Pharmacokinetics, Pharmacodynamics and Depletion of Florfenicol Applied in White Leg Shrimp (Litopenaeus vannamei) Aquaculture and Impact on Shrimp Hepatopancreas Histology
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Equipment
2.2. Bacterial Strains
2.2.1. Antibiotic Susceptibility Testing
2.2.2. Determination of the Minimum Inhibitory Concentration (MIC) of FF
2.3. Shrimp
2.4. Pharmacokinetics Experiment
2.4.1. Experimental Design
2.4.2. Sample Collection
2.5. Depletion of Florfenicol in Muscle Tissue and Hepatopancreas Histological Observation
2.5.1. Depletion of Florfenicol in Shrimp Muscle
2.5.2. Shrimp Hepatopancreas Histological Examination
2.6. Florfenicol and Florfenicol Amine Quantification
2.7. Pharmacokinetics Data Analysis
2.8. Withdrawal Times Calculation
3. Results
3.1. Antimicrobial Activity
3.2. Pharmacokinetics of Florfenicol in Plasma, Hepatopancreas and Muscle of White Leg Shrimp
3.3. Depletion and Withdrawal Time of Florfenicol in White Leg Shrimp Muscle
3.4. Hepatopancreas Histology of White Leg Shrimp
4. Discussion
4.1. Antimicrobial Activity of FF on Various V. parahaemolyticus Strains
4.2. Pharmacokinetics of FF in Shrimp
4.3. Depletion of Florfenicol in White Leg Shrimp Muscle
4.4. Impact on Shrimp Hepatopancreas Histology
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AHPND | Acute hepatopancreatic necrosis disease |
| AUC0−inf | Area under the plasma concentration-time curve from time 0 to infinity |
| Cl | Total body clearance |
| Cmax | Maximum concentration |
| EMA | European Medicine Agency guidelines |
| FF | Florfenicol |
| IS | Internal standard |
| kel | Elimination rate constant |
| MIC | Minimum inhibitory concentration |
| MRL | Maximum residue limit |
| PD | Pharmacodynamics |
| PK | Pharmacokinetic |
| PK/PD | Pharmacokinetics/Pharmacodynamics |
| T1/2el | Elimination half-life |
| Tmax | Time to maximal plasma concentration |
| Vd | Volume of distribution |
| WT | Withdrawal time |
References
- Vietnam Association of Seafood Exporters and Producers (VASEP). Vietnam Aquaculture and Fisheries Overview. 2024. Available online: https://vasep.com.vn/san-pham-xuat-khau/tom/tong-quan-nganh-tom (accessed on 20 November 2024).
- Nguyen, K.A.T.; Nguyen, T.A.T.; Jolly, C.; Nguelifack, B.M. Economic efficiency of extensive and intensive shrimp production under conditions of disease and natural disaster risks in Khánh Hòa and Trà Vinh Provinces, Vietnam. Sustainability 2020, 12, 2140. [Google Scholar] [CrossRef]
- Tran, L.; Nunan, L.; Redman, R.M.; Mohney, L.L.; Pantoja, C.R.; Fitzsimmons, K.; Lightner, D.V. Determination of the infectious nature of the agent of acute hepatopancreatic necrosis syndrome affecting penaeid shrimp. Dis. Aquat. Org. 2013, 105, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Flegel, T.W.; Sritunyaluucksana, K. Recent research on acute hepatopancreatic necrosis disease (AHPND) and Enterocytozoon hepatopenaei in Thailand. Asian Fish. Sci. 2018, 31, 257–269. [Google Scholar] [CrossRef]
- Thinh, N.Q.; Maita, M.; Phu, T.M. Chemical use in intensive white-leg shrimp aquaculture in Tra Vinh province, Vietnam. Int. J. Sci. Res. Publ. 2020, 56, 70–77. [Google Scholar] [CrossRef]
- Chanratchakool, P.; Phillips, M.J. Social and economic impacts and management of shrimp disease among small-scale farmers in Thailand and Viet Nam. FAO Fish. Tech. Paper 2002, 406, 177–189. [Google Scholar]
- Zhou, J.; Fang, W.; Yang, X.; Zhou, S.; Hu, L.; Li, X.; Qi, X.; Su, H.; Xie, L. A nonluminescent and highly virulent Vibrio harveyi strain is associated with “bacterial white tail disease” of Litopenaeus vannamei shrimp. PLoS ONE 2012, 7, e29961. [Google Scholar] [CrossRef]
- Rico, A.; Oliveira, R.; McDonough, S.; Matser, A.; Khatikarn, J.; Satapornvanit, K.; Nogueira, A.J.A.; Soares, A.M.V.M.; Domingues, I.; Van den Brink, P.J. Use, fate and ecological risks of antibiotics applied in tilapia cage farming in Thailand. Environ. Pollut. 2014, 191, 8–16. [Google Scholar] [CrossRef]
- Phu, T.M.; Phuong, N.T.; Dung, T.T.; Hai, D.M.; Son, V.N.; Rico, A.; Clausen, J.H.; Madsen, H.; Murray, F.; Dalsgaard, A. An evaluation of fish health-management practices and occupational health hazards associated with P angasius catfish (P angasianodon hypophthalmus) aquaculture in the M ekong D elta, V ietnam. Aquac. Res. 2016, 47, 2778–2794. [Google Scholar] [CrossRef]
- Chi, T.T.K.; Clausen, J.H.; Van, P.T.; Tersbøl, B.; Dalsgaard, A. Use practices of antimicrobials and other compounds by shrimp and fish farmers in Northern Vietnam. Aquac. Rep. 2017, 7, 40–47. [Google Scholar] [CrossRef]
- Ren, X.; Pan, L.; Wang, L. Tissue distribution, elimination of florfenicol and its effect on metabolic enzymes and related genes expression in the white shrimp Litopenaeus vannamei following oral administration. Aquac. Res. 2016, 47, 1584–1595. [Google Scholar] [CrossRef]
- Phu, T.M.; Vinh, P.Q.; Dao, N.L.A.; Viet, L.Q.; Thinh, N.Q. Chemical use in intensive white-leg shrimp aquaculture in Ben Tre province, Vietnam. Training 2019, 73, 46–47. [Google Scholar] [CrossRef]
- Lillehaug, A.; Børnes, C.; Grave, K. A pharmaco-epidemiological study of antibacterial treatments and bacterial diseases in Norwegian aquaculture from 2011 to 2016. Dis. Aquat. Org. 2018, 128, 117–125. [Google Scholar] [CrossRef]
- Wang, W.; Dai, X.; Li, Z.; Meng, Q. Tissue distribution and elimination of florfenicol in topmouth culter (Culter alburnus) after oral administration. Czech J. Food Sci. 2009, 27, 214–221. [Google Scholar] [CrossRef]
- Lambert, T. Antibiotics that affect the ribosome. Rev. Sci. Tech. (Int. Off. Epizoot.) 2012, 31, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Nejad, A.J.; Peyghan, R.; Varzi, H.N.; Shahriyari, A. Florfenicol pharmacokinetics following intravenous and oral administrations and its elimination after oral and bath administrations in common carp (Cyprinus carpio). Vet. Res. Forum 2017, 8, 327–331. [Google Scholar]
- Dowling, P.M. Chloramphenicol, thiamphenicol and florfenicol. In Antimicrobial Therapy in Veterinary Medicine, 4th ed.; Gigu, S., Prescott, J.F., Baggot, J.D., Walker, R.D., Dowling, P.M., Eds.; Blackwell Scientific Publications: Ames, Iowa, 2006; pp. 241–248. [Google Scholar] [CrossRef]
- Samuelsen, O.B.; Bergh, Ø. Efficacy of orally administered florfenicol and oxolinic acid for the treatment of vibriosis in cod (Gadus morhua). Aquaculture 2004, 235, 27–35. [Google Scholar] [CrossRef]
- Caipang, C.M.A.; Lazado, C.C.; Brinchmann, M.F.; Berg, I.; Kiron, V. In vivo modulation of immune response and antioxidant defense in Atlantic cod, Gadus morhua following oral administration of oxolinic acid and florfenicol. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 150, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Chen, S.J.; Huang, J.; Yang, L.; Ju, Y.; Zhu, X.R.; Peng, D.X. Analysis of antibiotic resistance of Salmonella isolated from animals and identification of its florfenicol resistant gene. China Anim. Husb. Vet. Med. 2015, 42, 459–466. [Google Scholar]
- Park, B.K.; Lim, J.H.; Kim, M.S.; Yun, H.I. Pharmacokinetics of florfenicol and its metabolite, florfenicol amine, in the Korean catfish (Silurus asotus). J. Vet. Pharmacol. Ther. 2006, 29, 37–40. [Google Scholar] [CrossRef]
- Gaunt, P.S.; Langston, C.; Wrzesinski, C.; Gao, D.; Adams, P.; Crouch, L.; Sweeney, D.; Endris, R. Single intravenous and oral dose pharmacokinetics of florfenicol in the channel catfish (Ictalurus punctatus). J. Vet. Pharmacol. Ther. 2012, 35, 503–507. [Google Scholar] [CrossRef]
- Yang, Q.; Xie, L.; Wu, Z.; Chen, X.; Yang, Y.; Liu, J.; Zhang, Q. Pharmacokinetics of florfenicol after oral administration in yellow catfish, Pelteobagrus fulvidraco. J. World Aquac. Soc. 2013, 44, 586–592. [Google Scholar] [CrossRef]
- Pham, Q.V.; Nguyen, Q.T.; Devreese, M.; Croubels, S.; Dang, T.H.O.; Dalsgaard, A.; Maita, M.; Tran, M.P. Pharmacokinetics and depletion of florfenicol in striped catfish Pangasianodon hypophthalmus after oral administration. Fish. Sci. 2023, 89, 357–365. [Google Scholar] [CrossRef]
- Martinsen, B.; Horsberg, T.E.; Varma, K.J.; Sams, R. Single dose pharmacokinetic study of florfenicol in Atlantic salmon (Salmo salar) in seawater at 11 C. Aquaculture 1993, 112, 1–11. [Google Scholar] [CrossRef]
- Horsberg, T.E.; Hoff, K.A.; Nordmo, R. Pharmacokinetics of florfenicol and its metabolite florfenicol amine in Atlantic salmon. J. Aquat. Anim. Health 1996, 8, 292–301. [Google Scholar] [CrossRef]
- Kverme, K.O.; Haugland, G.T.; Hannisdal, R.; Kallekleiv, M.; Colquhoun, D.J.; Lunestad, B.T.; Wergeland, H.I.; Samuelsen, O.B. Pharmacokinetics of florfenicol in lumpfish (Cyclopterus lumpus L.) after a single oral administration. Aquaculture 2019, 512, 734279. [Google Scholar] [CrossRef]
- Feng, J.B.; Huang, D.R.; Zhong, M.; Liu, P.; Dong, J.D. Pharmacokinetics of florfenicol and behaviour of its metabolite florfenicol amine in orange-spotted grouper (Epinephelus coioides) after oral administration. J. Fish Dis. 2016, 39, 833–843. [Google Scholar] [CrossRef]
- Samuelsen, O.B.; Bergh, Ø.; Ervik, A. Pharmacokinetics of florfenicol in cod Gadus morhua and in vitro antibacterial activity against Vibrio anguillarum. Dis. Aquat. Org. 2003, 56, 127–133. [Google Scholar] [CrossRef]
- Yang, F.; Yang, F.; Wang, G.; Kong, T.; Liu, B. Pharmacokinetics of florfenicol and its metabolite florfenicol amine in crucian carp (Carassius auratus) at three temperatures after single oral administration. Aquaculture 2019, 503, 446–451. [Google Scholar] [CrossRef]
- Lim, J.H.; Kim, M.S.; Hwang, Y.H.; Song, I.B.; Park, B.K.; Yun, H.I. Pharmacokinetics of florfenicol following intramuscular and intravenous administration in olive flounder (Paralichthys olivaceus). J. Vet. Pharmacol. Ther. 2011, 34, 206–208. [Google Scholar] [CrossRef]
- Pourmolaie, B.; Eshraghi, H.R.; Haghighi, M.; Mortazavi, S.A.; Rohani, M.S. Pharmacokinetics of florfenicol administrated to rainbow trout (Oncorhynchus mykiss) by oral gavages and medicated feed routes. Bull. Environ. Pharmacol. Life Sci. 2015, 4, 14–17. [Google Scholar]
- Mallik, S.K.; Shahi, N.; Pathak, R.; Kala, K.; Patil, P.K.; Singh, B.; Ravindran, R.; Krishna, N.; Pandey, P.K. Pharmacokinetics and biosafety evaluation of a veterinary drug florfenicol in rainbow trout, Oncorhynchus mykiss (Walbaum 1792) as a model cultivable fish species in temperate water. Front. Pharmacol. 2023, 14, 1033170. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.B.; Jia, X.P. Single dose pharmacokinetic study of flofenicol in tilapia (Oreochromis niloticus× O. aureus) held in freshwater at 22 C. Aquaculture 2009, 289, 129–133. [Google Scholar] [CrossRef]
- Rairat, T.; Hsieh, C.Y.; Thongpiam, W.; Sung, C.H.; Chou, C.C. Temperature-dependent pharmacokinetics of florfenicol in Nile tilapia (Oreochromis niloticus) following single oral and intravenous administration. Aquaculture 2019, 503, 483–488. [Google Scholar] [CrossRef]
- Fang, W.; Li, G.; Zhou, S.; Li, X.; Hu, L.; Zhou, J. Pharmacokinetics and tissue distribution of thiamphenicol and florfenicol in Pacific white shrimp Litopenaeus vannamei in freshwater following oral administration. J. Aquat. Anim. Health 2013, 25, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Tipmongkolsilp, N.; Limpanon, Y.; Patamalai, B.; Lusanandana, P.; Wongtavatchai, J. Oral medication with florfenicol for black tiger shrimps Penaeus monodon. Thai J. Vet. Med. 2006, 36, 39–47. [Google Scholar] [CrossRef]
- Feng, Y.; Zhai, Q.; Wang, J.; Li, J.; Li, J. Comparison of florfenicol pharmacokinetics in Exopalaemon carinicauda at different temperatures and administration routes. J. Vet. Pharmacol. Ther. 2019, 42, 230–238. [Google Scholar] [CrossRef]
- Toutain, P.L.; Ferran, A.; Bousquet-Mélou, A. Species differences in pharmacokinetics and pharmacodynamics. Comp. Vet. Pharmacol. 2010, 199, 19–48. [Google Scholar] [CrossRef]
- Manan, H.; Zhong, J.M.H.; Othman, F.; Ikhwanuddin, M.H.D. Histopathology of the hepatopancreas of pacific white shrimp, Penaeus vannamei from none early mortality syndrome (EMS) shrimp ponds. J. Fish. Aquat. Sci. 2015, 10, 562–568. [Google Scholar] [CrossRef]
- Bray, W.A.; Williams, R.R.; Lightner, D.V.; Lawrence, A.L. Growth, survival and histological responses of the marine shrimp, Litopenaeus vannamei, to three dosage levels of oxytetracycline. Aquaculture 2006, 258, 97–108. [Google Scholar] [CrossRef]
- Romano, N.; Koh, C.B.; Ng, W.K. Dietary microencapsulated organic acids blend enhances growth, phosphorus utilization, immune response, hepatopancreatic integrity and resistance against Vibrio harveyi in white shrimp, Litopenaeus vannamei. Aquaculture 2015, 435, 228–236. [Google Scholar] [CrossRef]
- Maftuch, K.A.; Eslfitri, D.; Sanoesi, E.; Prihanto, A.A. Enrofloxacin stimulates cell death in several tissues of vannamei shrimp (Litopenaeus vannamei). Comp. Clin. Pathol. 2017, 26, 249–254. [Google Scholar] [CrossRef]
- Avunje, S.; Patil, P.K.; Ezaz, W.; Praveena, E.; Ray, A.; Viswanathan, B.; Alavandi, S.V.; Puthiyedathu, S.K.; Vijayan, K.K. Effect of oxytetracycline on the biosafety, gut microbial diversity, immune gene expression and withdrawal period in Pacific whiteleg shrimp, Penaeus vannamei. Aquaculture 2021, 543, 736957. [Google Scholar] [CrossRef]
- Sotomayor, M.A.; Reyes, J.K.; Restrepo, L.; Domínguez-Borbor, C.; Maldonado, M.; Bayot, B. Efficacy assessment of commercially available natural products and antibiotics, commonly used for mitigation of pathogenic Vibrio outbreaks in Ecuadorian Penaeus (Litopenaeus) vannamei hatcheries. PLoS ONE 2019, 14, e0210478. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Jiang, H.; Yu, T.; Yang, Y.; Yu, S.; Wu, J.; Lin, R.; Li, Y.; Fang, J.; Zhu, C. Co-occurrence of antibiotic and heavy metal resistance and sequence type diversity of Vibrio parahaemolyticus isolated from Penaeus vannamei at freshwater farms, seawater farms, and markets in Zhejiang province, China. Front. Microbiol. 2020, 11, 1294. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 27th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017; p. 36. [Google Scholar]
- Ali, N.G.; Aboyadak, I.M.; El-Sayed, H.S. Chemotherapeutic control of Gram-positive infection in white sea bream (Diplodus sargus, Linnaeus 1758) broodstock. Vet. World 2019, 12, 316. [Google Scholar] [CrossRef]
- Huynh, T.K.D.; Scippo, M.L.; Devreese, M.; Croubels, S.; Nguyen, Q.T.; Douny, C.; Dang, T.H.O.; Le, Q.V.; Tran, M.P. Pharmacokinetics and Withdrawal Times of Cefotaxime in White Leg Shrimp (Litopenaeus vannamei) After Oral Administration. Fishes 2024, 9, 232. [Google Scholar] [CrossRef]
- Nima, N.; Duangsuwan, P.; Pongtippatee, P.; Kanjanasopa, D.; Withyachumnarnkul, B. Types of cells in the hepatopancreas of the Pacific whiteleg shrimp Litopenaeus vannamei being infected by Enterocytozoon hepatopenaei. Songklanakarin J. Sci. Technol. 2022, 44, 97–102. [Google Scholar] [CrossRef]
- United States Department of Agriculture Food Safety and Inspection Service (USDA). Office of Public Health Science Determination and Confirmation of Florfenicol; CLG-FLOR1.04; USDA: Washington, DC, USA, 2010; pp. 1–27. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). AOAC Guidelines for Single Laboratory Validation of Chemical Methods for Dietary Supplements and Botanicals. 2002, p. 26. Available online: https://s27415.pcdn.co/wp-content/uploads/2020/01/64ER20-7/Validation_Methods/d-AOAC_Guidelines_For_Single_Laboratory_Validation_Dietary_Supplements_and_Botanicals.pdf (accessed on 12 January 2025).
- European Commission (EC). Commission Decision 2002/657/EC of 12 August 2002 Implementing Council Directive 96/23/EC Concerning the Performance of Analytical Methods and the Interpretation of Results; OJEU: Luxembourg, Luxembourg, 2002; Volume 221, pp. 8–36. [Google Scholar]
- Hekman, P. Withdrawal-Time Calculation Program WT1. 4; BRD Agency for the Registration of Veterinary Medicinal Products: Wageningen, The Netherlands, 2004; pp. 1–8. [Google Scholar]
- European Medicine Agency (EMA). Guideline on Determination of Withdrawal Periods for Edible Tissues. 2022. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/adopted-guideline-determination-withdrawal-periods-edible-tissues-revision-2_en.pdf (accessed on 10 June 2024).
- Mohney, L.L.; Bell, T.A.; Lightner, D.V. Shrimp Antimicrobial Testing. I. In Vitro Susceptibility of Thirteen Gram-Negative Bacteria to Twelve Antimicrobials. J. Aquat. Anim. Health 1992, 4, 257–261. [Google Scholar] [CrossRef]
- Roque, A.; Molina-Aja, A.; Bolán-Mejıa, C.; Gomez-Gil, B. In vitro susceptibility to 15 antibiotics of vibrios isolated from penaeid shrimps in Northwestern Mexico. Int. J. Antimicrob. Agents 2001, 17, 383–387. [Google Scholar] [CrossRef]
- Vaseeharan, B.; Ramasamy, P.; Murugan, T.; Chen, J.C. In vitro susceptibility of antibiotics against Vibrio spp. and Aeromonas spp. isolated from Penaeus monodon hatcheries and ponds. Int. J. Antimicrob. Agents 2005, 26, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Rebouças, R.H.; de Sousa, O.V.; Lima, A.S.; Vasconcelos, F.R.; de Carvalho, P.B.; dos Fernandes Vieira, R.H.S. Antimicrobial resistance profile of Vibrio species isolated from marine shrimp farming environments (Litopenaeus vannamei) at Ceará, Brazil. Environ. Res. 2011, 111, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Al-Othrubi, S.M.Y.; Kqueen, C.Y.; Mirhosseini, H.; Hadi, Y.A.; Radu, S. Antibiotic resistance of Vibrio parahaemolyticus isolated from cockles and shrimp sea food marketed in Selangor, Malaysia. Clin. Microbiol. 2014, 3, 148. [Google Scholar] [CrossRef]
- Melo, L.M.R.D.; Almeida, D.; Hofer, E.; Reis, C.M.F.D.; Theophilo, G.N.D.; Santos, A.F.D.M.; Vieira, R.H.S.D.F. Antibiotic resistance of Vibrio parahaemolyticus isolated from pond-reared Litopenaeus vannamei marketed in Natal, Brazil. Braz. J. Microbiol. 2011, 42, 1463–1469. [Google Scholar] [CrossRef]
- Yano, Y.; Hamano, K.; Satomi, M.; Tsutsui, I.; Aue-umneoy, D. Diversity and characterization of oxytetracycline-resistant bacteria associated with non-native species, white-leg shrimp (Litopenaeus vannamei), and native species, black tiger shrimp (Penaeus monodon), intensively cultured in Thailand. J. Appl. Microbiol. 2011, 110, 713–722. [Google Scholar] [CrossRef]
- Zanetti, S.; Spanu, T.; Deriu, A.; Romano, L.; Sechi, L.A.; Fadda, G. In vitro susceptibility of Vibrio spp. isolated from the environment. Int. J. Antimicrob. Agents 2001, 17, 407–409. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.H.T.; Yanagawa, H.; Nguyen, K.T.; Hara-Kudo, Y.; Taniguchi, T.; Hayashidani, H. Prevalence of Vibrio parahaemolyticus in seafood and water environment in the Mekong Delta, Vietnam. J. Vet. Med. Sci. 2018, 80, 1737–1742. [Google Scholar] [CrossRef]
- Rocha, R.d.S.; de Sousa, O.V.; dos Fernandes Vieira, R.H.S. Multidrug-resistant Vibrio associated with an estuary affected by shrimp farming in Northeastern Brazil. Mar. Pollut. Bull. 2016, 105, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Saifedden, G.; Farinazleen, G.; Nor-Khaizura, A.; Kayali, A.Y.; Nakaguchi, Y.; Nishibuchi, M.; Son, R. Antibiotic Susceptibility profile of Vibrio parahaemolyticus isolated from shrimp in Selangor, Malaysia. Int. Food Res. J. 2016, 23, 2732–2736. [Google Scholar]
- Morales-Covarrubias, M.S.; Tlahuel-Vargas, L.; Martínez-Rodríguez, I.E.; Lozano-Olvera, R.; Palacios-Arriaga, J.M. Necrotising hepatobacterium (NHPB) infection in Penaeus vannamei with florfenicol and oxytetracycline: A comparative experimental study. Rev. Cient. 2012, 22, 72–80. [Google Scholar]
- Ferri, G.; Lauteri, C.; Vergara, A. Antibiotic resistance in the finfish aquaculture industry: A review. Antibiotics 2022, 11, 1574. [Google Scholar] [CrossRef]
- Tu, H.T.; Silvestre, F.; Phuong, N.T.; Kestemont, P. Effects of pesticides and antibiotics on penaeid shrimp with special emphases on behavioral and biomarker responses. Environ. Toxicol. Chem. 2010, 29, 929–938. [Google Scholar] [CrossRef]
- Verri, T.; Mandal, A.; Zilli, L.; Bossa, D.; Mandal, P.K.; Ingrosso, L.; Zonno, V.; Vilella, S.; Ahearn, G.A.; Storelli, C. D-glucose transport in decapod crustacean hepatopancreas. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001, 130, 585–606. [Google Scholar] [CrossRef]
- Faroongsarng, D.; Chandumpai, A.; Chiayvareesajja, S.; Theapparat, Y. Bioavailability and absorption analysis of oxytetracycline orally administered to the standardized moulting farmed Pacific white shrimps (Penaeus vannamei). Aquaculture 2007, 269, 89–97. [Google Scholar] [CrossRef]
- Ma, R.; Wang, Y.; Zou, X.; Fu, G.; Li, C.; Fan, P.; Fang, W. Pharmacokinetics of oxytetracycline in Pacific white shrimp, Penaeus vannamei, after oral administration of a single-dose and multiple-doses. Aquaculture 2019, 512, 734348. [Google Scholar] [CrossRef]
- Chiayvareesajja, S.; Chandumpai, A.; Theapparat, Y.; Faroongsarng, D. The complete analysis of oxytetracycline pharmacokinetics in farmed Pacific white shrimp (Litopenaeus vannamei). J. Vet. Pharmacol. Ther. 2006, 29, 409–414. [Google Scholar] [CrossRef]
- Ma, R.; Wang, Y.; Zou, X.; Hu, K.; Sun, B.; Fang, W.; Fu, G.; Yang, X. Pharmacokinetics of sulfamethoxazole and trimethoprim in Pacific white shrimp, Litopenaeus vannamei, after oral administration of single-dose and multiple-dose. Environ. Toxicol. Pharmacol. 2017, 52, 90–98. [Google Scholar] [CrossRef]
- Fang, X.; Zhou, J.; Liu, X. Pharmacokinetics and tissue distribution of enrofloxacin after single intramuscular injection in Pacific white shrimp. J. Vet. Pharmacol. Ther. 2018, 41, 148–154. [Google Scholar] [CrossRef]
- Ma, R.; Huang, L.; Wei, W.; Wang, Y.; Zou, X.; Zhou, J.; Li, X.; Fang, W. Pharmacokinetics of enrofloxacin and its metabolite ciprofloxacin in Pacific white shrimp Litopenaeus vannamei after multiple-dose oral administration. Fish. Sci. 2018, 84, 869–876. [Google Scholar] [CrossRef]
- Dowling, P.M. Chloramphenicol, thiamphenicol, and florfenicol. In Antimicrobial Therapy in Veterinary Medicine; Wiley: Hoboken, NJ, USA, 2013; pp. 269–277. [Google Scholar]
- Reed, M.D. Optimal antibiotic dosing. The pharmacokinetic-pharmacodynamic interface. Postgrad. Med. 2000, 108, 17–24. [Google Scholar] [CrossRef]
- AliAbadi, F.S.; Lees, P. Antibiotic treatment for animals: Effect on bacterial population and dosage regimen optimisation. Int. J. Antimicrob. Agents 2000, 14, 307–313. [Google Scholar] [CrossRef]
- European Commission (EC). Commission Regulation (EU) No 37/2010 of 22 December 2009 on Pharmacologically Active Substances and Their Classification Regarding Maximum Residue Limits in Foodstuffs of Animal Origin; OJEU: Luxembourg, 2010; pp. 1–72. [Google Scholar]
- Korean Ministry of Food and Drug Safety, MRLs Vet. Drugs, Republic of Korea. 2019. Available online: https://apps.fas.usda.gov/newgainapi/api/Report/DownloadReportByFileName?fileName=Korea%27s+Veterinary+Drug+MRL+Situation_Seoul_Korea+-+Republic+of_11-16-2011.pdf (accessed on 20 May 2024).
- Rairat, T.; Hsieh, M.K.; Ho, W.C.; Lu, Y.P.; Fu, Z.Y.; Chuchird, N.; Chou, C.C. Effects of temperature on the pharmacokinetics, optimal dosage, tissue residue, and withdrawal time of florfenicol in asian seabass (Lates calcarifer). Food Addit. Contam. Part A 2023, 40, 235–246. [Google Scholar] [CrossRef]
- Lin, H.C.; Chen, W.Y. Bayesian population physiologically-based pharmacokinetic model for robustness evaluation of withdrawal time in tilapia aquaculture administrated to florfenicol. Ecotoxicol. Environ. Saf. 2021, 210, 111867. [Google Scholar] [CrossRef]
- Wang, W.; Lin, H.; Xue, C.; Khalid, J. Elimination of chloramphenicol, sulphamethoxazole and oxytetracycline in shrimp, Penaeus chinensis following medicated-feed treatment. Environ. Int. 2004, 30, 367–373. [Google Scholar] [CrossRef]
- Caceci, T.; Neck, K.F.; Lewis, D.D.H.; Sis, R.F. Ultrastructure of the hepatopancreas of the pacific white shrimp, Penaeus vannamei (Crustacea: Decapoda). J. Mar. Biol. Assoc. UK 1988, 68, 323–337. [Google Scholar] [CrossRef]
- Pathak, M.S.; Reddy, A.K.; Kulkarni, M.V.; Harikrishna, V.; Srivastava, P.P.; Chadha, N.K.; Lakra, W.S. Histological alterations in the hepatopancreas and growth performance of Pacific white shrimp (Litopenaeus vannamei, Boone 1931) reared in potassium fortified inland saline ground water. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3531–3542. [Google Scholar] [CrossRef]
- Hu, K.J.; Leung, P.C. Food digestion by cathepsin L and digestion-related rapid cell differentiation in shrimp hepatopancreas. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2007, 146, 69–80. [Google Scholar] [CrossRef]
- Nunes, E.T.; Braga, A.A.; Camargo-Mathias, M.I. Histochemical study of the hepatopancreas in adult females of the pink-shrimp Farfantepenaeus brasiliensis Latreille, 1817. Acta Histochem. 2014, 116, 243–251. [Google Scholar] [CrossRef]
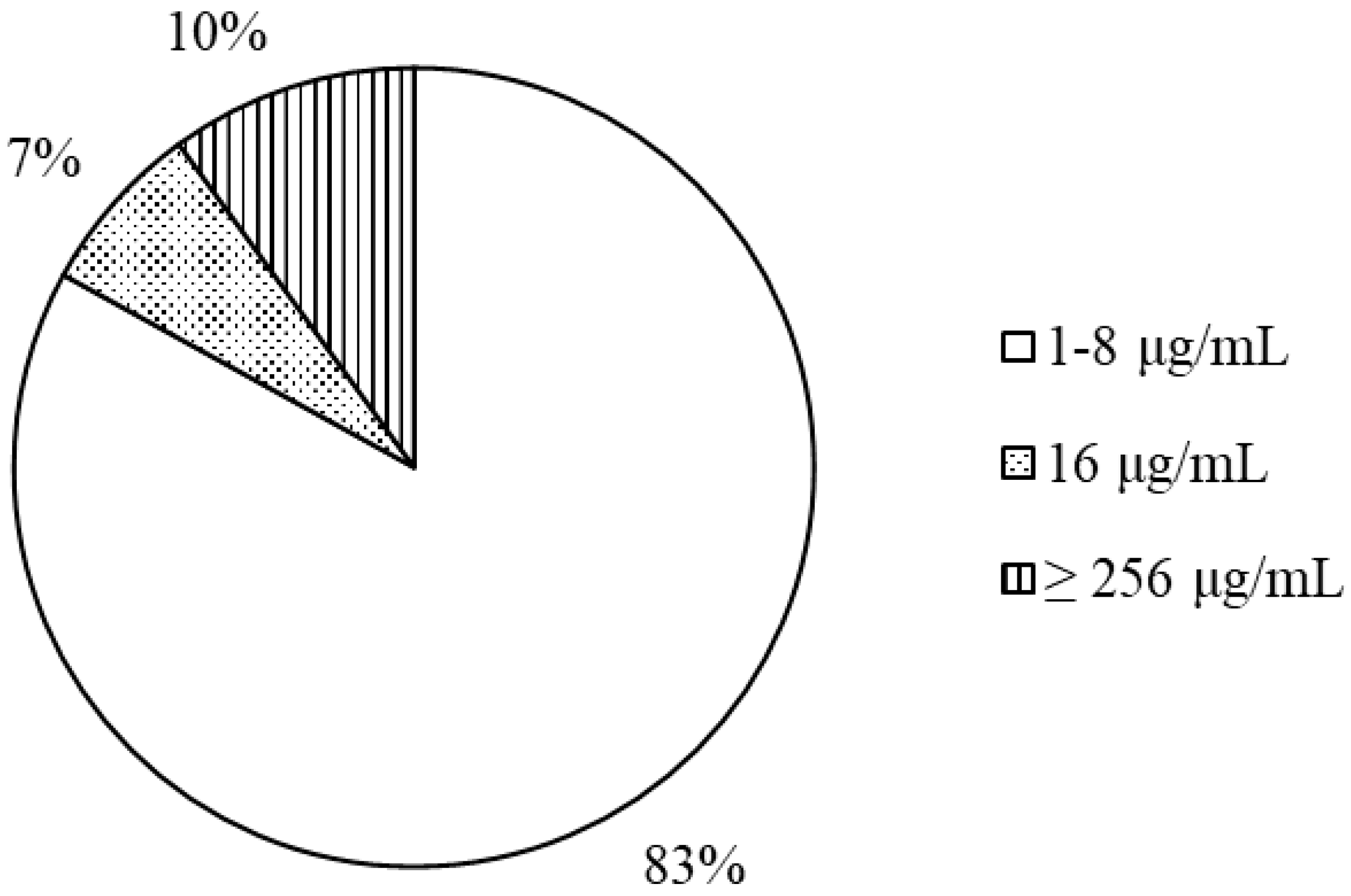
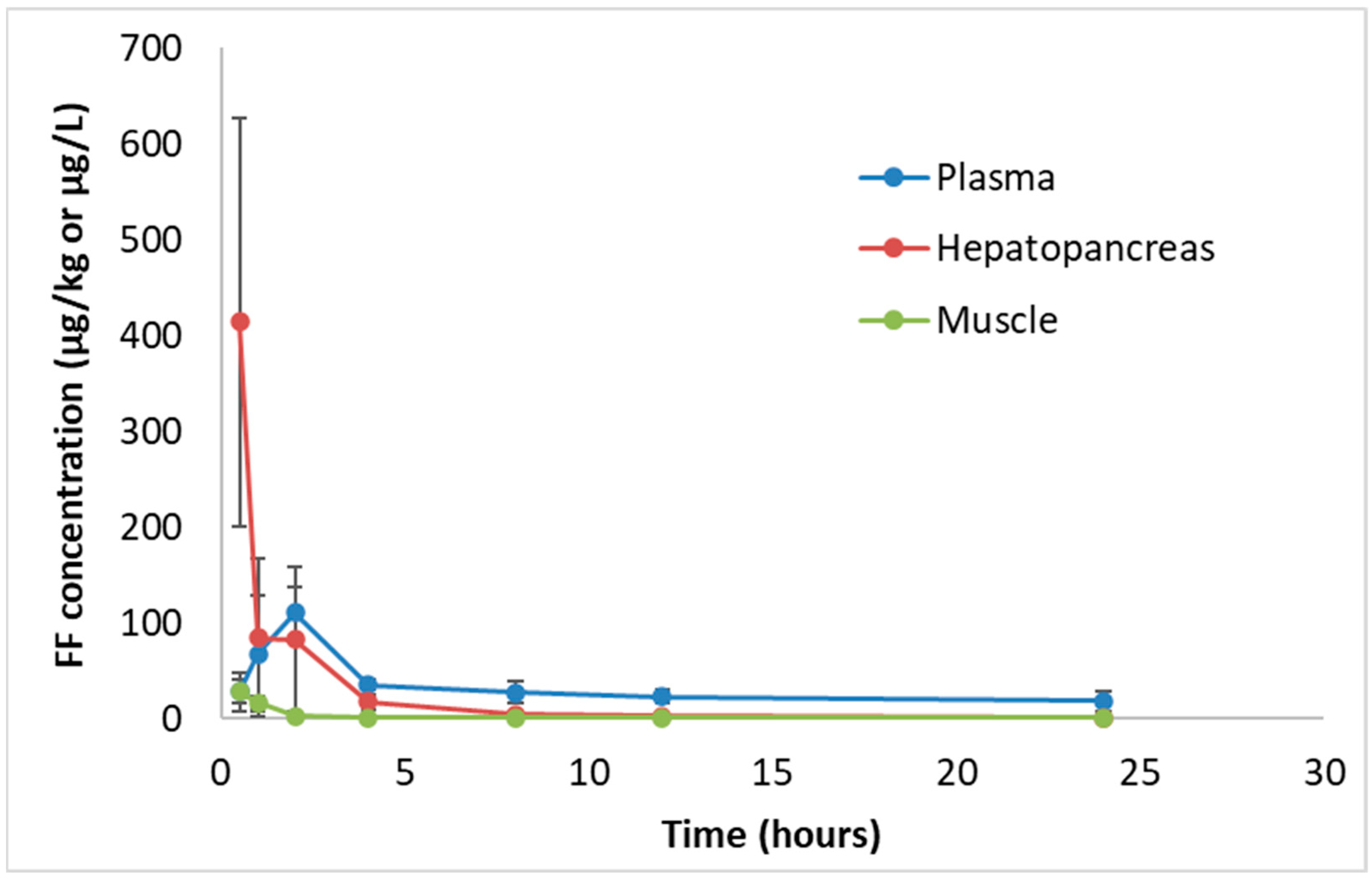
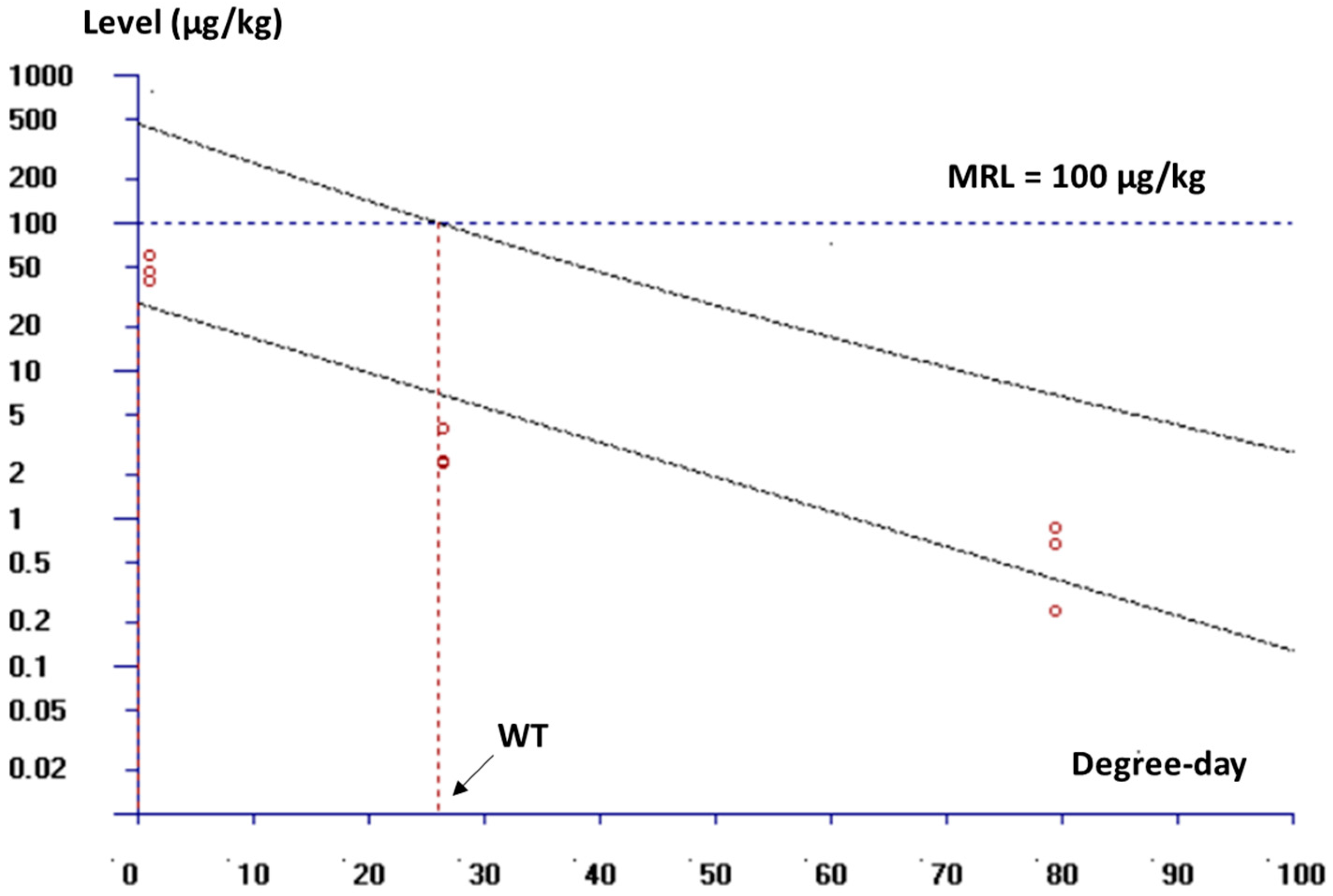
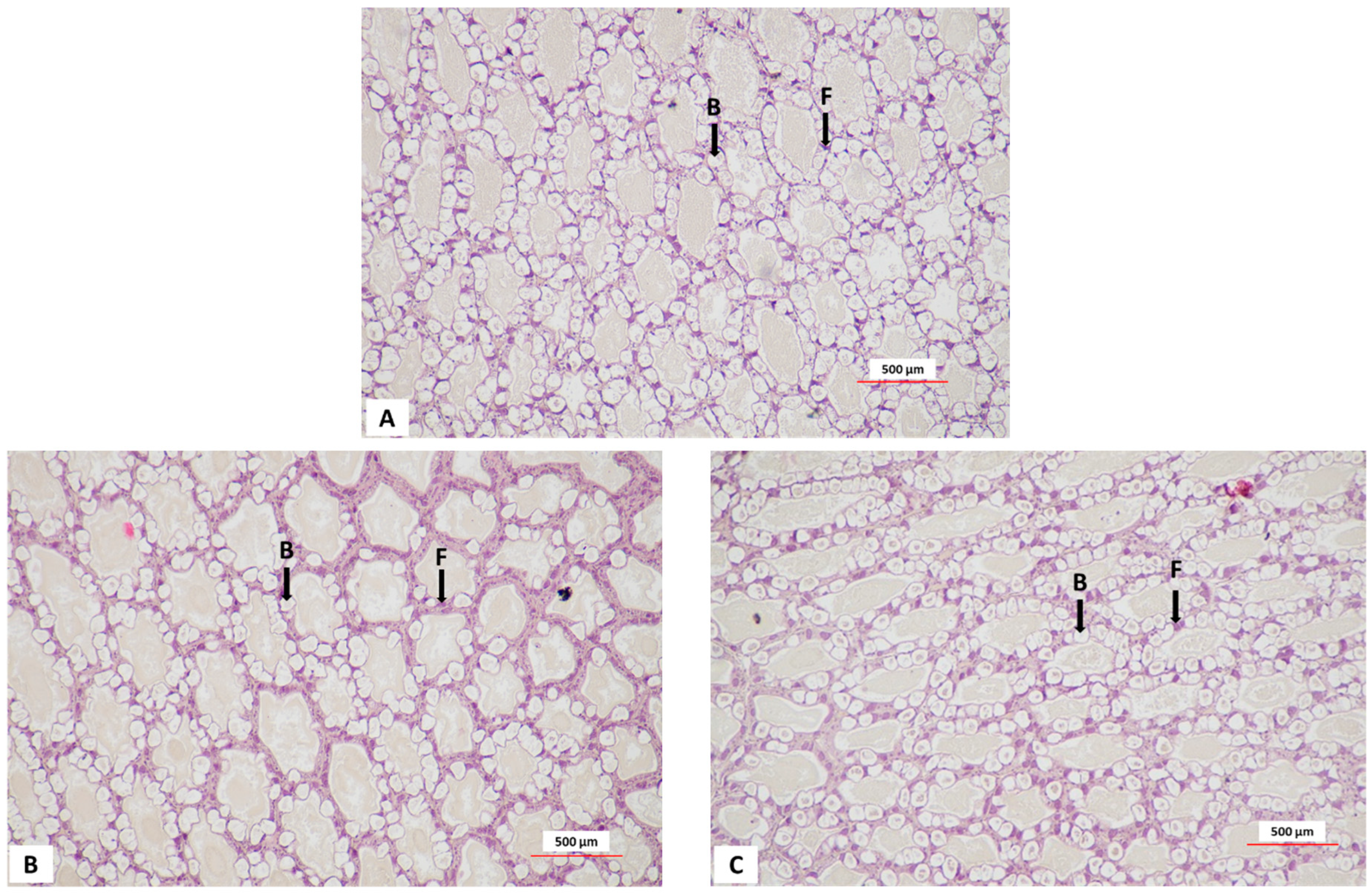
| Isolate Number | Inhibition Zone Diameter | MIC | Isolate Number | Inhibition Zone Diameter | MIC |
|---|---|---|---|---|---|
| 1 | 36 | 8 | 22 | 34 | 8 |
| 2 | 22 | 1 | 23 | 24 | 4 |
| 3 | 21 | 1 | 24 | 26 | 4 |
| 4 | 24 | 1 | 25 | 22 | 1 |
| 5 | 28 | 2 | 26 | 21 | 8 |
| 6 | 30 | 2 | 27 | 18 | 256 |
| 7 | 31 | 8 | 28 | 24 | 8 |
| 8 | 6 | >256 | 29 | 23 | 16 |
| 9 | 22 | 4 | 30 | 22 | 4 |
| 10 | 24 | 2 | 31 | 25 | 8 |
| 11 | 20 | 4 | 32 | 25 | 4 |
| 12 | 30 | 4 | 33 | 24 | 4 |
| 13 | 18 | 256 | 34 | 30 | 4 |
| 14 | 24 | 8 | 35 | 25 | 4 |
| 15 | 31 | 16 | 36 | 25 | 4 |
| 16 | 18 | 256 | 37 | 26 | 4 |
| 17 | 25 | 16 | 38 | 28 | 2 |
| 18 | 26 | 4 | 39 | 27 | 8 |
| 19 | 22 | 2 | 40 | 27 | 8 |
| 20 | 36 | 4 | 41 | 25 | 8 |
| 21 | 31 | 8 | 42 | 28 | 8 |
| PK Parameter | Unit | Plasma Estimate | CV% |
|---|---|---|---|
| ka | h−1 | 0.066 | 13.88 |
| T1/2abs | h | 10.44 | 13.88 |
| Cmax | µg/L | 60.56 | 7.45 |
| Tmax | h | 1.77 | 10.41 |
| Cl | L/kg/h | 9.75 | 13.85 |
| Vd | L/kg | 4.93 | 30.51 |
| AUC0−inf | µg·h/L | 1026.07 | 13.85 |
| kel | h−1 | 1.97 | 17.96 |
| T1/2el | h | 0.35 | 17.96 |
| PK Parameter | Unit | Hepatopancreas Estimate | Muscle Estimate |
|---|---|---|---|
| Cmax | µg/kg | 386.92 | 11.76 |
| Tmax | h | 0.19 | 0.20 |
| AUC0−inf | µg·h/kg | 1660 | 88.0 |
| Period | Sampling Time (Days) | FF Level (Once-a-Day Treatment) (µg/kg) | FF Level (Twice-a-Day Treatment) (µg/kg) |
|---|---|---|---|
| Medication | 1 | 3.65 ± 0.16 | 20.53 ± 4.52 |
| 3 | 9.35 ± 3.10 | 49.29 ± 10.1 | |
| After stopping medication | 1 | 3.03 ± 1.61 | 2.95 ± 0.95 |
| 3 | <LOD | <LOD | |
| 7 | <LOD | <LOD | |
| 14 | <LOD | <LOD | |
| 21 | <LOD | <LOD |
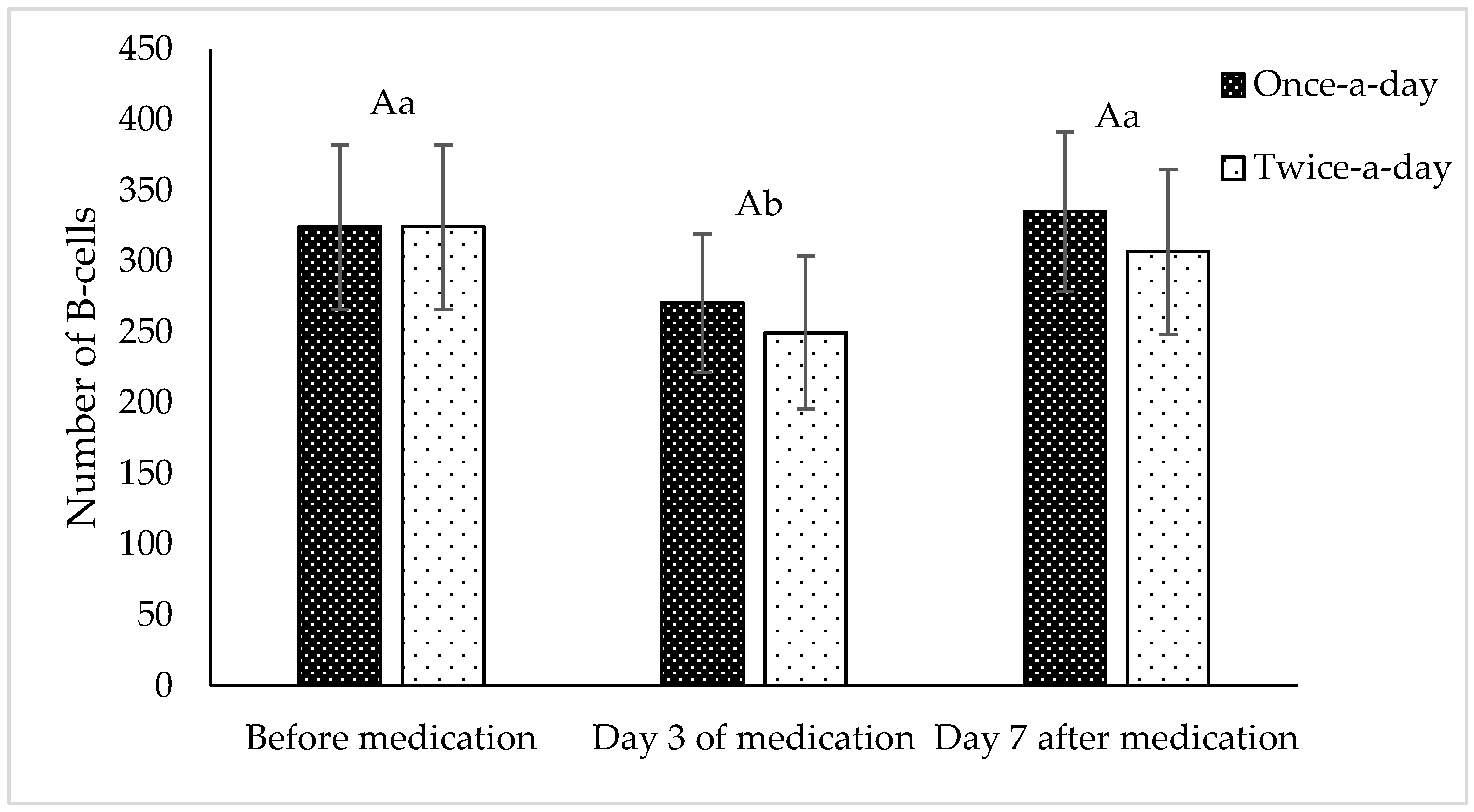
| Number of F-Cells | p-Value | |||
|---|---|---|---|---|
| Before Medication | Day 3 of Medication | Day 7 After Medication | ||
| Once-a-day | 164 ± 7 | 150 ± 20 | 160 ± 27 | 0.439 |
| Twice-a-day | 164 ± 7 | 153 ± 17 | 169 ± 21 | |
| p-value | 0.056 | |||
| Antibiotic | Muscle | Hepatopancreas | Plasma | References | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tmax (h) | Cmax (mg/kg) | AUC0-∞ (mg h/kg) | T1/2el (h) | Tmax (h) | Cmax (mg/kg) | AUC0-∞ (mg h/kg) | T1/2el (h) | Tmax (h) | Cmax (mg/L) | AUC0-∞ (mg·h/L) | T1/2el (h) | ||
| Single dose | |||||||||||||
| Oxytetracyline * 100 mg/kg | 6 | 0.73 | 32.63 | 23.53 | 1 | 149.5 | 1015 | 14.89 | 6 | 27.77 | 691.3 | 11.01 | [73] |
| Oxytetracyline ** 10 mg/kg | 6 | 7.94 | 166.5 | 20.90 | - | - | - | - | 0 | 32.22 | 266.1 | 20.74 | [74] |
| Thiamphenicol * 10 mg/kg | 2 | 2.98 | 29.33 | 6.84 | 1 | 204.2 | 1381 | 31.29 | 2 | 7.96 | 43.96 | 10.66 | [36] |
| Sulfamethoxazole * 83.3 mg/kg | 1 | 21.5 | 203.9 | 6.53 | 0.5 | 89.54 | 381.1 | 8.22 | 1 | 36.52 | 395.3 | 13.76 | [75] |
| Trimethoprim * 16.7 mg/kg | 1 | 0.78 | 1.90 | 2.12 | 0.5 | 73.17 | 321.3 | 4.53 | 1 | 0.89 | 4.82 | 7.38 | [75] |
| Enrofloxacin *** 10 mg/kg | 0.25 | 5.81 | 90.1 | 52.3 | 0.5 | 11.23 | 274.2 | 75.8 | 0.083 | 4.87 | 75.8 | 19.8 | [76] |
| Multiple dose | |||||||||||||
| Enrofloxacin * 30 mg/kg | 1 | 1.96 | 34.75 | 10.92 | 3 | 16.29 | 314.9 | 15.86 | 1 | 19.64 | 299.6 | 16.07 | [77] |
| Oxytetracyline * 100 mg/kg | 6 | 1.67 | 66.05 | 35.39 | 6 | 124.3 | 1858 | 18.89 | 6 | 68.03 | 2049 | 20.52 | [73] |
| Sulfamethoxazole * 83.3 mg/kg | 1 | 47.8 | 403.5 | 10.92 | 1 | 73.73 | 756.5 | 11.33 | 1 | 85.89 | 1493 | 11.03 | [75] |
| Trimethoprim * 16.7 mg/kg | 1 | 0.80 | 3.03 | 2.71 | 1 | 46.50 | 190.2 | 9.65 | 1 | 1.14 | 4.84 | 5.25 | [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huynh, T.K.D.; Nguyen, Q.T.; Scippo, M.-L.; Dang, T.H.O.; Devreese, M.; Douny, C.; Croubels, S.; Le, Q.V.; Tran, M.P. Pharmacokinetics, Pharmacodynamics and Depletion of Florfenicol Applied in White Leg Shrimp (Litopenaeus vannamei) Aquaculture and Impact on Shrimp Hepatopancreas Histology. Fishes 2025, 10, 318. https://doi.org/10.3390/fishes10070318
Huynh TKD, Nguyen QT, Scippo M-L, Dang THO, Devreese M, Douny C, Croubels S, Le QV, Tran MP. Pharmacokinetics, Pharmacodynamics and Depletion of Florfenicol Applied in White Leg Shrimp (Litopenaeus vannamei) Aquaculture and Impact on Shrimp Hepatopancreas Histology. Fishes. 2025; 10(7):318. https://doi.org/10.3390/fishes10070318
Chicago/Turabian StyleHuynh, Thi Kim Duyen, Quoc Thinh Nguyen, Marie-Louise Scippo, Thi Hoang Oanh Dang, Mathias Devreese, Caroline Douny, Siska Croubels, Quoc Viet Le, and Minh Phu Tran. 2025. "Pharmacokinetics, Pharmacodynamics and Depletion of Florfenicol Applied in White Leg Shrimp (Litopenaeus vannamei) Aquaculture and Impact on Shrimp Hepatopancreas Histology" Fishes 10, no. 7: 318. https://doi.org/10.3390/fishes10070318
APA StyleHuynh, T. K. D., Nguyen, Q. T., Scippo, M.-L., Dang, T. H. O., Devreese, M., Douny, C., Croubels, S., Le, Q. V., & Tran, M. P. (2025). Pharmacokinetics, Pharmacodynamics and Depletion of Florfenicol Applied in White Leg Shrimp (Litopenaeus vannamei) Aquaculture and Impact on Shrimp Hepatopancreas Histology. Fishes, 10(7), 318. https://doi.org/10.3390/fishes10070318







