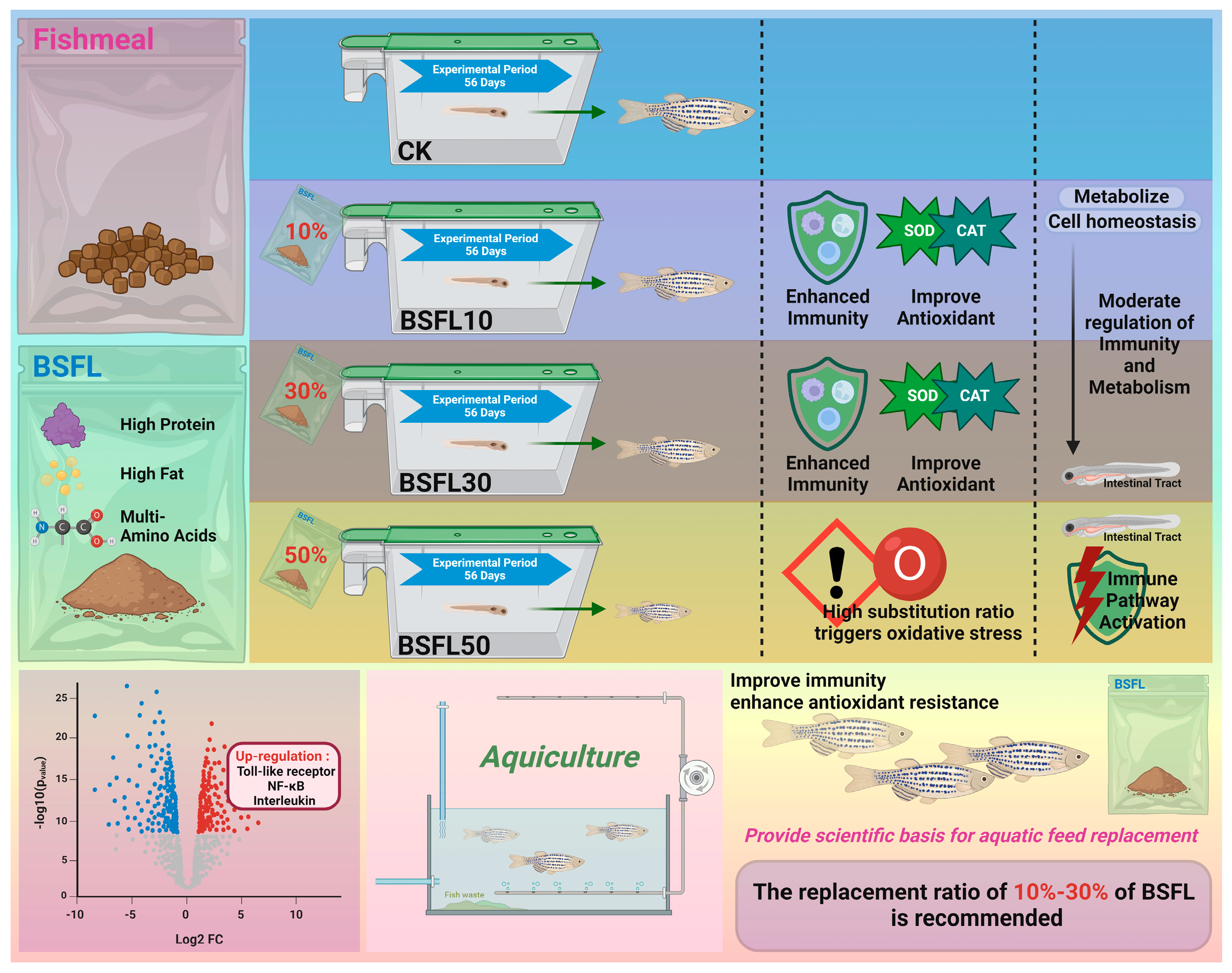
Black Soldier Fly Larvae Meal as a Sustainable Fishmeal Substitute for Juvenile Hybrid Grouper: Impacts on Growth, Immunity, and Gut Health
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Feeding Management and Experimental Design
2.3. Sample Collection
2.4. Indicator Measurement and Data Processing
- Survival Rate (SR, %) = (N1/N0) × 100%;
- Condition Factor (CF, g/cm3) = Wf/L3;
- Viscerosomatic Index (VSI, %) = (W2/Wf) × 100%;
- Hepatosomatic Index (HSI, %) = (W3/Wf) × 100%.
2.5. Serum Immunity and Antioxidant Indicator Measurement
2.6. Gut Transcriptome Sequencing and Data Analysis
2.7. Statistical Analysis
3. Results
3.1. Effects of Replacing Fishmeal with Black Soldier Fly Larvae Meal on Survival Rate and Final Weight in Grouper
3.2. Positive Effects of Serum Biochemical Indicators, Immune Response, and Antioxidant Capacity in Grouper by 30% BSFL Replacement
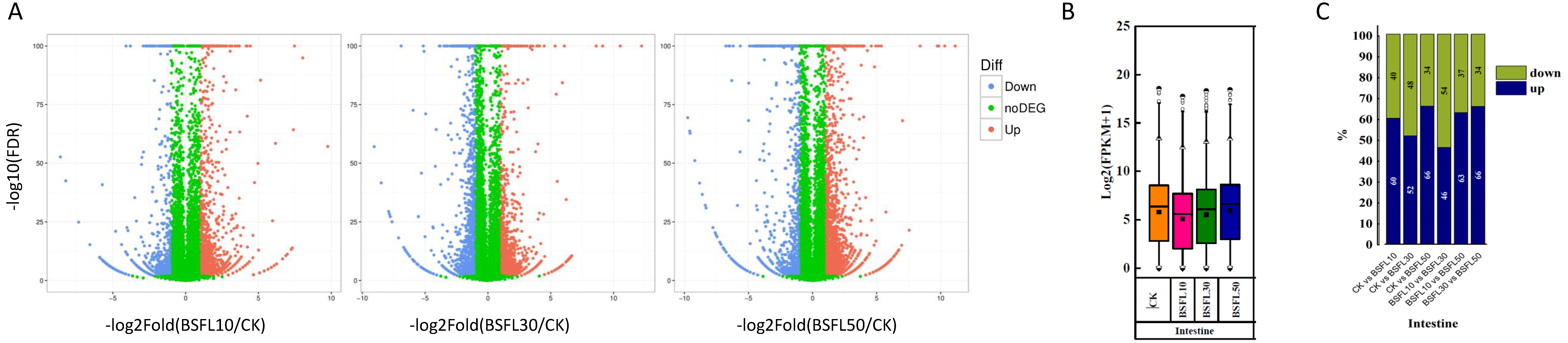
3.3. Impact of BSFL Substitution on Gut Gene Expression and Differential Analysis in Juvenile Hybrid Grouper
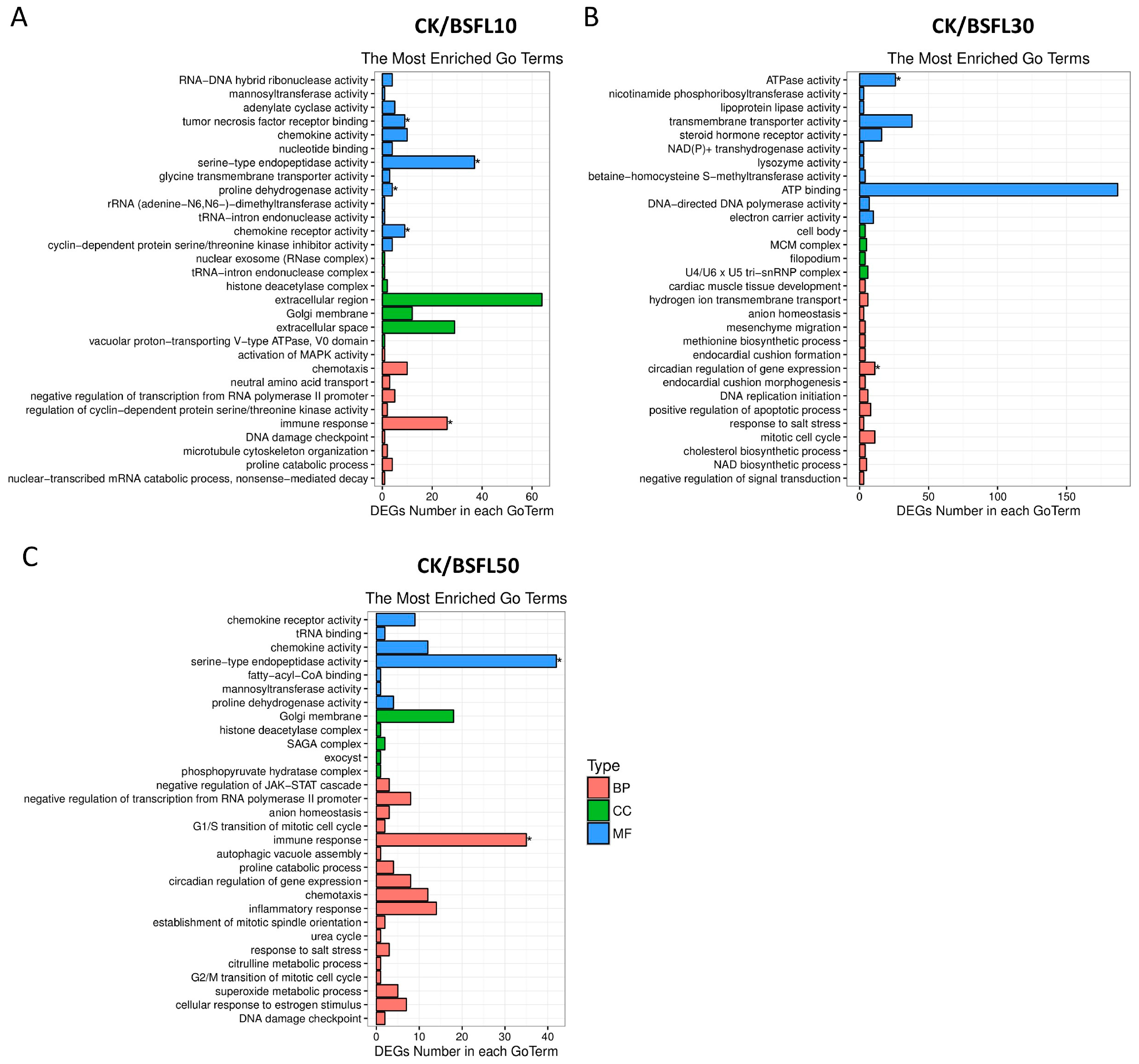
3.4. GO Functional Enrichment Analysis of DEGs in the Gut of Hybrid Grouper Following BSFL Substitution
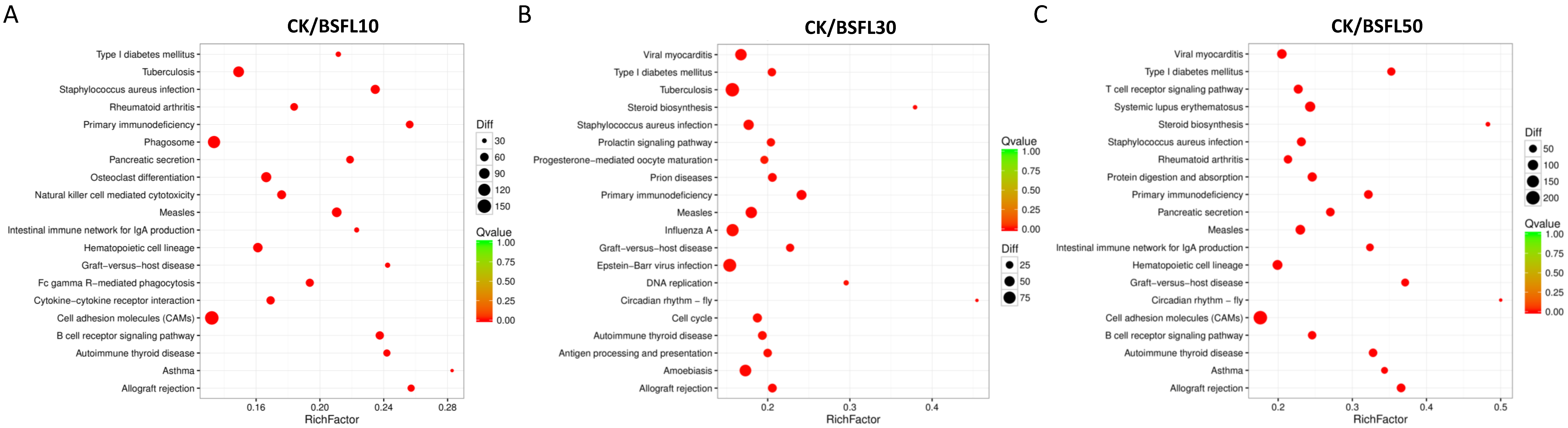
3.5. KEGG Pathway Enrichment Analysis of DEGs in the Gut of Hybrid Grouper Following BSFL Substitution
3.6. Tissue-Specific Regulation of Immune-Related Gene Expression in the Intestine of Hybrid Grouper Following BSFL Substitution
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALB | albumin |
| ANOVA | analysis of variance |
| BP | biological processes |
| BSF | black soldier fly |
| BSFL | black soldier fly larvae meal |
| CAT | catalase |
| CC | cellular components |
| CF | condition factor |
| CK | control group |
| DEGs | differentially expressed genes |
| GLB | globulin |
| GO | Gene Ontology |
| GSH-Px | glutathione peroxidase |
| HIS | hepatosomatic index |
| IPR | intraperitoneal fat ratio |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LZY | lysozyme |
| MDA | malondialdehyde |
| Mean ± SE | mean ± standard error |
| MF | molecular functions |
| nfb | NF-kappa B |
| noDEG | non-significantly differentially expressed genes |
| PAMPs | Pathogen-Associated Molecular Patterns |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
| SR | survival rate |
| tlr | Toll-like receptor |
| TNF | Tumor Necrosis Factor |
| TP | total protein |
| VSI | viscerosomatic index |
| Wi | initial weight |
| Wf | final weight |
References
- Ido, A.; Ali, M.-F.-Z.; Takahashi, T.; Miura, C.; Miura, T. Growth of Yellowtail (Seriola quinqueradiata) Fed on a Diet Including Partially or Completely Defatted Black Soldier Fly (Hermetia illucens) Larvae Meal. Insects 2021, 12, 722. [Google Scholar] [CrossRef] [PubMed]
- Goglio, P.; Van Den Burg, S.; Kousoulaki, K.; Skirtun, M.; Espmark, Å.M.; Kettunen, A.H.; Abbink, W. The Environmental Impact of Partial Substitution of Fish-Based Feed with Algae- and Insect-Based Feed in Salmon Farming. Sustainability 2022, 14, 12650. [Google Scholar] [CrossRef]
- Ferrer Llagostera, P.; Kallas, Z.; Reig, L.; Amores de Gea, D. The Use of Insect Meal as a Sustainable Feeding Alternative in Aquaculture: Current Situation, Spanish Consumers’ Perceptions and Willingness to Pay. J. Clean. Prod. 2019, 229, 10–21. [Google Scholar] [CrossRef]
- Malcorps, W.; Kok, B.; van‘t Land, M.; Fritz, M.; van Doren, D.; Servin, K.; van der Heijden, P.; Palmer, R.; Auchterlonie, N.; Rietkerk, M.; et al. The Sustainability Conundrum of Fishmeal Substitution by Plant Ingredients in Shrimp Feeds. Sustainability 2019, 11, 1212. [Google Scholar] [CrossRef]
- Belghit, I.; Liland, N.S.; Gjesdal, P.; Biancarosa, I.; Menchetti, E.; Li, Y.; Waagbø, R.; Krogdahl, Å.; Lock, E.-J. Black Soldier Fly Larvae Meal Can Replace Fish Meal in Diets of Sea-Water Phase Atlantic Salmon (Salmo salar). Aquaculture 2019, 503, 609–619. [Google Scholar] [CrossRef]
- Tippayadara, N.; Dawood, M.A.O.; Krutmuang, P.; Hoseinifar, S.H.; Doan, H.V.; Paolucci, M. Replacement of Fish Meal by Black Soldier Fly (Hermetia illucens) Larvae Meal: Effects on Growth, Haematology, and Skin Mucus Immunity of Nile Tilapia, Oreochromis Niloticus. Animals 2021, 11, 193. [Google Scholar] [CrossRef] [PubMed]
- Jahan, R.; Tipu, M.M.H.; Haque, M.M.; Salam, M.A. Black Soldier Fly (Hermetia illucens) Larvae Meal as a Fish Meal Replacement in Diets for 1 Nursing Common Carp (Cyprinus carpio) Fry. AgriRxiv 2021, 2021, 20210012413. [Google Scholar]
- Weththasinghe, P.; Rocha, S.D.C.; Øyås, O.; Lagos, L.; Hansen, J.Ø.; Mydland, L.T.; Øverland, M. Modulation of Atlantic Salmon (Salmo salar) Gut Microbiota Composition and Predicted Metabolic Capacity by Feeding Diets with Processed Black Soldier Fly (Hermetia illucens) Larvae Meals and Fractions. Anim Microbiome 2022, 4, 9. [Google Scholar] [CrossRef]
- Xia, J.; Ge, C.; Yao, H. Antimicrobial Peptides from Black Soldier Fly (Hermetia illucens) as Potential Antimicrobial Factors Representing an Alternative to Antibiotics in Livestock Farming. Animals 2021, 11, 1937. [Google Scholar] [CrossRef]
- Lu, S.; Taethaisong, N.; Meethip, W.; Surakhunthod, J.; Sinpru, B.; Sroichak, T.; Archa, P.; Thongpea, S.; Paengkoum, S.; Purba, R.A.P.; et al. Nutritional Composition of Black Soldier Fly Larvae (Hermetia illucens L.) and Its Potential Uses as Alternative Protein Sources in Animal Diets: A Review. Insects 2022, 13, 831. [Google Scholar] [CrossRef]
- Hender, A.; Siddik, M.; Howieson, J.; Fotedar, R. Black Soldier Fly, Hermetia Illucens as an Alternative to Fishmeal Protein and Fish Oil: Impact on Growth, Immune Response, Mucosal Barrier Status, and Flesh Quality of Juvenile Barramundi, Lates Calcarifer (Bloch, 1790). Biology 2021, 10, 505. [Google Scholar] [CrossRef]
- Lanes, C.F.C.; Pedron, F.A.; Bergamin, G.T.; Bitencourt, A.L.; Dorneles, B.E.R.; Villanova, J.C.V.; Dias, K.C.; Riolo, K.; Oliva, S.; Savastano, D.; et al. Black Soldier Fly (Hermetia illucens) Larvae and Prepupae Defatted Meals in Diets for Zebrafish (Danio rerio). Animals 2021, 11, 720. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lv, J.; Ma, Z.; Ma, J.; Chen, J. Advances in Antimicrobial Peptides: Mechanisms, Design Innovations, and Biomedical Potential. Molecules 2025, 30, 1529. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.R.T.; Guay, L.-D.; Fliss, I.; Biron, E. Structure–Activity Study of the Antimicrobial Lipopeptide Humimycin A and Screening Against Multidrug-Resistant Staphylococcus aureus. Antibiotics 2025, 14, 385. [Google Scholar] [CrossRef]
- Casillas-Vargas, G.; Ocasio-Malavé, C.; Medina, S.; Morales-Guzmán, C.; Del Valle, R.G.; Carballeira, N.M.; Sanabria-Ríos, D.J. Antibacterial Fatty Acids: An Update of Possible Mechanisms of Action and Implications in the Development of the next-Generation of Antibacterial Agents. Prog. Lipid Res. 2021, 82, 101093. [Google Scholar] [CrossRef] [PubMed]
- Obukhova, E.S.; Murzina, S.A. Mechanisms of the Antimicrobial Action of Fatty Acids: A Review. Appl. Biochem. Microbiol. 2024, 60, 1035–1043. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhu, L.; Liang, R.; Su, J.; Yang, J.; Cao, X.; Lu, Y.; Yu, Y.; Hu, J. A Meta-analysis of the Effects of Black Soldier Fly Meal on Fish Immune Response and Antioxidant Capacity. Comp. Immunol. Rep. 2024, 7, 200162. [Google Scholar] [CrossRef]
- Ruiz, A.; Andree, K.B.; Furones, D.; Holhorea, P.G.; Calduch-Giner, J.À.; Viñas, M.; Pérez-Sánchez, J.; Gisbert, E. Modulation of Gut Microbiota and Intestinal Immune Response in Gilthead Seabream (Sparus aurata) by Dietary Bile Salt Supplementation. Front. Microbiol. 2023, 14, 1123716. [Google Scholar] [CrossRef]
- Fadel, A.; Khafage, A.; Abdelsalam, M.; Abdel-Rahim, M.M. Comparative Evaluation of Three Herbal Extracts on Growth Performance, Immune Response, and Resistance against Vibrio Parahaemolyticus in Litopenaeus Vannamei. BMC Vet. Res. 2025, 21, 166. [Google Scholar] [CrossRef]
- Taklu, M.; Islami, H.R.; Shekarabi, S.P.H.; Mousavi, S.A.; Jourdehi, A.Y. Supplemental Effect of Dietary Nucleotides on Hematological Profile, Hepatic Biomarkers, Antioxidant Capacity, and Digestive Functions in Sterlet Sturgeon, Acipenser Ruthenus. Sci. Rep. 2025, 15, 11408. [Google Scholar] [CrossRef]
- Hervé, M.K.; Calice, M.D.; Dzepe, D.; Djouaka, R.F.; Chia, S.Y.; Efole, T.; Ndindeng, S.A. Black Soldier Fly (Hermetia illucens) Larvae Improve Growth Performance and Flesh Quality of African Catfish (Clarias gariepinus). Discov. Anim. 2025, 2, 9. [Google Scholar] [CrossRef]
- Muurmann, A.T.; Eriksen, N.T.; Rasmussen, J.A.; Limborg, M.T.; Tomberlin, J.K.; Gilbert, M.T.P.; Bahrndorff, S. Growth and Metabolic Performance of House Fly and Black Soldier Fly Larvae Differ across Densities and Waste-Based Growth Substrates. Waste Manag. 2025, 193, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Zarantoniello, M.; Bruni, L.; Randazzo, B.; Vargas, A.; Gioacchini, G.; Truzzi, C.; Annibaldi, A.; Riolo, P.; Parisi, G.; Cardinaletti, G.; et al. Partial Dietary Inclusion of Hermetia Illucens (Black soldier fly) Full-Fat Prepupae in Zebrafish Feed: Biometric, Histological, Biochemical, and Molecular Implications. Zebrafish 2018, 15, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Weththasinghe, P.; Lagos, L.; Cortés, M.; Hansen, J.Ø.; Øverland, M. Dietary Inclusion of Black Soldier Fly (Hermetia illucens) Larvae Meal and Paste Improved Gut Health but Had Minor Effects on Skin Mucus Proteome and Immune Response in Atlantic Salmon (Salmo salar). Front. Immunol. 2021, 12, 599530. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, G.; Liland, N.S.; Koch, M.W.; Lock, E.-J.; Philip, A.J.P.; Belghit, I. Evaluation of Black Soldier Fly Larvae Meal as a Functional Feed Ingredient in Atlantic Salmon (Salmo salar) under Farm-like Conditions. Front. Aquac. 2023, 2, 1239402. [Google Scholar] [CrossRef]
- Chen, Y.; Zhuang, Z.; Liu, J.; Wang, Z.; Guo, Y.; Chen, A.; Chen, B.; Zhao, W.; Niu, J. Effects of Hermetia Illucens Larvae Meal on the Pacific White Shrimp (Litopenaeus vannamei) Revealed by Innate Immunity and 16S RRNA Gene Sequencing Analysis. Comp. Biochem. Physiol. Part D Genom. Proteom. 2023, 46, 101080. [Google Scholar]
- Fatima, S.; Afzal, A.; Rashid, H.; Iqbal, S.; Zafar, R.; Khalid, K.; Rauf, A.; Majeed, M.; Malik, A.; Carter, C.G. Dietary Replacement of Soybean Meal with Black Soldier Fly Larvae Meal in Juvenile Labeo Rohita and Catla Catla: Effects on Growth, Nutritional Quality, Oxidative Stress Biomarkers and Disease Resistance. PLoS ONE 2023, 18, e0294452. [Google Scholar] [CrossRef]
- Kamalii, A.; Antony, C.; Ahilan, B.; Uma, A.; Prabu, E. Dietary Protein Replacement of Fish Meal with Black Soldier Fly Larvae Meal: Effects on Growth, Whole-Body Composition, Digestive Enzyme Activity, Muscle-Growth-Related Gene Expression and Haemato-Biochemical Responses of Juvenile Goldfish, Carassius Auratus. Turk. J. Fish. Aquat. Sci. 2022, 23, TRJFAS21837. [Google Scholar]
- Chen, Y.; Ma, J.; Yong, Y.-S.; Chen, Y.; Chen, B.; Cao, J.; Peng, K.; Wang, G.; Huang, H.; Loh, J.-Y. Impacts of Black Soldier Fly (Hermetia illucens) Larval Meal on Intestinal Histopathology and Microbiome Responses in Hybrid Grouper (Epinephelus fuscoguttatus ♀ × E. Lanceolatus ♂): A Comprehensive Analysis. Animals 2024, 14, 3596. [Google Scholar] [CrossRef]
- Nankervis, L.; Cobcroft, J.M.; Nguyen, N.V.; Rimmer, M.A. Advances in Practical Feed Formulation and Adoption for Hybrid Grouper (Epinephelus fuscoguttatus ♀ × E. Lanceolatus ♂) Aquaculture. Rev. Aquac. 2021, 14, 288–307. [Google Scholar] [CrossRef]
- Limbu, S.M.; Shoko, A.P.; Ulotu, E.E.; Luvanga, S.A.; Munyi, F.M.; John, J.O.; Opiyo, M.A. Black Soldier Fly (Hermetia illucens L.) Larvae Meal Improves Growth Performance, Feed Efficiency and Economic Returns of Nile Tilapia (Oreochromis niloticus L.) Fry. Aquac. Fish Fish. 2022, 2, 167–178. [Google Scholar] [CrossRef]
- Rawles, S.D.; Riche, M.; Gaylord, T.G.; Webb, J.; Freeman, D.W.; Davis, M. Evaluation of Poultry By-Product Meal in Commercial Diets for Hybrid Striped Bass (Morone chrysops ♀ × M. Saxatilis ♂) in Recirculated Tank Production. Aquaculture 2006, 259, 377–389. [Google Scholar] [CrossRef]
- Jiang, B.; Sun, Y.; Li, W.; Liu, C.; Wen, C.; Li, A.; Huang, Y.; Su, Y. Effects of Dietary Black Soldier Fly (Hermetia illucens linnaeus) on the Disease Resistance of Juvenile Grouper (Epinephelus coioides). Fish Amp. Shellfish. Immunol. 2022, 123, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Simões-Alves, A.C.; Costa-Silva, J.H.; Bassot, A.; Leandro, C.G.; Pirola, L.; Fernandes, M.P.; Morio, B. Diet Enriched in Saturated Fatty Acids Induces Liver Oxidative Stress and Elicits Inflammatory Pathways Prior to Metabolic Disruption in Perinatal Protein Undernutrition. Nutr. Res. 2023, 118, 104–115. [Google Scholar] [CrossRef]
- Xiao, X.; Jin, P.; Zheng, L.; Cai, M.; Yu, Z.; Yu, J.; Zhang, J. Effects of Black Soldier Fly (Hermetia illucens) Larvae Meal Protein as a Fishmeal Replacement on the Growth and Immune Index of Yellow Catfish (Pelteobagrus fulvidraco). Aquac. Res. 2018, 49, 1569–1577. [Google Scholar] [CrossRef]

| Items | Diets | |||
|---|---|---|---|---|
| CK | BSFL 10 | BSFL 30 | BSFL 50 | |
| Growth indices | ||||
| Wi (g) | 56.50 ± 0.00 | 56.47 ± 0.04 | 56.23 ± 0.05 | 56.58 ± 0.06 |
| Wf (g) | 108.70 ± 0.24 a | 107.19 ± 0.25 b | 95.36 ± 0.04 c | 76.55± 0.17 d |
| Survival (%) | 100.0 ± 0.00 | 100.0 ± 0.00 | 98.20 ± 1.55 | 99.10 ± 1.55 |
| Biometric indices | ||||
| CF (g cm−3) | 1.61 ± 0.11 | 1.58 ± 0.12 | 1.57 ± 0.14 | 1.55 ± 0.08 |
| HSI (%) | 3.38± 0.19 b | 5.51 ± 0.35 a | 5.99 ± 0.41 a | 5.77 ± 0.42 a |
| IPR (%) | 5.68 ± 0.33 a | 3.55 ± 0.46 b | 3.76 ± 0.42 b | 4.42 ± 0.40 b |
| Items | CK | BSFL 10 | BSFL 30 | BSFL 50 |
|---|---|---|---|---|
| Serum Chemistry | ||||
| TP (g/L) | 34.79 ± 1.26 b | 40.82 ± 2.90 ab | 53.31 ± 0.50 a | 36.39 ± 1.30 b |
| GLB (g/L) | 21.38 ± 0.07 c | 24.33 ± 0.34 b | 26.40 ± 0.68 a | 19.46 ± 0.56 d |
| ALB (g/L) | 13.41 ± 1.21 c | 16.49 ± 3.22 b | 26.91± 6.40 a | 16.94 ± 1.29 b |
| ALB/GLB | 0.63 ± 0.10 | 0.68 ± 0.15 | 1.02 ± 0.25 | 0.87 ± 0.07 |
| TC (mmol/L) | 5.81 ± 1.29 | 6.65 ± 1.3 | 5.47 ± 1.21 | 8.32 ± 1.91 |
| HDL (mmol/L) | 2.42 ± 0.07 | 2.34 ± 0.12 | 2.62 ± 0.16 | 2.30 ± 0.07 |
| LDL (mmol/L) | 2.89 ± 0.20 d | 3.63 ± 0.69 b | 3.00 ± 0.63 bc | 5.27 ± 0.22 a |
| Anti-oxidation | ||||
| SOD (U/mL) | 174.27 ± 2.36 a | 146.79 ± 2.76 b | 208.19 ± 12.25 a | 142.69± 3.40 b |
| LZY (U/mL) | 34.00 ± 1.81 b | 35.05 ± 1.46 a | 35.80± 1.64 a | 34.00± 2.11 b |
| MDA (nmol/mL) | 92.32 ± 2.63 d | 109.20 ± 4.83 b | 128.13 ± 2.41 a | 107.68 ± 1.83 c |
| GSH-Px (U/mL) | 1089.38 ± 171.11 b | 1100.31 ± 75.16 bc | 1246.88 ± 108.20 a | 1185.63 ± 27.79 c |
| CAT (U/mL) | 24.82 ± 8.46 c | 18.03 ± 8.50 d | 29.22 ± 3.82 b | 34.56 ± 3.93 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Li, W.; Zhong, M.; Ma, J.; Chen, B.; Cao, J.; Loh, J.-Y.; Huang, H. Black Soldier Fly Larvae Meal as a Sustainable Fishmeal Substitute for Juvenile Hybrid Grouper: Impacts on Growth, Immunity, and Gut Health. Fishes 2025, 10, 344. https://doi.org/10.3390/fishes10070344
Chen Y, Li W, Zhong M, Ma J, Chen B, Cao J, Loh J-Y, Huang H. Black Soldier Fly Larvae Meal as a Sustainable Fishmeal Substitute for Juvenile Hybrid Grouper: Impacts on Growth, Immunity, and Gut Health. Fishes. 2025; 10(7):344. https://doi.org/10.3390/fishes10070344
Chicago/Turabian StyleChen, Yan, Wenfeng Li, Minyi Zhong, Jun Ma, Bing Chen, Junming Cao, Jiun-Yan Loh, and Hai Huang. 2025. "Black Soldier Fly Larvae Meal as a Sustainable Fishmeal Substitute for Juvenile Hybrid Grouper: Impacts on Growth, Immunity, and Gut Health" Fishes 10, no. 7: 344. https://doi.org/10.3390/fishes10070344
APA StyleChen, Y., Li, W., Zhong, M., Ma, J., Chen, B., Cao, J., Loh, J.-Y., & Huang, H. (2025). Black Soldier Fly Larvae Meal as a Sustainable Fishmeal Substitute for Juvenile Hybrid Grouper: Impacts on Growth, Immunity, and Gut Health. Fishes, 10(7), 344. https://doi.org/10.3390/fishes10070344






