Spatial–Seasonal Shifts in Phytoplankton and Zooplankton Community Structure Within a Subtropical Plateau Lake: Interplay with Environmental Drivers During Rainy and Dry Seasons
Abstract
1. Introduction
2. Materials and Methods
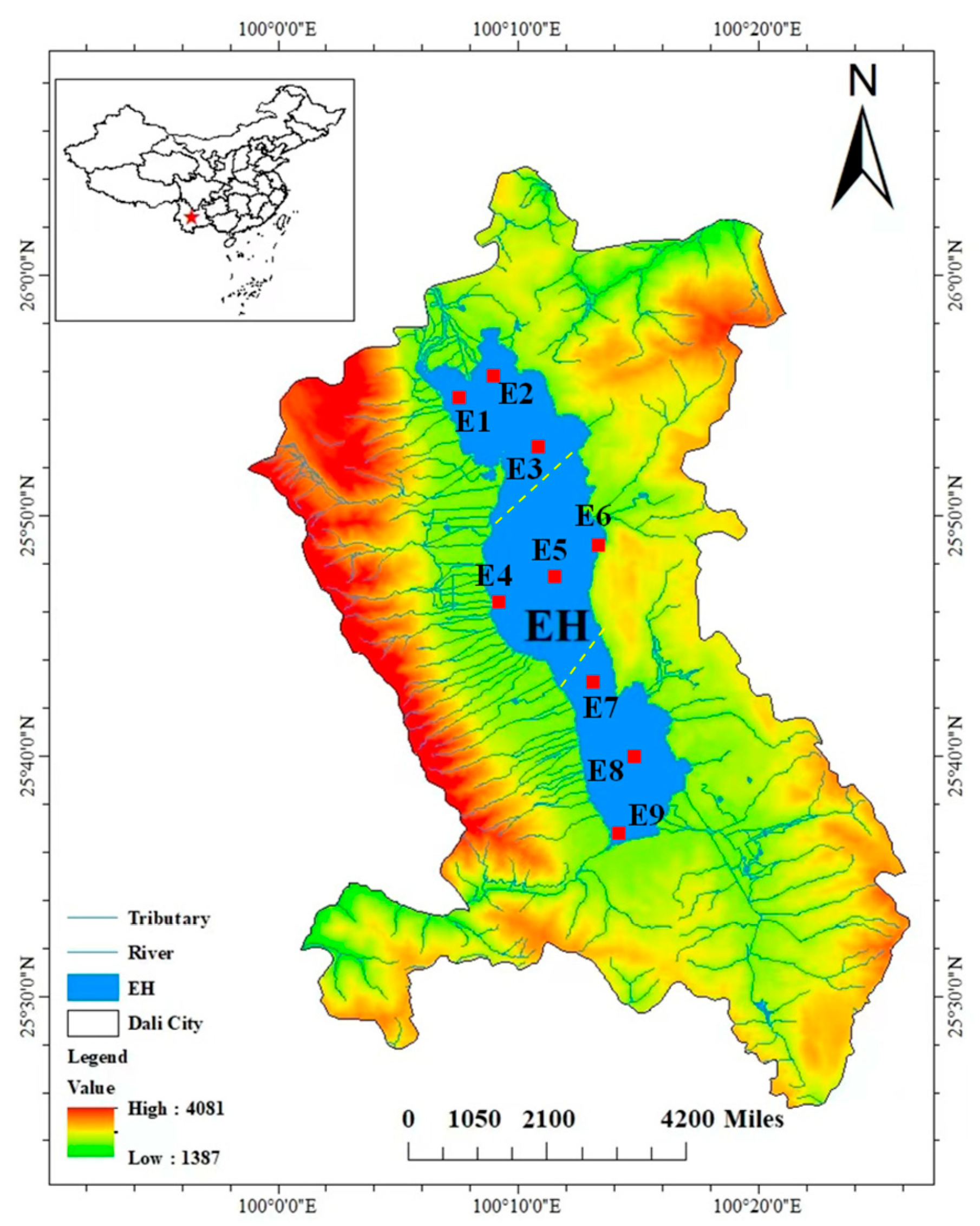
2.1. Study Area
2.2. Sampling Methods
2.3. Data Analysis
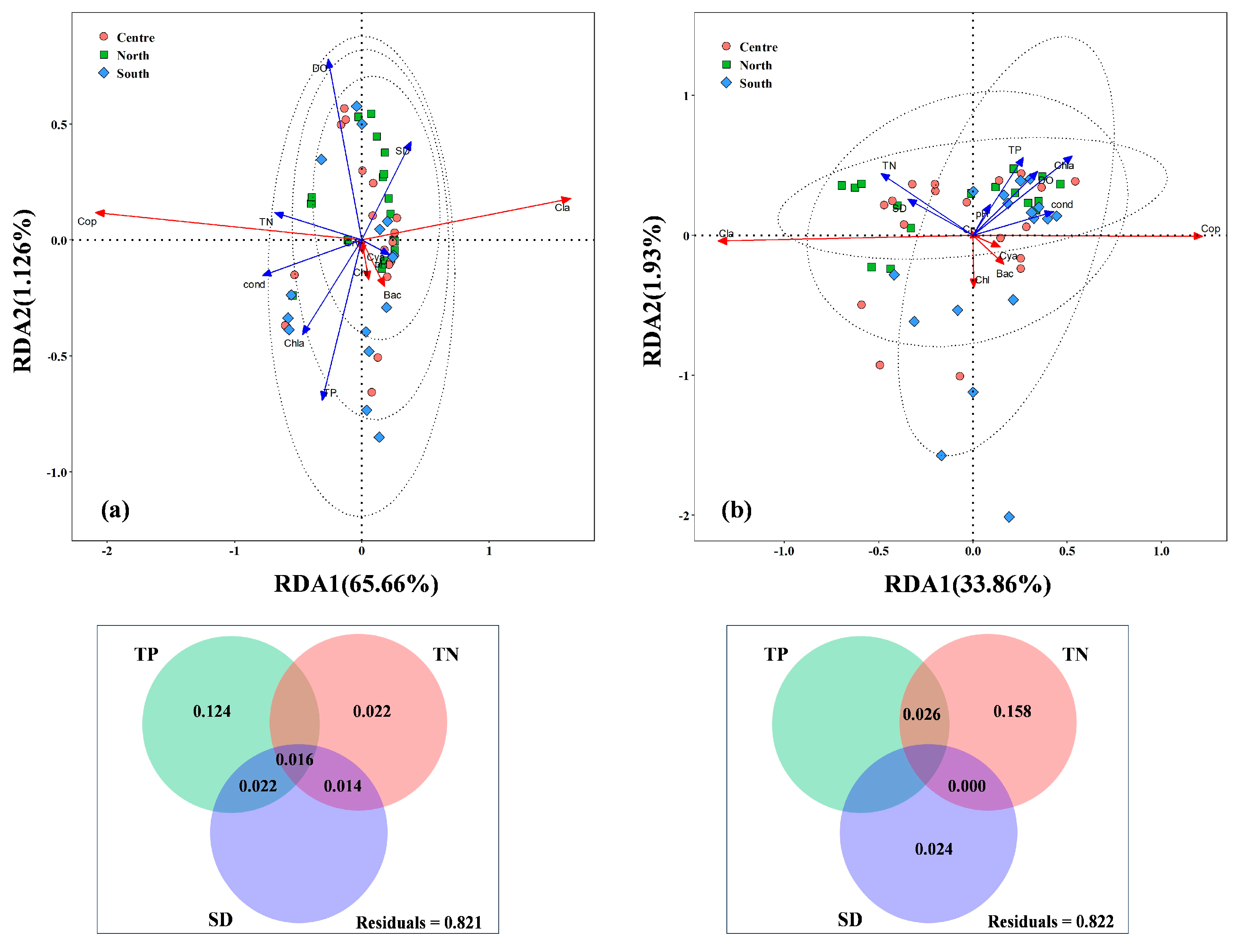
3. Results
3.1. Variations in Water Quality and Environmental Factors
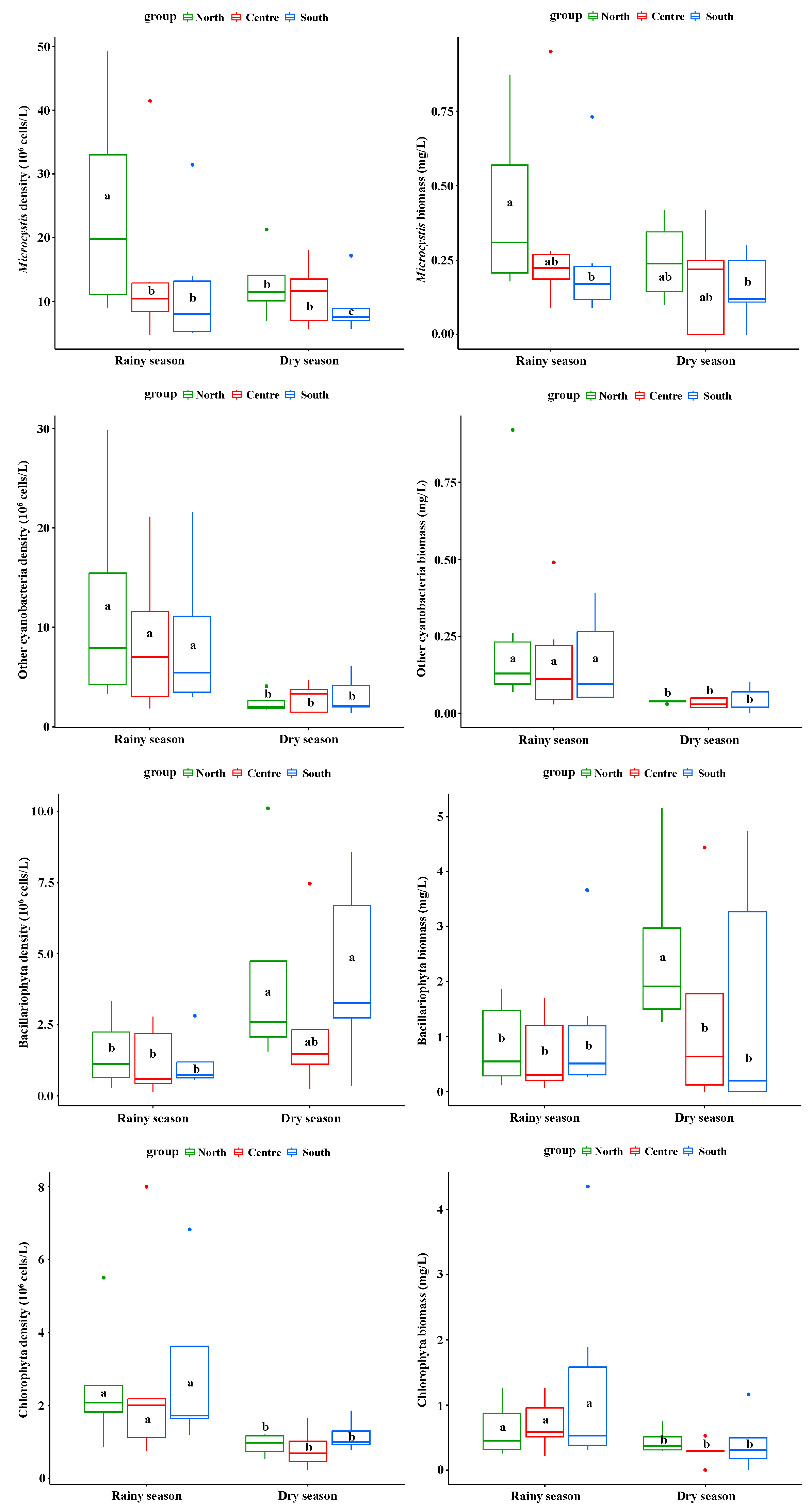
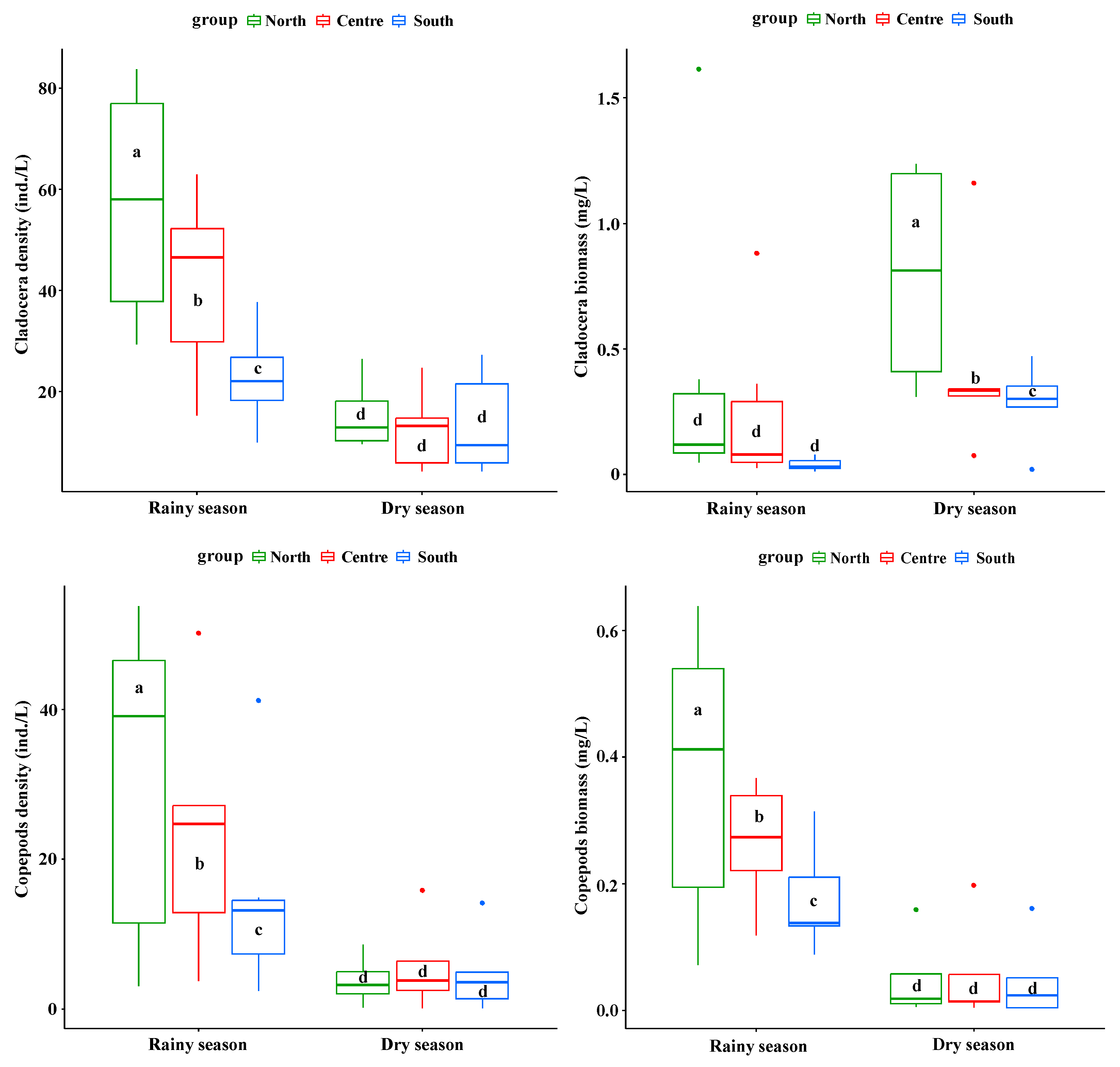
3.2. Spatial Distribution Differences of Plankton Density and Biomass in Rainy and Dry Seasons
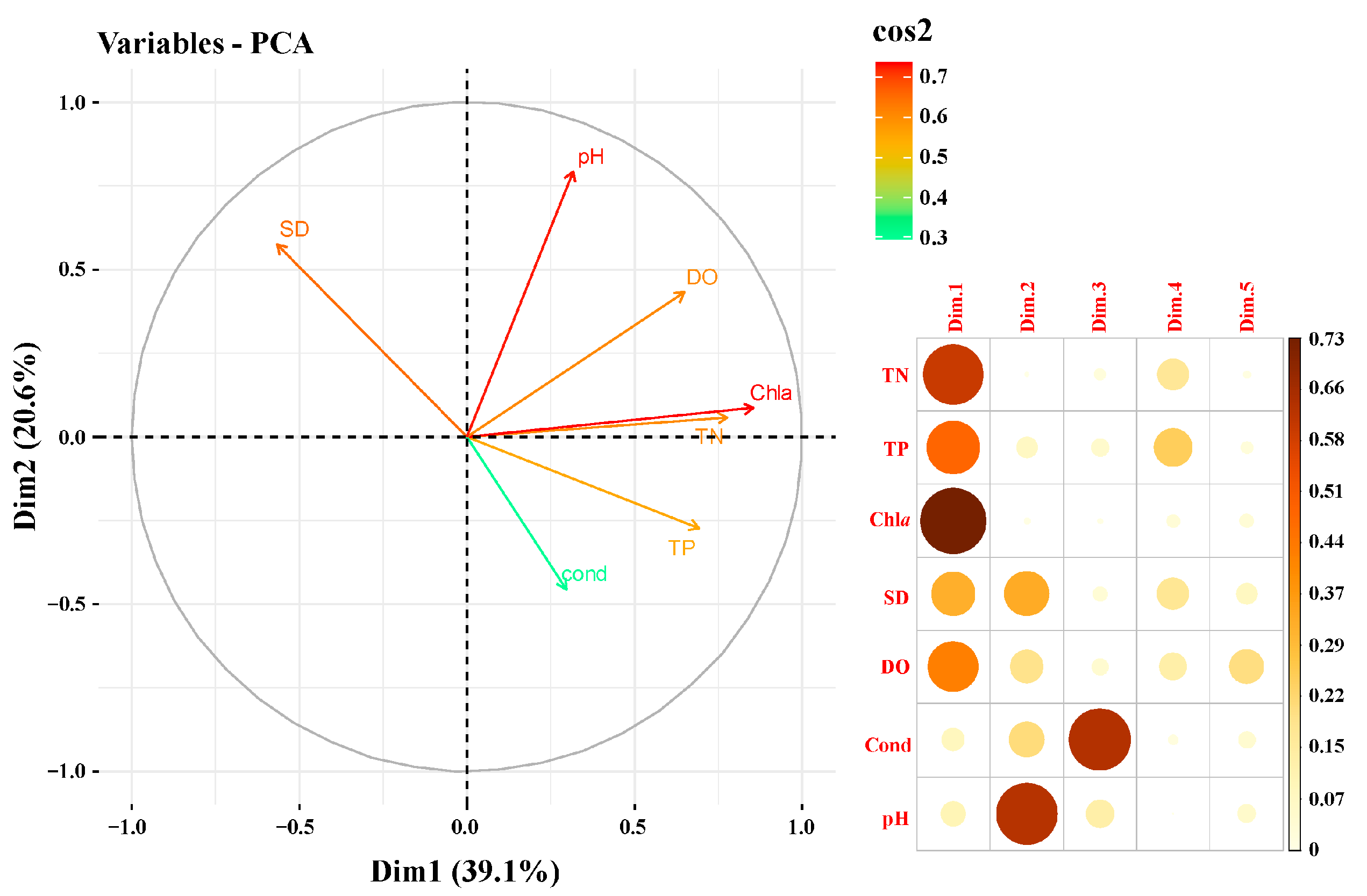
3.3. The Relationship Between Plankton Biomass and Environmental Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cadier, M.; Gorgues, T.; Sourisseau, M.; Edwards, C.A.; Aumont, O.; Marié, O.; Memery, L. Assessing spatial and temporal variability of phytoplankton communities’ composition in the Iroise Sea ecosystem (Brittany, France): A 3D modeling approach. Part 1: Biophysical control over plankton functional types succession and distribution. J. Mar. Syst. 2017, 165, 47–68. [Google Scholar] [CrossRef]
- Moe, S.J.; Hobæk, A.; Persson, J.; Skjelbred, B.; Løvik, J.E. Shifted dynamics of plankton communities in a restored lake: Exploring the effects of climate change on phenology through four decades. Clim. Res. 2022, 86, 125–143. [Google Scholar] [CrossRef]
- Xu, B.; Huang, X.F.; Xu, K.; Wang, X. Spatial and Seasonal Dynamics of Plankton Community and Its Relationship with Environmental Factors in an Urban River: A Case Study of Wuxi City, China. Water 2024, 17, 51. [Google Scholar] [CrossRef]
- Doi, H.; Chang, K.H.; Ando, T.; Imai, H.; Nakano, S.; Kajimoto, A.; Katano, I. Drifting plankton from a reservoir subsidize downstream food webs and alter community structure. Oecologia 2008, 155, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Bunnell, D.B.; Barbiero, R.P.; Ludsin, S.A.; Madenjian, C.P.; Warren, G.J.; Dolan, D.M.; Brenden, T.O.; Briland, R.; Gorman, O.T.; Hi, J.X.; et al. Changing ecosystem dynamics in the Laurentian Great Lakes: Bottom-up and top-down regulation. Bioscience 2014, 64, 26–39. [Google Scholar] [CrossRef]
- Cael, B.B.; Dutkiewicz, S.; Henson, S. Abrupt shifts in 21st-century plankton communities. Sci. Adv. 2021, 7, eabf8593. [Google Scholar] [CrossRef]
- Lin, S.J. Phosphate limitation and ocean acidification co-shape phytoplankton physiology and community structure. Nat. Commun. 2023, 14, 2699. [Google Scholar] [CrossRef] [PubMed]
- El Dine, Z.S.; Guinet, C.; Picard, B. Influence of the phytoplankton community structure on the southern elephant seals’ foraging activity within the Southern Ocean. Commun. Biol. 2025, 8, 620. [Google Scholar] [CrossRef]
- Rodríguez-Gómez, C.F.; Vázquez, G.; Papiol, V.; Mariño-Tapia, I.; Enriquez, C. Phytoplankton distribution and its ecological and hydrographic controls in two contrasting areas of a stratified oligotrophic system. Hydrobiologia 2022, 849, 3175–3195. [Google Scholar] [CrossRef]
- Xu, Y.P.; Xiang, Z.L.; Rizo, E.Z.; Naselli-Flores, L.; Han, B.P. Combination of linear and nonlinear multivariate approaches effectively uncover responses of phytoplankton communities to environmental changes at regional scale. J. Environ. Manag. 2022, 305, 114399. [Google Scholar] [CrossRef]
- Xu, Z.M.; Cheung, S.Y.; Endo, H.; Xia, X.M.; Wu, W.X.; Chen, B.Z.; Ernest Ho, N.H.; Suzuki, K.; Li, M.; Liu, H.B. Disentangling the ecological processes shaping the latitudinal pattern of phytoplankton communities in the Pacific Ocean. Msystems 2022, 7, e01203-21. [Google Scholar] [CrossRef]
- Jacobsen, B.A.; Simonsen, P. Disturbance events affecting phytoplankton biomass, composition and species-diversity in a shallow, eutrophic, temperate lake. Hydrobiologia 1993, 249, 9–14. [Google Scholar] [CrossRef]
- James, T.R.; Chimney, M.J.; Sharfstein, B.; Engstrom, D.R.; Schottler, S.P.; East, T.; Jin, K.R. Hurricane effects on a shallow lake ecosystem, Lake Okeechobee, Florida (USA). Fund. Appl. Limnol. 2008, 172, 273–287. [Google Scholar] [CrossRef]
- Han, X.; Pan, B.Z.; Zhao, G.N.; Li, D.B.; Sun, H.; Zhu, P.H.; Lu, Y. Local and geographical factors jointly drive elevational patterns of phytoplankton in the source region of the Yangtze River, China. River Res. Appl. 2021, 37, 1145–1155. [Google Scholar] [CrossRef]
- Yin, C.J.; He, W.C.; Guo, L.G.; Gong, L.; Yang, Y.L.; Yang, J.J.; Ni, L.Y.; Chen, Y.S.; Jeppesen, E. Can top-down effects of planktivorous fish removal be used to mitigate cyanobacterial blooms in large subtropical highland lakes? Water Res. 2022, 218, 118483. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.J.; Yang, Y.L.; Ni, L.Y.; Chen, Y.S.; Wen, Z.H.; Su, H.J.; Guo, L.G. Temperature, nutrients and planktivorous fish predation interact to drive crustacean zooplankton in a large plateau lake, southwest China. Aquat. Sci. 2023, 85, 22. [Google Scholar] [CrossRef]
- Becker, V.; Caputo, L.; Ordóñez, J.; Marcé, R.; Armengol, J.; Crossetti, L.O.; Huszar, V.L.M. Driving factors of the phytoplankton functional groups in a deep Mediterranean reservoir. Water Res. 2010, 44, 3345–3354. [Google Scholar] [CrossRef]
- Engels, S.; Cwynar, L.C. Changes in fossil chironomid remains along a depth gradient: Evidence for common faunal thresholds within lakes. Hydrobiologia 2011, 661, 15–38. [Google Scholar] [CrossRef]
- Fan, L.M.; Song, C.; Meng, S.L.; Qiu, L.P.; Zheng, Y.; Wu, W.; Qu, J.H.; Li, D.D.; Zhang, C.; Hu, G.D.; et al. Spatial distribution of planktonic bacterial and archaeal communities in the upper section of the tidal reach in Yangtze River. Sci. Rep. 2016, 6, 39147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Shi, X.L.; Yang, Z.; Yu, Y.; Shi, L.M.; Qin, B.Q. Long-term dynamics and drivers of phytoplankton biomass in eutrophic Lake Taihu. Sci. Total Environ. 2018, 645, 876–886. [Google Scholar] [CrossRef]
- Wang, J.Y.; Huo, D.; Guo, C.X.; Zhu, G.W.; Gong, Z.J.; Fan, Y.W.; Wang, J.J. Vertical distribution characteristics of phytoplankton communities and its influencing factors in Qiandao Lake, a deep-water reservoir. Environ. Sci. 2022, 43, 3575–3586. (In Chinese) [Google Scholar] [CrossRef]
- Tian, W.; Zhang, H.Y.; Zhao, L.; Huang, H. Responses of a phytoplankton community to seasonal and environmental changes in Lake Nansihu, China. Mar. Freshw. Res. 2022, 68, 1877–1886. [Google Scholar] [CrossRef]
- Gehlot, B.; Chandra, S.; Joshi, R.; Arya, M.; Chakrabarti, R. Temporal variations in plankton communities and environmental factors in the Shipra, a central Himalayan tributary of the Kosi River in Uttarakhand, India. Environ. Monit. Assess. 2024, 196, 326. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Liu, J.X.; Liu, W.W.; Dai, S.; Ke, Z.X.; Guo, J.; Lai, Y.J.; Tan, Y.H. Phytoplankton communities miniaturization driven by extreme weather in subtropical estuary under climate changes. Water Res. 2024, 245, 120588. [Google Scholar] [CrossRef]
- Gulati, R.D. Zooplankton and its grazing as indicators of trophic status in Dutch lakes. Environ. Monit. Assess. 1983, 3, 343–354. [Google Scholar] [CrossRef]
- Primo, A.L.; Azeiteiro, U.M.; Marques, S.C.; Martinho, F.; Pardal, M.A. Changes in zooplankton diversity and distribution pattern under varying precipitation regimes in a southern temperate estuary. Estuar. Coast. Shelf S. 2009, 82, 341–347. [Google Scholar] [CrossRef]
- de Jonge, V.N. Importance of temporal and spatial scales in applying biological and physical process knowledge in coastal management, an example for the Ems estuary. Cont. Shelf Res. 2000, 20, 1655–1686. [Google Scholar] [CrossRef]
- Nayak, A.R.; Jiang, H.S.; Byron, M.L.; Sullivan, J.M.; McFarland, M.N.; Murphy, D.W. Small scale spatial and temporal patterns in particles, plankton, and other organisms. Front. Mar. Sci. 2021, 8, 669530. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, P.; Chen, F.Z.; Li, Y.L.; Li, S.X.; Guo, N.C.; Qin, J.H. Present status and changes of the phytoplankton community after invasion of Neosalanx taihuensis since 1982 in a deep oligotrophic plateau lake, Lake Fuxian in the subtropical China. J. Environ. Sci. 2005, 17, 389–394. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, J.P.; Hou, Z.Y.; Tian, Z.B.; Chu, Z.S.; Wang, S.R. Seasonal dynamics of algal net primary production in response to phosphorus input in a mesotrophic subtropical plateau lake, southwestern China. Water 2022, 14, 835. [Google Scholar] [CrossRef]
- Tiberti, R.; Dory, F.; Arthaud, F.; Augé, V.; Birk, C.; Cavalli, L.; Fontaneto, D.; Napoleoni, R.; Perga, M.E.; Sabás, I.; et al. Long-term changes of zooplankton in alpine lakes result from a combination of local and global threats. Biol. Conserv. 2025, 308, 111222. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, X.; Li, Y.L.; Yang, L.J.; Li, Y.H.; Liu, Y.; Zhou, L.; Wang, P.Z.; Zhao, X.; Wang, H.J.; et al. Interactive effects of nutrients and salinity on phytoplankton in subtropical plateau lakes of contrasting water depths. Water 2023, 15, 69. [Google Scholar] [CrossRef]
- Chen, Z.D.; Huang, L.P.; Chen, L.; Liang, H.; Liu, Y.Y.; Chen, X.L.; Zhang, T.; Chen, G.J. Seasonal variation and driving factors of carbon and nitrogen stable isotope values of plankton in four lakes of Yunnan Province. J. Lake Sci. 2021, 33, 761–773. [Google Scholar] [CrossRef]
- Yin, C.J.; Gong, L.; Chen, Y.S.; Pitcher, T.J.; Kang, B.; Guo, L.G. Modeling ecosystem impacts of invasive Japanese smelt Hypomesus nipponensis in Lake Erhai, southwestern China. Ecol. Inform. 2022, 67, 101488. [Google Scholar] [CrossRef]
- Du, B.H. Study on hydrological characteristics and non-point source pollution load in the Erhai lake watershed. Yunnan Environ. Prot. 1992, 2, 25–33. (In Chinese) [Google Scholar]
- Duan, S.X.; Yang, Z.; Li, Y.L.; He, B.; Shi, J.C.; Song, W.Z. Progress of agricultural non-point source pollution in Erhai Lake Basin: A review. J. Ecol. Rural Environ. 2021, 37, 279–286. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.C.; Hu, M.M.; Li, Y.H.; Liu, Y.D.; Shen, Y.W.; Li, G.B.; Wang, G.H. Succession of the phytoplankton community in response to environmental factors in north Lake Erhai during 2009–2010. Fresen. Environ. Bull. 2011, 20, 2221–2231. [Google Scholar]
- Reichwaldt, E.S.; Ghadouani, A. Effects of rainfall patterns on toxic cyanobacterial blooms in a changing climate: Between simplistic scenarios and complex dynamics. Water Res. 2012, 46, 1372–1393. [Google Scholar] [CrossRef]
- Yu, G.L.; Jiang, Y.J.; Song, G.F.; Tan, W.H.; Zhu, M.L.; Li, R.H. Variation of Microcystis and microcystin coupling nitrogen and phosphorus nutrient in Lake Erhai, drinking water source in Southwest Plateau, China. Environ. Sci. Pollut. Res. 2014, 21, 9887–9898. [Google Scholar] [CrossRef]
- Chu, X.L.; Chen, Y.Y. The Fishes of Yunnan; Science Press: Beijing, China, 1990. (In Chinese) [Google Scholar]
- Lu, S.Y.; Zhang, W.T.; Xing, Y.; Qu, J.T.; Li, K.; Zhang, Q.; Xue, W. Spatial distribution of water quality parameters of rivers around Erhai Lake during the dry and rainy seasons. Environ. Earth Sci. 2015, 74, 7423–7430. [Google Scholar] [CrossRef]
- Cao, J.; Hou, Z.Y.; Li, Z.K.; Chu, Z.S.; Yang, P.P.; Zheng, B.H. Succession of phytoplankton functional groups and their driving factors in a subtropical plateau lake. Sci. Total Environ. 2018, 631–632, 1127–1137. [Google Scholar] [CrossRef]
- Xiang, S.; Pang, Y.; Chu, Z.S.; Hu, X.Z.; Sun, L.; Xue, L.Q. Response of inflow water quality to land use pattern in northern watershed of Lake Erhai. Environ. Sci. 2016, 37, 2947–2956. [Google Scholar] [CrossRef]
- Pang, Y.; Xiang, S.; Chu, Z.S.; Xue, L.Q.; Ye, B.B. Relationship between agricultural land and water quality of inflow river in Erhai Lake basin. Environ. Sci. 2015, 36, 4005–4011. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, K.C.; Wang, S.R.; Wang, S.G.; Li, Y.P.; Li, Q.C.; Meng, Z. Characteristics of dissolved organic nitrogen in overlying water of typical lakes of Yunnan Plateau, China. Ecol. Indic. 2017, 84, 727–737. [Google Scholar] [CrossRef]
- Li, Y.; Xie, P.; Zhao, D.D.; Zhu, T.S.; Guo, L.G.; Zhang, J. Eutrophication strengthens the response of zooplankton to temperature changes in a high-altitude lake. Ecol. Evol. 2016, 6, 6690–6701. [Google Scholar] [CrossRef]
- Greenberg, A.E.; Clesceri, L.S.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1992. [Google Scholar]
- Yang, J.R.; Lv, H.; Alain, I.; Liu, L.M.; Yu, X.P.; Chen, H.H.; Yang, J. Disturbance-induced phytoplankton regime shifts and recovery of cyanobacteria dominance in two subtropical reservoirs. Water Res. 2017, 120, 52–63. [Google Scholar] [CrossRef]
- Yin, C.J.; Guo, L.G.; Yi, C.L.; Luo, C.Q.; Ni, L.Y. Physiochemical process, crustacean zooplankton and Microcystis changes in water column after introduction of silver carp, an in-situ enclosure experiment to control cyanobacteria bloom at Meiliang Bay, Lake Taihu. Scientifica 2017, 2017, 9643234–9643243. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J. Principal component analysis, wiley interdisciplinary reviews. Computation. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- Yan, C.Z.; Jin, X.C.; Zhao, J.Z. Ecological protection and sustainable utilization of Erhai Lake, Yunnan. Environ. Sci. 2005, 26, 38–42. Available online: https://pubmed.ncbi.nlm.nih.gov/16366467/ (accessed on 6 June 2025).
- Hui, T.X.; Xie, P.; Guo, L.G.; Chu, Z.S.; Liu, M.H. Phytoplankton dynamics and their equilibrium phases in the Yanghe Reservoir, China. J. Freshwater Ecol. 2014, 29, 1–15. [Google Scholar] [CrossRef]
- Shaw, G.; Garnett, C.; Moore, M.R.; Florian, P. The predicted impact of climate change on toxic algal (Cyanobacterial) blooms and toxin production in Queensland. Environ. Health 2001, 1, 76–88. Available online: https://search.informit.org/doi/abs/10.3316/INFORMIT.229352650430349 (accessed on 6 June 2025).
- Ahn, C.Y.; Chung, A.S.; Oh, H.M. Rainfall, phycocyanin, and N:P ratios related to cyanobacterial blooms in a Korean large reservoir. Hydrobiologia 2002, 474, 117–124. [Google Scholar] [CrossRef]
- Prepas, E.E.; Charette, T. Worldwide Eutrophication of Water Bodies: Causes, Concerns, Controls. In Treatise on Geochemistry; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 311–331. [Google Scholar] [CrossRef]
- Zhang, Y.P. Urbanization and urban water environment. Urban Environ. Urban Ecol. 1998, 11, 20–22. (In Chinese) [Google Scholar]
- Fabbro, L.D.; Duivenvoorden, L.J. Profile of a bloom of the cyanobacterium Cylindrospermopsis raciborskii (Woloszynska) Seenaya and Subba Raju in the Fitzroy River in tropical central Queensland. Mar. Freshw. Res. 1996, 47, 685–694. [Google Scholar] [CrossRef]
- Kebede, E.; Belay, A. Species composition and phytoplankton biomass in a tropical African Lake (Lake Awassa, Ethiopia). Hydrobiologia 1994, 288, 13–32. [Google Scholar] [CrossRef]
- Van de Waal, D.B.; Verspagen, J.M.H.; Lurling, M.; Donk, E.V.; Visser, P.M.; Huisman, J. The ecological stoichiometry of toxins produced by harmful cyanobacteria: An experimental test of the carbon-nutrient balance hypothesis. Ecol. Lett. 2009, 12, 1326–1335. [Google Scholar] [CrossRef]
- Hou, Z.J.; Jiang, Y.; Liu, Q.; Tian, Y.L.; He, K.J.; Fu, L. Impacts of environmental variables on a phytoplankton community: A case study of the tributaries of a subtropical river, southern China. Water 2018, 10, 152. [Google Scholar] [CrossRef]
- Zhao, P.P.; Wei, Z.H.; Wu, Q.T.; Han, B.P.; Lin, Q.Q. Response of planktonic copepods to seasonal fishing moratorium in Erhai Lake, Yunnan, China. Chin. J. Appl. Environ. Biol. 2012, 18, 421–425. (In Chinese) [Google Scholar] [CrossRef]
- Lu, H.B.; Chen, G.J.; Cai, Y.F.; Wang, J.Y.; Chen, X.L.; Duan, L.Z.; Zhang, H.C. Cladoceran community responses to eutrophication, fish introduction and macrophyte degradation over the past century in Lake Erhai. J. Lake Sci. 2016, 28, 132–140. [Google Scholar] [CrossRef][Green Version]
- Paczkowska, J.; Rowe, O.F.; Figueroa, D.; Andersson, A. Drivers of phytoplankton production and community structure in nutrient-poor estuaries receiving terrestrial organic inflow. Mar. Environ. Res. 2019, 151, 104778. [Google Scholar] [CrossRef]
- Zhu, R.; Wang, H.; Chen, J.; Shen, H.; Deng, X.W. Use the predictive models to explore the key factors affecting phytoplankton succession in Lake Erhai, China. Environ. Sci. Pollut. Res. 2018, 25, 1283–1293. [Google Scholar] [CrossRef] [PubMed]
- Bradburn, M.; Lewis, W.M.; McCutchan, J.H. Comparative adaptations of Aphanizomenon and Anabaena for nitrogen fixation under weak irradiance. Freshwater Biol. 2012, 57, 1042–1049. [Google Scholar] [CrossRef]
- Elser, J.J.; Bracken, M.E.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Rainy | Dry | ||||
|---|---|---|---|---|---|---|
| North | Center | South | North | Center | South | |
| TN (mg/L) | 0.822 ± 0.072 | 0.645 ± 0.036 | 0.625 ± 0.034 | 0.630 ± 0.022 | 0.502 ± 0.041 | 0.578 ± 0.021 |
| DIN (mg/L) | 0.445 ± 0.042 | 0.427 ± 0.034 | 0.421 ± 0.026 | 0.381 ± 0.056 | 0.359 ± 0.050 | 0.323 ± 0.055 |
| NO3-N (mg/L) | 0.150 ± 0.008 | 0.155 ± 0.013 | 0.160 ± 0.017 | 0.154 ± 0.005 | 0.139 ± 0.006 | 0.133 ± 0.003 |
| NH4-N (mg/L) | 0.021 ± 0.007 | 0.029 ± 0.016 | 0.019 ± 0.008 | 0.024 ± 0.029 | 0.029 ± 0.006 | 0.018 ± 0.005 |
| TP (mg/L) | 0.042 ± 0.002 | 0.035 ± 0.001 | 0.033 ± 0.003 | 0.027 ± 0.003 | 0.025 ± 0.003 | 0.027 ± 0.003 |
| SRP (mg/L) | 0.016 ± 0.001 | 0.013 ± 0.001 | 0.015 ± 0.001 | 0.012 ± 0.002 | 0.011 ± 0.002 | 0.010 ± 0.002 |
| Chla (µg/L) | 23.32 ± 2.68 | 17.49 ± 2.16 | 13.84 ± 0.96 | 13.52 ± 1.02 | 11.98 ± 1.37 | 13.22 ± 2.09 |
| DO (mg/L) | 7.86 ± 0.51 | 6.76 ± 0.38 | 6.66 ± 0.27 | 6.89 ± 0.41 | 6.85 ± 0.41 | 7.63 ± 0.23 |
| pH | 8.92 ± 0.22 | 8.88 ± 0.26 | 9.04 ± 0.36 | 8.59 ± 0.054 | 8.57 ± 0.049 | 8.52 ± 0.048 |
| SD (cm) | 132.98 ± 8.52 | 170.5 ± 15.8 | 160 ± 14.8 | 206 ± 12.02 | 227.7 ± 14.5 | 214.6 ± 19.2 |
| Cond (µs/cm) | 324.2 ± 5.99 | 307.9 ± 8.39 | 309.5 ± 11 | 266.8 ± 7.3 | 258.2 ± 8.27 | 254.8 ± 8.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, C.; Gong, L.; Yang, J.; Yang, Y.; Guo, L. Spatial–Seasonal Shifts in Phytoplankton and Zooplankton Community Structure Within a Subtropical Plateau Lake: Interplay with Environmental Drivers During Rainy and Dry Seasons. Fishes 2025, 10, 343. https://doi.org/10.3390/fishes10070343
Yin C, Gong L, Yang J, Yang Y, Guo L. Spatial–Seasonal Shifts in Phytoplankton and Zooplankton Community Structure Within a Subtropical Plateau Lake: Interplay with Environmental Drivers During Rainy and Dry Seasons. Fishes. 2025; 10(7):343. https://doi.org/10.3390/fishes10070343
Chicago/Turabian StyleYin, Chengjie, Li Gong, Jiaojiao Yang, Yalan Yang, and Longgen Guo. 2025. "Spatial–Seasonal Shifts in Phytoplankton and Zooplankton Community Structure Within a Subtropical Plateau Lake: Interplay with Environmental Drivers During Rainy and Dry Seasons" Fishes 10, no. 7: 343. https://doi.org/10.3390/fishes10070343
APA StyleYin, C., Gong, L., Yang, J., Yang, Y., & Guo, L. (2025). Spatial–Seasonal Shifts in Phytoplankton and Zooplankton Community Structure Within a Subtropical Plateau Lake: Interplay with Environmental Drivers During Rainy and Dry Seasons. Fishes, 10(7), 343. https://doi.org/10.3390/fishes10070343






