1. Introduction
Input of 95Zr, 95Nb, 103Ru, and 106Ru into Aquatic Ecosystems Before the 1986 Chornobyl NPP Accident
95Zr,
95Nb,
103Ru, and
106Ru are among the environmentally significant radionuclides produced via neutron-induced fission of
235U,
239Pu, or
238U [
1]. These radionuclides have short half-lives:
95Zr, 64 days;
95Nb, 35 days;
103Ru, 39.3 days; and
106Ru, 374.5 days [
2]. The short half-life of these radionuclides restricts the duration available for experimental observation and data collection, thereby complicating long-term monitoring and impeding a comprehensive analysis of their behavior in aquatic ecosystems.
The IAEA-WOMARS study categorizes anthropogenic marine radionuclides into two groups based on their biogeochemical behavior: conservative and particle-reactive radionuclides [
3]. Conservative radionuclides (those mobile in water) are highly soluble in seawater, and their redistribution upon entering the ocean is primarily determined by physical processes related to mixing and diffusion (e.g.,
3H,
14C,
90Sr,
129I,
137Cs). In contrast, particle-reactive radionuclides (those associated with particles) penetrate more rapidly into deeper layers and underlying sediments. This group includes radionuclides such as
106Ru,
144Ce, and
239,240Pu.
The selection of radionuclides 95Zr, 95Nb, 103Ru, and 106Ru for investigation is driven by a set of key factors that determine their ecological, physicochemical, and biological relevance to aquatic ecosystems:
Their presence serves as an indicator of anthropogenic radioactive contamination of the aquatic environment, both during accidental releases and routine discharges from nuclear fuel cycle facilities.
They have differing biogeochemical behavior. All these radionuclides are particle-reactive, yet they exhibit varying degrees of solubility and bioavailability. Studying these differences enables the assessment of how contamination affects trophic interactions and radionuclide dynamics in aquatic environments.
The presence or absence of these radionuclides in different organs of aquatic organisms enables evaluation of the risk levels across various trophic levels, identification of uptake pathways, and assessment of the effectiveness of natural detoxification mechanisms.
Particular importance is attributed to the assessment of potential health risks associated with the consumption of fish and other aquatic organisms exposed to these radionuclides.
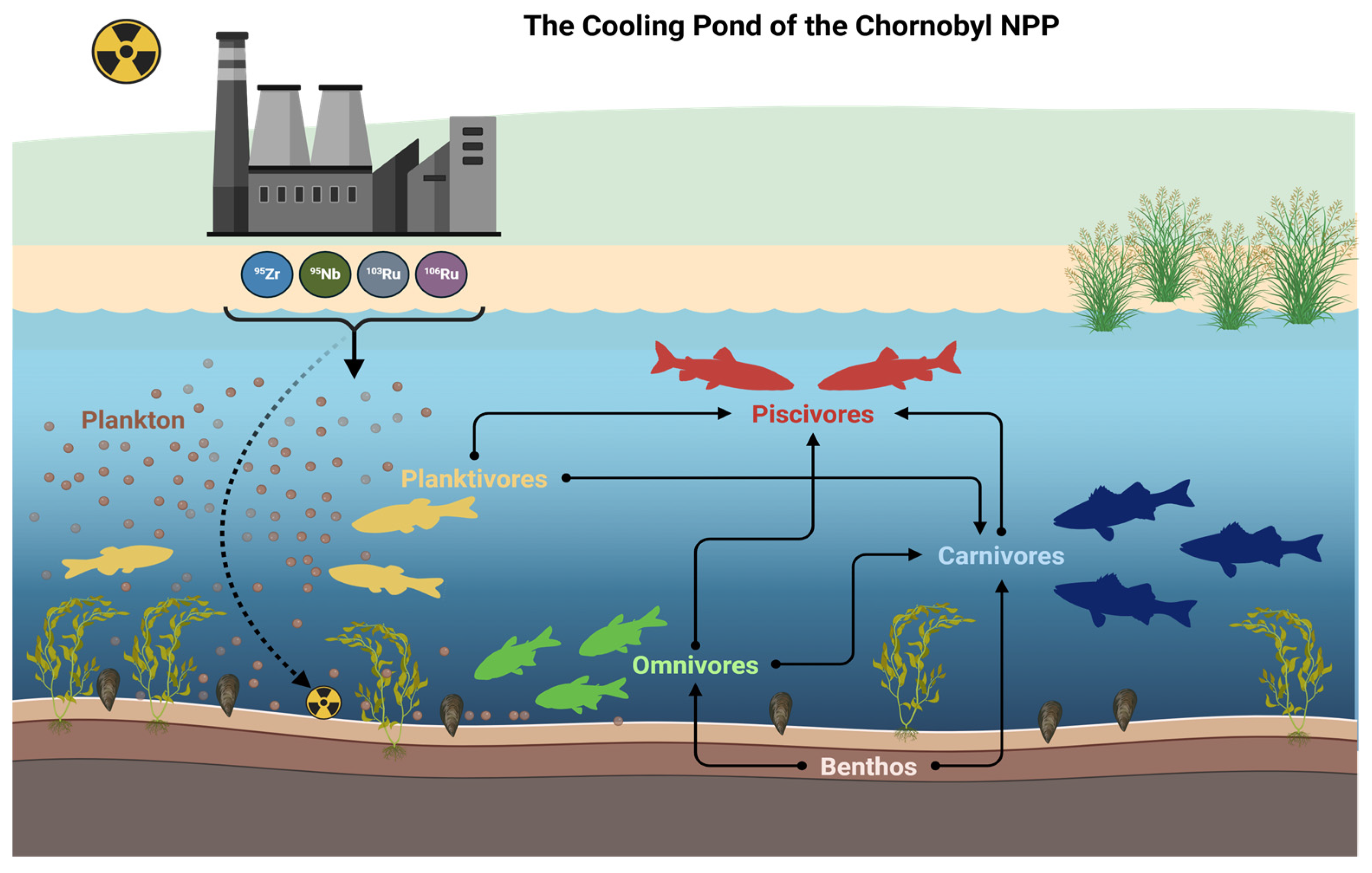
Upon entering aquatic ecosystems (primarily as particles),
95Zr,
95Nb,
103Ru, and
106Ru serve initially only as external sources of radiation exposure for aquatic plants. However, the digestive systems of invertebrates and fish are capable of breaking down such particles, allowing the radionuclides to become soluble and be incorporated into the tissues of organisms [
4].
Prior to the Chornobyl accident,
95Zr,
95Nb,
103Ru, and
106Ru entered aquatic ecosystems via multiple pathways: global fallout from nuclear weapons testing, nuclear facility accidents, authorized disposal of nuclear waste, and wastewater discharges from nuclear fuel cycle operations and weapons production. Approximately 85 × 10
15 Bq of various radionuclides were dumped into the oceans at more than 80 locations worldwide. Between 1946 and 1982, five countries—including Belgium, Japan, the former Soviet Union, the United Kingdom, and the United States—routinely used at-sea disposal as a method of nuclear waste management. During this period, approximately 46 × 10
15 Bq of low-level nuclear waste, including
95Zr,
95Nb, and
106Ru, were disposed of at sea [
1]. Another source of radionuclide input into aquatic ecosystems was the use of nuclear energy for excavations of harbors, dams, and reservoirs [
5]. The biological consequences of these nuclear releases depend on the quantity and nature of the radioactivity generated, which in turn depend on the design and size of the nuclear device, the specific detonation conditions (locally extremely high temperatures and pressures), and the characteristics of the detonation medium.
As expected, the impact of short-lived radionuclides on the ecosystem is most significant in the early stages, as their concentrations decrease significantly due to physical decay and environmental dispersion [
5].
Beginning in the late 1960s and early 1970s, research began to be conducted focusing on the ecological impact of 95Zr, 95Nb, 103Ru, and 106Ru, which had been introduced into aquatic ecosystems through global fallout, accidents, and waste disposal from nuclear facilities. However, there is a limited amount of available data regarding the accumulation of 95Zr, 95Nb, 103Ru, and 106Ru in fish. Therefore, we have systematically reviewed all the existing literature dedicated to the accumulation of these radionuclides in aquatic ecosystems.
Regular monitoring studies of radionuclides such as
137Cs and
106Ru in the surface and coastal waters of the British and Channel Islands were not initiated until 1967 [
6], even though radioactive liquid effluents from the British Nuclear Fuels reprocessing plant at Sellafield, Cumbria (UK), had been discharged into the Irish Sea via pipeline since 1952.
106Ru was the dominant radionuclide present in the effluent. The authors of this study reported that
95Zr,
95Nb, and
106Ru in the effluent are particle-reactive radionuclides.
As a result of these discharges,
106Ru activity exceeding 100,000 Bq/kg dry weight was recorded in surface sediments of the Irish Sea in 1968 and 1972. In bottom sediments, significant quantities of
95Zr and
95Nb were also detected, resulting from discharges in the late 1960s and early 1970s. In 1968, one sample contained more than 500,000 Bq/kg dry weight of these radionuclides [
7].
Analysis of radionuclide content in various organs and tissues of mollusks has shown that the parts of the animal exposed to the external environment are more contaminated than internal tissues [
8]. In 1969, near Windscale, only two isotopes of cesium with traces of
106Ru were detected in the flesh of
Pleuronectes platessa (Linnaeus, 1758) collected near the pipeline, whereas
106Ru along with traces of
95Zr/
95Nb was detected in the remnants of fish muscle tissue filets. Different species of benthic organisms were found to contain
106Ru in concentrations ranging from 6.3 to 591.5 pCi/g (233 to 21,900 Bq/kg) fresh weight and
95Zr/
95Nb in concentrations ranging from <1.0 to 1043.0 pCi/g (up to 38,600 Bq/kg) fresh weight. The authors also suggested that zirconium, niobium, and ruthenium do not accumulate through the food chain [
9]. In 1970, in the vicinity of Windscale, zirconium, niobium, and ruthenium were almost undetectable in fish of various species. In the muscle tissue of
P. platessa,
106Ru was detected at levels below 0.1 pCi/g (3.7 Bq/kg) fresh weight.
95Zr/
95Nb in amounts ranging from 9 to 281 pCi/g (330 to 10,400 Bq/kg) fresh weight and
106Ru in amounts ranging from 0.0 to 60 pCi/g (up to 2220 Bq/kg) fresh weight were found in the muscle tissue of crustaceans [
10].
The accumulation of gamma-emitting radionuclides by the mussel
Mytilus edulis (Linnaeus, 1758) and the periwinkle
Littorina littorea (Linnaeus, 1758) was investigated in the vicinity of the Sellafield plant [
11]. In Ravenglass mussels sampled in 1983, the concentration of
95Nb was lowest in the shell (scraped), measured at 17 Bq/kg dry weight, and highest in the byssal threads, measured at 6110 Bq/kg dry weight. The distribution of niobium among the organs can be ranked as follows: byssal threads > viscera > periostracum > gills > muscle > mantle edge > shell. The distribution pattern of
106Ru in the organs of Ravenglass mussels differed from that of niobium. The lowest concentration of
106Ru was recorded in the mantle at 770 Bq/kg dry weight, where
95Nb was not detected. The highest concentration was observed in the viscera, reaching 16,000 Bq/kg dry weight. The distribution of
106Ru in mussel organs followed this order: viscera > byssal threads > gills > periostracum > mantle edge > muscle > mantle. Ruthenium was not detected in the shell.
Berg and Ginsberg [
12], in 1972, investigated the accumulation of
106Ru by crayfish (
Orconectes obscurus (Hagen, 1870) and
Cambarus robustus (Girard, 1852)) in streams contaminated with radioactive waste in West Valley, New York (USA). In samples collected in 1972,
106Ru accounted for more than 90% of the total gamma radioactivity and was found in higher concentrations in crayfish compared to fish from the same sampling sites. Most of the ruthenium in crayfish organisms was associated with the exoskeleton, where it was distributed proportionally to the surface area of the integumentary system. Ruthenium levels in large muscle groups represented only a small fraction of the total body concentration. Although the digestive gland accounted for only 7% of body weight, it retained 29% of the total body burden of ruthenium. In Butlermilk Creek and Cattaraugus Creek,
106Ru concentrations in crayfish were 20 and 61 nCi/kg (740 and 2257 Bq/kg), respectively. Fish from these same water bodies did not accumulate ruthenium to the same extent as crayfish. More than half of the fish samples contained no detectable
106Ru, and the concentrations in fish ranged from 0 to 3.7 nCi/kg (0 to 137 Bq/kg).
Martin and Thomas summarized the transport of radionuclides from the Rhône River to the Mediterranean Sea during the period from 1982 to 1985 [
13]. The principal source of radioactive isotopes entering the river was the Marcoule reprocessing plant. It was found that, in suspended matter collected in the Rhône delta, the maximum concentration of
95Zr was <40.7 Bq/kg dry weight. Concentrations of
106Ru reached up to 180 Bq/kg dry weight, and
103Ru up to 22.2 Bq/kg dry weight. In sediments, the concentrations were significantly lower:
95Zr at 4.7 Bq/kg dry weight,
106Ru up to 109.2 Bq/kg dry weight, and
103Ru up to 2.29 Bq/kg dry weight. The authors noted that it is not always possible to distinguish between radionuclide inputs from the Marcoule reprocessing facility and those resulting from atmospheric deposition.
As reported in [
14], during a large-scale investigation of radionuclide contamination in the Bohai Sea and Yellow Sea in 1981–1982, the average concentration of
106Ru in coastal seawater was 16.7 Bq/m
3. The average concentrations of
106Ru in different marine organisms were as follows: 0.40 Bq/kg in fish muscle, 0.17 Bq/kg in mollusks, 0.20 Bq/kg in shrimp and crabs, and 0.89 Bq/kg in the algae
Laminaria japonica (Areschoug, 1851) (all values given in fresh weight). In the bottom sediments of the Bohai Sea in 1981, the average concentration of this radionuclide was 20 Bq/kg dry weight.
Coughtrey and Thorne [
15] indicated that
95Zr can be significantly accumulated by filter-feeding or sediment-dwelling invertebrates. Most of the accumulated activity is associated with the exoskeleton, followed by the gills and digestive glands. In
Anodonta cygnea (Linnaeus, 1758), 93.5% of the
95Nb activity in the whole organism was attributable to the shell, while 48% of the total activity in soft tissues was concentrated in the gills, 31.2% in the visceral mass, 12.5% in the mantle, and 8.3% in the muscle. Mollusks accumulate radioactive isotopes of ruthenium in their guts and external organs. More than 50% of all
103Ru and
106Ru accumulated by mollusks is retained in the exoskeleton, and likely less than 15% of the radionuclide load in soft tissues is retained in the muscle. In fish
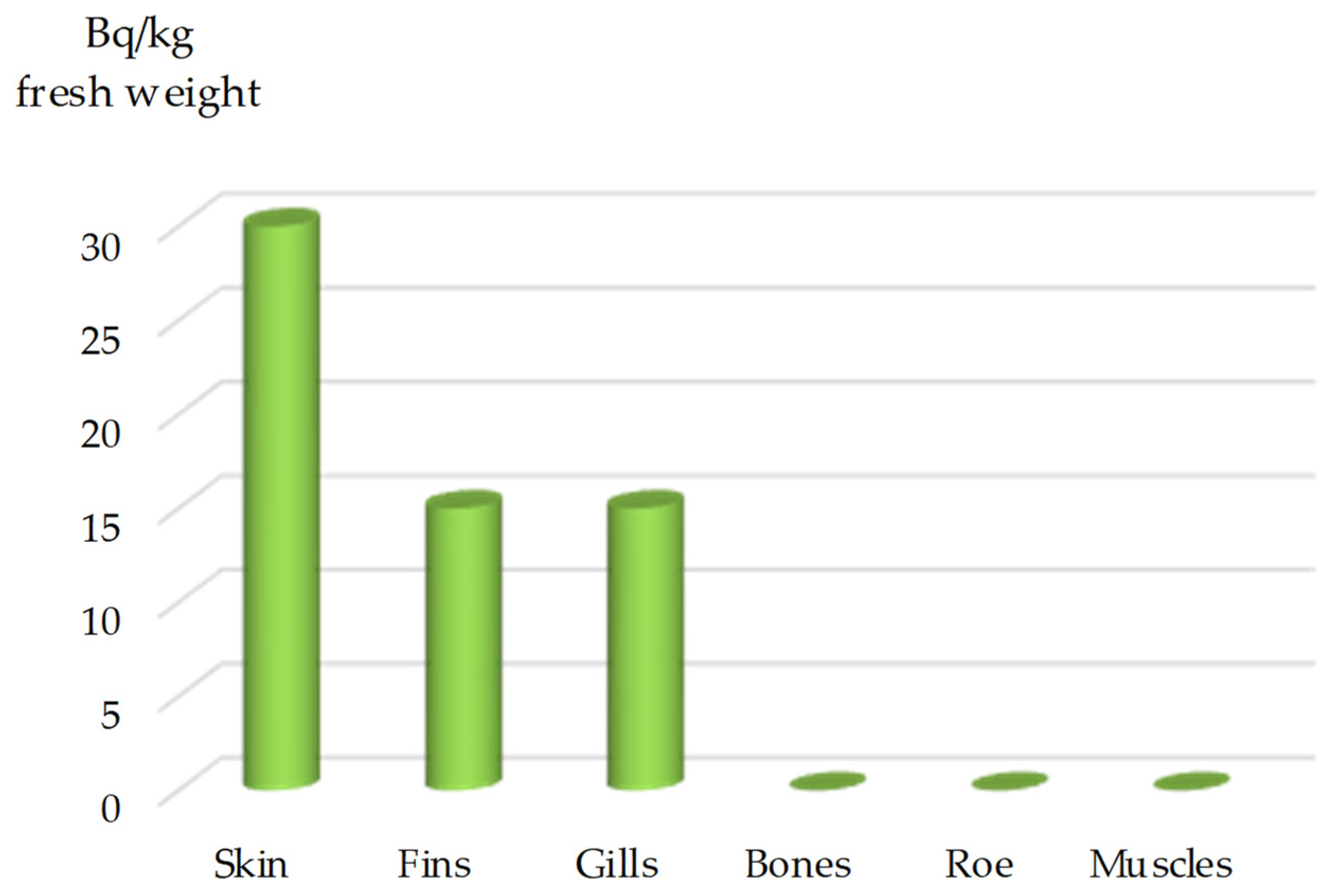
Thunnus thynnus (Linnaeus, 1758), the highest activity of
106Ru was recorded in the liver, measured at 139 pCi/g (5100 Bq/kg) fresh weight. The sequence of
106Ru content in
T. thynnus was as follows: liver > kidney > gills > bone > dark muscles > pyloric caeca > light muscles.
106Ru was not detected in the skin, scales, heart, stomach, spleen, intestinal contents, stomach contents, or gonads of this species.
Studies of the accumulation of
95Zr,
95Nb,
103Ru, and
106Ru were conducted under laboratory conditions before the Chornobyl accident. The authors of [
5] investigated the accumulation of
95Zr/
95Nb,
103Ru, and other radionuclides by aquatic organisms using a system of continuous recirculation of aquarium water through a column containing sediment material derived from the nuclear cratering event “Cabriolet” (solid debris from the 2-kiloton Plowshare crater explosion conducted in hard rock). Twenty days after the start of the experiment, the concentration of
103Ru in the soft tissues of freshwater bivalves reached 6200 pCi/kg (229.4 Bq/kg). This radionuclide was not recorded in the muscles of crustaceans; however, it was detected in the viscera at 39,000 pCi/kg (1443 Bq/kg). In freshwater fish,
103Ru was not recorded after 20 days. In marine animals,
103Ru was detected in the following concentrations: for bivalves, soft tissues—1700 pCi/kg (62.9 Bq/kg); for crustaceans, muscles—1400 pCi/kg (51.8 Bq/kg), internal organs—14,000 pCi/kg (518 Bq/kg); for fish, muscle—not detected, non-muscle tissues—1800 pCi/kg (66.7 Bq/kg), whole body—1700 pCi/kg (62.9 Bq/kg). The radionuclides
95Zr and
95Nb were rarely, if ever, detected in water samples or aquatic fauna.
The study [
16] presents experimental data on the bioaccumulation and depuration kinetics of
95Zr and
95Nb in a seawater/fish system under controlled laboratory conditions. More than 60% of the total content of
95Zr and
95Nb in
Acanthogobius flavimanus (Temminck & Schlegel, 1845) was concentrated in internal organs, mainly in the digestive tract, which may be considered an external organ since marine fishes must absorb significant amounts of seawater (8–55% of body weight per day) to maintain osmotic balance between their internal environment and the seawater. The
95Zr content in external tissues (gills, fins, skin) was significantly higher than in internal tissues (muscle, blood, liver, and bone).
Experimental studies on the uptake and accumulation of
106Ru by
Oncorhynchus mykiss (Walbaum, 1792) were conducted to obtain information on radioecological concentration during fish egg development and larval growth. The highest activity of
106Ru (30,000 cpm/g) was observed in eyed eggs treated with a solution of ruthenium-containing salt (
106Ru chloro complexes, product code RKS-2, from The Radiochemical Centre at Amersham, United Kingdom). In fry, the highest concentration was recorded in the visceral mass at 600 cpm/g, while the lowest was found in muscle tissue at 18 cpm/g. The sequence of
106Ru concentrations in fry was as follows: viscera (including digestive tract) > liver > gills > skin > bones > muscles [
17]. When the roe of
Salmo gairdneri irideus (a synonym for
O. mykiss) was separated into egg capsule, embryo, and yolk, the majority of
106Ru was associated with the egg capsule [
18].
The uptake of
106Ru by freshwater organisms was studied in an aquarium ecosystem consisting of natural river water, duckweeds, mud snails, river snails, freshwater mussels, and crucian carp (
Carassius carassius (Linnaeus, 1758)), as well as bottom sediments [
19]. The mean concentration ratios of
106Ru in the organisms demonstrated a decreasing trend in the following order: duckweeds > mud snails > river snails > freshwater mussels >
C. carassius. In
C. carassius, approximately 80% of the total radioactivity was concentrated in the visceral mass, including the digestive tract.
As previously discussed, 95Zr, 95Nb, 103Ru, and 106Ru can enter aquatic environments through various pathways. The contamination of aquatic ecosystems raises significant concerns regarding ecological integrity and public health. Particularly important is the need to assess the potential health risks associated with the consumption of fish and other aquatic organisms exposed to these radionuclides. This issue remains especially relevant in regions where local communities rely heavily on aquatic resources for nutrition and economic stability.
4. Radionuclide Contamination in Chornobyl NPP Cooling Pond and Kaniv Reservoir: Effects on Water and Aquatic Biota
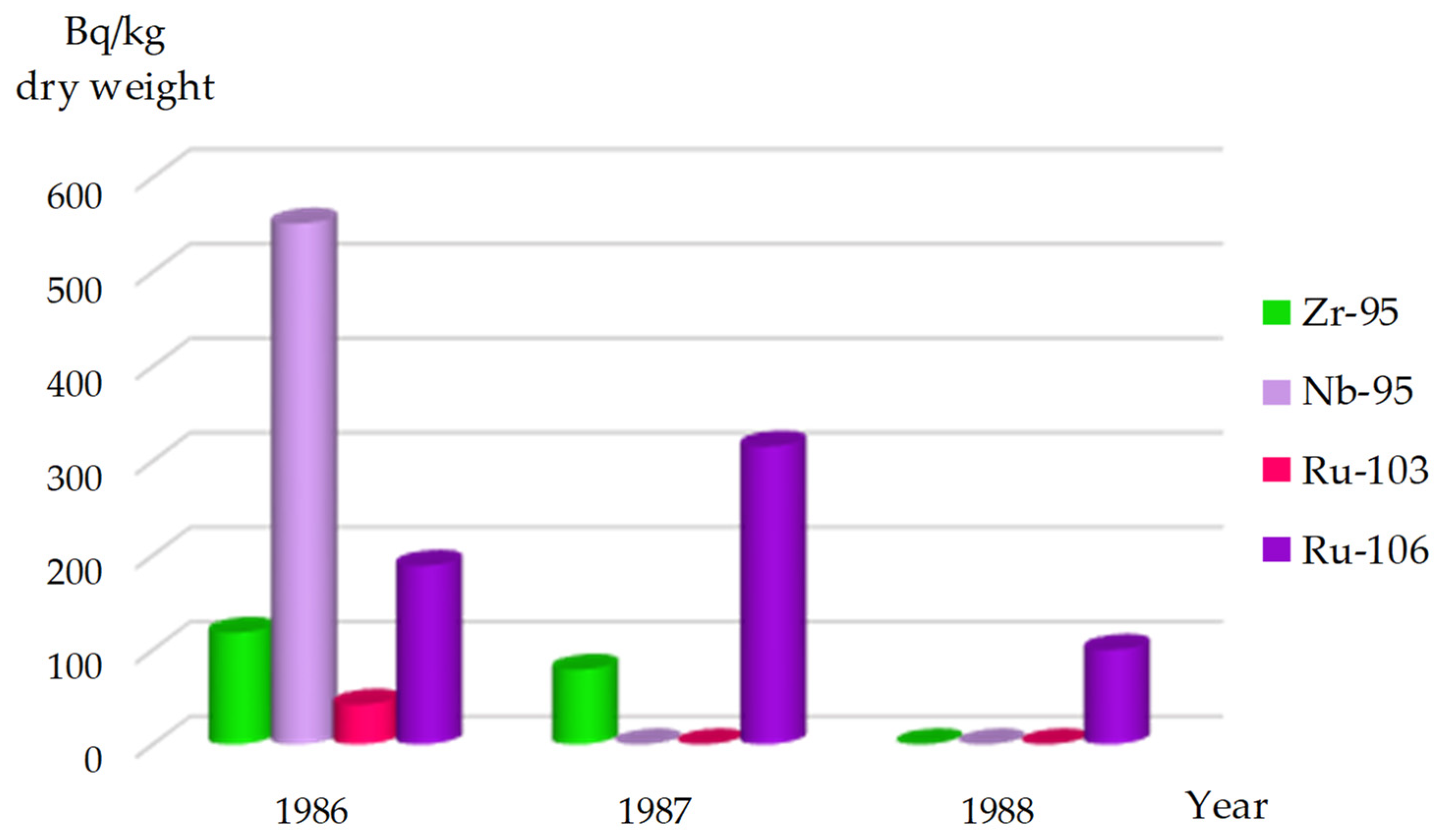
Zirconium (95Zr): Before 1986, this radionuclide was intermittently detected in vegetation and bottom sediments of the Chornobyl NPP Cooling Pond, with levels never exceeding 50 Bq/kg dry weight [
30].
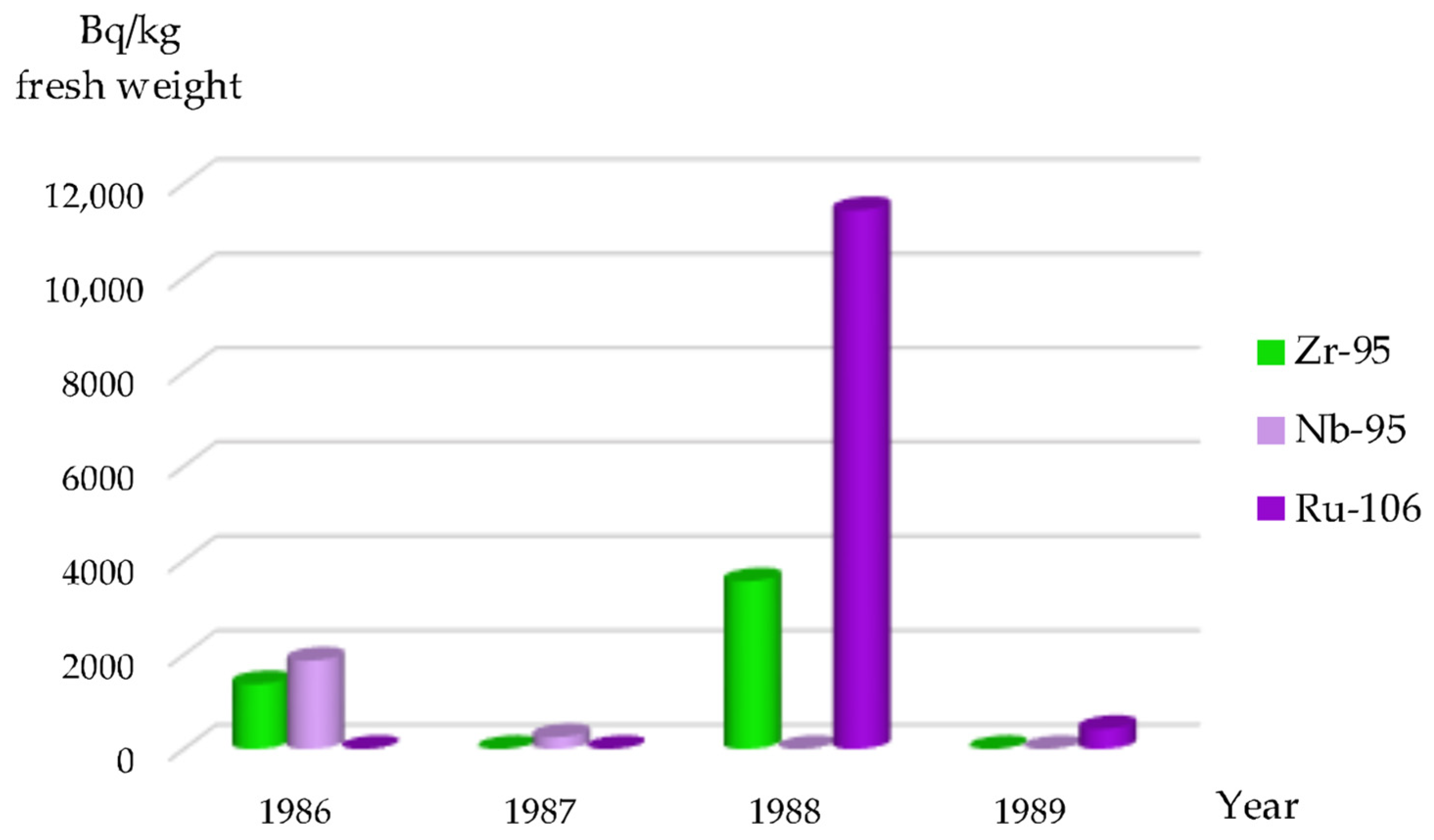
Following the Chornobyl accident, the highest concentrations of
95Zr were recorded in species of the genus
Potamogeton, reaching up to 1,000,000 Bq/kg dry weight in aquatic vegetation within the Cooling Pond. The concentration of this radionuclide in filamentous algae (
Cladophora spp.) was slightly lower, at 680,000 Bq/kg dry weight.
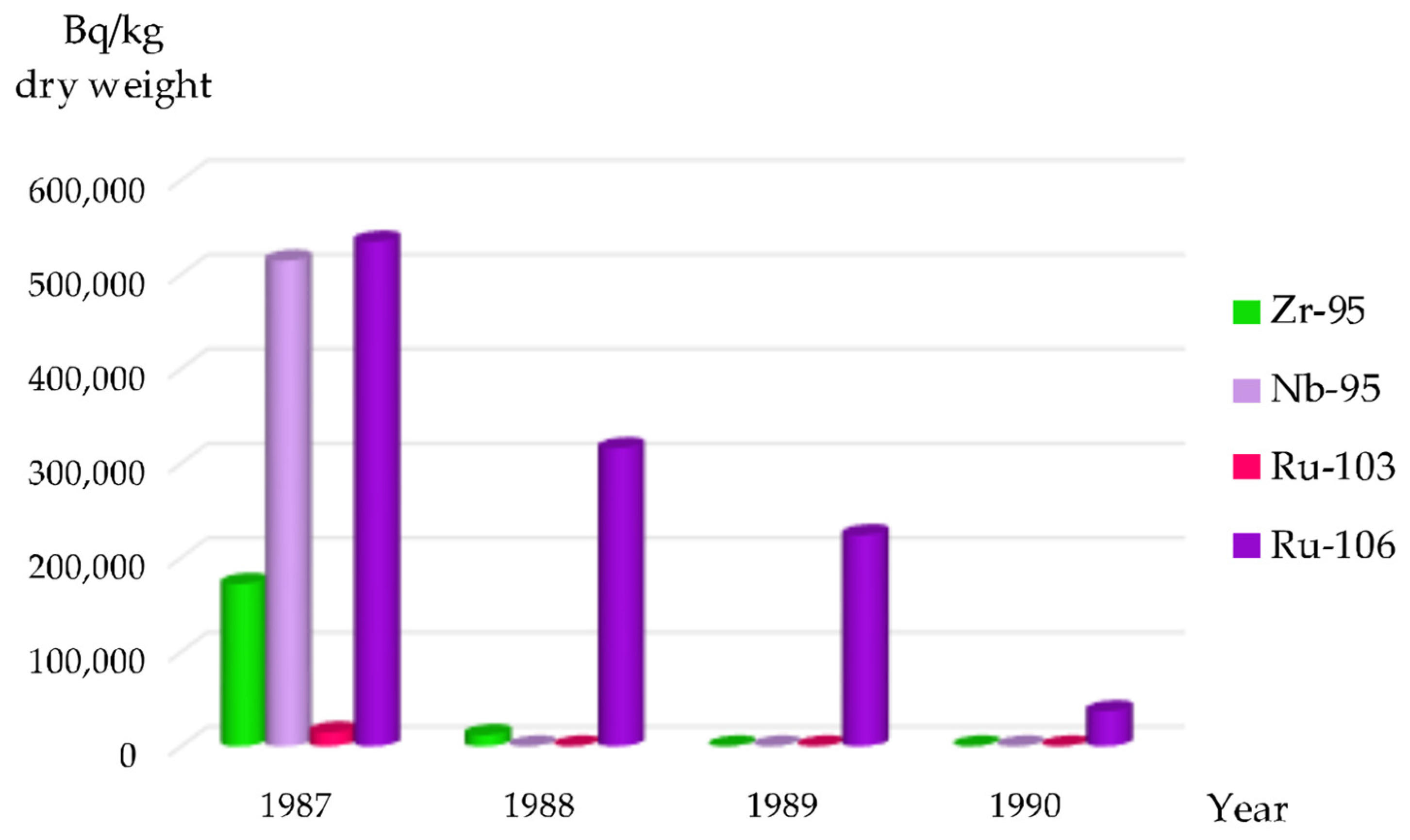
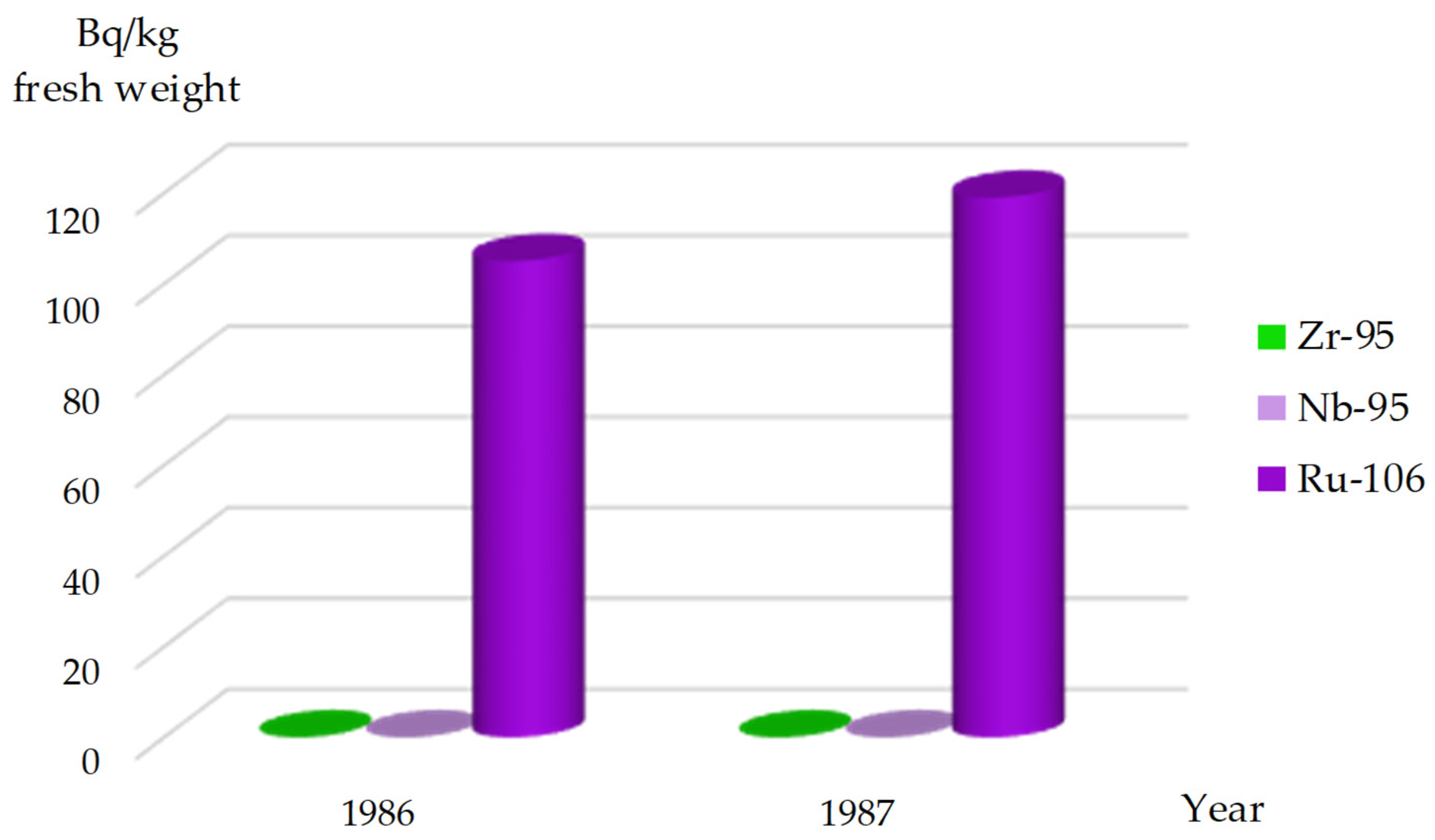
95Zr was identified in this algae species from 1986 to 1988 (
Figure 3), with the last detection of
95Zr in
Cladophora spp. in the Kaniv Reservoir occurring in 1987 (
Figure 4). The rapid decline of
95Zr activity in aquatic vegetation is likely due to its short physical half-life rather than biological decontamination processes within the plant tissues.
95Zr was generally detected in fish from the ChNPP Cooling Pond between 1986 and 1989 [
30]. In 1990,
95Zr was identified in a single fish sample, specifically in the internal organs of
Scardinius erythrophthalmus (Linnaeus, 1758), at a concentration of 27 ± 11 Bq/kg fresh weight (
Table 1).
95Zr was rarely found in piscivorous species from the ChNPP Cooling Pond, with the exception of a single sample of Leuciscus aspius (Linnaeus, 1758) showing 31 ± 12 Bq/kg fresh weight (carcass, May 1989). This radionuclide was more frequently detected in omnivorous and planktivorous fish (Cyprinus carpio (Linnaeus, 1758), Carassius carassius (Linnaeus, 1758), Blicca bjoerkna (Linnaeus, 1758), Hypophthalmichthys molitrix (Valenciennes, 1844), Abramis brama (Linnaeus, 1758)), and less frequently in carnivorous fish (Silurus glanis (Linnaeus, 1758)). This distribution may be attributed to the type of feeding in fish: omnivores may ingest bottom sediments and detritus where ‘hot particles’ containing radioactive zirconium have settled, while planktivores may accumulate this radionuclide directly from suspended sediments and plankton, which contain higher concentrations. The absence of detectable 95Zr in piscivores suggests that the radionuclide is poorly available for absorption into the bloodstream and further distribution within the fish organism when consumed.
95Zr was consistently found in fish organs and tissues that come into direct contact with water, such as the head, scales, mucus, and fins (
Figure 5 and
Figure 6,
Table 1). The lowest concentrations were recorded in muscle, bone, and fat tissues.
95Zr was not detected in the liver and kidneys of fish across various species.
Table 1.
Content of 95Zr in fish tissues and organs (Bq/kg, fresh weight) from the Cooling Pond of the ChNPP, with sampling periods in the early years following the Chornobyl accident.
Table 1.
Content of 95Zr in fish tissues and organs (Bq/kg, fresh weight) from the Cooling Pond of the ChNPP, with sampling periods in the early years following the Chornobyl accident.
| Sample Collection Period | Tissue or Organ | 95Zr min | 95Zr max |
|---|
| November 1986–March 1988 | Head | 117 | 7579 |
| February 1987–August 1987 | Gills | 12 | 429 |
| October 1987–March 1989 | Muscles | 68 | 128 |
| November 1986–March 1990 | Internal Organs | 27 | 59,177 |
| November 1986–March 1988 | Intestines with Content | 37 | 3062 |
| March 1987 | Roe | 22,225 |
| August 1987–March 1988 | Fat | 16 | 127 |
| November 1987 | Swimming Bladder | 866 |
| August 1987–October 1987 | Bones | 177 | 247 |
| November 1986–March 1988 | Fins | 97 | 2635 |
| November 1986–August 1987 | Scales | 67 | 1240 |
| November 1986–March 1987 | Whole Fish | 109 | 15,424 |
In the first month following the Chornobyl accident, studies conducted in the Kaniv Reservoir indicated that
95Zr contributed significantly to the radioactive contamination of aquatic vegetation [
4]. According to the report [
31],
95Zr and
95Nb were the predominant radionuclides contaminating higher aquatic plants in the Reservoir during July–August 1986.
In the Kaniv Reservoir,
95Zr was detected in fish only during 1986 and 1987 (
Table 2). Unlike the ChNPP Cooling Pond,
95Zr was more frequently found in piscivorous species (
L. aspius,
Sander lucioperca (Linnaeus, 1758)), as well as in carnivorous species (
S. glanis,
Perca fluviatilis (Linnaeus, 1758)) during 1986. This can be attributed to the increased bioavailability of the radionuclide at greater distances from the ChNPP. Therefore, in the Kaniv Reservoir, the trophic transfer and bioaccumulation of
95Zr likely became the primary factor influencing its distribution.
Table 2.
Content of radionuclides (95Zr, 95Nb, 103Ru, and 106Ru) in fish tissues and organs (Bq/kg, fresh weight) from the Kaniv Reservoir, with sampling periods in the early years following the Chornobyl accident.
Table 2.
Content of radionuclides (95Zr, 95Nb, 103Ru, and 106Ru) in fish tissues and organs (Bq/kg, fresh weight) from the Kaniv Reservoir, with sampling periods in the early years following the Chornobyl accident.
| Sample Collection Period | Tissue or Organ | 95Zr min | 95Zr max |
|---|
| October 1986 | Head | 58 |
| September 1986 | Skin | 29 |
| September 1986 | Internal Organs | 19 |
| 17–27 September 1986 | Fins | 15 | 31 |
| May 1986–June 1987 | Whole Fish | 11 | 1112 |
| | | 95Nb min | 95Nb max |
| September 1986–October 1986 | Head | 10 | 120 |
| September 1986 | Skin | 22 |
| October 1986 | Internal Organs | 54 |
| September 1986–November 1987 | Roe | 7 | 42 |
| September 1986 | Bones | 32 |
| September 1986 | Scales | 15 |
| September 1986–June 1987 | Whole Fish | 3 | 129 |
| | | 103Ru min | 103Ru max |
| September 1986 | Head | 12 |
| September 1986 | Skin | 19 |
| September 1986–October 1986 | Roe | 7 | 12 |
| September 1986 | Bones | 32 |
| September 1986 | Fins | 25 |
| May 1986 | Whole Fish | 630 |
| | | 106Ru min | 106Ru max |
| October 1986–July 1987 | Internal Organs | 59 | 143 |
| October 1986–August 1988 | Whole Fish | 60 | 361 |
In the Kaniv Reservoir, a similar accumulation pattern of
95Zr in fish was observed, with the highest concentrations detected in organs and tissues in contact with water, and the lowest in muscles, bones, and roe (
Figure 7).
Niobium (95Nb): Niobium was detected in omnivorous and planktivorous fish (C. carpio, C. carassius, B. bjoerkna, H. molitrix, Abramis brama (Linnaeus, 1758)), as well as in carnivorous fish (S. glanis and Ictalurus punctatus (Rafinesque, 1818)) from the ChNPP Cooling Pond, with a single exception of piscivore L. aspius (11 ± 5 Bq/kg, fresh weight, head, May 1989). In herbivorous fish (Scardinius erythrophthalmus (Linnaeus, 1758), 95Nb was detected only once in a “whole fish” sample at 224 ± 113 Bq/kg fresh weight. The bioaccumulation of niobium in fish was likely influenced by its initial introduction into the Cooling Pond primarily via ‘hot particle’ transport. Omnivores and carnivores likely obtained 95Nb from particles settled at the bottom of the Cooling Pond, while planktivores accumulated it from plankton and suspended matter.
The distribution of
95Nb in fish organs and tissues followed a similar pattern to that of
95Zr, but the specific activity levels of
95Nb were several times higher [
32]. The highest specific activity of
95Nb was observed in the internal organs of
H. molitrix, with a concentration of 204 ± 14 × 10
3 Bq/kg fresh weight in February 1987. Additionally, unlike
95Zr, niobium was detected in the kidneys and liver of
H. molitrix at various sampling periods, with concentrations approaching 1 kBq/kg in the liver (
Table 3). In the same species, the concentration of
95Nb in roe was the next highest. Significant levels of this radionuclide were also detected in muscles until spring 1989, nearly three years post-accident.
It is likely that 95Nb enters the fish organism solely through ingestion, is minimally processed in the digestive system, and is not significantly accumulated, except in a few instances. This is corroborated by the absence of its detection in piscivorous species. Furthermore, the presence of 95Nb in fish tissues that come into contact with water may indicate external contamination rather than percutaneous absorption.
Table 3.
Content of 95Nb in fish tissues and organs (Bq/kg, fresh weight) from the Cooling Pond of the ChNPP, with sampling periods in the early years following the Chornobyl accident.
Table 3.
Content of 95Nb in fish tissues and organs (Bq/kg, fresh weight) from the Cooling Pond of the ChNPP, with sampling periods in the early years following the Chornobyl accident.
| Sample Collection Period | Tissue or Organ | 95Nb min | 95Nb max |
|---|
| November 1986–May 1989 | Head | 11 | 25,287 |
| February 1987–August 1987 | Gills | 31 | 1454 |
| March 1987–August 1987 | Skin | 40 | 475 |
| October 1987–March 1989 | Muscles | 142 | 537 |
| November 1986–March 1988 | Internal Organs | 22 | 203,687 |
| November 1987 | Liver | 985 |
| August 1987 | Kidneys | 175 |
| April 1987–March 1989 | Intestines with Content | 52 | 7436 |
| March 1987 | Roe | 57,535 |
| February 1987–March 1988 | Fat | 21 | 133 |
| August 1987–October 1987 | Bones | 220 | 911 |
| November 1986–October 1987 | Fins | 41 | 7601 |
| November 1986–August 1987 | Scales | 117 | 2021 |
| November 1986–May 1989 | Whole Fish | 76 | 21,957 |
In contrast to the Cooling Pond, in the Kaniv Reservoir,
95Nb was more frequently detected in piscivores (
L. aspius,
S. lucioperca). In
L. aspius, this radionuclide was recorded in multiple years in various body parts, including the head, scales, skin, bones, whole samples, and repeatedly in roe.
95Nb was not detected in carnivorous species. In omnivorous and planktivorous species from the Kaniv Reservoir, this radionuclide was primarily found in the head and whole fish samples, but not in muscle tissue (
Table 2).
Ruthenium (103Ru): Elevated levels of
103Ru were recorded in higher aquatic vegetation in the Kaniv Reservoir (
Figure 4), while significantly lower concentrations were detected in fish (
Table 2). As in the Kaniv Reservoir, the highest specific activity of
103Ru in the ChNPP Cooling Pond was found in higher aquatic plants, predominantly represented by
Potamogeton spp.
In general, fish accumulate most radionuclides through the food chain. In the Kaniv Reservoir,
103Ru was more frequently detected in piscivores. In the ChNPP Cooling Pond, however,
103Ru was found only in omnivores and planktivores, with the exception of a single carnivorous sample (
S. glanis). This difference likely reflects the varying biological availability of
103Ru, influenced by the size of the ‘hot particles’ entering the ecosystem, particularly with increasing distance from the Chornobyl NPP [
33].
The presence of
103Ru in the internal organs and whole fish samples of omnivores and planktivores from the Chornobyl Cooling Pond can likely be attributed to the ingestion of this radionuclide via food (
Table 4).
Table 4.
Content of 103Ru in fish tissues and organs (Bq/kg, fresh weight) from the Cooling Pond of the ChNPP, with sampling periods in the early years following the Chornobyl accident.
Table 4.
Content of 103Ru in fish tissues and organs (Bq/kg, fresh weight) from the Cooling Pond of the ChNPP, with sampling periods in the early years following the Chornobyl accident.
| Sample Collection Period | Tissue or Organ | 103Ru min | 103Ru max |
|---|
| November 1986–April 1987 | Head | 330 | 1320 |
| November 1986–April 1987 | Internal Organs | 173 | 6687 |
| March 1987 | Roe | 2294 |
| April 1987 | Fins | 211 |
| November 1986–December 1986 | Whole Fish | 366 | 3487 |
The accumulation intensity of
103Ru in fish increases with distance from the Chornobyl NPP, likely due to the changes in the size of
103Ru-containing ‘hot particles’ [
33].
Ruthenium (106Ru): In the Kaniv Reservoir,
106Ru was primarily detected in omnivorous fish (
Figure 8).
The highest number of samples containing
106Ru were from whole fish, with this radionuclide detected until August 1988 (
Table 2).
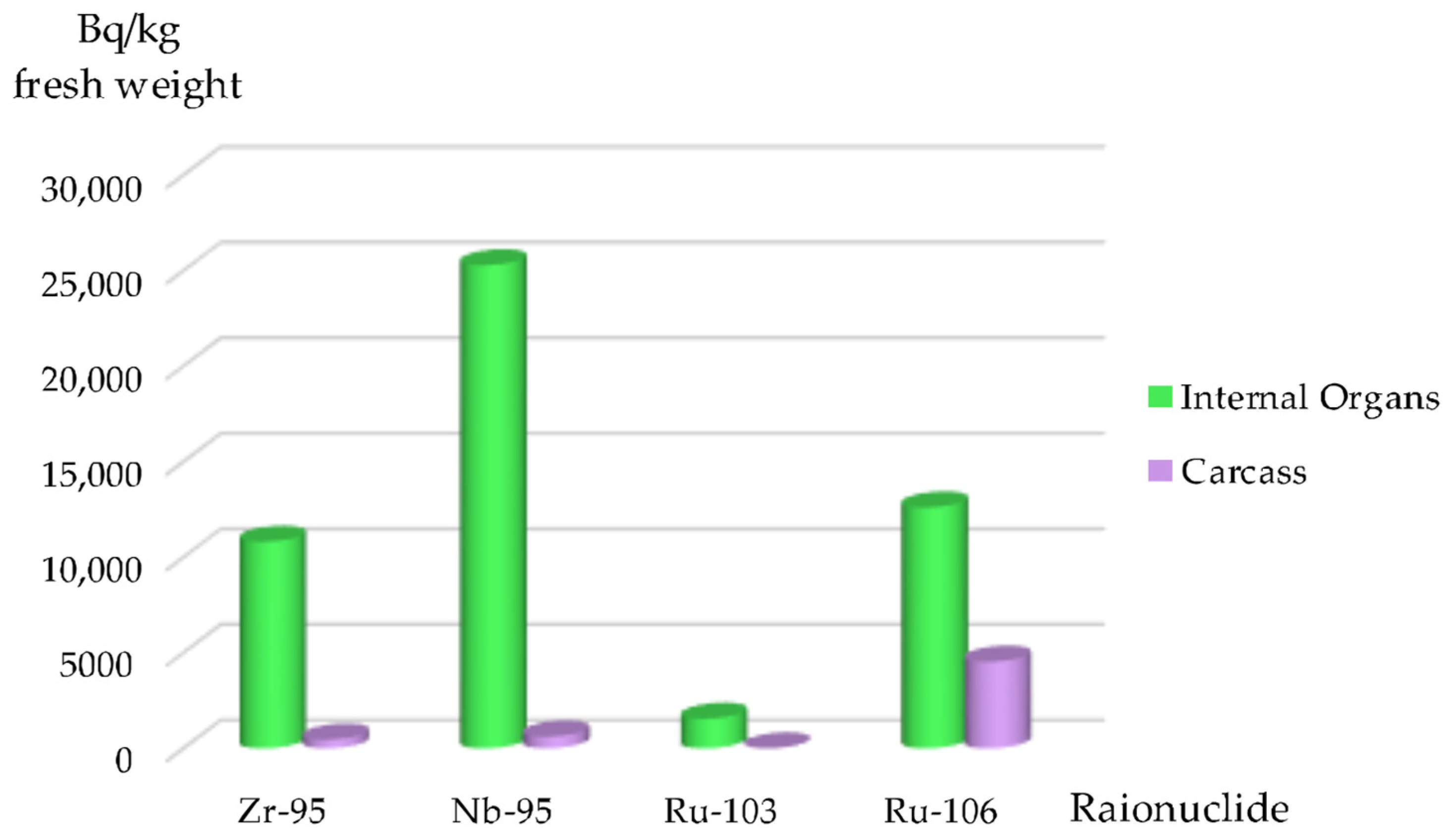
In contrast to the Kaniv Reservoir,
106Ru was found in fish belonging to different ecological groups in the ChNPP Cooling Pond, predominantly in omnivores, planktivores, and herbivores (
Figure 9).
106Ru was also detected in carnivores (S. glanis and I. punctatus). Piscivores, including L. aspius and S. lucioperca, also exhibited detectable levels of 106Ru.
The highest specific activities of
106Ru were predominantly found in internal organs, suggesting that the radionuclide entered the fish organism via food (
Table 5).
Table 5.
Content of 106Ru in fish tissues and organs (Bq/kg, fresh weight) from the Cooling Pond of the ChNPP, with sampling periods in the early years following the Chornobyl accident.
Table 5.
Content of 106Ru in fish tissues and organs (Bq/kg, fresh weight) from the Cooling Pond of the ChNPP, with sampling periods in the early years following the Chornobyl accident.
| Sample Collection Period | Tissue or Organ | 106Ru min | 106Ru max |
|---|
| November 1986–March 1990 | Head | 135 | 13,278 |
| March 1987–April 1989 | Gills | 25 | 3700 |
| October 1987–February 1990 | Skin | 190 | 933 |
| October 1987–March 1989 | Muscles | 535 | 106,875 |
| November 1986–March 1990 | Internal Organs | 92 | 2,769,433 |
| August 1987 | Liver | 379 |
| November 1987–March 1990 | Intestines with Content | 316 | 22,906 |
| March 1987–March 1990 | Roe | 107 | 66,857 |
| August 1987–March 1988 | Fat | 62 | 758 |
| October 1987–September 1988 | Swimming Bladder | 217 | 643 |
| November 1987–March 1990 | Bones | 185 | 1019 |
| April 1987–March 1990 | Fins | 76 | 32,106 |
| February 1987–March 1990 | Scales | 74 | 513,088 |
| November 1986–May 1989 | Whole Fish | 50 | 21,139 |
The maximum
106Ru content (2,769,433 ± 234,254 Bq/kg fresh weight) was recorded in the internal organs of
H. molitrix in October 1987. Lower levels were found in fat, liver, and other organs not in contact with water [
34].
However, 106Ru was present in large quantities in the muscles of various ecological fish groups, including planktivores, piscivores, and carnivores in 1987–1989, i.e., not immediately after the accident.
The majority of the
106Ru-containing samples were fins and scales, suggesting that surface fixation of
106Ru particles on these tissues plays a significant role in fish contamination [
34].
5. Post-Chornobyl Accumulation of 95Zr, 95Nb, 103Ru, and 106Ru in Fish
Since 1986, studies have been conducted in various countries of the Northern Hemisphere to assess the impact of the Chornobyl accident on aquatic ecosystems. Data on the post-Chornobyl accumulation of
95Zr,
95Nb,
103Ru, and
106Ru in air, soil, vegetation, and agricultural products remain limited [
35,
36,
37,
38,
39,
40,
41,
42,
43,
44]. Moreover, the available literature provides few measurements of specific activity levels in fish.
Concentrations of
103Ru and
106Ru were measured in the water column and sediments of the North Basin of Windermere and Esthwaite Water (Cumbria, England) after the Chornobyl accident [
45]. Observations indicated that Ru and Cs exhibited similar behavior in both lake systems with respect to their sedimentation rates.
Large particles collected at a depth of 200 m, 15 nautical miles off the coast of Calvi (Corsica), on 22 May 1986, contained Chornobyl-derived
95Zr,
95Nb,
103Ru, and
106Ru. Zooplankton sampled on 6 May contained
95Nb at 12 Bq/kg,
103Ru at 280 Bq/kg, and
106Ru at 70 Bq/kg (all based on dry weight);
95Zr was not detected [
46]. The authors suggested that zooplankton, by ingesting radioactive fallout particles, produced fecal pellets that rapidly transported radionuclides to deeper waters within days, much faster than the expected years-long particle sedimentation model.
In the same region of the Mediterranean Sea, near Monaco, the accumulation of radionuclides from the Chornobyl release in the mollusk
Mytilus galloprovincialis (Lamarck, 1819) was investigated [
47]. Peak concentrations of
103Ru temporarily reached 480 Bq/kg (soft tissues, fresh weight). The soft tissues, including the gut, concentrated radionuclides approximately ten times more than the shells. In
Patella lusitanica (Gmelin, 1791), the concentrations were 20–100 times higher than those observed in mussels.
The effects of the Chornobyl accident on aquatic biota were also examined in northern Scotland [
48]. Significant levels of
137Cs,
134Cs,
106Ru,
103Ru, and
110mAg were detected in the marine environment. Both the brown alga
Fucus vesiculosus (Linnaeus, 1753) and the clam
Patella vulgata (Linnaeus, 1758) demonstrated rapid accumulation of Cs and Ru radionuclides, with peak values recorded within days of radioactive fallout reaching the UK.
Due to the Chornobyl accident, intensified monitoring was implemented around the Tokai Works of the Power Reactor and Nuclear Fuel Development Corporation (PNC), located 120 km north of Tokyo, Japan [
49]. Marine fish species such as
Pleuronectes platessa (Linnaeus, 1758), as well as algae, clams, shrimp, squid, and seawater samples, were collected.
131I,
103Ru, and
106Ru were detected in algae;
103Ru,
106Ru, and
110mAg were found in cephalopods and clams.
Radionuclides, including
103Ru and
106Ru, were detected shortly after the Chornobyl accident in the Mediterranean seagrass
Posidonia oceanica ((Linnaeus) Delile, 1813). Samples were collected along the northwestern French Mediterranean coastline, including Corsica. The species showed selective distribution of radionuclides among tissues, with adult leaves exhibiting the highest contamination [
50].
The brown alga
F. vesiculosus served as a bioindicator for assessing the spatial and temporal distribution of radionuclides in the Baltic Sea following the Chornobyl accident [
51]. In samples collected along the eastern, southern, and southwestern coasts of Sweden in July 1986,
103Ru ranged from 12 ± 0.2 to 181 ± 14 Bq/kg,
106Ru from 0 to 136 ± 18 Bq/kg, and
95Zr from 0.0 to 60 ± 4 Bq/kg (all dry weight). By August–September 1987,
95Zr was no longer detected, and
106Ru activity had decreased to ≤22 ± 5 Bq/kg. No ruthenium isotopes were recorded in 1988.
Snoeijs and Notter [
52] reported extremely high concentrations of fission products in diatom algae in and around the Biotest Basin (northern Baltic Sea, Sweden) within a week after the Chornobyl accident. On 6 May 1986, epilithic diatom communities contained
95Zr ranging from 14 to 864 kBq/kg,
95Nb from 18 to 1022 kBq/kg, and
103Ru from 16 to 540 kBq/kg (all dry weight). After April 1987, only
106Ru,
134Cs, and
137Cs were detectable. The radionuclides were likely bound to the organic envelope surrounding the diatom shells.
From 1990 to 2009, low concentrations of
106Ru were observed in the marine environment near the Channel Islands [
6]. In
Fucus seaweed, levels were <6 Bq/kg fresh weight; in mollusks (
Osilinus lineatus (da Costa, 1778) and
Patella vulgate (Linnaeus, 1758)), <10 Bq/kg; in sediment, <100 Bq/kg dry weight. The highest concentrations of
106Ru were recorded at the beginning of monitoring (1990), with a sharp decline by 1998. The presence of radionuclides in this region is not attributed to Chornobyl fallout but is believed to result from discharges from the La Hague nuclear fuel reprocessing plant in France. No detectable impact from radioactive waste disposal at the Hurd Deep site (1950–1963) was reported.
The bioavailability of
95Zr was examined in aquarium experiments using the edible shallow-water marine fish
Bostrichthys sinensis (Lacepède, 1801) [
53]. The highest accumulation was found in internal organs, gills, and fins. Concentrations in muscle, bone, liver, and roe were only slightly above detection limits. The authors noted that zirconium, being a non-essential element, is not readily transported to these tissues following ingestion or adsorption by visceral organs, gills, or fins.
An artificial aquatic ecosystem composed of water, sediment, aquatic plants (
Eichhornia crassipes (Kunth, 1843)), snails (
Bellamya purificata (Heude, 1890)), and fish (
Carassius auratus (Linnaeus, 1758)) was established under laboratory conditions [
54]. Results indicated that the specific activity of
95Nb in different fish tissues decreased in the following order: viscera > gills > fins > skin and scales > bones, head, roe > muscle. Specific activity in muscle, bones, liver, and roe remained low and only slightly above background levels.
95Nb persisted in the intestine, stomach, gills, and fins, with limited transport to internal organs. Maximum activity levels in skin and scales were 990 and 710 Bq/kg, respectively. The highest enrichment of
95Nb from water occurred in
E. crassipes, followed by
B. purificata, and then
C. auratus.
Contaminated soil samples from the Kapikule Customs Grounds in Edirne (Turkey), affected by the Chornobyl accident, were used in aquarium experiments to investigate radionuclide accumulation in duckweed (
Spirodela punctata (G. Mey.) C.H. Thomps) and a mixture including
S. punctata,
Lemna minor (Linnaeus, 1758), and
Lemna gibba (Linnaeus, 1758). It was found that
106Ru leached into water more readily than cesium, although it was detected only in mixed duckweed samples, indicating a relatively low transfer coefficient compared to
134Cs and
137Cs [
55].
The uptake of radionuclides by
C. carpio from water sourced from the Rhône River (southeastern France) was examined in [
56]. The experiment was conducted in a semi-natural 3 m
3 pond located downstream of the Marcoule fuel reprocessing facility, continuously supplied with Rhône water. The highest concentration of
106Ru was detected in the digestive tract of fish. Muscle tissue showed the lowest concentrations and, despite its mass, accounted for only 1–5% of total radioactivity.
These observations strongly support the hypothesis that the transport mechanisms of such radionuclides in aquatic environments are not only influenced by their chemical properties, but also significantly shaped by ecological interactions and organism-specific metabolic processes, thus requiring further multidisciplinary studies to fully understand the long-term implications for both environmental and human health, particularly in post-accident scenarios where exposure routes may vary.
6. Summary
This study presents a comprehensive analysis of the scientific literature and original data concerning the input, distribution, and accumulation of 95Zr, 95Nb, 103Ru, and 106Ru in aquatic ecosystems. These isotopes exhibit varying bioavailability, different accumulation mechanisms in fish tissues, and show diverse abilities to participate in the trophic chain.
Table 6 provides a summarized analysis of the content of these radionuclides in fish based on literature sources and original data from the authors.
Particular attention should be drawn to the fact that all radionuclides were detected in significant concentrations in the fish roe from the Cooling Pond of the Chornobyl Nuclear Power Plant. Additionally, 95Nb and 103Ru were also found in fish roe from the Kaniv Reservoir. This situation poses a potential risk both to aquatic organisms and to humans. For fish, high concentrations of radionuclides in roe can have mutagenic or lethal effects, impacting embryonic development, reducing reproductive capacity, and disrupting natural population processes. On the other hand, human consumption of contaminated roe may lead to internal radiation exposure, which could cause serious health disorders over time. Thus, the presence of radionuclides in the reproductive tissues of fish signals potential threats to ecological safety and human health.
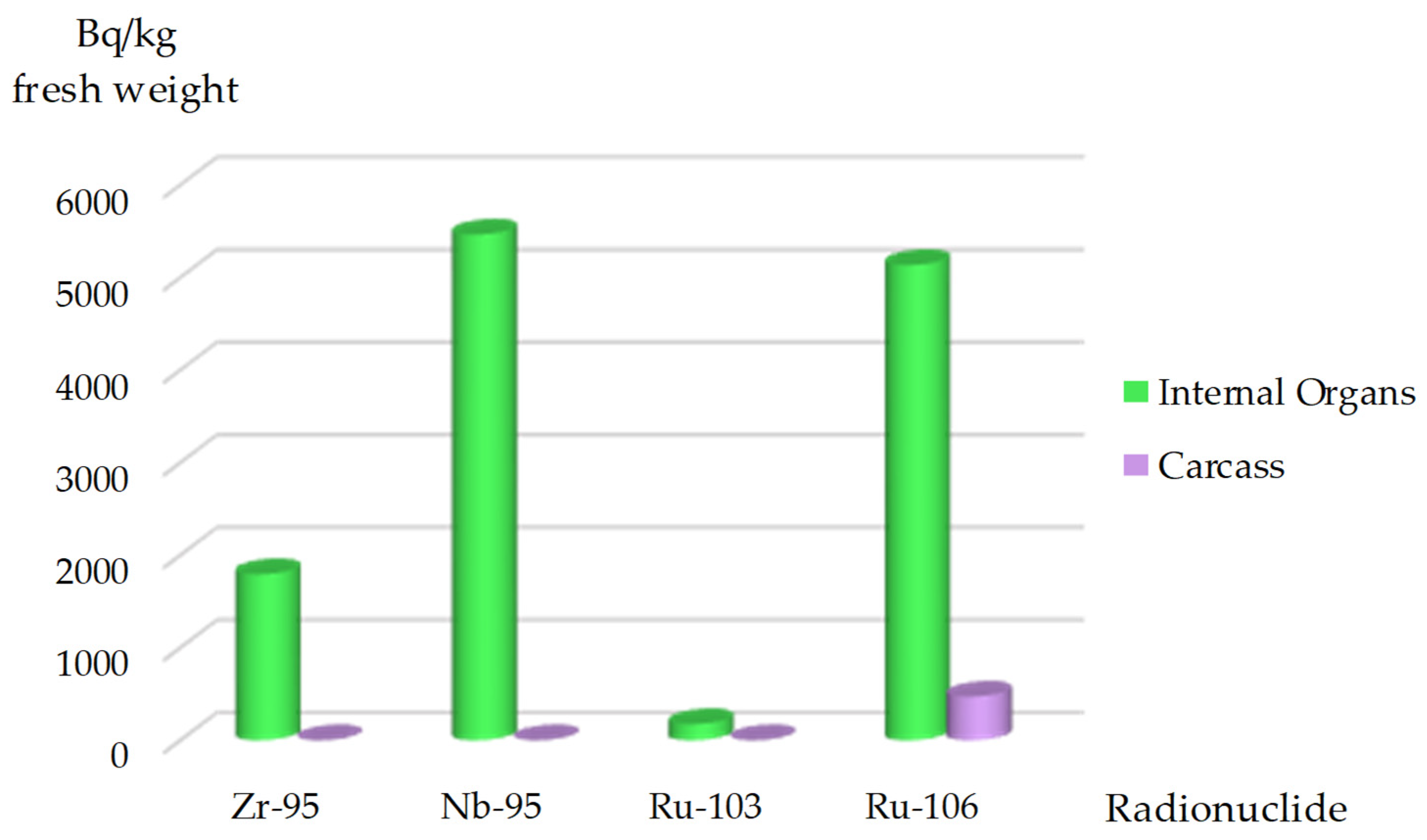
When comparing the accumulation of
95Zr,
95Nb,
103Ru, and
106Ru in fish from various ecological groups, omnivores and planktivores showed the highest accumulation levels of
95Zr and
95Nb. This may be due to these fish species consuming benthos and plankton that contained ‘hot particles’. In carnivores, nearly all isotopes were observed, with particular sensitivity to
95Nb and
106Ru.
95Zr,
95Nb,
103Ru, and
106Ru were also detected in piscivores, with high levels of
106Ru found in the muscle tissues of piscivores, suggesting possible trophic transfer of this radionuclide (
Figure 10).
The ability of 106Ru to penetrate fish muscle tissue deserves special attention. In 1986, this radionuclide was not detected in the muscle tissues of fish from the Cooling Pond. The maximum concentration of 106Ru (106,875 Bq/kg fresh weight) was recorded in March 1989, nearly three years after the accident. This suggests that the redistribution of 106Ru in fish bodies occurs very slowly, with its arrival in muscle tissue taking more than a year after ecosystem contamination. 106Ru was found in the muscle tissues of fish from various ecological groups: planktivores (H. molitrix), piscivores (S. lucioperca, L. aspius), and carnivores (S. glanis). This indicates an extraordinarily high biological availability of 106Ru, regardless of the type of nutrition. It also confirms that this isotope has the ability to penetrate the “most protected” tissues of fish, which was previously considered unlikely.
Unfortunately, a comparison of the
106Ru accumulation data with another significant accident, the Fukushima Dai-ichi nuclear disaster, is not possible, as traditional fish surveys focused on two cesium isotopes (
134Cs and
137Cs) [
57], although
106Ru was recorded.
International recommendations classify two ruthenium isotopes (
103Ru and
106Ru) under the group with a standard of 100 Bq/kg for “other radionuclides” [
58,
59,
60]. According to these norms, a
106Ru concentration exceeding 100 Bq/kg is considered non-compliant. The levels of
106Ru in the Chornobyl Cooling Pond exceed international standards (≤100 Bq/kg) by hundreds to thousands of times, while in the Kaniv Reservoir, they exceed the standard several times. This provides sufficient grounds to assess the serious risk of direct consumption of such fish—the dose load significantly exceeds 1 mSv/year (for fish from the Chornobyl Cooling Pond).
When comparing the accumulation of
95Zr,
95Nb,
103Ru, and
106Ru (particle-reactive radionuclides) with the accumulation of biogenic short-lived radionuclides
54Mn,
60Co, and
65Zn in fish from the Chornobyl Cooling Pond [
61], the primary mechanism of entry for all these radionuclides is dietary, with some contribution from percutaneous absorption. Muscle tissue generally exhibits low concentrations of all these radionuclides. The only one that accumulates in high concentrations in muscle tissue is
106Ru. This makes this radionuclide critical from a food safety perspective.
To reduce risks, it is necessary to strengthen ecological monitoring of water bodies, particularly in regions affected by radioactive contamination or in critical areas for potential accidents. Systematic fish checks for dangerous radionuclide content (including 106Ru) should be introduced, along with restrictions on fishing and consuming fish from critical zones. Public awareness of potential risks and support for scientific research in radioecology are equally important for making effective decisions at the state level.
The release of radionuclides into the environment, even in small quantities, poses a significant threat to both ecosystems and human health, as evidenced by numerous cases of radioactive contamination. Among these incidents, the release of the isotope
106Ru in 2017 is particularly concerning. Regrettably, the impact of this incident on aquatic ecosystems has yet to be comprehensively addressed in the scientific literature. From late September to early October 2017, air monitoring stations across Europe detected
106Ru [
62]. Extraction experiments on air filters indicated a substantial presence of soluble
106Ru. In some regions of central and eastern Europe, daily average concentrations ranged from tens to over 100 mBq/m
3. Analysis of diurnal variations in total beta activity suggested airborne
106Ru contamination, indicative of a radioactive plume passage [
63]. Simultaneously,
103Ru was also detected at various European stations. Most atmospheric dispersion models trace the source to the Southern Urals (Russia) [
62]. Specific studies [
64] pinpoint two potential sources of
106Ru: (i) the Research Institute of Atomic Reactors, JSC “SSC Research Institute of Atomic Reactors” in Dimitrovgrad, Russia, and/or (ii) the Mayak Production Association (Ozersk, Russia). While trajectory calculations implicate the Southern Urals, official confirmation remains pending [
62].
Historically, emissions of radionuclides have occurred repeatedly at the Mayak Production Association (MPA). Hamilton [
1] documented that from 1949 to 1956, the operation of this facility resulted in the discharge of approximately 76 million m
3 of liquid radioactive waste into the Techa River. This waste contained an estimated 100 × 10
15 Bq of radioactivity, primarily consisting of ruthenium isotopes (
103Ru,
106Ru) and rare earth elements. The Kyshtym disaster in 1957, caused by an explosion at a Mayak waste tank, released 74 × 10
15 Bq of radioactivity into the atmosphere, primarily short-lived fission products such as
95Zr,
95Nb, and
144Ce, alongside smaller amounts of
137Cs. Additional emissions occurred through wind dispersal from Lake Karachay. Mayak’s production reactors operated until 1990, with spent nuclear fuel sent to Tomsk-7 for reprocessing. Radioactive waste injected into the ground at Tomsk-7 accounts for nearly 90% of the total waste released, approximately 37,000 × 10
15 Bq. The most severe radiation accident at this facility occurred on 6 April 1993, when a waste storage tank exploded, releasing approximately 0.03 × 10
15 Bq of radioactivity, predominantly short-lived beta- and gamma-emitting radionuclides, including
95Nb,
106Ru, and
95Zr.
Given this historical context, the recurrence of man-made incidents with cross-border consequences is plausible. Despite the absence of immediate threats to the population, the imperceptible and uncontrolled spread of artificial radionuclides over vast territories underscores the high risks associated with unauthorized or accidental releases that may remain undetected. Particularly alarming is the lack of transparency and reliable information from the source state, heightening the likelihood of future incidents. Amidst systematic opacity, the prospect of new radioactive leaks poses not only environmental but also global security concerns. With substantial volumes of radioactive materials and inadequate storage oversight, the potential for catastrophic environmental and public health consequences looms large.
This development reinforces the notion that even short-lived radionuclides, when released in significant and uncontrolled quantities, can have enduring environmental impacts and pose potential risks to public health. Therefore, continuous radiation monitoring, enhanced international cooperation in nuclear safety, and strengthened oversight of facilities involved in nuclear fuel production and processing are urgent imperatives that demand attention at both national and global levels.
7. Conclusions
Based on the analysis of the scientific literature and observational data, several general conclusions can be drawn regarding the entry, distribution, and accumulation of the short-lived radionuclides 95Zr, 95Nb, 103Ru, and 106Ru in aquatic ecosystems, with particular emphasis on their behavior in fish.
95Zr predominantly accumulates in external organs such as the skin, fins, gills, mucus, and scales. It is only occasionally detected in muscle and bone tissues, and in low concentrations. Its presence in fish is primarily associated with external contamination or the ingestion of contaminated particles through food and water.
95Nb exhibits a distribution pattern similar to that of 95Zr; however, its activity levels are notably higher in internal organs, especially in the liver, kidneys, roe, and gastrointestinal tract. This suggests a greater capacity for systemic accumulation within the organism. 95Nb is also found in muscle tissue, although at relatively low concentrations.
103Ru accumulates primarily in internal organs. In piscivorous fish from the Kaniv Reservoir, its presence is more pronounced, which may indicate increased bioavailability through trophic transfer at a distance from the contamination source.
106Ru is also concentrated mainly in internal organs, particularly within the digestive system, and less frequently in external tissues such as fins, skin, and scales. Unlike the other radionuclides studied, 106Ru is present in significant concentrations in muscle tissue. In general, this isotope has been detected in the internal organs of omnivorous, herbivorous, planktivorous, and carnivorous fish. However, its substantial accumulation in both muscle and reproductive tissues (roe) suggests it has exceptionally high bioavailability within the aquatic food web.
These summarized findings provide a foundation for further environmental monitoring, for forecasting the ecological consequences of radionuclide contamination in aquatic systems, and for assessing potential health risks to the human population in the event of fish consumption contaminated with these radionuclides.