Habitat Matters: Behavior and Activity of Round Goby (Neogobius melanostomus) at Different Substrates
Abstract
1. Introduction
2. Materials and Methods
- Bare smooth substrate and bare pebbles (bare substrate vs. rocky substrate);
- Bare smooth substrate and substrate with fouling organisms in the early stage of succession (bare substrate vs. colonized substrate);
- Substrate with fouling organisms in the early stage of succession and bare pebbles (colonized substrate vs. rocky substrate).

3. Results
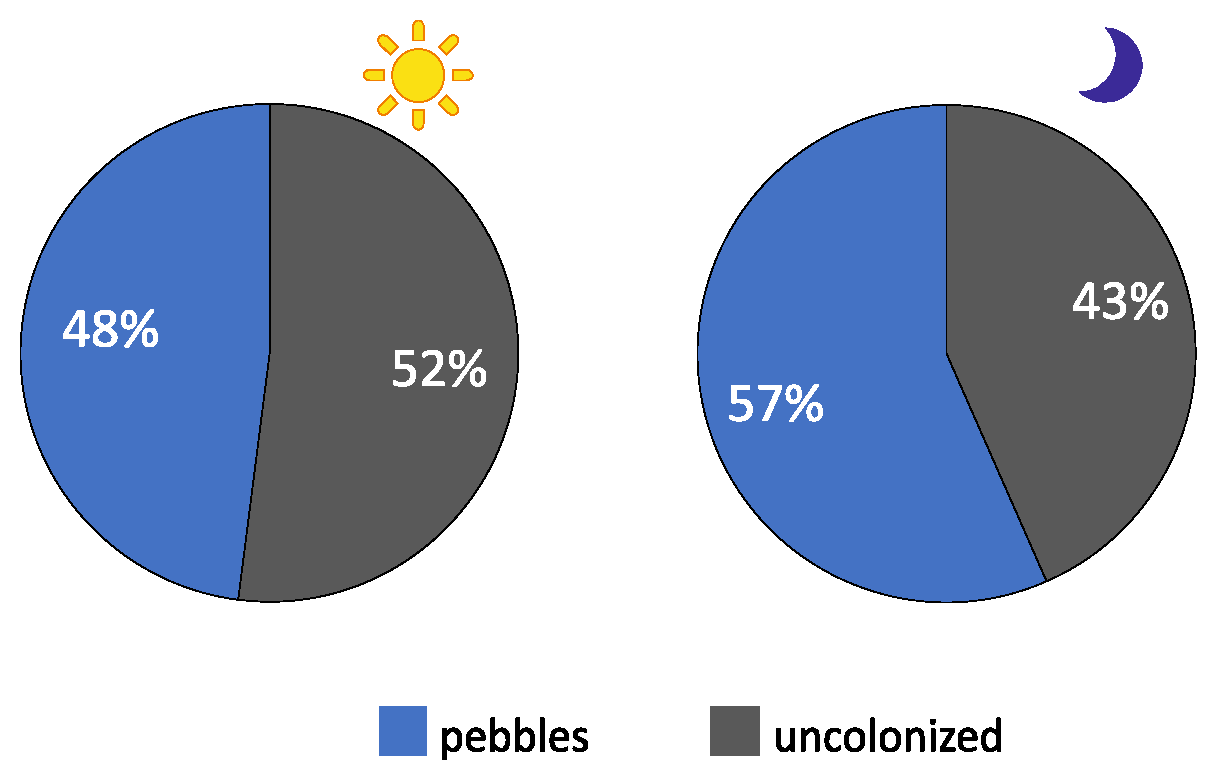
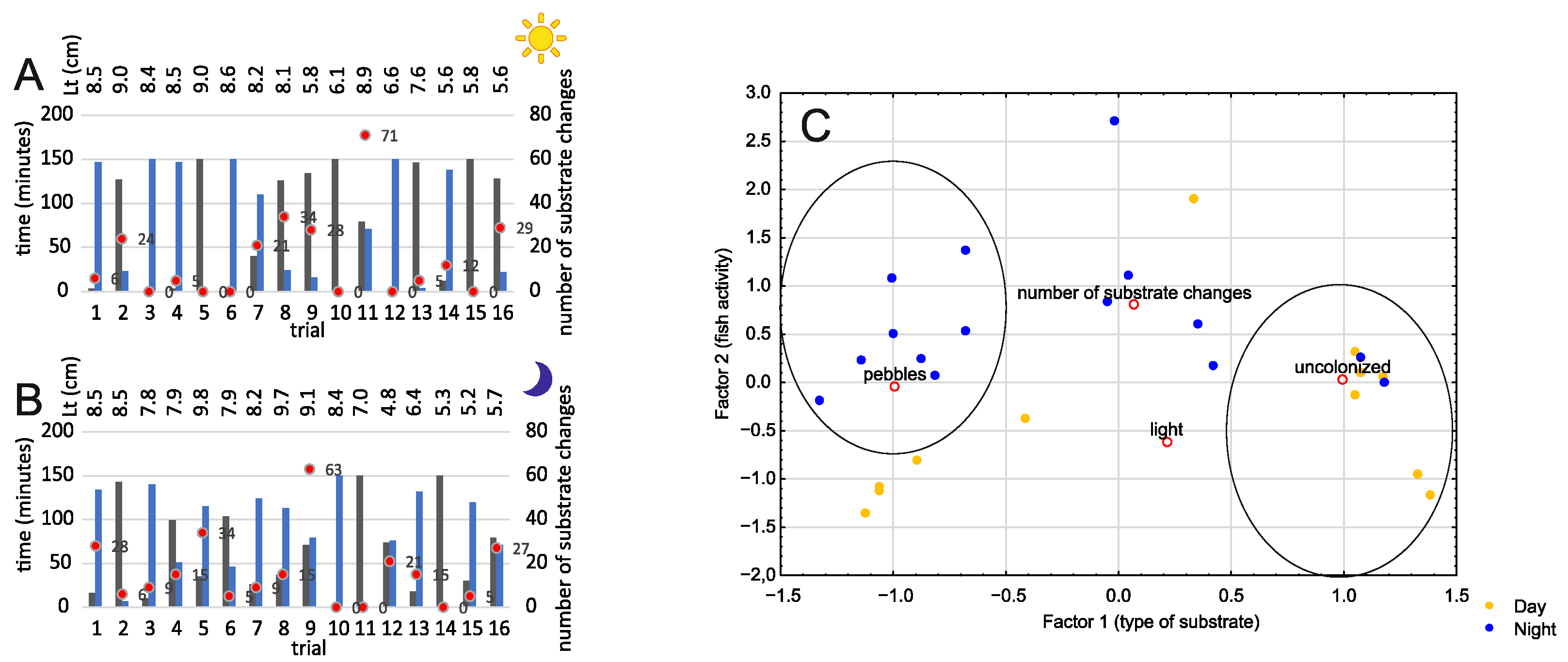
3.1. Bare Substrate vs. Rocky Substrate
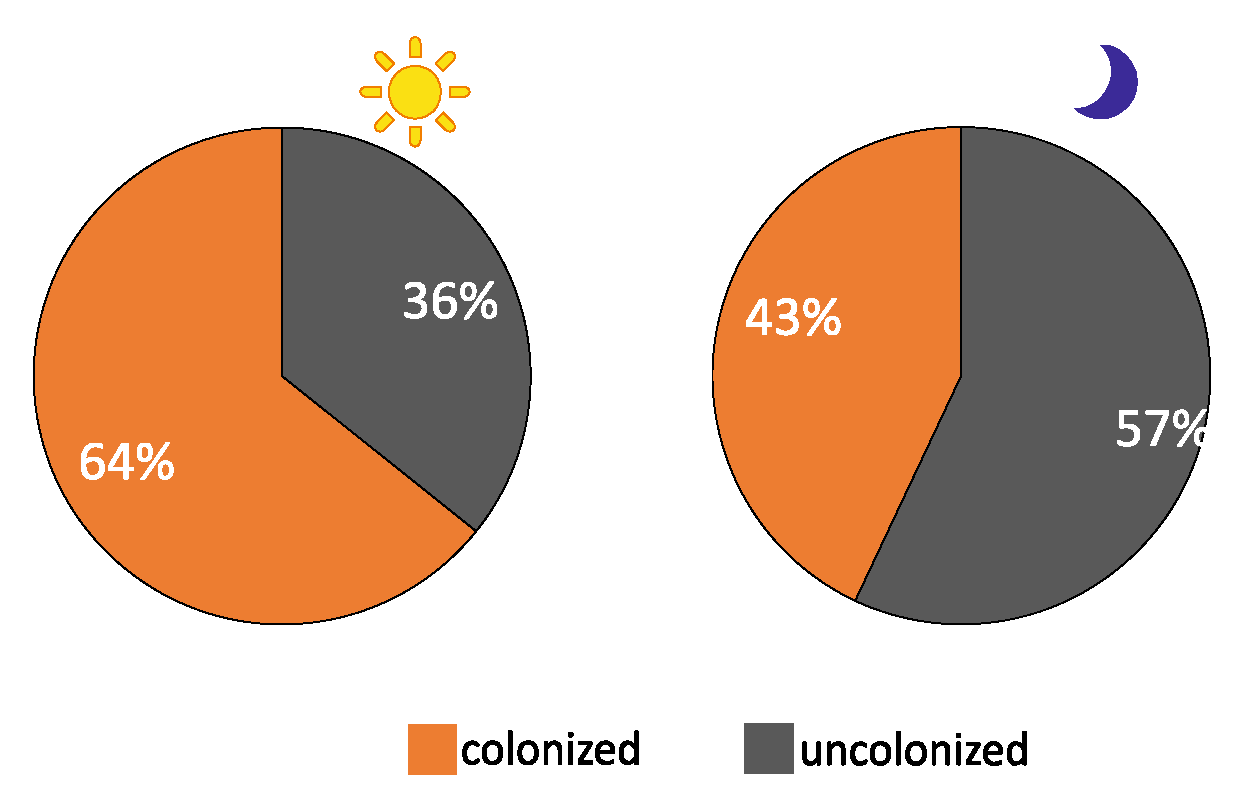
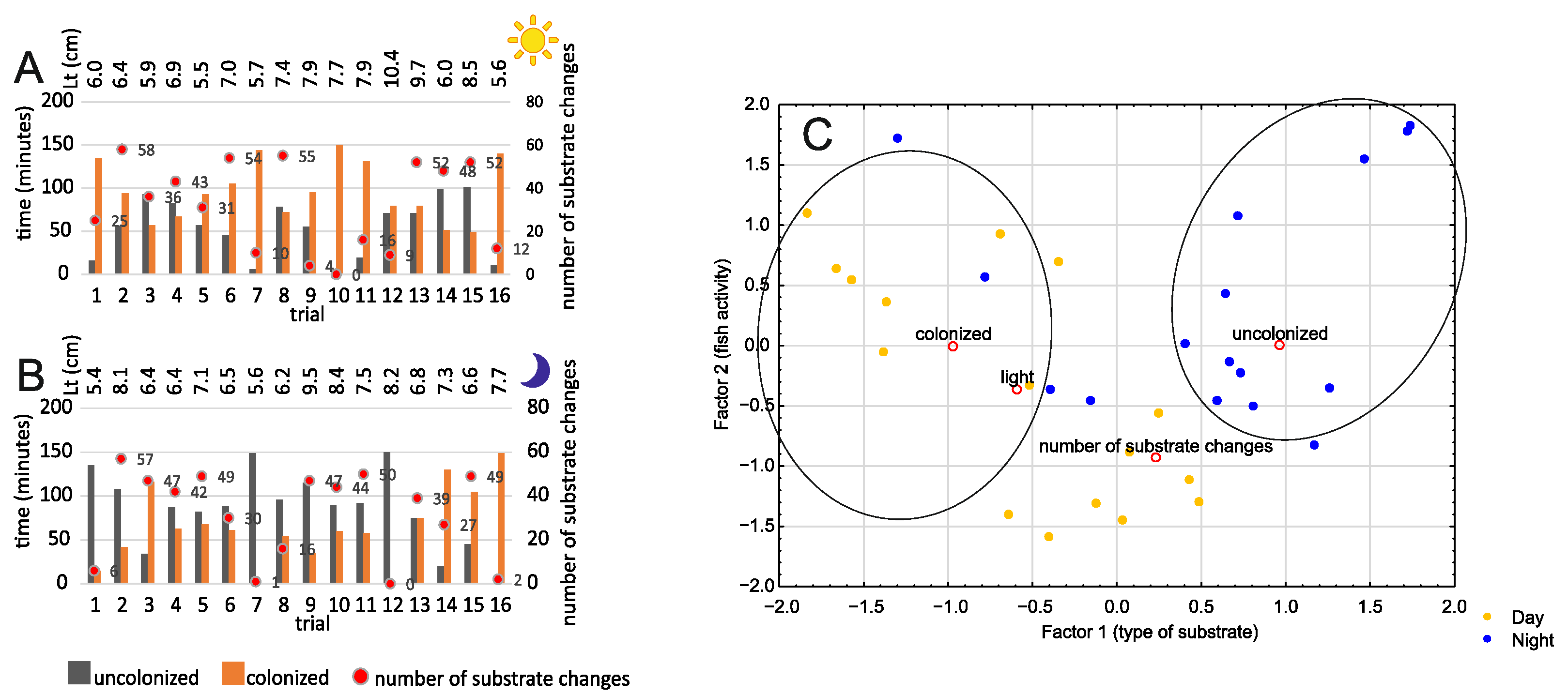
3.2. Bare Substrate vs. Colonized Substrate
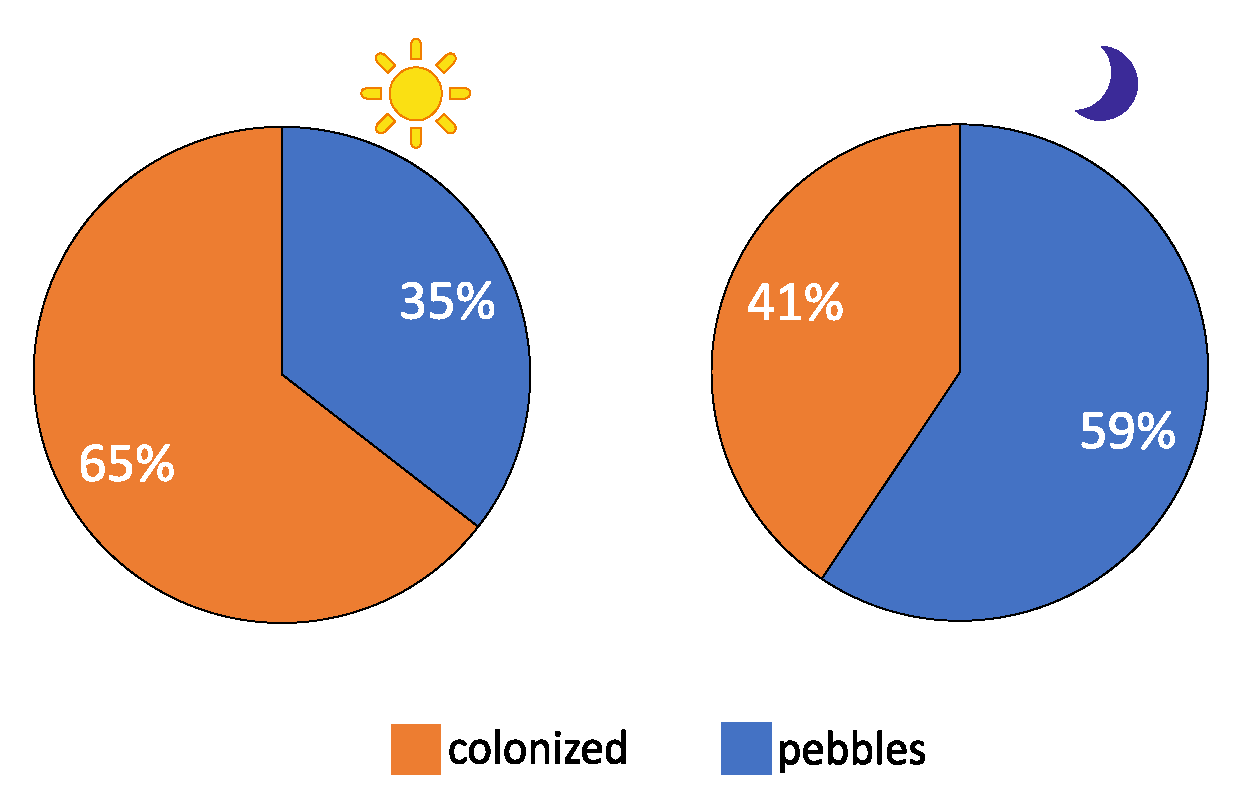
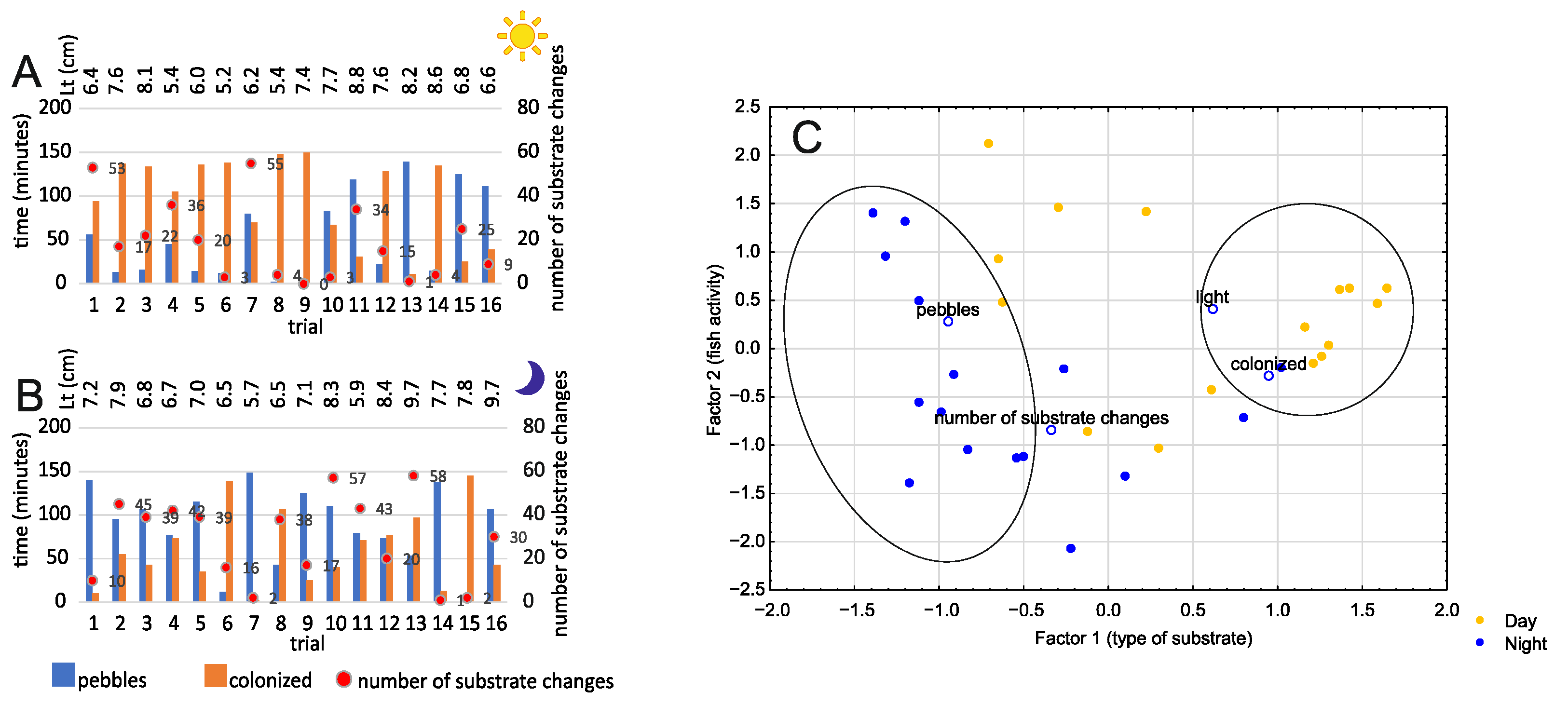
3.3. Colonized Substrate vs. Rocky Substrate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Szcześniak, M.; Kokociński, M.; Jagodziński, R.; Pleskot, K.; Zajączkowski, M.; Szczuciński, W. Late Holocene Vistula River floods recorded in grain size distributions and diatom assemblages of marine sediments of the Gulf of Gdańsk (Baltic Sea). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2023, 617, 111499. [Google Scholar] [CrossRef]
- Young, J.A.M.; Marentette, J.R.; Gross, C.; McDonald, J.I.; Verma, A.; Marsh-Rollo, S.E.; Macdonald, P.D.M.; Earn, D.J.D.; Balshine, S. Demography and Substrate Affinity of the Round Goby (Neogobius melanostomus) in Hamilton Harbour. J. Great Lakes Res. 2010, 36, 115–122. [Google Scholar] [CrossRef]
- Sapota, M.R.; Skóra, K.E. Spread of alien (non-indigenous) fish species Neogobius melanostomus in the Gulf of Gdansk (south Baltic). Biol. Invasions 2005, 7, 157–164. [Google Scholar] [CrossRef]
- Cerwenka, A.F.; Brandner, J.; Dashinov, D.; Geist, J. Small but Mighty: The Round Goby (Neogobius melanostomus) as a Model Species of Biological Invasions. Diversity 2023, 15, 528. [Google Scholar] [CrossRef]
- Sapota, M.R. Biologia i Ekologia Babki Byczej Neogobius Melanostomus (Pallas 1811) Gatunku Inwazyjnego w Zatoce Gdańskiej; Wydawnictwo Uniwersytetu Gdańskiego: Sopot, Poland, 2005; ISBN 8373262814. [Google Scholar]
- Kornis, M.S.; Mercado-Silva, N.; Vander Zanden, M.J. Twenty years of invasion: A review of Round Goby Neogobius melanostomus biology, spread and ecological implications. J. Fish. Biol. 2012, 80, 235–285. [Google Scholar] [CrossRef]
- Clark, K.H.; Wisor, J.M.; Mueller, S.J.; Bradshaw-Wilson, C.; Boyer, E.W.; Stauffer, J.R., Jr. Status of Freshwater Mussels (Unionidae) in the French Creek Watershed, USA at the Onset of Invasion by Round Goby, Neogobius melanostomus. Water 2021, 13, 3064. [Google Scholar] [CrossRef]
- Stauffer, J.R.; Schnars, J.; Wilson, C.; Taylor, R.; Murray, C.K. Status of Exotic Round Goby and Tubenose Goby in Pennsylvania. Northeast. Nat. 2016, 23, 395–407. [Google Scholar] [CrossRef]
- Demchenko, V.O.; Tkachenko, M.Y. Biological characteristics of the Round Goby, Neogobius melanostomus (Pallas, 1814), from different water bodies. Fish. Aquat. Life 2017, 25, 51–61. [Google Scholar] [CrossRef]
- Marentette, J.R.; Wang, G.; Tong, S.; Sopinka, N.M.; Taves, M.D.; Koops, M.A.; Balshine, S. Laboratory and field evidence of sex-biased movement in the invasive Round Goby. Behav. Ecol. Sociobiol. 2011, 65, 2239–2249. [Google Scholar] [CrossRef]
- Hirsch, P.E.; N’Guyen, A.; Adrian-Kalchhauser, I.; Burkhardt-Holm, P. What do we really know about the impacts of one of the 100 worst invaders in Europe? A reality check. Ambio 2016, 45, 267–279. [Google Scholar] [CrossRef]
- Tierney, K.B.; Kereliuk, M.; Katare, Y.K.; Scott, A.P.; Loeb, S.J.; Zielinski, B. Invasive male round gobies (Neogobius melanostomus) release pheromones in their urine to attract females. Can. J. Fish. Aquat. Sci. 2013, 70, 3. [Google Scholar] [CrossRef]
- Brownscombe, J.W.; Fox, M.G. Range expansion dynamics of the invasive round goby (Neogobius melanostomus) in a river system. Aquat. Ecol. 2012, 46, 175–189. [Google Scholar] [CrossRef]
- Piraino, M.N.; Copp, G.H. Temporal and spatial patterns in the diel activity of invasive Ponto-Caspian gobies (Neogobius spp.) in the River Thames, England. Aquat. Invasions 2014, 9, 319–331. [Google Scholar] [CrossRef]
- Ray, W.J.; Corkum, L.D. Habitat and Site Affinity of the Round Goby. J. Great Lakes Res. 2001, 27, 329–334. [Google Scholar] [CrossRef]
- Jude, D.J.; DeBoe, S.F. Possible impact of gobies and other introduced species on habitat restoration efforts. Can. J. Fish. Aquat. Sci. 1996, 53 (Suppl. S1), 136–141. [Google Scholar] [CrossRef]
- Balshine, S.; Verma, A.; Chant, V.; Theysmeyer, T. Competitive Interactions between Round Gobies and Logperch. J. Great Lakes Res. 2005, 31, 68–77. [Google Scholar] [CrossRef]
- Janssen, J.; Jude, D.J. Recruitment Failure of Mottled Sculpin Cottus bairdi in Calumet Harbor, Southern Lake Michigan, Induced by the Newly Introduced Round Goby Neogobius melanostomus. J. Great Lakes Res. 2001, 27, 319–328. [Google Scholar] [CrossRef]
- Cooper, M.J.; Ruetz, C.R.; Uzarski, D.G.; Krueger, D.M. Habitat use and movement of round gobies (Neogobius melanostomus) in coastal areas of eastern Lake Michigan and Lake Huron. J. Great Lakes Res. 2007, 33, 690–697. [Google Scholar] [CrossRef]
- Meunier, B.; Yavno, S.; Ahmed, S.; Corkum, L.D. First documentation of spawning and nest guarding in the laboratory by the invasive fish, the round goby (Neogobius melanostomus). J. Great Lakes Res. 2009, 35, 608–612. [Google Scholar] [CrossRef]
- Roje, S.; Drozd, B.; Richter, L.; Kubec, J.; Polívka, Z.; Worischka, S.; Buřič, M. Comparison of Behavior and Space Use of the European Bullhead Cottus gobio and the Round Goby Neogobius melanostomus in a Simulated Natural Habitat. Biology 2021, 10, 821. [Google Scholar] [CrossRef]
- Jude, D.J.; Janssen, J.; Crawford, G. Ecology, distribution, and impact of the newly introduced round and tubenose gobies on the biota of the St. Clair and Detroit Rivers. In The Lake Huron Ecosystem: Ecology, Fisheries and Management Munawar; Edsall, M., Leach, J., Eds.; SPB Academic Publishing: Amsterdam, The Netherlands, 1995; pp. 447–460. [Google Scholar] [CrossRef]
- Dubs, D.O.; Corkum, L.D. Behavioral Interactions Between Round Gobies (Neogobius melanostomus) and Mottled Sculpins (Cottus bairdi). J. Great Lakes Res. 1996, 22, 838–844. [Google Scholar] [CrossRef]
- MacInnis, A.J.; Corkum, L.D. Fecundity and Reproductive Season of the Round Goby Neogobius melanostomus in the Upper Detroit River. Trans. Am. Fish. Soc. 2000, 129, 136–144. [Google Scholar] [CrossRef]
- Henseler, C.; Kotterba, P.; Bonsdorff, E.; Nordström, M.C.; Oesterwind, D. Habitat utilization and feeding ecology of small round goby in a shallow brackish lagoon. Mar. Biodivers. 2020, 50, 88. [Google Scholar] [CrossRef]
- Behrens, J.W.; von Friesen, L.W.; Brodin, T.; Ericsson, P.; Hirsch, P.E.; Persson, A.; Sundelin, A.; van Deurs, M.; Nilsson, P.A. Personality-and size-related metabolic performance in invasive Round Goby (Neogobius melanostomus). Physiol. Behav. 2020, 215, 112777. [Google Scholar] [CrossRef]
- Galli, A.; Behrens, J.W.; Gesto, M.; Moran, N.P. Boldness and physiological variation in Round Goby populations along their Baltic Sea invasion front. Physiol. Behav. 2023, 269, 114261. [Google Scholar] [CrossRef]
- Neff, B.D.; Knapp, R. Paternity, Parental Behavior and Circulating Steroid Hormone Concentrations in Nest-Tending Male Bluegill. Horm. Behav. 2009, 56, 239–245. [Google Scholar] [CrossRef]
- Brandner, J.; Cerwenka, A.F.; Schliewen, U.K.; Geist, J. Invasion strategies in round goby (Neogobius melanostomus): Is bigger really better? PloS ONE 2018, 13, e0190777. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dziubińska, A.; Sapota, M.; Socha, E. Habitat Matters: Behavior and Activity of Round Goby (Neogobius melanostomus) at Different Substrates. Fishes 2025, 10, 319. https://doi.org/10.3390/fishes10070319
Dziubińska A, Sapota M, Socha E. Habitat Matters: Behavior and Activity of Round Goby (Neogobius melanostomus) at Different Substrates. Fishes. 2025; 10(7):319. https://doi.org/10.3390/fishes10070319
Chicago/Turabian StyleDziubińska, Anna, Mariusz Sapota, and Emilia Socha. 2025. "Habitat Matters: Behavior and Activity of Round Goby (Neogobius melanostomus) at Different Substrates" Fishes 10, no. 7: 319. https://doi.org/10.3390/fishes10070319
APA StyleDziubińska, A., Sapota, M., & Socha, E. (2025). Habitat Matters: Behavior and Activity of Round Goby (Neogobius melanostomus) at Different Substrates. Fishes, 10(7), 319. https://doi.org/10.3390/fishes10070319






