.40, control—263.83 + 2.58; differences are reliable at p ≤ 0.05). Observations showed that when placed in water with increasing temperature, locomotor activity gradually increased (phase 1). The fry then showed the maximum activity accompanied by rapid respiration (phase 2). Some individuals adapted to the new temperature, while those that subsequently died sunk to the bottom of the fish tank. They showed a complete suppression of locomotor activity and suppression of respiratory function, which manifested as an absence of movements of opercular (gill) lids, eventually leading to mortality (phase 3).
The juveniles from the experimental groups showed higher survival rates of 40.0–46.67%, which were 20–26.67% higher than the control group (
Figure 4). This indicates a good adaptation of juvenile fish fed with experimental probiotics to high temperatures. After completion of the 24 h experiment, some of the juveniles in all variants adapted to temperature, which was also observed in previous studies. In them, it was shown that juvenile steelhead salmon are able to survive and grow at temperatures above 20 °C (up to 28 °C) [
19], but mortality increases when the temperature rises to 25–26 °C [
20].
The results showed that juvenile fish that received probiotics throughout the rearing period had a better ability to adapt to such stressors than fish from the control group.
The study conducted overnight showed a high tolerance of juvenile steelhead salmon to a salinity of 18‰. It was found that in all experimental groups, the survival rate was 90.9%, indicating a sufficient osmoregulatory capacity of the juveniles. A mortality rate of 10.1% was observed 16 h after the start of the experiment. Earlier studies have shown that during gradual acclimatization in salt water, juvenile steelhead salmon adapt to a salinity of 6‰, tolerating increases up to 11.6‰. Preliminary adaptation activates the body’s osmoregulatory mechanisms.
However, even prolonged adaptation to this salinity did not guarantee survival when transferred to water with a salinity of 17‰. When directly transferred to water with a salinity of 6‰, the survival rate remained at 100% for 60 h. At a salinity 11.6‰, 100% mortality was observed after 36 h, and at a salinity 17‰, 50% of juveniles died within 23 h [
21]. It seems that longer-term experiments are needed to investigate the resistance of juvenile steelhead salmon consuming probiotic-supplemented feeds to sublethal salinity.
Examination of the intestinal contents of the fish showed that they contained a high number of bacilli morphologically identical to the given probiotics (
Table 3). Thus, in experiment 1, the vast majority of bacilli had a morphology identical to
B. velezensis MT14 and
B. velezensis MT42 strains, with single colonies of other morphotypes. In variant 2, the vast majority of bacilli had a colony morphology identical to
B. subtilis MT74 and
B. subtilis MT48 strains, with single colonies of other morphotypes. In the control group, a diversity of
Bacillus sp. colony morphotypes (more than 12) was observed, with some of them corresponding to the commercial probiotic present in the feed. These colonies were counted as colonies potentially belonging to the industrial probiotic present in the feed.
The number of cells of probiotic bacteria in the experimental groups corresponded to the number presented in the feed, which means that the strains passed the gastric conditions well. However, only 68% of cells for variant 1 and 40% for variant 2 were found in vegetative form, i.e., only some of the cells were in a metabolically active state. This may indicate an overly high number of probiotic cells, i.e., further reduction in the amount of probiotic in the feed would not weaken its properties.
Among the extraneous bacilli, 80–86% of the cells were in a vegetative form in all cases.
The content of industrial probiotic in the intestinal contents was significantly lower than in the original feed—6.7 × 104 CFU/g, while in the original feed it was 2.0 × 107 CFU/g. At the same time, 93% of cells were in a vegetative, metabolically active form.
4. Conclusions
As a result of the conducted research work, the developed feed formulations with the introduction of probiotic preparations were tested on juvenile steelhead salmon in the conditions of the basin farm. The grown objects were evaluated in terms of growth, mass accumulation, and microbiological research indicators. The reared objects were tested for viability. The obtained data were processed by methods of variation statistics.
Experimental groups of fish that received feeds enriched with probiotics showed a statistically significant improvement in growth indicators compared to the control group. In particular, the weight of individuals in the first experimental group was 10.55% higher than that of the control group, averaging 4.17 g. The second experimental variant also showed a 6.8% increase in mass, with a final fish weight of 4.02 g.
Statistical analysis of the obtained data confirmed the significance of the results with a significance level of p < 0.05, indicating the reliability of the differences detected. Absolute weight gains in the experimental groups amounted to 3.13 g and 2.29 g, respectively, which also exceeded that of the control group. In addition, the average daily weight gain of fish receiving probiotic supplements was higher by 13.75% on average, indicating a more efficient assimilation of nutrients and improved the general health of individuals in these groups.
This study conducted on juvenile steelhead salmon showed that feeding experimental feeds resulted in notable changes in their behavior. According to the data presented, juveniles from the experimental groups show an improved ability to adapt to changing environmental conditions and a higher tolerance to stressors. These observations suggest that experimental feeds have a positive effect on the physiological state of fish, which may contribute to the improvement of their housing and rearing conditions. In addition, the results emphasize the importance of selecting an optimal diet to ensure the healthy development of juvenile fish.
This study found that juvenile salmonids reared with probiotic-supplemented feeds had an improved ability to survive in high salinity and sublethal temperatures. This indicates that probiotics may play a significant role in enhancing the adaptive system of fish.
The results of this study showed that the cell counts of probiotic bacteria in the experimental groups matched the amount present in the feed (2.0–107 CFU/g), indicating that the bacteria successfully passed through the digestive tract. However, only 68% and 40% of the cells in Experiment 1 and Experiment 2, respectively, were in a metabolically active state, which indicates a possible excess of probiotic cells in the feed.
The examination of the intestinal contents of the fish showed that they contained a high number of bacilli morphologically identical to the given probiotics. However, among the extraneous bacilli, 80–86% of the cells were in a vegetative form in all cases, which may indicate the need to further investigate the effect of probiotics on the intestinal microflora of fish.
Overall, the findings demonstrate the potential of probiotics in increasing the resistance of fish to unfavorable conditions and improving their health and growth. However, further studies are needed to optimize the probiotic concentration in feed and to investigate the effect of probiotics on fish gut microflora.
The results emphasize the efficacy of probiotics as a means to improve the quality of farmed fish and may serve as a basis for further development of nutritional strategies in aquaculture. They also indicate the potential of probiotics to improve the productivity and resistance of fish to stressors, ultimately contributing to an improved quality and quantity of aquaculture products.
In this way, the technological foundations are being laid for the development of targeted probiotics designed not only to suppress pathogens but also to manage host adaptive systems.