Stock Assessment of Long Whisker Catfish (Mystus gulio): Ensuring the Fisheries’ Sustainability in the Sundarbans Mangrove, Bangladesh
Abstract
1. Introduction
2. Materials and Methods
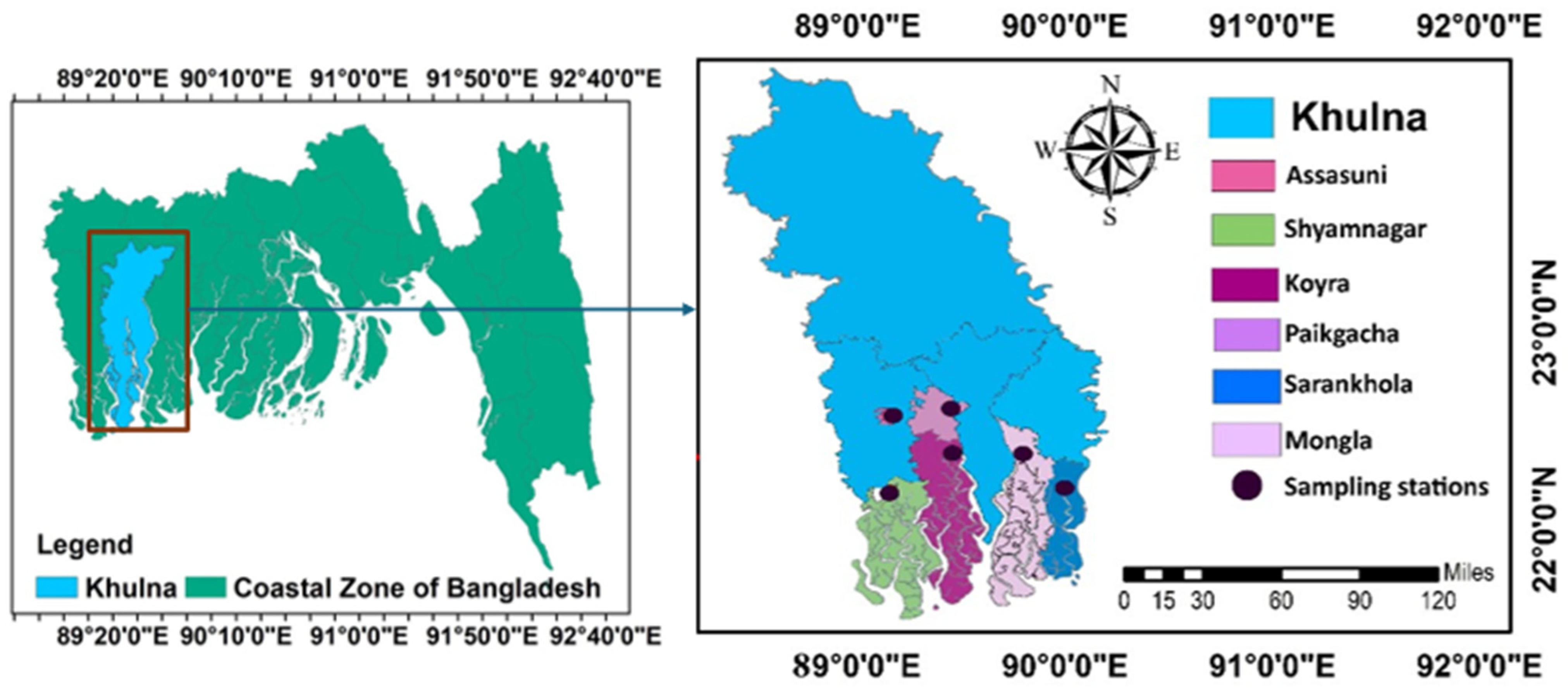
2.1. Sampling Sites
2.2. Data Collection and Analysis
2.3. Description of Length-Based Bayesian Biomass (LBB) Method
2.4. Description of Length-Based Spawning Potential Ratio (LB-SPR)
3. Results
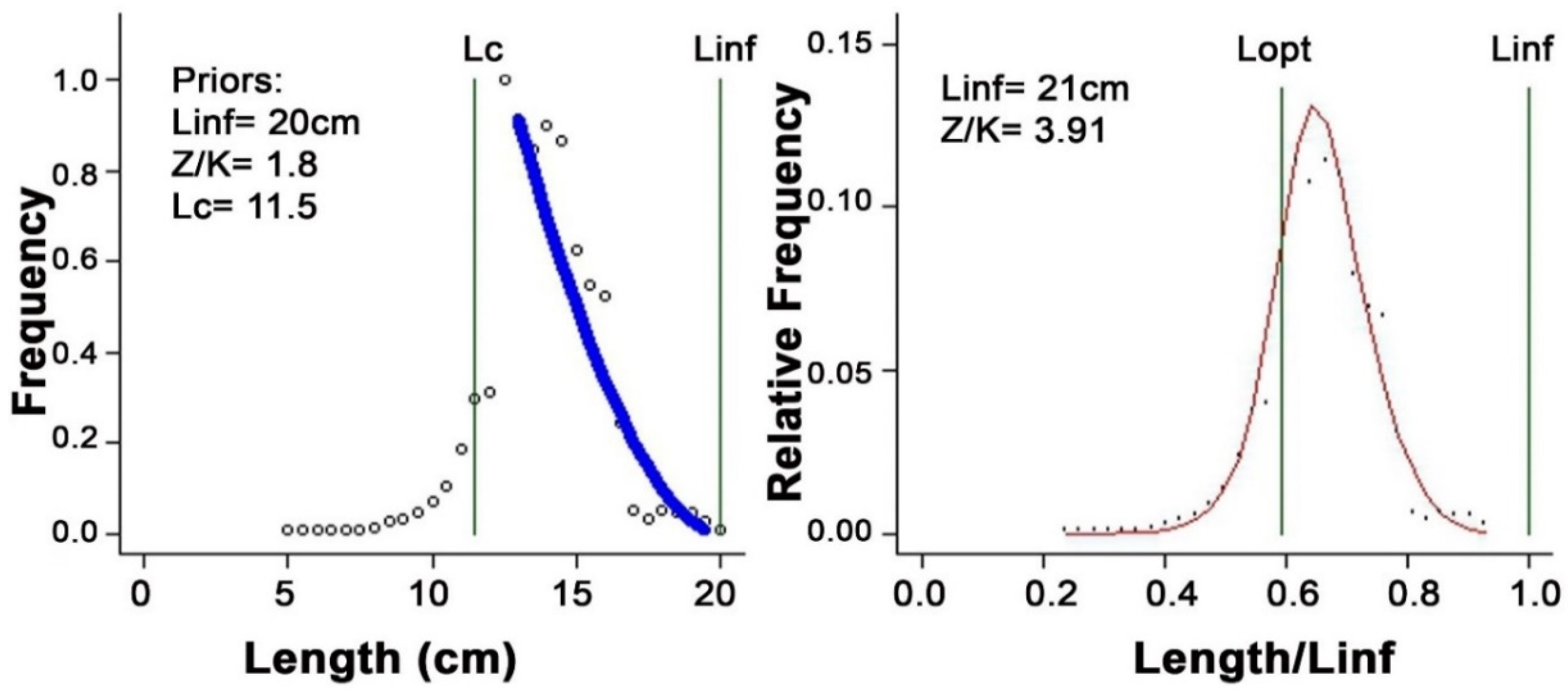
3.1. Length-Based Reference Points of Mystus gulio
3.2. Stock Status and Exploitation of Mystus gulio
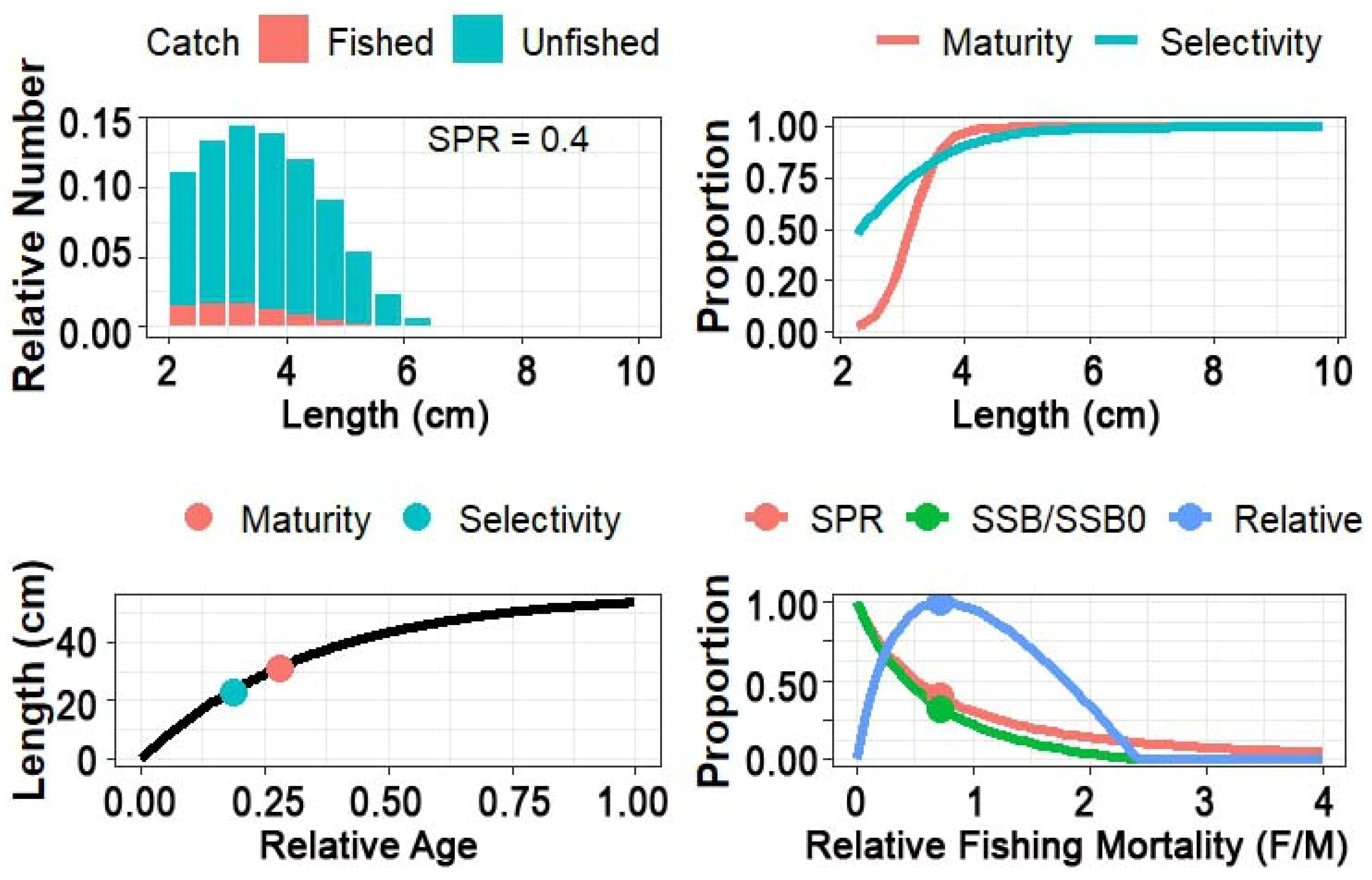
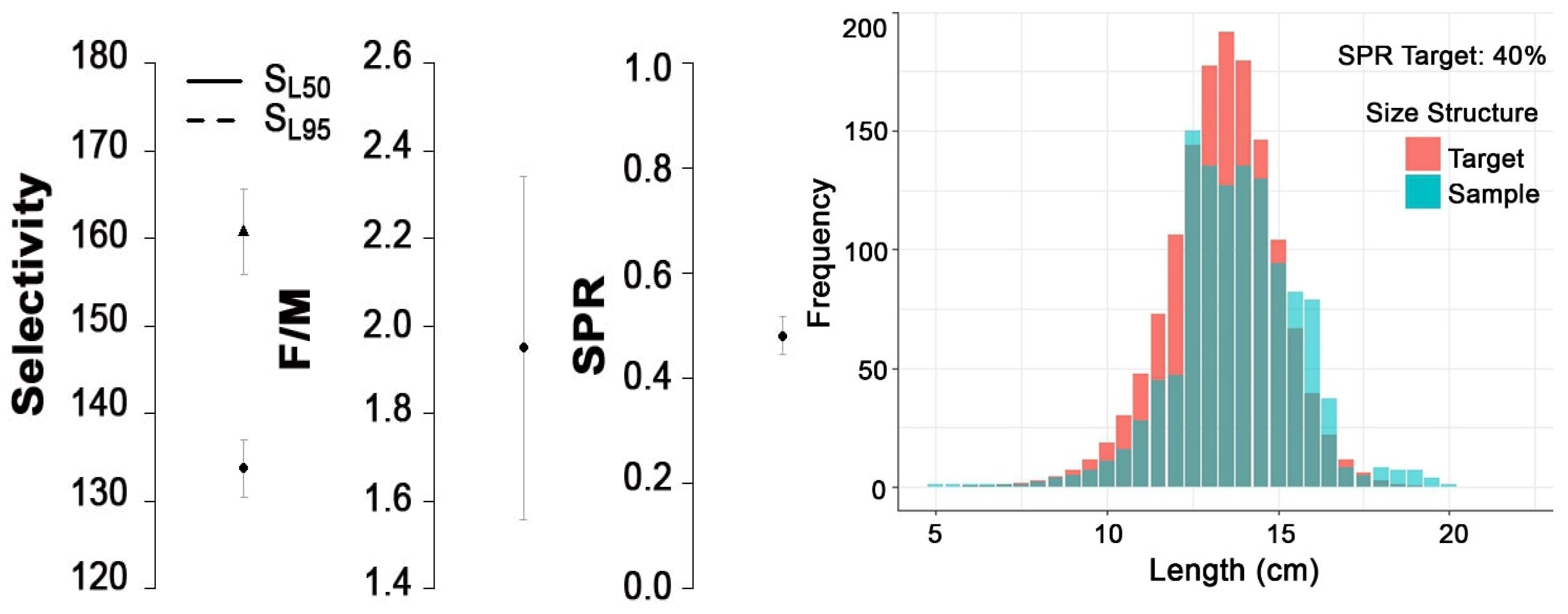
3.3. Life History, Selectivity, Maturity, and SPR of Mystus gulio
4. Discussion
4.1. Suitability of LBB and LB-SPR for Mystus gulio
4.2. Length-Based Reference Points of Mystus gulio
| Study Location | L∞ | K | Lc | Lc_opt | Reference |
|---|---|---|---|---|---|
| Sundarbans, Bangladesh | 21.0 | - | 12.8 | 10.0 | Present Study (2025) |
| The Malancha River, Bangladesh | 19.3 | 0.94 | - | - | [19] |
| Sundarbans, Bangladesh | 23.0 | 0.75 | 8.53 | - | [46] |
| The Meghna estuary, Bangladesh | 18.5 | 0.80 | 9.80 | 9.50 | [47] |
| The Krishna estuary, India | 21.0 | 0.68 | 10.5 | 10.0 | [48] |
| The Ganga delta, India | 20.2 | 0.70 | 10.2 | 10.0 | [49] |
| The Hoogly estuary, India | 17.6 | 0.85 | 9.50 | 9.20 | [50] |
4.3. Stock Status and Exploitation of Mystus gulio
4.4. Spawning Potential Ratio of Mystus gulio
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoq, M.E. Sundarbans fisheries: A livelihood option for the coastal people of Bangladesh. In Proceedings of the 8th Asian Fisheries Forum, Kochi, India, 20–23 November 2007; Bangladesh Fisheries Research Institute: Mymensingh, Bangladesh, 2008. [Google Scholar]
- Habib, A.; Hossain, M.S.; Islam, M.R.; Das, N.G. Fish biodiversity of the Sundarbans mangrove ecosystem in Bangladesh: Present status and challenges. Aquat. Ecosyst. Health Manag. 2020, 23, 261–273. [Google Scholar]
- Department of Fisheries (DoF). Yearbook of Fisheries Statistics of Bangladesh 2022–2023; Ministry of Fisheries and Livestock, Government of the People’s Republic of Bangladesh: Dhaka, Bangladesh, 2023.
- Shafi, M.; Quddus, M.M.A. Bangladesh: The Living Fisheries; University of Dhaka: Dhaka, Bangladesh, 2001. [Google Scholar]
- Huda, M.S.; Haque, M.M.; Sultana, M. Fisheries of the Sundarbans. In The Ecosystem of the Sundarbans and Management Options; Islam, M.A., Ed.; BFRI & DoF: Dhaka, Bangladesh, 2003; pp. 125–138. [Google Scholar]
- Ray, S.; Barman, S.; Sarma, D. Small indigenous fish species of India: Significance, conservation and utilization. J. Bioresour. Sustain. Dev. 2014, 1, 1–10. [Google Scholar]
- Tripathi, S.D. Present status and future prospects of catfish culture in India. Aquac. Asia 1996, 1, 13–15. [Google Scholar]
- Sarker, M.N.; Rahman, M.M.; Dewan, S. Food and feeding habits of Mystus gulio (Hamilton) in the River Padma. Bangladesh J. Fish. Res. 2002, 6, 47–54. [Google Scholar]
- Begum, M.; Alam, M.J.; Islam, M.A.; Pal, H.K. Artificial breeding and nursery management of indigenous catfish, Mystus gulio. Progress. Agric. 2008, 19, 117–124. [Google Scholar]
- Haniffa, M.A. Breeding and culture of indigenous catfishes of India. Aquac. Asia Mag. 2009, 14, 14–17. [Google Scholar]
- Islam, S.M.M.; Bhuiyan, A. Sundarbans mangrove forest of Bangladesh: Causes of degradation and sustainable management options. Environ. Sustain. 2018, 1, 113–131. [Google Scholar] [CrossRef]
- Mahmood, N.; Rahman, M.M.; Hossain, M.S. Declining fish biodiversity in the Sundarbans mangrove ecosystem: Status, causes and management. Reg. Stud. Mar. Sci. 2021, 45, 101838. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Roy, P. Fisheries decline in the Sundarbans: A socio-ecological assessment of vulnerabilities and adaptation. Ocean Coast. Manag. 2022, 225, 106254. [Google Scholar]
- Patra, R.W.; Azad, S.A.; Alam, M.J. Studies on catch trends and production decline of Mugil cephalus in the Sundarbans. Bangladesh J. Fish. Res. 2005, 9, 15–22. [Google Scholar]
- Kibria, M.G. Growth performance and feed utilization of Mystus gulio under different feeding regimes. Aquac. Res. Lett. 2024, 5, 12–20. [Google Scholar]
- Sabbir, W.; Islam, M.N.; Hasan, M.M. Food and feeding habits of Mystus gulio (Hamilton, 1822) from the Meghna estuary, Bangladesh. J. Fish. 2017, 5, 55–60. [Google Scholar]
- Hossain, M.A.R.; Nahiduzzaman, M.; Saha, D.; Alam, M.T. Gonadal development and spawning season of Mystus gulio in the southwestern region of Bangladesh. J. Bangladesh Agric. Univ. 2016, 14, 47–54. [Google Scholar]
- Hossain, M.A.R.; Nahiduzzaman, M.; Saha, D. Length-weight relationship and condition factor of Mystus gulio in different coastal habitats of Bangladesh. Egypt. J. Aquat. Res. 2018, 44, 135–139. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.S.; Ahmed, K. Preliminary stock assessment of Mystus gulio in the Satkhira region using surplus production models. Reg. Stud. Fish. Manag. 2024, 9, 88–96. [Google Scholar]
- Froese, R.; Winker, H.; Coro, G.; Demirel, N.; Tsikliras, A.C.; Dimarchopoulou, D.; Scarcella, G.; Probst, W.N.; Dureuil, M.; Pauly, D. A new approach for estimating stock status from length frequency data. ICES J. Mar. Sci. 2018, 75, 2004–2015, Erratum in ICES J. Mar. Sci. 2019, 76, 50–60. [Google Scholar] [CrossRef]
- Hordyk, A.R.; Ono, K.; Valencia, S.R.; Loneragan, N.R.; Prince, J.D. A novel length-based empirical estimation method of spawning potential ratio (SPR), and tests of its performance, for small-scale, data-poor fisheries. ICES J. Mar. Sci. 2015, 72, 217–231. [Google Scholar] [CrossRef]
- von Bertalanffy, L. A quantitative theory of organic growth (inquiries on growth laws II). Hum. Biol. 1938, 10, 181–213. [Google Scholar]
- Beverton, R.J.H.; Holt, S.J. On the Dynamics of Exploited Fish Populations; Chapman and Hall: London, UK, 1957; p. 179. [Google Scholar]
- Quinn, T.J.; Deriso, R.B. Quantitative Fish Dynamics; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Sparre, P.; Venema, S.C. Introduction to Tropical Fish Stock Assessment. Part 1: Manual; FAO Fisheries Technical Paper No. 306/1, Rev. 2; FAO: Rome, Italy, 1998. [Google Scholar]
- Beverton, R.J.H. Patterns of reproductive strategy parameters in some marine teleost fishes. J. Fish Biol. 1992, 41, 137–160. [Google Scholar] [CrossRef]
- Chong, L.; Hordyk, A.R.; Pilling, G.M.; Davies, C.R. Performance evaluation of a length-based method for data-limited fisheries. ICES J. Mar. Sci. 2020, 77, 483–496. [Google Scholar] [CrossRef]
- Dowling, N.A.; Dichmont, C.M.; Smith, D.C. Simple methods for estimating stock status from length frequency data. Fish Fish. 2019, 20, 1051–1071. [Google Scholar] [CrossRef]
- Pons, M.; Prince, J.D.; Hordyk, A.R.; Kell, L.T.; Hillary, R.M. Developing empirical harvest strategies for data-poor fisheries: A case study from Uruguay. Fish Fish. 2019, 20, 183–200. [Google Scholar] [CrossRef]
- Babcock, E.A.; Hordyk, A.R.; Prince, J.D.; Walters, C.J. Even simple fisheries models can have complex dynamics: A cautionary tale for data-poor assessment. Mar. Coast. Fish. 2018, 10, 517–531. [Google Scholar] [CrossRef]
- Prince, J.D.; Hordyk, A.R.; Valencia, S.R.; Loneragan, N.R.; Sainsbury, K.J. Revisiting the concept of Beverton–Holt life history invariants with the aim of informing data-poor fisheries assessment. ICES J. Mar. Sci. 2015, 72, 194–203. [Google Scholar] [CrossRef]
- Froese, R.; Binohlan, C. Empirical relationships to estimate asymptotic length, length at first maturity and length at maximum yield per recruit in fishes, with a simple method to evaluate length frequency data. J. Fish Biol. 2000, 56, 758–773. [Google Scholar] [CrossRef]
- Prince, J.D.; Hordyk, A. Length-based spawning potential ratio: A new approach for estimating fish stock reproductive potential and sustainability using length data. Fish Fish. 2019, 20, 135–152. [Google Scholar] [CrossRef]
- Cope, J.M.; Punt, A.E. Length-based reference points for data-limited situations: Applications and restrictions. Mar. Coast. Fish. 2009, 1, 169–186. [Google Scholar] [CrossRef]
- Costello, C.; Ovando, D.A.; Hilborn, R.; Gaines, S.D.; Deschenes, O.; Lester, S.E. Status and solutions for the world’s unassessed fisheries. Science 2012, 338, 517–520. [Google Scholar] [CrossRef]
- Kleisner, K.M.; Pauly, D.; Froese, R. Using length-frequency data to estimate fishing mortality and stock status of marine fishes. ICES J. Mar. Sci. 2013, 70, 217–226. [Google Scholar] [CrossRef]
- Palomares, M.L.D.; Froese, R.; Pauly, D. Length-based methods in fisheries research: Applications and recommendations. Fish. Res. 2018, 204, 203–212. [Google Scholar] [CrossRef]
- Froese, R. Keep it simple: Three indicators to deal with overfishing. Fish Fish. 2004, 5, 86–91. [Google Scholar] [CrossRef]
- Winker, H.; Carvalho, F.; Kapur, M. JABBA: Just Another Bayesian Biomass Assessment. Fish. Res. 2018, 204, 275–288. [Google Scholar] [CrossRef]
- Froese, R.; Winker, H.; Gascuel, D.; Sumaila, U.R.; Pauly, D. Bottom-up, fisheries-independent indicators of the status of marine fish stocks and ecosystems: Evaluating a pilot set. ICES J. Mar. Sci. 2012, 69, 1065–1073. [Google Scholar] [CrossRef]
- Shin, Y.-J.; Rochet, M.-J.; Jennings, S.; Field, J.G.; Gislason, H. Using size-based indicators to evaluate the ecosystem effects of fishing. ICES J. Mar. Sci. 2005, 62, 384–396. [Google Scholar] [CrossRef]
- Millar, R.B.; Fryer, R.J. Estimating the size-selection curves of towed gears, traps, nets and hooks. Rev. Fish Biol. Fish. 1999, 9, 89–116. [Google Scholar] [CrossRef]
- Punt, A.E.; Smith, A.D.M.; Cui, G. Review of progress in the introduction of management strategy evaluation (MSE) for Australia’s south east fishery. Mar. Freshw. Res. 2000, 52, 719–726. [Google Scholar] [CrossRef]
- Maunder, M.N.; Punt, A.E. A review of integrated analysis in fisheries stock assessment. Fish. Res. 2013, 142, 61–74. [Google Scholar] [CrossRef]
- Froese, R.; Winker, H.; Gascuel, D.; Sumaila, U.R.; Pauly, D. Minimizing the impact of fishing. Fish Fish. 2016, 17, 785–802. [Google Scholar] [CrossRef]
- Mustafa, M.G.; Hossain, M.A.R.; Kabir, K.M.R. Assessment of the fish stock status in the Sundarbans mangrove ecosystem, Bangladesh. Indian J. Geo-Mar. Sci. 2019, 48, 187–193. [Google Scholar]
- Islam, M.A.; Alam, M.T.; Hossain, M.B. Population dynamics and stock assessment of Coilia dussumieri from the Meghna estuary, Bangladesh. J. Appl. Ichthyol. 2018, 34, 615–621. [Google Scholar] [CrossRef]
- Srinivasa, A.R.; Rao, G.R.; Kumar, V. Length-based stock assessment of Coilia dussumieri in the Krishna estuary, India. J. Aquat. Biol. Fish. 2021, 9, 35–42. [Google Scholar]
- Naser, N.M.; Das, B.K.; Paul, S.K. Biological parameters and exploitation level of Coilia dussumieri in the Ganga delta region, India. Indian J. Fish. 2020, 67, 78–84. [Google Scholar]
- Bhakta, J.N.; Ghosh, A.R.; Datta, N.C. Length-based population parameters and exploitation status of Coilia dussumieri from the Hooghly estuary, India. Int. J. Fish. Aquat. Stud. 2016, 4, 10–15. [Google Scholar]
- Froese, R.; Coro, G.; Kleisner, K.M.; Demirel, N. Revisiting safe biological limits in fisheries. Fish Fish. 2017, 18, 758–771. [Google Scholar] [CrossRef]
- Pauly, D.; Ingles, J. Aspects of the growth and natural mortality of exploited coral reef fishes. ICLARM Conf. Proc. 1981, 4, 89–98. [Google Scholar]
- Pauly, D. Some Simple Methods for the Assessment of Tropical Fish Stocks; FAO Fisheries Technical Paper No. 234; FAO: Rome, Italy, 1983. [Google Scholar]
- Barman, S.K.; Hossain, M.J.; Shesir, M.A.R.; Hossain, S.; Barman, P.P. Stock status and spawning potential ratio of orange mud crab (Scylla olivacea, Herbst 1796) in the southwestern coastal waters of Bangladesh. Heliyon 2024, 10, e39283. [Google Scholar] [CrossRef]
- Barman, P.P.; Liu, Q.; Al-Mamun, M.A.; Schneider, P.; Mozumder, M.M.H. Stock assessment of exploited sardine populations from northeastern Bay of Bengal water, Bangladesh using the length-based Bayesian biomass (LBB) method. J. Mar. Sci. Eng. 2021, 9, 1137. [Google Scholar] [CrossRef]
- Rosenberg, A.A.; Restrepo, V.R. Uncertainty and risk evaluation in stock assessment advice for U.S. marine fisheries. Can. J. Fish. Aquat. Sci. 1994, 51, 2715–2720. [Google Scholar] [CrossRef]
- Goodyear, C.P. Spawning stock biomass per recruit in fisheries management: Foundation and current use. Can. Spec. Publ. Fish. Aquat. Sci. 1993, 120, 67–81. [Google Scholar]
- Gulland, J.A. Fish Stock Assessment: A Manual of Basic Methods; FAO/Wiley Series on Food and Agriculture: Rome, Italy, 1983. [Google Scholar]
- Legault, C.M.; Restrepo, V.R. A Flexible Forward Age-Structured Population Dynamics Model; NOAA Technical Memorandum NMFS-F/NEC-104; National Marine Fisheries Service: Silver Spring, MD, USA, 1993.

| Methods | Parameters | Values |
|---|---|---|
| Input values for LBB | Min (mm) | 50 |
| Max (mm) | 200 | |
| Class Interval (mm) | 5.0 | |
| Individual Number | 1180 | |
| Linf Prior (cm) | 20.0 | |
| Z/K Prior | 1.80 | |
| M/K Prior | 1.50 | |
| F/K Prior | 0.27 | |
| Lc Prior | 11.7 | |
| Alpha Prior | 44.7 | |
| Input values for LB-SPR | L∞ (cm) | 21.0 |
| M/K | 1.98 | |
| L50 (cm)/Lm | 9.16 | |
| L95 (cm) | 16.07 |
| Length Parameters cm | Different Length Ratios | Stock Information | |||
|---|---|---|---|---|---|
| Lmean | 14.4 | Lc_opt/Linf | 0.49 | M/K | 1.98 |
| Linf | 21.0 | Lopt/Linf | 0.57 | F/M | 0.90 |
| Lc | 12.8 | Lc/Linf | 0.61 | F/K | 1.80 |
| Lc_opt | 10.0 | Lmean/Lopt | 1.20 | Z/K | 3.90 |
| Lc50 | 12.8 | Lc/Lc_opt | 1.30 | B/B0 | 0.43 |
| Lc95 | 15.0 | L95th/Linf | 0.93 | B/BMSY | 1.20 |
| L95th | 19.5 | E | 0.46 | BMSY/B0 | 0.36 |
| Lopt | 12.0 | Lc/Lopt | 1.28 | ||
| Stock Status | Healthy stock and safe exploitation of M. gulio in Bangladesh. | ||||
| Parameters | Values |
|---|---|
| SL50% (cm) | 13.37 |
| SL95% (cm) | 16.07 |
| F/M | 1.95 |
| SPR | 0.48 |
| Study Location | M/K | Z/K | F/K | F/M | E | Reference |
|---|---|---|---|---|---|---|
| Sundarbans, Bangladesh | 1.98 | 3.9 | 1.8 | 0.9 | 0.46 | Present Study (2025) |
| The Malancha River, Bangladesh | 1.60 | 3.6 | 2.9 | 1.8 | 0.63 | [19] |
| Sundarbans, Bangladesh | 2.12 | 4.0 | 1.9 | 0.9 | 0.47 | [46] |
| The Meghna estuary, Bangladesh | 1.20 | 2.3 | 1.1 | 0.9 | 0.48 | [47] |
| The Krishna estuary, India | 1.00 | 1.6 | 0.9 | 0.9 | 0.40 | [48] |
| The Ganga delta, India | 1.10 | 1.9 | 1.0 | 0.9 | 0.47 | [49] |
| The Hoogly estuary, India | 1.3 | 2.5 | 1.2 | 0.9 | 0.49 | [50] |
| Study Location | Stock Status | Reference |
|---|---|---|
| Sundarbans, Bangladesh | Healthy | Present Study (2025) |
| The Malancha River, Bangladesh | Overexploited | [19] |
| Sundarbans, Bangladesh | Fully exploited | [46] |
| The Meghna estuary, Bangladesh | Fully exploited | [47] |
| The Krishna estuary, India | Healthy | [48] |
| The Ganga delta, India | Healthy | [49] |
| The Hoogly estuary, India | Fully exploited | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ovi, M.T.R.; Sarkar, T.; Gope, D.; Ahmod, R.; Barman, S.K.; Shamsuzzaman, M.M.; Mozumder, M.M.H.; Schneider, P.; Barman, P.P. Stock Assessment of Long Whisker Catfish (Mystus gulio): Ensuring the Fisheries’ Sustainability in the Sundarbans Mangrove, Bangladesh. Fishes 2025, 10, 300. https://doi.org/10.3390/fishes10070300
Ovi MTR, Sarkar T, Gope D, Ahmod R, Barman SK, Shamsuzzaman MM, Mozumder MMH, Schneider P, Barman PP. Stock Assessment of Long Whisker Catfish (Mystus gulio): Ensuring the Fisheries’ Sustainability in the Sundarbans Mangrove, Bangladesh. Fishes. 2025; 10(7):300. https://doi.org/10.3390/fishes10070300
Chicago/Turabian StyleOvi, Md. Tanvir Rahman, Tanni Sarkar, Dwipika Gope, Rayhan Ahmod, Sanzib Kumar Barman, Md. Mostafa Shamsuzzaman, Mohammad Mojibul Hoque Mozumder, Petra Schneider, and Partho Protim Barman. 2025. "Stock Assessment of Long Whisker Catfish (Mystus gulio): Ensuring the Fisheries’ Sustainability in the Sundarbans Mangrove, Bangladesh" Fishes 10, no. 7: 300. https://doi.org/10.3390/fishes10070300
APA StyleOvi, M. T. R., Sarkar, T., Gope, D., Ahmod, R., Barman, S. K., Shamsuzzaman, M. M., Mozumder, M. M. H., Schneider, P., & Barman, P. P. (2025). Stock Assessment of Long Whisker Catfish (Mystus gulio): Ensuring the Fisheries’ Sustainability in the Sundarbans Mangrove, Bangladesh. Fishes, 10(7), 300. https://doi.org/10.3390/fishes10070300










