Stress in Fish: Neuroendocrine and Neurotransmitter Responses
Abstract
1. Introduction
2. Stressors in Aquaculture
3. Neurotransmitters and Neuroendocrine Systems Respond to Stress in Fish
3.1. Neurotransmitter During Stress
3.1.1. Acetylcholine (ACh)
3.1.2. Glutamate (Glu) and Gamma-Aminobutyric Acid (GABA)
3.1.3. Catecholamine (CA)
3.1.4. Serotonin
3.2. Neuroendocrine Systems During Stress
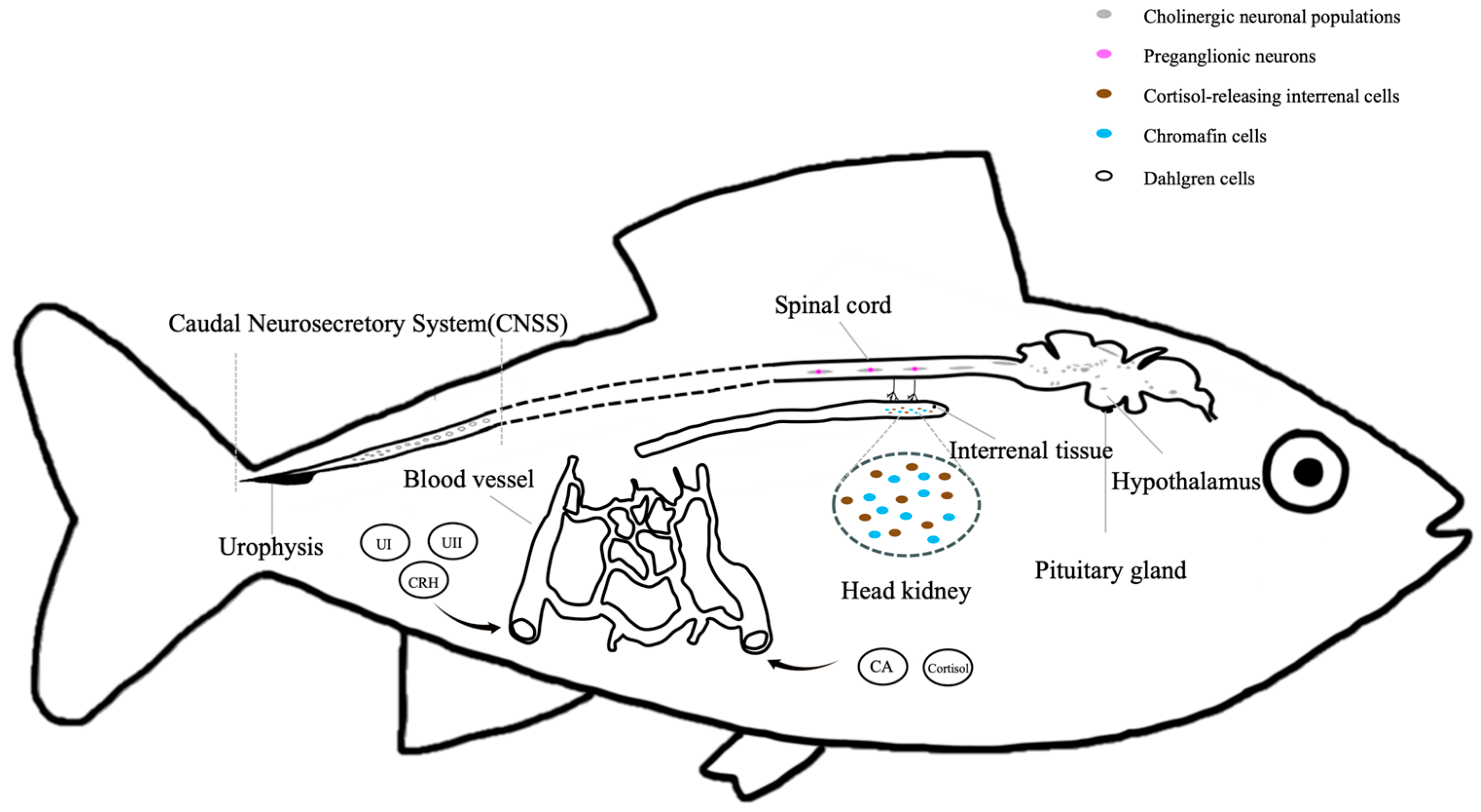
3.2.1. Brain–Sympathetic–Chromaffin Cell (BSC) Axis
3.2.2. Hypothalamus–Pituitary–Interrenal (HPI) Axis
3.2.3. Caudal Neurosecretory System (CNSS)
4. Dietary Supplements on Stress Mitigation Through Neuroendocrine and Neurotransmitter Regulations
4.1. Nutritional Supplements
4.2. Non-Nutritional Supplements
5. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Boyd, C.E.; McNevin, A.A.; Davis, R.P. The contribution of fisheries and aquaculture to the global protein supply. Food Secur. 2022, 14, 805–827. [Google Scholar] [CrossRef] [PubMed]
- Naylor, R.L.; Hardy, R.W.; Buschmann, A.H.; Bush, S.R.; Cao, L.; Klinger, D.H.; Little, D.C.; Lubchenco, J.; Shumway, S.E.; Troell, M. A 20-year retrospective review of global aquaculture. Nature 2021, 591, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Agorastos, A.; Chrousos, G.P. The neuroendocrinology of stress: The stress-related continuum of chronic disease development. Mol. Psychiatry 2022, 27, 502–513. [Google Scholar] [CrossRef] [PubMed]
- Koolhaas, J.M.; Bartolomucci, A.; Buwalda, B.; de Boer, S.F.; Flügge, G.; Korte, S.M.; Meerlo, P.; Murison, R.; Olivier, B.; Palanza, P.; et al. Stress revisited: A critical evaluation of the stress concept. Neurosci. Biobehav. Rev. 2011, 35, 1291–1301. [Google Scholar] [CrossRef]
- Urbinati, E.C.; Zanuzzo, F.S.; Biller, J.D. Stress and Immune System in Fish, Biology and Physiology of Freshwater Neotropical Fish; Elsevier: Amsterdam, The Netherlands, 2020; pp. 93–114. [Google Scholar]
- Nardocci, G.; Navarro, C.; Cortés, P.P.; Imarai, M.; Montoya, M.; Valenzuela, B.; Jara, P.; Acuña-Castillo, C.; Fernández, R. Neuroendocrine mechanisms for immune system regulation during stress in fish. Fish Shellfish Immunol. 2014, 40, 531–538. [Google Scholar] [CrossRef]
- Dhabhar, F.S. The short-term stress response—Mother nature’s mechanism for enhancing protection and performance under conditions of threat, challenge, and opportunity. Front. Neuroendocr. 2018, 49, 175–192. [Google Scholar] [CrossRef]
- Boonstra, R. Fox, Reality as the leading cause of stress: Rethinking the impact of chronic stress in nature. Funct. Ecol. 2012, 27, 11–23. [Google Scholar] [CrossRef]
- Canosa, L.F.; Bertucci, J.I. The effect of environmental stressors on growth in fish and its endocrine control. Front. Endocrinol. 2023, 14, 1109461. [Google Scholar] [CrossRef]
- Gao, X.; Ke, L.; Wang, L.; Zheng, S.; Liu, X.; Hu, W.; Tong, G.; Li, Z.; Hu, G. Low-temperature-induced disruption of reproductive axis and sperm vitality via stress axis in Monopterus albus. Gen. Comp. Endocrinol. 2024, 359, 114617. [Google Scholar] [CrossRef]
- Vargas-Chacoff, L.; Regish, A.M.; Weinstock, A.; McCormick, S.D. Effects of elevated temperature on osmoregulation and stress responses in Atlantic salmon Salmo salar smolts in fresh water and seawater. J. Fish Biol. 2018, 93, 550–559. [Google Scholar] [CrossRef]
- Evans, D.H.; Claiborne, J.B.; Currie, S. The Physiology of Fishes, 4th ed.; CRC Press: New York, NY, USA, 2013. [Google Scholar]
- Raman, R.; Prakash, C.; Makesh, M.; Pawar, N. Environmental stress mediated diseases of fish: An overview. Adv. Fish Res. 2013, 5, 141–158. [Google Scholar]
- Kazmi, S.S.U.H.; Wang, Y.Y.L.; Cai, Y.-E.; Wang, Z. Temperature effects in single or combined with chemicals to the aquatic organisms: An overview of thermo-chemical stress. Ecol. Indic. 2022, 143, 109354. [Google Scholar] [CrossRef]
- Chang, C.-H.; Wang, Y.-C.; Lee, T.-H. Hypothermal stress-induced salinity-dependent oxidative stress and apoptosis in the livers of euryhaline milkfish, Chanos chanos. Aquaculture 2021, 534, 736280. [Google Scholar] [CrossRef]
- Hassenruck, C.; Reinwald, H.; Kunzmann, A.; Tiedemann, I.; Gardes, A. Effects of Thermal Stress on the Gut Microbiome of Juvenile Milkfish (Chanos chanos). Microorganisms 2020, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Topal, A.; Ozdemir, S.; Arslan, H.; Comakli, S. How does elevated water temperature affect fish brain? (A neurophysiological and experimental study: Assessment of brain derived neurotrophic factor, cFOS, apoptotic genes, heat shock genes, ER-stress genes and oxidative stress genes). Fish Shellfish Immunol. 2021, 115, 198–204. [Google Scholar] [CrossRef]
- Li, X.; Wei, P.; Liu, S.; Tian, Y.; Ma, H.; Liu, Y. Photoperiods affect growth, food intake and physiological metabolism of juvenile European Sea Bass (Dicentrachus labrax L.). Aquacult. Rep. 2021, 20, 100656. [Google Scholar] [CrossRef]
- Konkal, P.; Ganesh, C.B. Continuous Exposure to Light Suppresses the Testicular Activity in Mozambique Tilapia Oreochromis mossambicus (Cichlidae). J. Ichthyol. 2020, 60, 660–667. [Google Scholar] [CrossRef]
- Suzuki, S.; Takahashi, E.; Nilsen, T.O.; Kaneko, N.; Urabe, H.; Ugachi, Y.; Yamaha, E.; Shimizu, M. Physiological changes in off-season smolts induced by photoperiod manipulation in masu salmon (Oncorhynchus masou). Aquaculture 2020, 526, 735353. [Google Scholar] [CrossRef]
- Sapozhnikova, Y.P.; Koroleva, A.G.; Yakhnenko, V.M.; Tyagun, M.L.; Glyzina, O.Y.; Coffin, A.B.; Makarov, M.M.; Shagun, A.N.; Kulikov, V.A.; Gasarov, P.V.; et al. Molecular and cellular responses to long-term sound exposure in peled (Coregonus peled). J. Acoust. Soc. Am. 2020, 148, 895. [Google Scholar] [CrossRef] [PubMed]
- Kusku, H.; Ergun, S.; Yilmaz, S.; Guroy, B.; Yigit, M. Impacts of Urban Noise and Musical Stimuli on Growth Performance and Feed Utilization of Koi fish (Cyprinus carpio) in Recirculating Water Conditions. Turk. J. Fish. Aquat. Sc. 2019, 19, 513–523. [Google Scholar]
- Kusku, H.; Yigit, Ü.; Yilmaz, S.; Yigit, M.; Ergün, S. Acoustic effects of underwater drilling and piling noise on growth and physiological response of Nile tilapia (Oreochromis niloticus). Aquac. Res. 2020, 51, 3166–3174. [Google Scholar] [CrossRef]
- Falahatkar, B.; Amlashi, A.S.; Kabir, M. Ventilation frequency in juvenile beluga sturgeon Huso huso L. exposed to clay turbidity. J. Exp. Mar. Biol. Ecol. 2019, 513, 10–12. [Google Scholar] [CrossRef]
- Phan, T.C.T.; Manuel, A.V.; Tsutsui, N.; Yoshimatsu, T. Impacts of short-term salinity and turbidity stress on the embryonic stage of red sea bream Pagrus major. Fish. Sci. 2019, 86, 119–125. [Google Scholar] [CrossRef]
- Hasenbein, M.; Fangue, N.A.; Geist, J.; Komoroske, L.M.; Truong, J.; McPherson, R.; Connon, R.E. Assessments at multiple levels of biological organization allow for an integrative determination of physiological tolerances to turbidity in an endangered fish species. Conserv. Physiol. 2016, 4, cow004. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, M.D.D.; Maltez, L.C.; Rodrigues, R.V.; Planas, M.; Sampaio, L.A. Does acidification lead to impairments on oxidative status and survival of orange clownfish Amphiprion percula juveniles? Fish Physiol. Biochem. 2021, 47, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, M.D.D.; Garcia-Mesa, S.; Sampaio, L.A.; Planas, M. Primary, secondary, and tertiary stress responses of juvenile seahorse Hippocampus reidi exposed to acute acid stress in brackish and seawater. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2021, 255, 110592. [Google Scholar] [CrossRef]
- Pellegrin, L.; Nitz, L.F.; Maltez, L.C.; Copatti, C.E.; Garcia, L. Alkaline water improves the growth and antioxidant responses of pacu juveniles (Piaractus mesopotamicus). Aquaculture 2020, 519, 734713. [Google Scholar] [CrossRef]
- Bal, A.; Pati, S.G.; Panda, F.; Mohanty, L.; Paital, B. Low salinity induced challenges in the hardy fish Heteropneustes fossilis; future prospective of aquaculture in near coastal zones. Aquaculture 2021, 543, 737007. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Noreldin, A.E.; Sewilam, H. Long term salinity disrupts the hepatic function, intestinal health, and gills antioxidative status in Nile tilapia stressed with hypoxia. Ecotoxicol. Environ. Environ. Saf. 2021, 220, 112412. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Saad, M.F.; Shukry, M.; El-Keredy, A.M.S.; Nasif, O.; Van Doan, H.; Dawood, M.A.O. Physiological and ion changes of Nile tilapia (Oreochromis niloticus) under the effect of salinity stress. Aquacult. Rep. 2021, 19, 100567. [Google Scholar] [CrossRef]
- Zeng, J.; Herbert, N.A.; Lu, W. Differential Coping Strategies in Response to Salinity Challenge in Olive Flounder. Front. Physiol. 2019, 10, 1378. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-S.; Guo, Z.-X.; Zhang, J.-D.; Wang, W.-Z.; Wang, Z.-L.; Amenyogbe, E.; Chen, G. Effects of hypoxia-reoxygenation conditions on serum chemistry indicators and gill and liver tissues of cobia (Rachycentron canadum). Aquacult. Rep. 2021, 20, 100692. [Google Scholar] [CrossRef]
- Schafer, N.; Matousek, J.; Rebl, A.; Stejskal, V.; Brunner, R.M.; Goldammer, T.; Verleih, M.; Korytar, T. Effects of Chronic Hypoxia on the Immune Status of Pikeperch (Sander lucioperca Linnaeus, 1758). Biology 2021, 10, 649. [Google Scholar] [CrossRef]
- Zheng, X.; Fu, D.; Cheng, J.; Tang, R.; Chu, M.; Chu, P.; Wang, T.; Yin, S. Effects of hypoxic stress and recovery on oxidative stress, apoptosis, and intestinal microorganisms in Pelteobagrus vachelli. Aquaculture 2021, 543, 736945. [Google Scholar] [CrossRef]
- Su, H.; Ma, D.; Zhu, H.; Liu, Z.; Gao, F. Transcriptomic response to three osmotic stresses in gills of hybrid tilapia (Oreochromis mossambicus female × O. urolepis hornorum male). BMC Genom. 2020, 21, 110. [Google Scholar]
- Zhao, Y.; Zhang, C.; Zhou, H.; Song, L.; Wang, J.; Zhao, J. Transcriptome changes for Nile tilapia (Oreochromis niloticus) in response to alkalinity stress. Comp. Biochem. Physiol. Part. D Genom. Proteom. 2020, 33, 100651. [Google Scholar] [CrossRef]
- Noor, N.M.; De, M.; Cob, Z.C.; Das, S.K. Welfare of scaleless fish, Sagor catfish (Hexanematichthys sagor) juveniles under different carbon dioxide concentrations. Aquac. Res. 2021, 52, 2980–2987. [Google Scholar] [CrossRef]
- Mota, V.C.; Nilsen, T.O.; Gerwins, J.; Gallo, M.; Kolarevic, J.; Krasnov, A.; Terjesen, B.F. Molecular and physiological responses to long-term carbon dioxide exposure in Atlantic salmon (Salmo salar). Aquaculture 2020, 519, 734715. [Google Scholar] [CrossRef]
- Machado, M.; Arenas, F.; Svendsen, J.C.; Azeredo, R.; Pfeifer, L.J.; Wilson, J.M.; Costas, B. Effects of Water Acidification on Senegalese Sole Solea senegalensis Health Status and Metabolic Rate: Implications for Immune Responses and Energy Use. Front. Physiol. 2020, 11, 26. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, X.-F.; He, S.; Li, L. Effects of long-term low-concentration nitrite exposure and detoxification on growth performance, antioxidant capacities, and immune responses in Chinese perch (Siniperca chuatsi). Aquaculture 2021, 533, 736123. [Google Scholar] [CrossRef]
- Yu, J.; Xiao, Y.; Wang, Y.; Xu, S.; Zhou, L.; Li, J.; Li, X. Chronic nitrate exposure cause alteration of blood physiological parameters, redox status and apoptosis of juvenile turbot (Scophthalmus maximus). Environ. Environ. Pollut. 2021, 283, 117103. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.Q.; Fei, F.; Huo, H.H.; Huang, B.; Meng, X.S.; Zhang, T.; Liu, B.L. Impact of nitrite exposure on plasma biochemical parameters and immune-related responses in Takifugu rubripes. Aquat. Toxicol. 2020, 218, 105362. [Google Scholar] [CrossRef]
- Xue, S.; Lin, J.; Han, Y.; Han, Y. Ammonia stress–induced apoptosis by p53–BAX/BCL-2 signal pathway in hepatopancreas of common carp (Cyprinus carpio). Aquacult Int. 2021, 29, 1895–1907. [Google Scholar] [CrossRef]
- Xue, S.; Lin, J.; Zhou, Q.; Wang, H.; Han, Y. Effect of ammonia stress on transcriptome and endoplasmic reticulum stress pathway for common carp (Cyprinus carpio) hepatopancreas. Aquacult. Rep. 2021, 20, 100694. [Google Scholar] [CrossRef]
- Cao, S.; Zhao, D.; Huang, R.; Xiao, Y.; Xu, W.; Liu, X.; Gui, Y.; Li, S.; Xu, J.; Tang, J.; et al. The influence of acute ammonia stress on intestinal oxidative stress, histology, digestive enzymatic activities and PepT1 activity of grass carp (Ctenopharyngodon idella). Aquacult. Rep. 2021, 20, 100722. [Google Scholar] [CrossRef]
- Kiemer, M.C.B.; Black, K.D.; Lussot, D.; Bullock, A.M.; Ezzi, I. The effects of chronic and acute exposure to hydrogen sulphide on Atlantic salmon (Salmo salar L.). Aquaculture 1995, 135, 311–327. [Google Scholar] [CrossRef]
- Bagarinao, T.; Lantin-Olaguer, I. The sulfide tolerance of milkfish and tilapia in relation to fish kills in farms and natural waters in the Philippines. Hydrobiologia 1998, 382, 137–150. [Google Scholar] [CrossRef]
- Valenzuela-Muñoz, V.; Valenzuela-Miranda, D.; Gonçalves, A.T.; Novoa, B.; Figueras, A.; Gallardo-Escárate, C. Induced-iron overdose modulate the immune response in Atlantic salmon increasing the susceptibility to Piscirickettsia salmonis infection. Aquaculture 2020, 521, 735058. [Google Scholar] [CrossRef]
- Singh, M.; Barman, A.S.; Devi, A.L.; Devi, A.G.; Pandey, P.K. Iron mediated hematological, oxidative and histological alterations in freshwater fish Labeo rohita. Ecotoxicol. Environ. Environ. Saf. 2019, 170, 87–97. [Google Scholar] [CrossRef]
- Asif, S.; Javed, M.; Abbas, S.; Ambreen, F.; Iqbal, S. Growth responses of carnivorous fish species under the chronic stress of water-borne copper. Iran. J. Fish. Sci. 2021, 20, 773–788. [Google Scholar]
- Paul, J.S.; Small, B.C. Chronic exposure to environmental cadmium affects growth and survival, cellular stress, and glucose metabolism in juvenile channel catfish (Ictalurus punctatus). Aquat. Toxicol. 2021, 230, 105705. [Google Scholar] [CrossRef] [PubMed]
- Kakade, A.; Salama, E.S.; Pengya, F.; Liu, P.; Li, X. Long-term exposure of high concentration heavy metals induced toxicity, fatality, and gut microbial dysbiosis in common carp, Cyprinus carpio. Environ. Pollut. 2020, 266 Pt 3, 115293. [Google Scholar] [CrossRef]
- Kong, Y.; Li, M.; Shan, X.; Wang, G.; Han, G. Effects of deltamethrin subacute exposure in snakehead fish, Channa argus: Biochemicals, antioxidants and immune responses. Ecotoxicol. Environ. Environ. Saf. 2021, 209, 111821. [Google Scholar] [CrossRef]
- Zhao, L.; Tang, G.; Xiong, C.; Han, S.; Yang, C.; He, K.; Liu, Q.; Luo, J.; Luo, W.; Wang, Y.; et al. Chronic chlorpyrifos exposure induces oxidative stress, apoptosis and immune dysfunction in largemouth bass (Micropterus salmoides). Environ. Environ. Pollut. 2021, 282, 117010. [Google Scholar] [CrossRef]
- Baldissera, M.D.; Souza, C.F.; Zanella, R.; Prestes, O.D.; Meinhart, A.D.; Da Silva, A.S.; Baldisserotto, B. Behavioral impairment and neurotoxic responses of silver catfish Rhamdia quelen exposed to organophosphate pesticide trichlorfon: Protective effects of diet containing rutin. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021, 239, 108871. [Google Scholar] [CrossRef] [PubMed]
- Zimba, P.V.; Khoo, L.; Gaunt, P.S.; Brittain, S.; Carmichael, W.W. Confirmation of catfish, Ictalurus punctatus (Rafinesque), mortality from Microcystis toxins. J. Fish Dis. 2008, 24, 41–47. [Google Scholar] [CrossRef]
- Bakke, M.J.; Horsberg, T.E. Effects of algal-produced neurotoxins on metabolic activity in telencephalon, optic tectum and cerebellum of Atlantic salmon (Salmo salar). Aquat. Toxicol. 2007, 85, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.O.; Moustafa, E.M.; Gewaily, M.S.; Abdo, S.E.; AbdEl-Kader, M.F.; SaadAllah, M.S.; Hamouda, A.H. Ameliorative effects of Lactobacillus plantarum L-137 on Nile tilapia (Oreochromis niloticus) exposed to deltamethrin toxicity in rearing water. Aquat. Toxicol. 2020, 219, 105377. [Google Scholar] [CrossRef]
- Chen, X.; Yi, H.; Liu, S.; Zhang, Y.; Su, Y.; Liu, X.; Bi, S.; Lai, H.; Zeng, Z.; Li, G. Promotion of pellet-feed feeding in mandarin fish (Siniperca chuatsi) by Bdellovibrio bacteriovorus is influenced by immune and intestinal flora. Aquaculture 2021, 542, 736864. [Google Scholar] [CrossRef]
- Zaineldin, A.I.; Hegazi, S.; Koshio, S.; Ishikawa, M.; Dawood, M.A.O.; Dossou, S.; Yukun, Z.; Mzengereza, K. Singular effects of Bacillus subtilis C-3102 or Saccharomyces cerevisiae type 1 on the growth, gut morphology, immunity, and stress resistance of red sea bream (Pagrus major). Ann. Anim. Sci. 2021, 21, 589–608. [Google Scholar] [CrossRef]
- Kolek, L.; Szczygieł, J.; Napora-Rutkowski, Ł.; Irnazarow, I. Effect of Trypanoplasma borreli infection on sperm quality and reproductive success of common carp (Cyprinus carpio L.) males. Aquaculture 2021, 539, 736623. [Google Scholar] [CrossRef]
- Xie, X.; Kong, J.; Huang, J.; Zhou, L.; Jiang, Y.; Miao, R.; Yin, F. Integration of metabolomic and transcriptomic analyses to characterize the influence of the gill metabolism of Nibea albiflora on the response to Cryptocaryon irritans infection. Vet. Parasitol. 2021, 298, 109533. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.P.; Marin, S.L.; Mancilla, M.; Canon-Jones, H.; Vargas-Chacoff, L. Fin Erosion of Salmo salar (Linnaeus 1758) Infested with the Parasite Caligus rogercresseyi (Boxshall & Bravo 2000). Animals 2020, 10, 1166. [Google Scholar] [CrossRef] [PubMed]
- Filipsson, K.; Bergman, E.; Greenberg, L.; Osterling, M.; Watz, J.; Erlandsson, A. Temperature and predator-mediated regulation of plasma cortisol and brain gene expression in juvenile brown trout (Salmo trutta). Front. Zool. 2020, 17, 25. [Google Scholar] [CrossRef]
- Kortet, R.; Laakkonen, M.V.M.; Tikkanen, J.; Vainikka, A.; Hirvonen, H. Size-dependent stress response in juvenile Arctic charr (Salvelinus alpinus) under prolonged predator conditioning. Aquac. Res. 2019, 50, 1482–1490. [Google Scholar] [CrossRef]
- Ling, H.; Fu, S.J.; Zeng, L.Q. Predator stress decreases standard metabolic rate and growth in juvenile crucian carp under changing food availability. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2019, 231, 149–157. [Google Scholar] [CrossRef]
- Barreto, T.N.; Boscolo, C.N.P.; Gonçalves-de-Freitas, E. Homogeneously sized groups increase aggressive interaction and affect social stress in Thai strain Nile tilapia (Oreochromis niloticus). Mar. Freshw. Behav. Phy 2015, 48, 309–318. [Google Scholar] [CrossRef]
- Gomez-Laplaza, L.M.; Morgan, E. The influence of social rank in the angelfish, Pterophyllum scalare, on locomotor and feeding activities in a novel environment. Lab. Anim. 2003, 37, 108–120. [Google Scholar] [CrossRef]
- Sloman, K.A.; Gilmour, K.M.; Taylor, A.C.; Metcalfe, N.B. Physiological effects of dominance hierarchies within groups of brown trout, Salmo trutta, held under simulated natural conditions. Fish Physiol. Biochem. 2000, 22, 11–20. [Google Scholar] [CrossRef]
- Zhang, Z.; Fu, Y.; Guo, H.; Zhang, X. Effect of environmental enrichment on the stress response of juvenile black rockfish Sebastes schlegelii. Aquaculture 2021, 533, 736088. [Google Scholar] [CrossRef]
- Zhang, Z.; Fu, Y.; Zhang, Z.; Zhang, X.; Chen, S. A Comparative Study on Two Territorial Fishes: The Influence of Physical Enrichment on Aggressive Behavior. Animals 2021, 11, 1868. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, X.; Wang, Y.; Zhang, X. Effects of environmental enrichment on growth performance, aggressive behavior and stress-induced changes in cortisol release and neurogenesis of black rockfish Sebastes schlegelii. Aquaculture 2020, 528, 735483. [Google Scholar] [CrossRef]
- Li, L.; Shen, Y.; Yang, W.; Xu, X.; Li, J. Effect of different stocking densities on fish growth performance: A meta-analysis. Aquaculture 2021, 544, 737152. [Google Scholar] [CrossRef]
- Poltronieri, C.; Laura, R.; Bertotto, D.; Negrato, E.; Simontacchi, C.; Guerrera, M.C.; Radaelli, G. Effects of exposure to overcrowding on rodlet cells of the teleost fish Dicentrarchus labrax (L.). Vet. Res. Commun. 2009, 33, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Caipang, C.M.A.; Brinchmann, M.F.; Berg, I.; Iversen, M.; Eliassen, R.; Kiron, V. Changes in selected stress and immune-related genes in Atlantic cod, Gadus morhua, following overcrowding. Aquac. Res. 2008, 39, 1533–1540. [Google Scholar] [CrossRef]
- Baldissera, M.D.; de Freitas Souza, C.; Val, A.L.; Baldisserotto, B. Involvement of purinergic signaling in the Amazon fish Pterygoplichthys pardalis subjected to handling stress: Relationship with immune response. Aquaculture 2020, 514, 734481. [Google Scholar] [CrossRef]
- Sun, P.; Yin, F.; Tang, B. Effects of Acute Handling Stress on Expression of Growth-Related Genes in Pampus argenteus. J. World Aquacult Soc. 2016, 48, 166–179. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, Y.; Yang, D.; Chen, J.; He, Y.; Li, X.; Feng, X.; Xiong, B. Cage-cultured Largemouth Bronze Gudgeon, Coreius guichenoti: Biochemical Profile of Plasma and Physiological Response to Acute Handling Stress. J. World Aquacult Soc. 2013, 44, 628–640. [Google Scholar] [CrossRef]
- de Magalhães, C.R.; Schrama, D.; Nakharuthai, C.; Boonanuntanasarn, S.; Revets, D.; Planchon, S.; Kuehn, A.; Cerqueira, M.; Carrilho, R.; Farinha, A.P.; et al. Metabolic Plasticity of Gilthead Seabream Under Different Stressors: Analysis of the Stress Responsive Hepatic Proteome and Gene Expression. Front. Mar. Sci. 2021, 8, 676189. [Google Scholar] [CrossRef]
- Takahashi, K.; Masuda, R. Net-chasing training improves the behavioral characteristics of hatchery-reared red sea bream (Pagrus major) juveniles. Can. J. Fish. Aquat. Sci. 2018, 75, 861–867. [Google Scholar] [CrossRef]
- Ziegelbecker, A.; Sefc, K.M. Growth, body condition and contest performance after early-life food restriction in a long-lived tropical fish. Ecol. Evol. 2021, 11, 10904–10916. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Luo, S.; Qiu, N.; Xiong, X.; Wang, J. The Reproductive Strategy of the Rare Minnow (Gobiocypris rarus) in Response to Starvation Stress. Zool. Stud. 2020, 59, e1. [Google Scholar]
- Varju-Katona, M.; Muller, T.; Bokor, Z.; Balogh, K.; Mezes, M. Effects of various lengths of starvation on body parameters and meat composition in intensively reared pikeperch (Sander lucioperca L.). Iran. J. Fish. Sci. 2020, 19, 2062–2076. [Google Scholar]
- Bortoletti, M.; Maccatrozzo, L.; Radaelli, G.; Caberlotto, S.; Bertotto, D. Muscle Cortisol Levels, Expression of Glucocorticoid Receptor and Oxidative Stress Markers in the Teleost Fish Argyrosomus regius Exposed to Transport Stress. Animals 2021, 11, 1160. [Google Scholar] [CrossRef]
- Vanderzwalmen, M.; McNeill, J.; Delieuvin, D.; Senes, S.; Sanchez-Lacalle, D.; Mullen, C.; McLellan, I.; Carey, P.; Snellgrove, D.; Foggo, A.; et al. Monitoring water quality changes and ornamental fish behaviour during commercial transport. Aquaculture 2021, 531, 735860. [Google Scholar] [CrossRef]
- Grausgruber, E.E.; Weber, M.J. Effects of Stocking Transport Duration on Age-0 Walleye. J. Fish. Wild. Manag. 2021, 12, 70–82. [Google Scholar] [CrossRef]
- Pfau, M.L.; Russo, S.J. Peripheral and Central Mechanisms of Stress Resilience. Neurobiol. Stress 2015, 1, 66–79. [Google Scholar] [CrossRef]
- Rima, M.; Lattouf, Y.; Younes, M.A.; Bullier, E.; Legendre, P.; Mangin, J.M.; Hong, E. Dynamic regulation of the cholinergic system in the spinal central nervous system. Sci. Rep. 2020, 10, 15338. [Google Scholar] [CrossRef]
- Gu, X.; Wang, X. An overview of recent analysis and detection of acetylcholine. Anal. Biochem. 2021, 632, 114381. [Google Scholar] [CrossRef]
- Mineur, Y.S.; Mose, T.N.; Vanopdenbosch, L.; Etherington, I.M.; Ogbejesi, C.; Islam, A.; Pineda, C.M.; Crouse, R.B.; Zhou, W.; Thompson, D.C.; et al. Hippocampal acetylcholine modulates stress-related behaviors independent of specific cholinergic inputs. Mol. Psychiatry 2022, 27, 1829–1838. [Google Scholar] [CrossRef]
- Yang, S.; Yan, T.; Zhao, L.; Wu, H.; Du, Z.; Yan, T.; Xiao, Q. Effects of temperature on activities of antioxidant enzymes and Na+/K+-ATPase, and hormone levels in Schizothorax prenanti. J. Therm. Biol. 2018, 72, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Sarasamma, S.; Audira, G.; Juniardi, S.; Sampurna, B.P.; Liang, S.-T.; Hao, E.; Lai, Y.-H.; Hsiao, C.-D. Zinc Chloride Exposure Inhibits Brain Acetylcholine Levels, Produces Neurotoxic Signatures, and Diminishes Memory and Motor Activities in Adult Zebrafish. Int. J. Mol. Sci. 2018, 19, 3195. [Google Scholar] [CrossRef] [PubMed]
- Szabo, A.; Nemcsok, J.; Kasa, P.; Budai, D. Comparative study of acetylcholine synthesis in organs of freshwater teleosts. Fish Physiol. Biochem. 1991, 9, 93–99. [Google Scholar] [CrossRef]
- Bejaoui, S.; Chetoui, I.; Ghribi, F.; Belhassen, D.; Abdallah, B.B.; Fayala, C.B.; Boubaker, S.; Mili, S.; Soudani, N. Exposure to different cobalt chloride levels produces oxidative stress and lipidomic changes and affects the liver structure of Cyprinus carpio juveniles. Env. Sci. Pollut. Res. Int. 2024, 31, 51658–51672. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.J.; Chung, J.K. Effects of trichlorfon on oxidative stress, neurotoxicity, and cortisol levels in common carp, Cyprinus carpio L., at different temperatures. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020, 229, 108698. [Google Scholar] [CrossRef]
- Ghosh, S.; Bhattacharya, R.; Pal, S.; Saha, N.C. Benzalkonium chloride induced acute toxicity and its multifaceted implications on growth, hematological metrics, biochemical profiles, and stress-responsive biomarkers in tilapia (Oreochromis mossambicus). Env. Sci. Pollut. Res. Int. 2024, 31, 52147–52170. [Google Scholar] [CrossRef]
- Deb, N.; Das, S. Acetylcholine esterase and antioxidant responses in freshwater teleost, Channa punctata exposed to chlorpyrifos and urea. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021, 240, 108912. [Google Scholar] [CrossRef]
- Renick, V.C.; Weinersmith, K.; Vidal-Dorsch, D.E.; Anderson, T.W. Effects of a pesticide and a parasite on neurological, endocrine, and behavioral responses of an estuarine fish. Aquat. Toxicol. 2016, 170, 335–343. [Google Scholar] [CrossRef]
- Villalba, A.M.; De la Llave-Propin, A.; De la Fuente, J.; Perez, C.; de Chavarri, E.G.; Diaz, M.T.; Cabezas, A.; Gonzalez-Garoz, R.; Torrent, F.; Villarroel, M.; et al. Using underwater currents as an occupational enrichment method to improve the stress status in rainbow trout. Fish Physiol. Biochem. 2024, 50, 463–475. [Google Scholar] [CrossRef]
- Kar, S.; Senthilkumaran, B. Recent advances in understanding neurotoxicity, behavior and neurodegeneration in siluriformes. Aquac. Fish 2024, 9, 404–410. [Google Scholar] [CrossRef]
- Jifa, W.; Yu, Z.; Xiuxian, S.; You, W. Response of integrated biomarkers of fish (Lateolabrax japonicus) exposed to benzo[a]pyrene and sodium dodecylbenzene sulfonate. Ecotoxicol. Environ. Saf. 2006, 65, 230–236. [Google Scholar] [CrossRef]
- Olivares-Rubio, H.F.; Espinosa-Aguirre, J.J. Acetylcholinesterase activity in fish species exposed to crude oil hydrocarbons: A review and new perspectives. Chemosphere 2021, 264, 128401. [Google Scholar] [CrossRef]
- Zhao, T.; Zheng, L.; Zhang, Q.; Wang, S.; Zhao, Q.; Su, G.; Zhao, M. Stability towards the gastrointestinal simulated digestion and bioactivity of PAYCS and its digestive product PAY with cognitive improving properties. Food Funct. 2019, 10, 2439–2449. [Google Scholar] [CrossRef]
- Alzualde, A.; Jaka, O.; Latino, D.; Alijevic, O.; Iturria, I.; de Mendoza, J.H.; Pospisil, P.; Frentzel, S.; Peitsch, M.C.; Hoeng, J.; et al. Effects of nicotinic acetylcholine receptor-activating alkaloids on anxiety-like behavior in zebrafish. J. Nat. Med. 2021, 75, 926–941. [Google Scholar] [CrossRef]
- da Rosa, J.G.S.; Barcellos, H.H.A.; Fagundes, M.; Variani, C.; Rossini, M.; Kalichak, F.; Koakoski, G.; Oliveira, T.A.; Idalencio, R.; Frandoloso, R.; et al. Muscarinic receptors mediate the endocrine-disrupting effects of an organophosphorus insecticide in zebrafish. Environ. Toxicol. 2017, 32, 1964–1972. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Li, X.H.; Li, Y.J. Glutamate in peripheral organs: Biology and pharmacology. Eur. J. Pharmacol. 2016, 784, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Matsuoka, H.; Fujihara, H.; Ueta, Y.; Yanagawa, Y.; Inoue, M. GABA Signaling and Neuroactive Steroids in Adrenal Medullary Chromaffin Cells. Front. Cell. Neurosci. 2016, 10, 100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Hua, Z.; Li, Z. The role of glutamate and glutamine metabolism and related transporters in nerve cells. CNS Neurosci. Ther. 2024, 30, e14617. [Google Scholar] [CrossRef]
- Sprengel, R.; Eltokhi, A. Ionotropic Glutamate Receptors and Their Role in Health and Disease; Pfaff, D.W., Volkow, N.D., Rubenstein, J.L., Eds.; Neuroscience in the 21st Century; Springer International Publishing: Cham, Switzerland, 2022; pp. 57–86. [Google Scholar]
- Lee, K.T.; Liao, H.S.; Hsieh, M.H. Glutamine Metabolism, Sensing and Signaling in Plants. Plant Cell Physiol. 2023, 64, 1466–1481. [Google Scholar] [CrossRef]
- Heli, Z.; Hongyu, C.; Dapeng, B.; Shin, T.Y.; Yejun, Z.; Xi, Z. Yingying, Recent advances of gamma-aminobutyric acid: Physiological and immunity function, enrichment, and metabolic pathway. Front. Nutr. 2022, 9, 1076223. [Google Scholar] [CrossRef]
- Hossein-Javaheri, N.; Buck, L.T. GABA receptor inhibition and severe hypoxia induce a paroxysmal depolarization shift in goldfish neurons. J. Neurophysiol. 2021, 125, 321–330. [Google Scholar] [CrossRef]
- Allen, N.J.; Attwell, D. The effect of simulated ischaemia on spontaneous GABA release in area CA1 of the juvenile rat hippocampus. J. Physiol. 2004, 561 Pt 2, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Wu, X.Y.; Zhou, X.Q.; Feng, L.; Liu, Y.; Jiang, W.D.; Wu, P.; Zhao, Y. Glutamate ameliorates copper-induced oxidative injury by regulating antioxidant defences in fish intestine. Br. J. Nutr. 2016, 116, 70–79. [Google Scholar] [CrossRef]
- Wang, M.; Li, E.; Huang, Y.; Liu, W.; Wang, S.; Li, W.; Chen, L.; Wang, X. Dietary supplementation with glutamate enhanced antioxidant capacity, ammonia detoxification and ion regulation ability in Nile tilapia (Oreochromis niloticus) exposed to acute alkalinity stress. Aquaculture 2025, 594, 741360. [Google Scholar] [CrossRef]
- Li, L.; Liu, Z.; Quan, J.; Lu, J.; Zhao, G.; Sun, J. Metabonomics analysis reveals the protective effect of nano-selenium against heat stress of rainbow trout (Oncorhynchus mykiss). J. Proteom. 2022, 259, 104545. [Google Scholar] [CrossRef]
- Sun, Y.; Geng, C.; Liu, W.; Liu, Y.; Ding, L.; Wang, P. Investigating the Impact of Disrupting the Glutamine Metabolism Pathway on Ammonia Excretion in Crucian Carp (Carassius auratus) under Carbonate Alkaline Stress Using Metabolomics Techniques. Antioxidants 2024, 13, 170. [Google Scholar] [CrossRef]
- El-Naggar, K.; El-Kassas, S.; Abdo, S.E.; Kirrella, A.A.K.; Al Wakeel, R.A. Role of gamma-aminobutyric acid in regulating feed intake in commercial broilers reared under normal and heat stress conditions. J. Therm. Biol. 2019, 84, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Sohrabipour, S.; Sharifi, M.R.; Talebi, A.; Sharifi, M.; Soltani, N. GABA dramatically improves glucose tolerance in streptozotocin-induced diabetic rats fed with high-fat diet. Eur. J. Pharmacol. 2018, 826, 75–84. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Wang, C.; Song, Y.; Pan, J.; Shi, Q.; Qin, J.; Chen, L. Gamma-aminobutyric acid regulates glucose homeostasis and enhances the hepatopancreas health of juvenile Chinese mitten crab (Eriocheir sinensis) under fasting stress. Gen. Comp. Endocrinol. 2021, 303, 113704. [Google Scholar] [CrossRef]
- Ncho, C.M.; Goel, A.; Gupta, V.; Jeong, C.M.; Choi, Y.H. Embryonic manipulations modulate differential expressions of heat shock protein, fatty acid metabolism, and antioxidant-related genes in the liver of heat-stressed broilers. PLoS ONE 2022, 17, e0269748. [Google Scholar] [CrossRef]
- Motiejunaite, J.; Amar, L.; Vidal-Petiot, E. Adrenergic receptors and cardiovascular effects of catecholamines. Ann. Endocrinol. 2021, 82, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Barsagade, V.G. Dopamine system in the fish brain: A review on current knowledge. J. Entomol. Zool. Stud. 2020, 8, 2549–2555. [Google Scholar] [CrossRef]
- Joyce, W.; Warwicker, J.; Shiels, H.A.; Perry, S.F. Evolution and divergence of teleost adrenergic receptors: Why sometimes ‘the drugs don’t work’ in fish. J. Exp. Biol. 2023, 226, jeb245859. [Google Scholar] [CrossRef] [PubMed]
- Lapish, C.C.; Ahn, S.; Evangelista, L.M.; So, K.; Seamans, J.K.; Phillips, A.G. Tolcapone enhances food-evoked dopamine efflux and executive memory processes mediated by the rat prefrontal cortex. Psychopharmacology 2009, 202, 521–530. [Google Scholar] [CrossRef]
- Jhou, T.C. Dopamine and anti-dopamine systems: Polar opposite roles in behavior. FASEB J. 2013, 27, 80.2. [Google Scholar] [CrossRef]
- Domschke, K.; Winter, B.; Gajewska, A.; Unterecker, S.; Warrings, B.; Dlugos, A.; Notzon, S.; Nienhaus, K.; Markulin, F.; Gieselmann, A.; et al. Multilevel impact of the dopamine system on the emotion-potentiated startle reflex. Psychopharmacology 2015, 232, 1983–1993. [Google Scholar] [CrossRef]
- Aerts, J. Quantification of a Glucocorticoid Profile in Non-pooled Samples Is Pivotal in Stress Research Across Vertebrates. Front. Endocrinol. 2018, 9, 635. [Google Scholar] [CrossRef]
- Vargas-Chacoff, L.; Muñoz, J.L.P.; Ocampo, D.; Paschke, K.; Navarro, J.M. The effect of alterations in salinity and temperature on neuroendocrine responses of the Antarctic fish Harpagifer antarcticus. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2019, 235, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Sreelekshmi, S.; Manish, K.; Peter, M.C.S.; Inbaraj, R.M. Analysis of neuroendocrine factors in response to conditional stress in zebrafish Danio rerio (Cypriniformes: Cyprinidae). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022, 252, 109242. [Google Scholar] [CrossRef]
- Joyce, W.; Williams, C.J.A.; Iversen, S.; Henriksen, P.G.; Bayley, M.; Wang, T. The effects of endogenous and exogenous catecholamines on hypoxic cardiac performance in red-bellied piranhas. J. Exp. Zool. A Ecol. Integr. Physiol. 2019, 331, 27–37. [Google Scholar] [CrossRef]
- Schoen, A.N.; Weinrauch, A.M.; Bouyoucos, I.A.; Treberg, J.R.; Anderson, W.G. Hormonal effects on glucose and ketone metabolism in a perfused liver of an elasmobranch, the North Pacific spiny dogfish, Squalus suckleyi. Gen. Comp. Endocrinol. 2024, 352, 114514. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Patino, M.A.; Skrzynska, A.K.; Naderi, F.; Mancera, J.M.; Miguez, J.M.; Martos-Sitcha, J.A. High Stocking Density and Food Deprivation Increase Brain Monoaminergic Activity in Gilthead Sea Bream (Sparus aurata). Animals 2021, 11, 1503. [Google Scholar] [CrossRef]
- Roy, J.; Terrier, F.; Marchand, M.; Herman, A.; Heraud, C.; Surget, A.; Lanuque, A.; Sandres, F.; Marandel, L. Effects of Low Stocking Densities on Zootechnical Parameters and Physiological Responses of Rainbow Trout (Oncorhynchus mykiss) Juveniles. Biology 2021, 10, 1040. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Narang, R.K.; Singh, S. AlCl(3) induced learning and memory deficit in zebrafish. Neurotoxicology 2022, 92, 67–76. [Google Scholar] [CrossRef]
- Bedrossiantz, J.; Bellot, M.; Dominguez-García, P.; Faria, M.; Prats, E.; Gómez-Canela, C.; López-Arnau, R.; Escubedo, E.; Raldúa, D. A Zebrafish Model of Neurotoxicity by Binge-Like Methamphetamine Exposure. Front. Pharmacol. 2021, 12, 770319. [Google Scholar] [CrossRef] [PubMed]
- Amador, M.H.B.; McDonald, M.D. Is serotonin uptake by peripheral tissues sensitive to hypoxia exposure? Fish Physiol. Biochem. 2022, 48, 617–630. [Google Scholar] [CrossRef]
- Mardones, O.; Oyarzun-Salazar, R.; Labbe, B.S.; Miguez, J.M.; Vargas-Chacoff, L.; Munoz, J.L.P. Intestinal variation of serotonin, melatonin, and digestive enzymes activities along food passage time through GIT in Salmo salar fed with supplemented diets with tryptophan and melatonin. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2022, 266, 111159. [Google Scholar] [CrossRef]
- Hoglund, E.; Overli, O.; Winberg, S. Tryptophan Metabolic Pathways and Brain Serotonergic Activity: A Comparative Review. Front. Endocrinol. 2019, 10, 158. [Google Scholar] [CrossRef]
- Lillesaar, C. The serotonergic system in fish. J. Chem. Neuroanat. 2011, 41, 294–308. [Google Scholar] [CrossRef]
- Backstrom, T.; Winberg, S. Serotonin Coordinates Responses to Social Stress-What We Can Learn from Fish. Front. Neurosci. 2017, 11, 595. [Google Scholar] [CrossRef]
- Khan, N.; Deschaux, P. Role of serotonin in fish immunomodulation. J. Exp. Biol. 1997, 200 Pt 13, 1833–1838. [Google Scholar] [CrossRef]
- Lim, C.H.; Soga, T.; Parhar, I.S. Social stress-induced serotonin dysfunction activates spexin in male Nile tilapia (Oreochromis niloticus). Proc. Natl. Acad. Sci. USA 2023, 120, e2117547120. [Google Scholar] [CrossRef]
- Shams, S.; Seguin, D.; Facciol, A.; Chatterjee, D.; Gerlai, R. Effect of social isolation on anxiety-related behaviors, cortisol, and monoamines in adult zebrafish. Behav. Neurosci. 2017, 131, 492–504. [Google Scholar] [CrossRef]
- Higuchi, Y.; Soga, T.; Parhar, I.S. Social Defeat Stress Decreases mRNA for Monoamine Oxidase A and Increases 5-HT Turnover in the Brain of Male Nile Tilapia (Oreochromis niloticus). Front. Pharmacol. 2018, 9, 1549. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.; Luzio, A.; Felix, L.; Bellas, J.; Monteiro, S.M. Oxidative stress, apoptosis and serotonergic system changes in zebrafish (Danio rerio) gills after long-term exposure to microplastics and copper. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022, 258, 109363. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wang, X.; Wang, X.; Fang, Y.; Cao, S.; Huang, B.; Chen, H.; Xing, R.; Liu, B. Toxicity in Takifugu rubripes exposed to acute ammonia: Effects on immune responses, brain neurotransmitter levels, and thyroid endocrine hormones. Ecotoxicol. Environ. Environ. Saf. 2022, 244, 114050. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, Y.; Inahata, M.; Komori, M.; Kagawa, N. Reduction of Tryptophan Hydroxylase Expression in the Brain of Medaka Fish After Repeated Heat Stress. Zool. Sci. 2019, 36, 223–230. [Google Scholar] [CrossRef]
- Schjolden, J.; Pulman, K.G.; Pottinger, T.G.; Tottmar, O.; Winberg, S. Serotonergic characteristics of rainbow trout divergent in stress responsiveness. Physiol. Behav. 2006, 87, 938–947. [Google Scholar] [CrossRef]
- Hou, Z.S.; Liu, M.Q.; Wen, H.S.; Gao, Q.F.; Li, Z.; Yang, X.D.; Xiang, K.W.; Yang, Q.; Hu, X.; Qian, M.Z.; et al. Identification, characterization, and transcription of serotonin receptors in rainbow trout (Oncorhynchus mykiss) in response to bacterial infection and salinity changes. Int. J. Biol. Macromol. 2023, 249, 125930. [Google Scholar] [CrossRef]
- Martorell-Ribera, J.; Venuto, M.T.; Otten, W.; Brunner, R.M.; Goldammer, T.; Rebl, A.; Gimsa, U. Time-Dependent Effects of Acute Handling on the Brain Monoamine System of the Salmonid Coregonus maraena. Front. Neurosci. 2020, 14, 591738. [Google Scholar] [CrossRef]
- Shi, M.; Rupia, E.J.; Jiang, P.; Lu, W. Switch from fight-flight to freeze-hide: The impacts of severe stress and brain serotonin on behavioral adaptations in flatfish. Fish. Physiol. Biochem. 2024, 50, 891–909. [Google Scholar] [CrossRef] [PubMed]
- Shapouri, S.; Sharifi, A.; Folkedal, O.; Fraser, T.W.K.; Vindas, M.A. Behavioral and neurophysiological effects of buspirone in healthy and depression-like state juvenile salmon. Front. Behav. Neurosci. 2024, 18, 1285413. [Google Scholar] [CrossRef]
- Silva, R.X.D.C.; Nascimento, B.G.D.; Gomes, G.C.V.; da Silva, N.A.H.; Pinheiro, J.S.; da Silva Chaves, S.N.; Pimentel, A.F.N.; Costa, B.P.D.; Herculano, A.M.; Lima-Maximino, M.; et al. 5-HT2C agonists and antagonists block different components of behavioral responses to potential, distal, and proximal threat in zebrafish. Pharmacol. Biochem. Behav. 2021, 210, 173276. [Google Scholar]
- Medeiros, L.R.; Cartolano, M.C.; McDonald, M.D. Crowding stress inhibits serotonin 1A receptor-mediated increases in corticotropin-releasing factor mRNA expression and adrenocorticotropin hormone secretion in the Gulf toadfish. J. Comp. Physiol. B 2014, 184, 259–271. [Google Scholar] [CrossRef]
- Varga, Z.K.; Pejtsik, D.; Biro, L.; Zsigmond, A.; Varga, M.; Toth, B.; Salamon, V.; Annus, T.; Mikics, E.; Aliczki, M. Conserved Serotonergic Background of Experience-Dependent Behavioral Responsiveness in Zebrafish (Danio rerio). J. Neurosci. 2020, 40, 4551–4564. [Google Scholar] [CrossRef] [PubMed]
- Hoglund, E.; Moltesen, M.; Castanheira, M.F.; Thornqvist, P.O.; Silva, P.I.M.; Overli, O.; Martins, C.; Winberg, S. Contrasting neurochemical and behavioral profiles reflects stress coping styles but not stress responsiveness in farmed gilthead seabream (Sparus aurata). Physiol. Behav. 2020, 214, 112759. [Google Scholar] [CrossRef]
- Ulrich-Lai, Y.M.; Herman, J.P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009, 10, 397–409. [Google Scholar] [CrossRef]
- Scott-Solomon, E.; Boehm, E.; Kuruvilla, R. The sympathetic nervous system in development and disease. Nat. Rev. Neurosci. 2021, 22, 685–702. [Google Scholar] [CrossRef]
- Won, E.; Kim, Y.K. Stress, the Autonomic Nervous System, and the Immune-kynurenine Pathway in the Etiology of Depression. Curr. Neuropharmacol. 2016, 14, 665–673. [Google Scholar] [CrossRef]
- Kulkarni, S.S.; Mischel, N.A.; Mueller, P.J. Revisiting differential control of sympathetic outflow by the rostral ventrolateral medulla. Front. Physiol. 2022, 13, 1099513. [Google Scholar] [CrossRef]
- Zhou, J.J.; Pachuau, J.; Li, D.P.; Chen, S.R.; Pan, H.L. Group III metabotropic glutamate receptors regulate hypothalamic presympathetic neurons through opposing presynaptic and postsynaptic actions in hypertension. Neuropharmacology 2020, 174, 108159. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.J.; Zeng, Q.H.; Wang, W.Z.; Wang, W. GABA(A) and GABA(B) receptor-mediated inhibition of sympathetic outflow in the paraventricular nucleus is blunted in chronic heart failure. Clin. Exp. Pharmacol. Physiol. 2009, 36, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Perez-Rodriguez, R.; Olivan, A.M.; Roncero, C.; Moron-Oset, J.; Gonzalez, M.P.; Oset-Gasque, M.J. Glutamate triggers neurosecretion and apoptosis in bovine chromaffin cells through a mechanism involving NO production by neuronal NO synthase activation. Free Radic. Biol. Med. 2014, 69, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Harada, K.; Matsuoka, H.; Warashina, A. Paracrine role of GABA in adrenal chromaffin cells. Cell Mol. Neurobiol. 2010, 30, 1217–1224. [Google Scholar] [CrossRef]
- Brindley, R.L.; Bauer, M.B.; Blakely, R.D.; Currie, K.P.M. Serotonin and Serotonin Transporters in the Adrenal Medulla: A Potential Hub for Modulation of the Sympathetic Stress Response. ACS Chem. Neurosci. 2017, 8, 943–954. [Google Scholar] [CrossRef]
- Bauer, M.B.; Brindley, R.L.; Currie, K.P. Serotonergic regulation of catecholamine exocytosis from adrenal chromaffin cells involves two mechanistically and temporally distinct pathways. Biophys. J. 2024, 123, 382a. [Google Scholar] [CrossRef]
- Kalamarz-Kubiak, H. Cortisol in Correlation to Other Indicators of Fish Welfare, Corticosteroids; IntechOpen: London, UK, 2018; pp. 155–183. [Google Scholar]
- Skrzynska, A.K.; Maiorano, E.; Bastaroli, M.; Naderi, F.; Miguez, J.M.; Martinez-Rodriguez, G.; Mancera, J.M.; Martos-Sitcha, J.A. Impact of Air Exposure on Vasotocinergic and Isotocinergic Systems in Gilthead Sea Bream (Sparus aurata): New Insights on Fish Stress Response. Front. Physiol. 2018, 9, 96. [Google Scholar] [CrossRef]
- Chelebieva, E.S.; Kladchenko, E.S.; Mindukshev, I.V.; Gambaryan, S.; Andreyeva, A.Y. ROS formation, mitochondrial potential and osmotic stability of the lamprey red blood cells: Effect of adrenergic stimulation and hypoosmotic stress. Fish Physiol. Biochem. 2024, 50, 1341–1352. [Google Scholar] [CrossRef]
- Zhao, N.; Jiang, K.; Ge, X.; Huang, J.; Wu, C.; Chen, S.X. Neurotransmitter norepinephrine regulates chromatosomes aggregation and the formation of blotches in coral trout Plectropomus leopardus. Fish Physiol. Biochem. 2024, 50, 705–719. [Google Scholar] [CrossRef]
- Shaughnessy, C.A.; Myhre, V.D.; Hall, D.J.; McCormick, S.D.; Dores, R.M. Hypothalamus-pituitary-interrenal (HPI) axis signaling in Atlantic sturgeon (Acipenser oxyrinchus) and sterlet (Acipenser ruthenus). Gen. Comp. Endocrinol. 2023, 339, 114290. [Google Scholar] [CrossRef]
- Faught, E.; Schaaf, M.J.M. Molecular mechanisms of the stress-induced regulation of the inflammatory response in fish. Gen. Comp. Endocrinol. 2024, 345, 114387. [Google Scholar] [CrossRef] [PubMed]
- Torres-Martinez, A.; Hattori, R.S.; Fernandino, J.I.; Somoza, G.M.; Hung, S.D.; Masuda, Y.; Yamamoto, Y.; Strussmann, C.A. Temperature- and genotype-dependent stress response and activation of the hypothalamus-pituitary-interrenal axis during temperature-induced sex reversal in pejerrey Odontesthes bonariensis, a species with genotypic and environmental sex determination. Mol. Cell. Endocrinol. 2024, 582, 112114. [Google Scholar] [CrossRef]
- Virtanen, M.I.; Iversen, M.H.; Patel, D.M.; Brinchmann, M.F. Daily crowding stress has limited, yet detectable effects on skin and head kidney gene expression in surgically tagged atlantic salmon (Salmo salar). Fish Shellfish. Immunol. 2024, 152, 109794. [Google Scholar] [CrossRef]
- Whitehouse, L.M.; Faught, E.; Vijayan, M.M.; Manzon, R.G. Hypoxia affects the ontogeny of the hypothalamus-pituitary-interrenal axis functioning in the lake whitefish (Coregonus clupeaformis). Gen. Comp. Endocrinol. 2020, 295, 113524. [Google Scholar] [CrossRef]
- Saiz, N.; Gomez-Boronat, M.; De Pedro, N.; Delgado, M.J.; Isorna, E. The Lack of Light-Dark and Feeding-Fasting Cycles Alters Temporal Events in the Goldfish (Carassius auratus) Stress Axis. Animals 2021, 11, 669. [Google Scholar] [CrossRef] [PubMed]
- Ghaedi, G.; Falahatkar, B.; Yavari, V.; Sheibani, M.T.; Broujeni, G.N. The onset of stress response in rainbow trout Oncorhynchus mykiss embryos subjected to density and handling. Fish Physiol. Biochem. 2015, 41, 485–493. [Google Scholar] [CrossRef]
- Madaro, A.; Olsen, R.E.; Kristiansen, T.S.; Ebbesson, L.O.; Nilsen, T.O.; Flik, G.; Gorissen, M. Stress in Atlantic salmon: Response to unpredictable chronic stress. J. Exp. Biol. 2015, 218 Pt 16, 2538–2550. [Google Scholar] [CrossRef]
- Arab-Bafrani, Z.; Zabihi, E.; Hoseini, S.M.; Sepehri, H.; Khalili, M. Silver nanoparticles modify the hypothalamic-pituitary-interrenal axis and block cortisol response to an acute stress in zebrafish, Danio rerio. Toxicol. Ind. Health 2022, 38, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Tellis, M.S.; Alsop, D.; Wood, C.M. Effects of copper on the acute cortisol response and associated physiology in rainbow trout. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2012, 155, 281–289. [Google Scholar] [CrossRef]
- Zhuo, M.Q.; Chen, X.; Gao, L.; Zhang, H.T.; Zhu, Q.L.; Zheng, J.L.; Liu, Y. Early life stage exposure to cadmium and zinc within hour affected GH/IGF axis, Nrf2 signaling and HPI axis in unexposed offspring of marine medaka Oryzias melastigma. Aquat. Toxicol. 2023, 261, 106628. [Google Scholar] [CrossRef]
- Nesan, D.; Vijayan, M.M. Maternal Cortisol Mediates Hypothalamus-Pituitary-Interrenal Axis Development in Zebrafish. Sci. Rep. 2016, 6, 22582. [Google Scholar] [CrossRef]
- Plotsky, P.M.; Cunningham, E.T., Jr.; Widmaier, E.P. Catecholaminergic modulation of corticotropin-releasing factor and adrenocorticotropin secretion. Endocr. Rev. 1989, 10, 437–458. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.W.; Strachan, M.W.; Lightly, E.R.; Williams, B.C.; Bird, I.M. Acetylcholine stimulates cortisol secretion through the M3 muscarinic receptor linked to a polyphosphoinositide-specific phospholipase C in bovine adrenal fasciculata/reticularis cells. Mol. Cell. Endocrinol. 1990, 72, 227–238. [Google Scholar] [CrossRef]
- Ulrich-Lai, Y.M.; Engeland, W.C. Sympatho-Adrenal Activity and Hypothalamic–Pituitary–Adrenal Axis Regulation; Steckler, T., Kalin, N.H., Reul, J.M.H.M., Eds.; Handbook of Stress and the Brain—Part 1: The Neurobiology of Stress; Elsevier: Amsterdam, The Netherlands, 2005; pp. 419–435. [Google Scholar]
- Ormaechea, E.B.; Cornide-Petronio, M.E.; Negrete-Sánchez, E.; De León, C.G.Á.; Álvarez-Mercado, A.I.; Gulfo, J.; Castro, M.B.J.; Gracia-Sancho, J.; Peralta, C. Effects of Cortisol-Induced Acetylcholine Accumulation on Tissue Damage and Regeneration in Steatotic Livers in the Context of Partial Hepatectomy Under Vascular Occlusion. Transplantation 2018, 102, S699. [Google Scholar] [CrossRef]
- Ulrich-Lai, Y.M.; Jones, K.R.; Ziegler, D.R.; Cullinan, W.E.; Herman, J.P. Forebrain origins of glutamatergic innervation to the rat paraventricular nucleus of the hypothalamus: Differential inputs to the anterior versus posterior subregions. J. Comp. Neurol. 2011, 519, 1301–1319. [Google Scholar] [CrossRef] [PubMed]
- Evanson, N.K.; Herman, J. Role of Paraventricular Nucleus Glutamate Signaling in Regulation of HPA Axis Stress Responses. Interdiscip. Inf. Sci. 2015, 21, 253–260. [Google Scholar] [CrossRef][Green Version]
- Godonu, S.S.; Francis-Lyons, N. Role of Cortisol in The Synthesis of Glutamate During Oxidative Stress. Int. J. Sci. R. Technol. 2025, 2, 100–104. [Google Scholar][Green Version]
- Jezová, D.; Juránková, E.; Vigas, M. Glutamate neurotransmission, stress and hormone secretion. Bratisl. Lek. Listy 1995, 96, 588–596. [Google Scholar][Green Version]
- Maguire, J. The relationship between GABA and stress: ‘it’s complicated’. J. Physiol. 2018, 596, 1781–1782. [Google Scholar] [CrossRef]
- Colmers, P.L.W.; Bains, J.S. Balancing tonic and phasic inhibition in hypothalamic corticotropin-releasing hormone neurons. J. Physiol. 2018, 596, 1919–1929. [Google Scholar] [CrossRef]
- Kaminski, K.L.; Watts, A.G. Intact catecholamine inputs to the forebrain are required for appropriate regulation of corticotrophin-releasing hormone and vasopressin gene expression by corticosterone in the rat paraventricular nucleus. J. Neuroendocr. Neuroendocrinol. 2012, 24, 1517–1526. [Google Scholar] [CrossRef]
- Douma, E.H.; de Kloet, E.R. Stress-induced plasticity and functioning of ventral tegmental dopamine neurons. Neurosci. Biobehav. Rev. 2020, 108, 48–77. [Google Scholar] [CrossRef]
- Stanwood, G.D. Dopamine and Stress. Stress: Physiology, Biochemistry, and Pathology; Fink, G., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 105–114. [Google Scholar]
- Hanley, N.R.; Van de Kar, L.D. Serotonin and the neuroendocrine regulation of the hypothalamic—Pituitary-adrenal axis in health and disease. Vitam. Horm. 2003, 66, 189–255. [Google Scholar] [PubMed]
- Jørgensen, H.; Knigge, U.; Kjaer, A.; Møller, M.; Warberg, J. Serotonergic stimulation of corticotropin-releasing hormone and pro-opiomelanocortin gene expression. J. Neuroendocr. Neuroendocrinol. 2002, 14, 788–795. [Google Scholar] [CrossRef]
- Wu, S.V.; Yuan, P.Q.; Lai, J.; Wong, K.; Chen, M.C.; Ohning, G.V.; Taché, Y. Activation of Type 1 CRH receptor isoforms induces serotonin release from human carcinoid BON-1N cells: An enterochromaffin cell model. Endocrinology 2011, 152, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Donner, N.C.; Siebler, P.H.; Johnson, D.T.; Villarreal, M.D.; Mani, S.; Matti, A.J.; Lowry, C.A. Serotonergic systems in the balance: CRHR1 and CRHR2 differentially control stress-induced serotonin synthesis. Psychoneuroendocrinology 2016, 63, 178–190. [Google Scholar] [CrossRef]
- Dahlgren, U. The Electric Motor Nerve Centers in the Skates (Rajidae). Science 1914, 40, 862–863. [Google Scholar] [CrossRef] [PubMed]
- Cioni, C.; Angiulli, E.; Toni, M. Nitric Oxide and the Neuroendocrine Control of the Osmotic Stress Response in Teleosts. Int. J. Mol. Sci. 2019, 20, 489. [Google Scholar] [CrossRef]
- Yuan, M.; Li, X.; Lu, W. The caudal neurosecretory system: A novel thermosensitive tissue and its signal pathway in olive flounder (Paralichthys olivaceus). J. Neuroendocrinol. 2020, 32, e12876. [Google Scholar] [CrossRef]
- Bernier, N.J.; Alderman, S.L.; Bristow, E.N. Heads or tails? Stressor-specific expression of corticotropin-releasing factor and urotensin I in the preoptic area and caudal neurosecretory system of rainbow trout. J. Endocrinol. 2008, 196, 637–648. [Google Scholar] [CrossRef]
- O’Brien, J.P.; Kriebel, R.M. Brain stem innervation of the caudal neurosecretory system. Cell Tissue Res. 1982, 227, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Lan, Z.; Zhang, W.; Xu, J.; Zhou, M.; Chen, Y.; Zou, H.; Lu, W. Modulatory effect of dopamine receptor 5 on the neurosecretory Dahlgren cells of the olive flounder, Paralichthys olivaceus. Gen. Comp. Endocrinol. 2018, 266, 67–77. [Google Scholar] [CrossRef]
- Shi, M.; Liu, C.; Qin, Y.; Yv, L.; Lu, W. alpha1 and beta3 adrenergic receptor-mediated excitatory effects of adrenaline on the caudal neurosecretory system (CNSS) in olive flounder, Paralichthys olivaceus. Gen. Comp. Endocrinol. 2024, 349, 114468. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Fang, S.; Huang, N.; Lu, W. The excitatory effect of 5-HT(1A) and 5-HT(2B) receptors on the caudal neurosecretory system Dahlgren cells in olive flounder, Paralichthys olivaceus. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2023, 283, 111457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lan, Z.; Li, K.; Liu, C.; Jiang, P.; Lu, W. Inhibitory role of taurine in the caudal neurosecretory Dahlgren cells of the olive flounder, Paralichthys olivaceus. Gen. Comp. Endocrinol. 2020, 299, 113613. [Google Scholar] [CrossRef]
- Lan, Z.; Xu, J.; Wang, Y.; Lu, W. Modulatory effect of glutamate GluR2 receptor on the caudal neurosecretory Dahlgren cells of the olive flounder, Paralichthys olivaceus. Gen. Comp. Endocrinol. 2018, 261, 9–22. [Google Scholar] [CrossRef]
- Lan, Z.; Zhang, W.; Xu, J.; Lu, W. GABA(A) receptor-mediated inhibition of Dahlgren cells electrical activity in the olive flounder, Paralichthys olivaceus. Gen. Comp. Endocrinol. 2021, 306, 113753. [Google Scholar] [CrossRef]
- Gozdowska, M.; Ślebioda, M.; Kulczykowska, E. Neuropeptides isotocin and arginine vasotocin in urophysis of three fish species. Fish Physiol. Biochem. 2013, 39, 863–869. [Google Scholar] [CrossRef]
- Zhou, H.; Ge, C.; Chen, A.; Lu, W. Dynamic Expression and Regulation of Urotensin I and Corticotropin-Releasing Hormone Receptors in Ovary of Olive Flounder Paralichthys olivaceus. Front. Physiol. 2019, 10, 1045. [Google Scholar] [CrossRef]
- Li, X.; Zhou, H.; Ge, C.; Li, K.; Chen, A.; Lu, W. Dynamic changes of urotensin II and its receptor during ovarian development of olive flounder Paralichthys olivaceus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2023, 263, 110782. [Google Scholar] [CrossRef]
- Lu, W.; Jin, Y.; Xu, J.; Greenwood, M.P.; Balment, R.J. Molecular characterisation and expression of parathyroid hormone-related protein in the caudal neurosecretory system of the euryhaline flounder, Platichthys flesus. Gen. Comp. Endocrinol. 2017, 249, 24–31. [Google Scholar] [CrossRef]
- Lu, W.; Dow, L.; Gumusgoz, S.; Brierley, M.J.; Warne, J.M.; McCrohan, C.R.; Balment, R.J.; Riccardi, D. Coexpression of corticotropin-releasing hormone and urotensin i precursor genes in the caudal neurosecretory system of the euryhaline flounder (Platichthys flesus): A possible shared role in peripheral regulation. Endocrinology 2004, 145, 5786–5797. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Greenwood, M.; Dow, L.; Yuill, J.; Worthington, J.; Brierley, M.J.; McCrohan, C.R.; Riccardi, D.; Balment, R.J. Molecular characterization and expression of urotensin II and its receptor in the flounder (Platichthys flesus): A hormone system supporting body fluid homeostasis in euryhaline fish. Endocrinology 2006, 147, 3692–3708. [Google Scholar] [CrossRef]
- Qin, Y.; Shi, M.; Wei, Y.; Lu, W. The role of NMDA receptors in fish stress response: Assessments based on physiology of the caudal neurosecretory system and defensive behavior. J. Neuroendocrinol. 2024, 36, e13448. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Li, X.; Long, T.; Chen, Y.; Lu, W. Dynamic Responses of the Caudal Neurosecretory System (CNSS) Under Thermal Stress in Olive Flounder (Paralichthys olivaceus). Front Physiol. 2019, 10, 1560. [Google Scholar] [CrossRef]
- Lu, W.; Zhu, G.; Chen, A.; Li, X.; McCrohan, C.R.; Balment, R. Gene expression and hormone secretion profile of urotensin I associated with osmotic challenge in caudal neurosecretory system of the euryhaline flounder, Platichthys flesus. Gen. Comp. Endocrinol. 2019, 277, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Kelsall, C.J.; Balment, R.J. Native urotensins influence cortisol secretion and plasma cortisol concentration in the euryhaline flounder, Platichthys flesus. Gen. Comp. Endocrinol. 1998, 112, 210–219. [Google Scholar] [CrossRef]
- Arnold-Reed, D.E.; Balment, R.J. Peptide hormones influence in vitro interrenal secretion of cortisol in the trout, Oncorhynchus mykiss. Gen. Comp. Endocrinol. 1994, 96, 85–91. [Google Scholar] [CrossRef]
- Rousseau, K.; Girardot, F.; Parmentier, C.; Tostivint, H. The Caudal Neurosecretory System: A Still Enigmatic Second Neuroendocrine Complex in Fish. Neuroendocrinology 2025, 115, 154–194. [Google Scholar] [CrossRef]
- Herrera, M.; Mancera, J.M.; Costas, B. The Use of Dietary Additives in Fish Stress Mitigation: Comparative Endocrine and Physiological Responses. Front. Endocrinol 2019, 10, 447. [Google Scholar] [CrossRef]
- Herrera, M.; Herves, M.A.; Giraldez, I.; Skar, K.; Mogren, H.; Mortensen, A.; Puvanendran, V. Effects of amino acid supplementations on metabolic and physiological parameters in Atlantic cod (Gadus morhua) under stress. Fish. Physiol. Biochem. 2017, 43, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Hoseini, S.M.; Khan, M.A.; Yousefi, M.; Costas, B. Roles of arginine in fish nutrition and health: Insights for future researches. Rev. Aquac. 2020, 12, 2091–2108. [Google Scholar] [CrossRef]
- Salamanca, N.; Giráldez, I.; Morales, E.; de La Rosa, I.; Herrera, M. Phenylalanine and Tyrosine as Feed Additives for Reducing Stress and Enhancing Welfare in Gilthead Seabream and Meagre. Animals 2021, 11, 45. [Google Scholar] [CrossRef]
- Costas, B.; Aragao, C.; Soengas, J.L.; Miguez, J.M.; Rema, P.; Dias, J.; Afonso, A.; Conceicao, L.E. Effects of dietary amino acids and repeated handling on stress response and brain monoaminergic neurotransmitters in Senegalese sole (Solea senegalensis) juveniles. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2012, 161, 18–26. [Google Scholar] [CrossRef]
- Salamanca, N.; Moreno, O.; Giráldez, I.; Morales, E.; de la Rosa, I.; Herrera, M. Effects of Dietary Phenylalanine and Tyrosine Supplements on the Chronic Stress Response in the Seabream (Sparus aurata). Front. Physiol. 2021, 12, 775771. [Google Scholar] [CrossRef]
- Li, W.; Feng, L.; Liu, Y.; Jiang, W.D.; Kuang, S.Y.; Jiang, J.; Li, S.H.; Tang, L.; Zhou, X.Q. Effects of dietary phenylalanine on growth, digestive and brush border enzyme activities and antioxidant capacity in the hepatopancreas and intestine of young grass carp (Ctenopharyngodon idella). Aquac. Nutr. 2015, 21, 913–925. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, C.A.; Liu, S.; Wang, Y.; Lu, S.; Han, S.; Jiang, H.; Liu, H.; Yang, Y. Effect of dietary phenylalanine on growth performance and intestinal health of triploid rainbow trout (Oncorhynchus mykiss) in low fishmeal diets. Front. Nutr. 2023, 10, 1008822. [Google Scholar] [CrossRef]
- Xiao, W.; Zou, Z.; Li, D.; Zhu, J.; Yue, Y.; Yang, H. Effect of dietary phenylalanine level on growth performance, body composition, and biochemical parameters in plasma of juvenile hybrid tilapia, Oreochromis niloticus × Oreochromis aureus. J. World Aquacult Soc. 2020, 51, 437–451. [Google Scholar] [CrossRef]
- Kumar, P.; Saurabh, S.; Pal, A.K.; Sahu, N.P.; Arasu, A.R. Stress mitigating and growth enhancing effect of dietary tryptophan in rohu (Labeo rohita, Hamilton, 1822) fingerlings. Fish Physiol. Biochem. 2014, 40, 1325–1338. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Yousefi, M.; Mirghaed, A.T.; Paray, B.A.; Hoseinifar, S.H.; Van Doan, H. Effects of rearing density and dietary tryptophan supplementation on intestinal immune and antioxidant responses in rainbow trout (Oncorhynchus mykiss). Aquaculture 2020, 528, 735537. [Google Scholar] [CrossRef]
- Peixoto, D.; Carvalho, I.; Machado, M.; Aragao, C.; Costas, B.; Azeredo, R. Dietary tryptophan intervention counteracts stress-induced transcriptional changes in a teleost fish HPI axis during inflammation. Sci. Rep. 2024, 14, 7354. [Google Scholar] [CrossRef]
- Wen, H.; Feng, L.; Jiang, W.; Liu, Y.; Jiang, J.; Li, S.; Tang, L.; Zhang, Y.; Kuang, S.; Zhou, X. Dietary tryptophan modulates intestinal immune response, barrier function, antioxidant status and gene expression of TOR and Nrf2 in young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2014, 40, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.; Azeredo, R.; Domingues, A.; Fernandez-Boo, S.; Dias, J.; Conceição, L.E.C.; Costas, B. Dietary tryptophan deficiency and its supplementation compromises inflammatory mechanisms and disease resistance in a teleost fish. Sci. Rep. 2019, 9, 7689. [Google Scholar] [CrossRef] [PubMed]
- Hoseini, S.M.; Pérez-Jiménez, A.; Costas, B.; Azeredo, R.; Gesto, M. Physiological roles of tryptophan in teleosts: Current knowledge and perspectives for future studies. Rev. Aquac. 2019, 11, 3–24. [Google Scholar] [CrossRef]
- Gupta, O.P.; Lahlou, B.; Botella, J.; Porthe-Nibelle, J. In vivo and in vitro studies on the release of cortisol from interrenal tissue in trout. I. Effects of ACTH and prostaglandins. Exp. Biol. 1985, 43, 201–212. [Google Scholar]
- Niu, J.; Tian, L.X.; Liu, Y.J.; Mai, K.S.; Yang, H.J.; Ye, C.X.; Gao, W. Nutrient values of dietary ascorbic acid (l-ascorbyl-2-polyphosphate) on growth, survival and stress tolerance of larval shrimp, Litopenaeus vannamei. Aquac. Nutr. 2009, 15, 194–201. [Google Scholar] [CrossRef]
- Ming, J.; Xie, J.; Xu, P.; Ge, X.; Liu, W.; Ye, J. Effects of emodin and vitamin C on growth performance, biochemical parameters and two HSP70s mRNA expression of Wuchang bream (Megalobrama amblycephala Yih) under high temperature stress. Fish Shellfish Immunol. 2012, 32, 651–661. [Google Scholar] [CrossRef]
- Liu, B.; Xu, P.; Xie, J.; Ge, X.; Xia, S.; Song, C.; Zhou, Q.; Miao, L.; Ren, M.; Pan, L.; et al. Effects of emodin and vitamin E on the growth and crowding stress of Wuchang bream (Megalobrama amblycephala). Fish Shellfish Immunol. 2014, 40, 595–602. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S. Vitamin C supplementation to optimize growth, health and stress resistance in aquatic animals. Rev. Aquac. 2016, 10, 334–350. [Google Scholar] [CrossRef]
- Ciji, A.; Akhtar, M.S. Stress management in aquaculture: A review of dietary interventions. Rev. Aquac. 2021, 13, 2190–2247. [Google Scholar] [CrossRef]
- Rahimnejad, S.; Dabrowski, K.; Izquierdo, M.; Hematyar, N.; Imentai, A.; Steinbach, C.; Policar, T. Effects of Vitamin C and E Supplementation on Growth, Fatty Acid Composition, Innate Immunity, and Antioxidant Capacity of Rainbow Trout (Oncorhynchus mykiss) Fed Oxidized Fish Oil. Front. Mar. Sci. 2021, 8, 760587. [Google Scholar] [CrossRef]
- Sherif, A.H.; Mahfouz, M.E. Immune status of Oreochromis niloticus experimentally infected with Aeromonas hydrophila following feeding with 1, 3 β-glucan and levamisole immunostimulants. Aquaculture 2019, 509, 40–46. [Google Scholar] [CrossRef]
- Meshkini, S.; Delirezh, N.; Tafi, A.A. Effects of levamisole on immune responses and resistance against density stress in rainbow trout fingerling (Oncorhynchus mykiss). J. Vet. Res. 2017, 72, 129–136. [Google Scholar]
- Pahor-Filho, E.; Castillo, A.S.C.; Pereira, N.L.; Pilarski, F.; Urbinati, E.C. Levamisole enhances the innate immune response and prevents increased cortisol levels in stressed pacu (Piaractus mesopotamicus). Fish Shellfish Immunol. 2017, 65, 96–102. [Google Scholar] [CrossRef]
- Jiang, L.; Dasgupta, I.; Hurcombe, J.A.; Colyer, H.F.; Mathieson, P.W.; Welsh, G.I. Levamisole in steroid-sensitive nephrotic syndrome: Usefulness in adult patients and laboratory insights into mechanisms of action via direct action on the kidney podocyte. Clin. Sci. 2015, 128, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Shukry, M.; Kamal, T.; Ali, R.; Farrag, F.; Almadaly, E.; Saleh, A.A.; El-Magd, M.A. Pinacidil and levamisole prevent glutamate-induced death of hippocampal neuronal cells through reducing ROS production. Neurol. Res. 2015, 37, 916–923. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Eweedah, N.M.; Moustafa, E.M.; Farahat, E.M. Probiotic effects of Aspergillus oryzae on the oxidative status, heat shock protein, and immune related gene expression of Nile tilapia (Oreochromis niloticus) under hypoxia challenge. Aquaculture 2020, 520, 734669. [Google Scholar] [CrossRef]
- Chudzik, A.; Orzylowska, A.; Rola, R.; Stanisz, G.J. Probiotics, Prebiotics and Postbiotics on Mitigation of Depression Symptoms: Modulation of the Brain-Gut-Microbiome Axis. Biomolecules 2021, 11, 1000. [Google Scholar] [CrossRef]
- Gomes, L.C.; Brinn, R.P.; Marcon, J.L.; Dantas, L.A.; Brandão, F.R.; de Abreu, J.S.; Lemos, P.E.M.; McComb, D.M.; Baldisserotto, B. Benefits of using the probiotic Efinol®L during transportation of cardinal tetra, Paracheirodon axelrodi (Schultz), in the Amazon. Aquac. Res. 2009, 40, 157–165. [Google Scholar] [CrossRef]
- Eissa, N.; Wang, H.P.; Yao, H.; Abou-ElGheit, E. Mixed Bacillus Species Enhance the Innate Immune Response and Stress Tolerance in Yellow Perch Subjected to Hypoxia and Air-Exposure Stress. Sci. Rep. 2018, 8, 6891. [Google Scholar] [CrossRef]
- Xie, J.; Liu, B.; Zhou, Q.; Su, Y.; He, Y.; Pan, L.; Ge, X.; Xu, P. Effects of anthraquinone extract from rhubarb Rheum officinale Bail on the crowding stress response and growth of common carp Cyprinus carpio var. Jian. Aquaculture 2008, 281, 5–11. [Google Scholar] [CrossRef]
- Song, C.; Liu, B.; Xie, J.; Ge, X.; Zhao, Z.; Zhang, Y.; Zhang, H.; Ren, M.; Zhou, Q.; Miao, L.; et al. Comparative proteomic analysis of liver antioxidant mechanisms in Megalobrama amblycephala stimulated with dietary emodin. Sci. Rep. 2017, 7, 40356. [Google Scholar] [CrossRef] [PubMed]
- Tadese, D.A.; Song, C.; Sun, C.; Liu, B.; Liu, B.; Zhou, Q.; Xu, P.; Ge, X.; Liu, M.; Xu, X.; et al. The role of currently used medicinal plants in aquaculture and their action mechanisms: A review. Rev. Aquac. 2021, 14, 816–847. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Gupta, S.K.; Yousefi, M.; Kulikov, E.V.; Drukovsky, S.G.; Petrov, A.K.; Mirghaed, A.T.; Hoseinifar, S.H.; Van Doan, H. Mitigation of transportation stress in common carp, Cyprinus carpio, by dietary administration of turmeric. Aquaculture 2022, 546, 737380. [Google Scholar] [CrossRef]

| Type of Stressor | Example | References |
|---|---|---|
| Physical | Water temperature; | [15,16,17] |
| Photoperiod; | [18,19,20] | |
| Sound; | [21,22,23] | |
| Turbidity | [24,25,26] | |
| … | ||
| Chemical | pH; | [27,28,29] |
| Salinity; | [30,31,32,33] | |
| Dissolved oxygen; | [34,35,36] | |
| Alkalinity; | [37,38] | |
| Carbon dioxide complexities; | [39,40,41] | |
| Nitrite; | [42,43,44] | |
| Ammonia; | [45,46,47] | |
| Hydrogen sulfide; | [48,49] | |
| Iron; | [50,51] | |
| Heavy metal; | [52,53,54] | |
| Pesticides | [55,56,57] | |
| … | ||
| Biological | Algal toxicosis; | [58,59] |
| Microorganisms; | [60,61,62] | |
| Parasites; | [63,64,65] | |
| Predators; | [66,67,68] | |
| Social rank; | [69,70,71] | |
| Environmental enrichment | [72,73,74] | |
| … | ||
| Procedural | Overcrowding; | [75,76,77] |
| Handling; | [78,79,80] | |
| Netting | [81,82] | |
| Feeding; | [83,84,85] | |
| Transportation | [86,87,88] | |
| … |
| System | Primary Component | Response Timing | Magnitude/Duration |
|---|---|---|---|
| Brain–Sympathetic–Chromaffin Cell (BSC) Axis | Brain (sympathetic neurons); Sympathetic nerves; Chromaffin cells (in head kidney; catecholamines) | Immediate (<1 min to hours) | Rapid onset; Short duration (minutes to hours); High peak magnitude |
| Hypothalamus–Pituitary–Interrenal (HPI) Axis | Hypothalamus (CRH/ACTH-releasing factors); Pituitary (ACTH); Interrenal tissue (cortisol/corticosterone) | Delayed (hours to days) | Slower onset; Long-lasting (days to weeks); Moderate-to-high magnitude (sustained) |
| Caudal Neurosecretory System (CNSS) | Dahlgren cell (urotensins, isotocin, CRH); Urophysis; | Variable (minutes to hours, depending on stimulus) | Moderate onset; Moderate duration (hours); Target-specific magnitude |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, M.; Fang, Q.; Lu, W.; Wang, X.; Hao, T.; Chong, C.-M.; Chen, S. Stress in Fish: Neuroendocrine and Neurotransmitter Responses. Fishes 2025, 10, 307. https://doi.org/10.3390/fishes10070307
Yuan M, Fang Q, Lu W, Wang X, Hao T, Chong C-M, Chen S. Stress in Fish: Neuroendocrine and Neurotransmitter Responses. Fishes. 2025; 10(7):307. https://doi.org/10.3390/fishes10070307
Chicago/Turabian StyleYuan, Mingzhe, Qian Fang, Weiqun Lu, Xubo Wang, Tianwei Hao, Cheong-Meng Chong, and Shan Chen. 2025. "Stress in Fish: Neuroendocrine and Neurotransmitter Responses" Fishes 10, no. 7: 307. https://doi.org/10.3390/fishes10070307
APA StyleYuan, M., Fang, Q., Lu, W., Wang, X., Hao, T., Chong, C.-M., & Chen, S. (2025). Stress in Fish: Neuroendocrine and Neurotransmitter Responses. Fishes, 10(7), 307. https://doi.org/10.3390/fishes10070307







