Abstract
The presence of pesticides in surface waters has been widely reported worldwide and represents a significant problem that raises concerns on local, regional, national, and international scales. Among these, metolachlor is one of the most widely used herbicides to control annual grasses and broadleaf weeds in various crops. Despite the existing research, data on the effects of metolachlor on the nervous system of fishes, remain limited. The present study aims to investigate the impact of metolachlor during embryonic development on the formation of the nervous system and the subsequent inflammatory response in zebrafish (Danio rerio), focusing specifically on larvae at 24 h post-fertilization (hpf). To achieve this, transgenic zebrafish lines marking neuronal populations Tg(Hu:GFP), glial cells Tg(gfap:GFP), and circulating macrophages Tg(mpeg:GFP) were employed. Following exposure to sub-lethal doses of metolachlor, we observed a significant decrease in GPF-positive cells marking the neuronal population, accompanied by an increase in apoptotic cells within the brain region. Additionally, treated embryos exhibited a marked neuroinflammatory response, characterized by astrogliosis and the specific accumulation of microglia/macrophage-positive cells in the head region. In situ hybridization and real-time PCR analyses revealed a significant downregulation of the neurogenin-1 (ngn1) transcript and a noticeable upregulation of the pro-inflammatory cytokine interleukin-1 beta (il1b). Our findings contribute to the growing body of evidence suggesting that metolachlor, even at early developmental stages, can have detrimental effects on both the formation of the nervous system and the regulation of immune responses.
Key Contribution:
Metolachlor exposure increases astrogliosis and cell death in the brain of zebrafish embryos. Metolachlor exposure inhibits embryonic neurogenesis.
1. Introduction
Pesticides are a group of chemical compounds widely employed in agriculture to protect crops from pests, diseases, and weeds, thereby increasing productivity and food security. The use of pesticides has continued to increase as it is still considered the most effective method to reduce pests and increase crop growth. However, their extensive use is detrimental for the environment, wildlife, and public health, and the adverse risks involved are not well investigated [1,2].
Pesticides are directly dispersed in the soil or in the surrounding waters [3]. In the soil, pesticides may bind to particles, degrade through microbial or chemical processes forming different metabolites, or leach into groundwater. Rainfall and irrigation can also transport pesticides and their metabolites into surface waters, such as rivers and lakes or allow them to infiltrate into aquifers [4]. The presence of pesticides in surface waters has been widely reported worldwide and represents a significant problem that raises concerns on local, regional, national, and international scales [1,5].
Among these, metolachlor (IUPAC: 2-chloro-N-(2-ethyl-6-methylphenyl)-N-(1-methoxypropan-2-yl) acetamide) is one of the most widely used herbicides to control annual grasses and broadleaf weeds in various crops, including corn, soybeans, and sorghum. It belongs to the chloroacetanilide herbicide family, and its mechanism of action in target plants determines the inhibition of protein synthesis and cell division by interfering with the formation of microtubules, which are essential for mitosis, thereby preventing germinating seeds’ growth [6]. The herbicide exists as a racemic mixture of R- and S-enantiomers, with S-metolachlor being the more biologically active form [7]. Consequently, S-metolachlor is often preferred in agricultural applications to achieve effective weed control with a lower environmental load. Its effectiveness in inhibiting weed germination and early development has made it a staple in modern agriculture [8]. However, the extensive application of metolachlor has raised significant environmental concerns, particularly regarding its persistence and impact on aquatic ecosystems [7]. Once applied, metolachlor can enter aquatic environments through runoff, leaching, and spray drift. Its chemical properties contribute to moderate persistence in soil and water, leading to detectable concentrations in surface and groundwater. In aquatic environments, metolachlor and its metabolites exhibit varying degrees of persistence; for instance, their half-life in water can range from days to weeks, influenced by factors such as photolysis, hydrolysis, and microbial degradation [9]. Its presence in aquatic environments raises preoccupations about potential chronic exposure to aquatic organisms and poses risks to a variety of non-target organisms, affecting them at both morphological and cellular levels [10].
In fishes, at the cellular level, metolachlor exposure has been associated with oxidative stress, endocrine disruption, and cytotoxicity. In common carp (Cyprinus carpio), subchronic exposure to S-metolachlor resulted in significant changes in antioxidant enzyme activities and increased lipid peroxidation, suggesting heightened oxidative stress [11]. A recent study demonstrated that increasing exposure to S-metolachlor, through the implantation of a slow-release delivery system, induced physiological and cellular alterations [12]. Specifically, it was observed that exposure to metolachlor for two weeks led to an increase in abnormal erythrocytes and a higher neutrophil/lymphocyte ratio in bullhead fishes (Cottus perifretum). Additionally, an increase in body mass was recorded in metolachlor-exposed fish compared to untreated controls, indicating an obesogenic effect following chronic exposure to metolachlor [12]. Other studies have shown that environmentally relevant concentrations can adversely affect the early life stages of aquatic species. For instance, exposure to metolachlor metabolites has been reported to negatively impact the development of marbled crayfish, leading to reduced growth and delayed ontogenetic development [13]. Moreover, a decreased activity of antioxidant enzymes was observed, indicating an increasing of the oxidative stress [7,13].
Model organisms and in vitro systems have been instrumental in elucidating the toxicological effects of metolachlor. In vitro experiments using human liver cells (HepG2) with metolachlor exposure at environmentally relevant concentrations (50–100 ppb) led to alterations in cell cycle regulation, notably through the decreased expression of retinoblastoma protein (Rb) and its phosphorylated forms, suggesting a cell cycle arrest without inducing apoptosis or necrosis [6].
In another study, isolated rat liver mitochondria were employed to evaluate the potential toxicity of metolachlor at a subcellular level. Here, metolachlor showed inhibitory effects on mitochondrial respiration, including reduced respiratory control ratio and membrane potential, pointing to mitochondrial dysfunction as a key mechanism of toxicity [14].
For the in vivo analyses, Zebrafish (Danio rerio) is widely recognized as a key model organism in toxicology studies due to its distinct advantages [15,16]. The transparency of its embryos allows for the real-time observation of biological processes, making it an excellent model to study the effects of chemicals during development. Additionally, its rapid embryonic development and high fecundity enable researchers to obtain results in a relatively short time frame, making zebrafish ideal for high-throughput toxicity screening.
Particularly, zebrafish embryos exposed to S-metolachlor showed significant developmental malformations, including craniofacial deformities [10], reduced swim bladder inflation, an altered expression of genes related to the swim bladder, and affected larval locomotor activity [17]. The morphological changes were accompanied by alterations in gene expression related to the hypothalamic–pituitary–thyroid (HPT) axis, suggesting endocrine disruption. Quintaneiro and colleagues observed that zebrafish embryos exposed to linuron and S-metolachlor showed impairment in neurotransmission and energy production, as well as the induction of steroidogenesis, indicating potential endocrine-disrupting effects [18]. Moreover, recent studies have investigated the effects of metolachlor exposure in zebrafish, with a particular focus on oxidative stress and inflammatory responses. In one study, adult zebrafish exposed to 300 μg/L of metolachlor for 28 days showed significant inhibition of the key antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT), and glutathione S-transferase (GST), along with evidence of hepatic developmental abnormalities. These findings suggest that metolachlor disrupts redox homeostasis and induces liver toxicity in adult animals [19].
Together, these findings highlight metolachlor’s ability to induce oxidative stress and inflammatory pathways in zebrafish, reinforcing the concerns about its ecotoxicological impact on aquatic vertebrates and the need for further mechanistic studies.
Despite the existing research, data on the effects of metolachlor on the nervous system, particularly in relation to neuroinflammation, remain limited. The present study aims to investigate the impact of metolachlor during embryonic development on the formation of the nervous system and the subsequent inflammatory response in zebrafish, focusing particularly on larvae at 24 h post-fertilization (hpf). To achieve this, transgenic zebrafish lines marking neuronal populations Tg(Hu:GFP), glial cells Tg(gfap:GFP), and macrophages (mpeg:GFP) were employed, enabling the direct visualization of morphological alterations in the nervous system as well as inflammatory activity. In addition, more detailed analyses were conducted to assess whether the observed morphological defects could be linked to the molecular pathways associated with apoptosis triggered by metolachlor exposure.
In conclusion, our findings contribute to the growing body of evidence suggesting that metolachlor, even at early developmental stages, can have detrimental effects on both the formation of the nervous system and the regulation of immune responses. The identification of potential links to apoptotic pathways further supports the hypothesis that this compound interferes with fundamental cellular processes. These results underscore the importance of considering the broader systemic impact of agrochemical exposure during early life stages and its implications for aquatic health and reinforce the utility of zebrafish as a sensitive and versatile model for environmental toxicology studies.
2. Materials and Methods
2.1. Zebrafish Husbandry
In the present study, we used zebrafish wild-type AB* and transgenic reporter lines: Tg(huC:GFP) [20]; Tg(mpeg:GFP) [21], and Tg(gfap:GFP) [22]. They were maintained in a 14/10 h light/dark cycle at 28 °C. For the experiments, fertilized eggs with normal development were used. We performed the experiments in triplicate. All fish were accommodated following the European guidelines (FELASA). We employed embryos within 120 h post-fertilization (hpf), which are not subject to animal experimentation regulation according to the European directives (2010/63/UE).
2.2. Metolachlor Treatment
Embryos were obtained as described previously [23]. After dechorionation, the embryos were exposed from 2 to 72 hpf to several metolachlor concentrations (0, 1, 10, 50, 100, 250, 500, 1000 µM) dissolved in E3 zebrafish medium (34.8 g of NaCl, 1.6 g of KCl, 5.8 g of CaCl2, 2H2O, 9.78 g of MgCl2, 6H2O). After the treatment, the embryos were observed in order to classify the morphology defects and establish the survival rate.
2.3. RNA Purification and Reverse Transcription
The total RNA was extracted using 30 zebrafish embryos at 24 hpf. We dissociated the embryos using RLT buffer from the RNAeasy kit (Qiagen, Frankfurt, Germany), according to the manufacturer’s protocol. We repeated this in three independent experiments (in total we used 90 embryos). Next, we performed reverse transcription. We incubated 0.5 μg of the total RNA with a buffer and an enzyme mix for 10 min at 25 °C, 30 min at 50 °C, and 5 min at 85 °C, according to the Superscript III First-Strand Synthesis System kit instructions (Invitrogen, Boston, MA, USA). Finally, we treated the samples using RNase-H for 30 min at 37 °C and stored them at −20 °C.
2.4. Quantitative Real-Time PCR
We obtained cDNA using reverse transcription (as described in the previous paragraph). Next, we performed quantitative polymerase chain reaction (qPCR) employing a thermocycler equipped with an MyiQ detector (Bio-Rad, Hercules, Dallas, TX, USA). We prepared the PCR mix: cDNA, specific forward and reverse primers, SYBR-Green (Bio-Rad Hercules, Dallas, TX, USA), and RNase-free water according to the manufacturer’s protocol. The PCR mix was incubated for 15 min at 95 °C, for 15 s at 95 °C for 40 cycles; for 30 s at 60 °C for 40 cycles; and for 30 s at 72 °C for 40 cycles. To detect il1b and ngn1, we used forward and reverse primers which were validated and published in previous studies [15,24].
Data are represented as the fold change in the target mRNA levels for the ef1a normalizer gene. The absolute quantification was calculated using 2−ΔΔCt. To confirm the correct amplification, melting curve analysis was performed, and the PCR efficiency was verified. In the qPCR analyses, each n represents the average of biological triplicates from a single experiment. The experiments were repeated at least three times. All primer pair sequences used for the experiments are listed in Table 1:

Table 1.
Primer sequences for qPCR.
2.5. Synthesis of Riboprobes for ngn1
To generate digoxigenin (DIG)-labeled antisense and sense riboprobe we used the protocol described in our previous studies [25,26,27]. Briefly, ngn1 riboprobe for zebrafish were synthetized by using the primers validated by a previous study [28], namely F:5′-CACAACCTTAACGACGCAT-3′ and R:5′-TGAGCGAAGCGCAGAGTCTCA-3′. After PCR amplification from fish brains, each insert was cloned in TOPO-TA vector (Invitrogen, Boston, MA, USA) and amplified via transformation into thermocompetent bacteria. Subsequently, plasmid DNA was purified by using the Quick Plasmid Miniprep Kit (Invitrogen, Boston, MA, USA) according to the manufacturer’s instructions. To confirm the antisense and sense orientation of DNA, we sequenced the plasmid. Next, we linearized the plasmids using the specific restriction enzymes. Finally, for transcription, we used SP6 polymerase and T7 polymerase (Roche-Diagnostic, Barrington, IL, USA) and a specific DIG-RNA Labelling Mix (Roche Diagnostic, Indianapolis, IN, USA). For riboprobe purification, we employed NucleoSpin RNA Clean-up columns (Qiagen, Frankfurt, Germany).
2.6. Whole-Mount In Situ Hybridization
All the embryos were fixed in methanol at −20 °C for 24 h. The next day, the embryos were rehydrated by soaking them in decreasing solutions of methanol and increasing solutions of PBT at room temperature. For embryo permeabilization, we used proteinase K (2 mg/mL) at RT for 10 min. Next, we fixed the embryos in 4% paraformaldehyde at RT for 20 min. All the embryos were washed in PBS 3 times for 5 min each. Next, we incubated the embryos with the probe (2 µg/mL) diluted in a specific medium (Denhart 5×; SSC 2×; 50% formamide; ethylenediamine-tetra acetic acid 4 mM; 5% dextran sulphate; yeast tRNA 50 µg/mL) at 63 °C for 24 h. After 24 h, the embryos were washed with SSC 2×; 50% formamide/SSC 2×; SSC 0.2× and SSC 0.1×. The embryos were immersed in a buffer of 100 mM Tris-HCl plus 150 mM NaCl and washed in the same buffer containing 0.5% milk powder plus 0.1% Triton. For staining, all the embryos were incubated with anti-digoxigenin alkaline phosphatase Fab fragments (1:5000) (Roche Diagnostic, Chicago, IL, USA) overnight at room temperature. Staining was performed using NBT/BCIP buffer (pH 9.5) (ThermoFischer, Waltham, MA, USA). The images were acquired through selecting the bright-field option of the Olympus Microscopy Series CX43 (Tokyo, Japan).
2.7. TUNEL Assay
We used the TUNEL assay to identify the apoptotic cells. We fixed all the embryos using 4% paraformaldehyde at RT for 1 h. We removed the yolk and included the head in agar. Next, we cut the brain into sections. We washed the sections three times in PBS. We prepared the TUNEL mix solution following the manufacturer’s instructions (In Situ Cell Death TUNEL Detection Kit, Roche Diagnostic, Chicago, IL, USA); we mixed: 90 µL of labeling solution plus 10 µL of enzyme solution, and we incubated the sections for 30 min at 37 °C. Finally, we washed the sections in PBS three times (5 min for each wash), and we acquired the images using a fluorescence microscope (Olympus CX43) equipped with cellSens Standard 4.3 Software, or via confocal microscopy.
2.8. Fiji Software Analysis
To quantify the TUNEL and/or GFP-positive cells, all sections or embryos were mounted using DAPI (1:500, Roche); next, we used the automated ImageJ free version 1 software, and specific platform tool for measurement analysis, also known as Fiji (version 2.9.0) [29].
2.9. Statistical Analysis
For statistical analysis we used the GraphPad Prism version 10.4.1 software (GraphPad Inc., San Diego, CA, USA); data are normally distributed, and we used one-way ANOVA for statistical comparison (multiple comparisons performed using the Tukey–Kramer post hoc test). p-values lower than 0.05 were considered statistically significant.
3. Results
3.1. Survival Rate and Morphological Analysis of Zebrafish Embryos Exposed to Metolachlor
To identify a sub-lethal dosage of metolachlor, we collected 400 embryos at 2 hpf (50 for each treatment) and exposed them to metolachlor using several concentrations (0; 1 µM; 10 µM; 50 µM; 100 µM; 250 µM; 500 µM; 1 mM). Their survival was monitored every 24 h until the 72 hpf developmental stage. We calculated the survival rate as the percentage of dead fishes compared to the non-treated embryos. We noted a significant decrease in survival of the embryos exposed to high concentrations of metolachlor (100 µM to 1 mM), as reported in Figure 1a–c. Next, we evaluated the morphology of these embryos, and we observed the presence of several defects after exposure to 100 µM, such as: development delay, head anomalies, and a severe reduction of blood circulation in embryos treated with metolachlor 100 µM (see Supplementary Figure S1). The morphology and survival rate were normal in the groups exposed to 1 µM, 10 µM, and 50 µM doses. For the further experiments, we employed these sub-lethal doses.
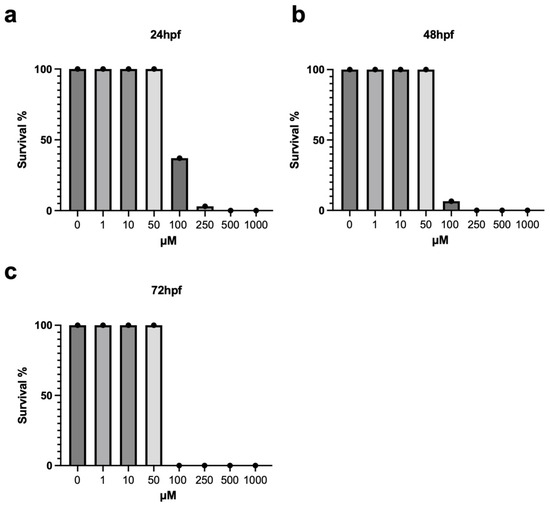
Figure 1.
(a) Survival rate of zebrafish embryos (50 embryos for each group) treated with different concentrations of metolachlor (1, 10, 50, 100, 250, 500, 1000 µM) and non-treated at 24 hpf. (b) Survival rate of zebrafish embryos (50 embryos for each group) treated with different concentrations of metolachlor (1, 10, 50, 100, 250, 500, 1000 µM) and non-treated at 48 hpf. (c) Survival rate of zebrafish embryos (50 embryos per group) treated with different concentrations of metolachlor (1, 10, 50, 100, 250, 500, 1000 µM) and non-treated at 72 hpf.
3.2. Metolachlor Exposure Affects the Expression of Neurogenin-1 (ngn1), a Proneural bHLH Transcription Factor in Zebrafish Embryos
To determine the effect of metolachlor during embryonic neurogenesis, first, we treated the embryos using the following concentrations: 1 µM, 10 µM, and 50 µM (non-lethal as reported in Figure 1a–c), and we fixed embryos at 24 hpf to perform whole-mount in situ hybridization for ngn1. As reported in a large number of published studies, in Drosophila, Xenopus, Zebrafish, mouse, and human models, the neurogenin-1 (ngn1) is an essential transcription factor in promoting embryonic neurogenesis [28,30,31]. We observed a reduction in the ngn1 transcript in the brain and spinal cord of zebrafish embryos exposed to metolachlor at 10 µM and 50 µM (Figure 2a), compared to non-treated embryos. Next, to validate our observations, we used a quantitative real-time PCR approach. We found a significative decrease in ngn1 expression levels in zebrafish embryos exposed to metolachlor at 10 µM and 50 µM (Figure 2b).
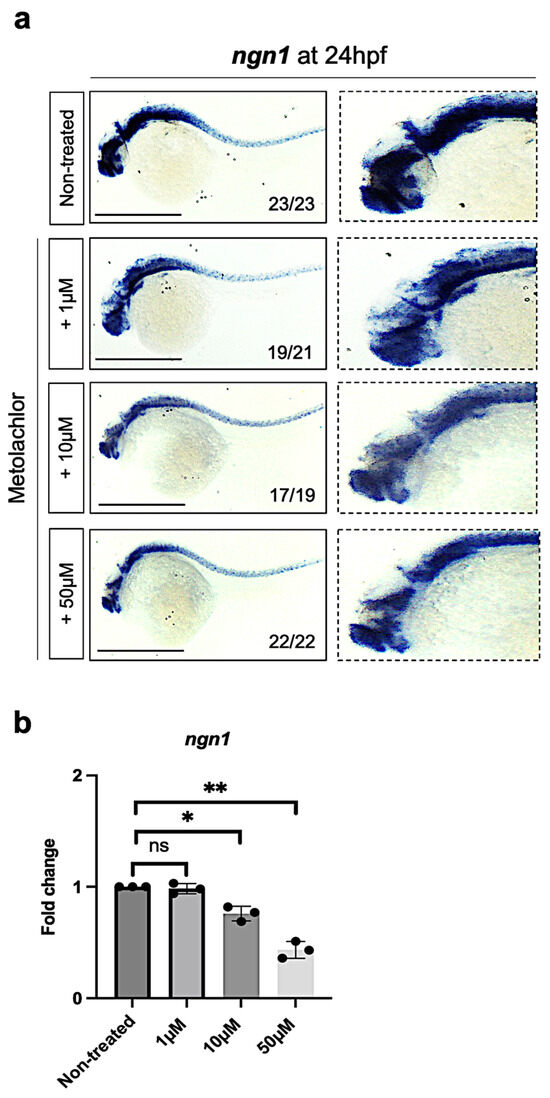
Figure 2.
(a) Whole-mount in situ hybridization and chromogenic revelation for ngn1 transcript in zebrafish embryos non-treated and exposed to metolachlor at 24 hpf. (b) qPCR analysis for ngn1. A pool of 30 embryos at 24 hpf was used for each group. Statistical analysis was performed via one-way ANOVA (multiple comparison—Tukey–Kramer post hoc test) (* p < 0.01; ** p < 0.001; ns: not-significant). All results are represented as the means ± SD of three independent experiments. Scale bar: 200 µm.
3.3. Metolachlor Exposure Induces Apoptotic Cell Death and Affects Neuronal Development
Based on our previous results, we also analyzed the neuronal cells in zebrafish embryos non-treated and after metolachlor exposure at 24 hpf by using a fluorescence live-imaging approach. We used a zebrafish transgenic line that expresses GFP under the Hu promoter tg(Hu:GFP) useful for labeling neuronal populations. We used the automated ImageJ software specific tool for measurement analysis to count the number of GFP-positive cells in the controls and embryos after metolachlor exposure. We found a significative decrease in GFP-positive cells in embryos exposed to metolachlor at 10 µM and 50 µM (Figure 3a,b).
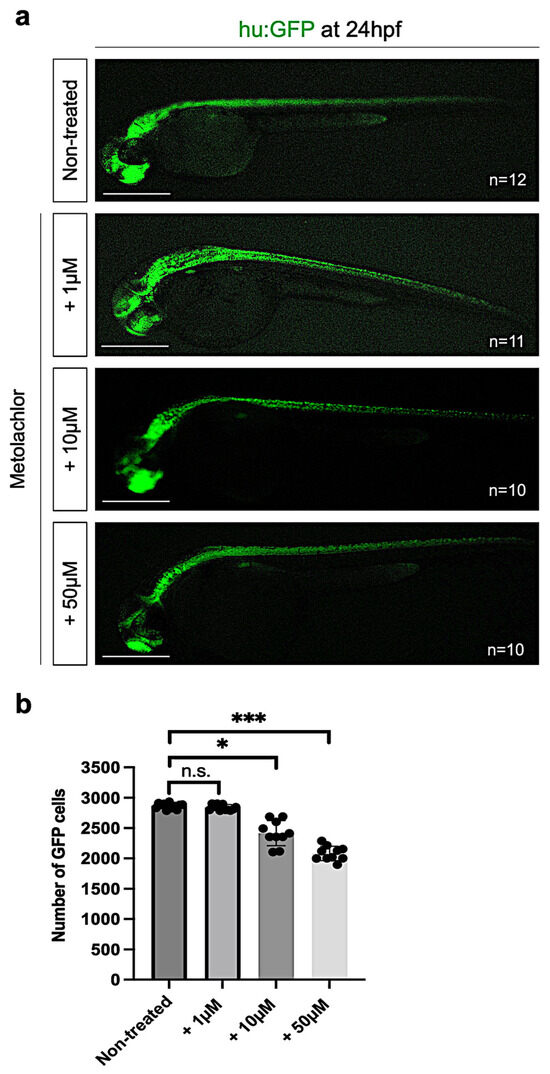
Figure 3.
(a) Fluorescence live imaging of hu:GFP zebrafish embryos at 24 hpf non-treated and exposed to metolachlor at 1, 10, and 50 µM. (b) Statistical analysis was performed via one-way ANOVA (multiple comparison—Tukey–Kramer post hoc test) (* p < 0.01; *** p < 0.0001; ns: not-significant). Scale bar: 200 µm.
In addition, we evaluated the presence of apoptotic cells at 24 hpf on brain transversal sections by using the TUNEL assay kit. We found that embryos treated with metolachlor at 10 and 50 µM showed a significant increase in the number of TUNEL-positive cells compared to non-treated controls (Figure 4a,b).
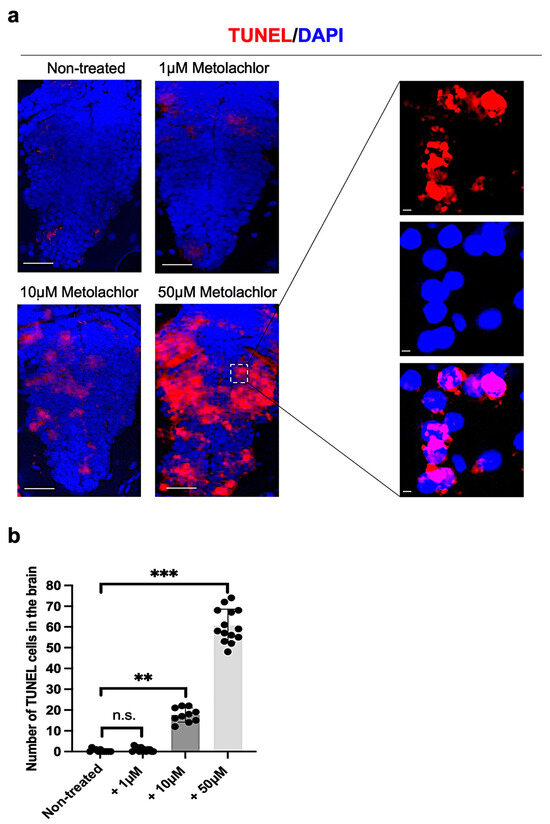
Figure 4.
(a) TUNEL and DAPI staining of brain apoptotic cells on transversal section of the brain embryos from controls and treated with different concentrations of metolachlor (1, 10, and 50 µM) at 24 hpf; microscope’s magnification: 20× and 60×; (b) counting of TUNEL-positive cell number. Each experiment was repeated independently three times. Statistical significance was calculated via one-way ANOVA (multiple comparison Tukey–Kramer post hoc test) (** p < 0.001; *** p < 0.0001; ns: not-significant). Center values denote the mean ± SD. Scale bar: 25 µm.
3.4. Metolachlor Exposure Increases Astrogliosis and Inflammation in Zebrafish Embryos
Next, we evaluated the effects of metolachlor on the glial cell population at 24 hpf by using a fluorescence live-imaging approach. We used a zebrafish transgenic line that expresses GFP under the glial fibrillary acidic protein promoter tg(gfap:GFP) labeling glial cells. We used the automated ImageJ software specific tool for measurement analysis to count the number of GFP-positive cells in the controls and embryos after metolachlor exposure. To identify the cell nucleus, we used DAPI staining. We found a significative increase in GFP+ cells in embryos exposed to metolachlor at 10 µM and 50 µM (Figure 5a,b). Our results confirm a cellular process extensively described in previous studies known as astrogliosis, which is a pathological hallmark of the CNS after injuries leading to an abnormal increase in the number of astrocytes and glial cells [32].
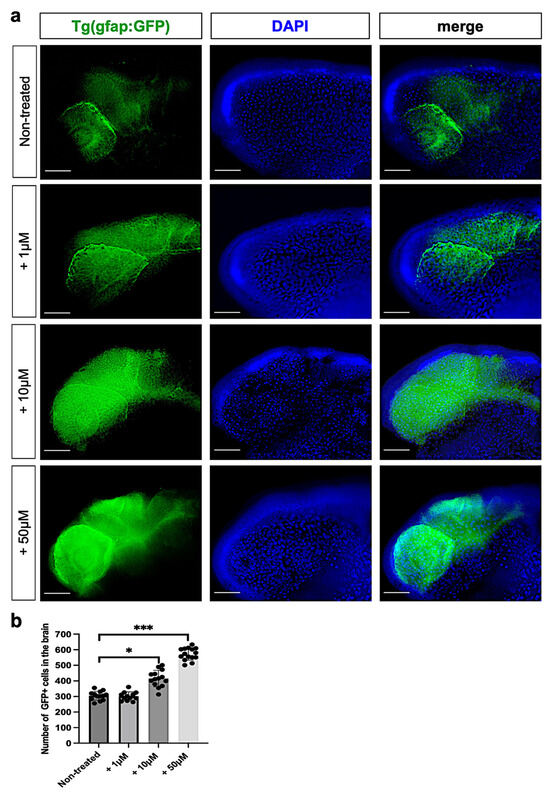
Figure 5.
(a) Confocal live imaging (lateral view) of gfap:GFP zebrafish brain embryos at 24 hpf non-treated and exposed to metolachlor at 1, 10, and 50 µM. (b) Statistical analysis was performed via one-way ANOVA (multiple comparison Tukey–Kramer post hoc test) (* p < 0.01; *** p < 0.0001; ns: not-significant). Scale bar: 50 µm.
As reported in different published studies, excessive glial activation is a key process in nervous system disorders involving the release of strong pro-inflammatory cytokines. To verify this hypothesis, we analyzed the transcript level of il1b (interleukin-1beta), a pro-inflammatory cytokine, performing qPCR in controls and embryos after metolachlor exposure. We found a significative increase in il1b in embryos treated with metolachlor at 10 µM and 50 µM (Supplementary Figure S2). To confirm these results, we used a zebrafish transgenic line, which expresses GFP under the macrophage-expressed gene 1 promoter tg(mpeg:GFP) to label microglia/macrophages (Figure 6a).
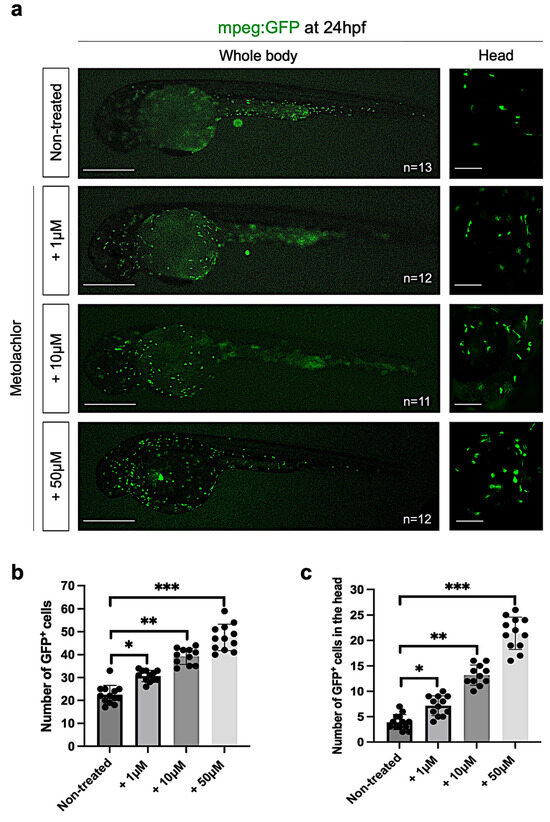
Figure 6.
(a) Fluorescence live imaging of mpeg:GFP zebrafish embryos at 24 hpf non-treated and exposed to metolachlor at 1, 10, and 50 µM. Statistical analyses to quantify the number of GFP+ cells in the (b) whole body and (c) head were performed via one-way ANOVA (multiple comparison Tukey–Kramer post hoc test) (* p < 0.01; ** p < 0.001; *** p < 0.0001; ns: not-significant). Scale bar: 100 µm; 50 µm.
Interestingly, we observed that, after metolachlor exposure at all the tested concentrations, macrophages were mainly accumulated in the head and yolk region if compared to the controls in which the cells were distributed along the dorsal aorta and in the tail portion (Figure 6a). These results confirmed an increase in the inflammatory activity driven by microglia/macrophages in the cephalic region, which was the most affected after metolachlor treatment. Next, we counted the total number of GFP+ cells in the controls and embryos treated with metolachlor observing a significative increase in the total number of mpeg:GFP+ cells in embryos exposed to metolachlor (Figure 6b), and in the head (Figure 6c).
4. Discussion
The present study elucidates the effects of metolachlor exposure on zebrafish (Danio rerio) embryonic development, with a particular focus on neurogenesis, gliogenesis, and neuroinflammatory responses. By employing a combination of morphological assessments, gene expression analyses, and fluorescence live imaging in transgenic reporter lines, we provide comprehensive insights into the cellular and molecular perturbations induced by metolachlor at sub-lethal concentrations. Initial survival assays revealed a dose-dependent toxicity of metolachlor, with significant embryonic lethality observed at concentrations ≥100 µM. Embryos exposed to the higher doses exhibited pronounced morphological defects, including developmental delays, craniofacial anomalies, and impaired blood circulation. These findings are consistent with previous studies demonstrating the teratogenic potential of metolachlor and its analogs in zebrafish models. Indeed, Rozmánková et al. [10] observed no statistically significant effects after exposures to the lower concentrations (1 and 50 μg/L) of S-metolachlor. The following statistically significant effects were observed: S-metolachlor induced non-inflation of the swim bladder (100 μg/L), malabsorption of the yolk sac (300 μg/L), and induced craniofacial malformations in zebrafish embryos treated from 4 hpf to 24 hpf. Again, Yang et al. [17] described a significant number of deformities that occurred in zebrafish embryos treated with metolachlor from 6 hpf to 24 hpf at concentrations of 100 µM or more. Concentrations above 200 µM resulted in 100% deformities. Similar to these previous studies, in our results, embryos treated with 1 µM, 10 µM, and 50 µM metolachlor did not exhibit significant mortality or noticeable morphological abnormalities, thereby establishing these doses as sub-lethal and suitable for subsequent analyses. Whole-mount in situ hybridization and quantitative real-time PCR analyses revealed a significant downregulation of neurogenin1 (ngn1) expression in the brain and spinal cord of embryos exposed to 10 µM and 50 µM metolachlor. We investigated ngn1 as a molecular marker for evaluating neurogenesis due to its fundamental and well-characterized role in the early differentiation of neuronal precursors during vertebrate development, including in zebrafish [30,31]. Ngn1 encodes a proneural basic helix–loop–helix (bHLH) transcription factor that is transiently expressed at the onset of neurogenesis and is essential for driving the commitment of neural progenitor cells toward a neuronal lineage [33,34]. Its expression is widely regarded as an early and sensitive indicator of neurogenic activity [35,36]. In addition, ngn1 has been extensively employed in zebrafish models to investigate how genetic and environmental factors influence neural development, making it a robust and reproducible marker in neurotoxicity studies [37,38]. The observed reduction in ngn1 expression suggests that metolachlor disrupts the initiation of neuronal differentiation pathways, potentially leading to long-term deficits in neural circuitry formation. These findings align with prior research indicating that environmental contaminants can interfere with neurogenic processes by modulating the expression of the key transcription factors with consequent alterations in the development of the neuronal system, of the neuron functionality, and the behavioral aspects [39,40]. However, the specific mechanisms through which metolachlor exerts its effects on ngn1 expression remain to be elucidated. The potential pathways may involve oxidative stress, endocrine disruption, or interference with signaling cascades essential for neurogenesis.
Fluorescence live imaging of Tg(Hu:GFP) transgenic embryos demonstrated a significant decrease in GFP-positive neuronal cells following exposure to 10 µM and 50 µM metolachlor. This reduction was corroborated via TUNEL assays, which revealed increased apoptotic cell death in the treated embryos. Apoptosis plays a pivotal role in sculpting the developing nervous system; however, excessive or untimely neuronal apoptosis can lead to neurodevelopmental disorders [41,42]. The induction of apoptosis via metolachlor treatments may be attributed to the activation of intrinsic apoptotic pathways, possibly through mitochondrial dysfunction or the generation of reactive oxygen species (ROS). The increase in oxidative stress is a well-documented mechanism induced by pesticide exposure and their subsequent toxicity [1]. The oxidative stress hypothesis is also supported by other studies, demonstrating a lipid peroxidation increase and a decrease in the antioxidant enzyme activity, indicating oxidative imbalance and mitochondrial impairment [14,43,44]. However, further studies employing caspase activity assays and ROS detection methods could provide deeper insights into the apoptotic mechanisms following the metolachlor exposure during zebrafish embryonic development. Interestingly, the analysis of Tg(gfap:GFP) embryos revealed a significant increase in GFP-positive glial cells in the central nervous system after metolachlor treatment. Glial fibrillary acidic protein (GFAP) is a well-established marker of astrocytes, and its upregulation is indicative of astrogliosis, a reactive process characterized by the proliferation and hypertrophy of astrocytes in response to CNS injury [22,45].
Astrogliosis is often accompanied by the release of pro-inflammatory cytokines and can contribute to the formation of glial scars, which impede neuronal regeneration [46]. The observed glial activation in metolachlor-treated embryos suggests that even sub-lethal concentrations of this herbicide can elicit neuroinflammatory responses, potentially disrupting the delicate balance between neuroprotection and neurotoxicity.
Quantitative PCR analyses demonstrated a significant increase in interleukin-1 beta (il1b) expression in embryos exposed to 10 µM and 50 µM metolachlor. IL-1β is a key pro-inflammatory cytokine involved in the initiation and propagation of inflammatory responses within the CNS [47,48]. Elevated levels of IL-1β can exacerbate neuronal damage by promoting apoptosis, disrupting the blood–brain barrier integrity, and impairing neurogenesis [49,50]. The upregulation of il1b in metolachlor-treated embryos underscores the herbicide’s potential to induce neuroinflammation, which may have long-term consequences for neural development and function. It also raises concerns about the broader implications of environmental exposure to metolachlor, particularly during critical periods of neurodevelopment. Moreover, we observed a notable redistribution and increase in microglia/macrophage populations in the Tg(mpeg:GFP) transgenic line exposed to metolachlor. Specifically, there was a pronounced accumulation of mpeg:GFP-positive cells in the head and yolk regions, contrasting with their distribution in the dorsal aorta and tail portion observed in the control embryos. Microglia are the resident immune cells of the CNS and play essential roles in maintaining neural homeostasis, responding to injury, and modulating neuroinflammation [51]. The altered distribution and increased density of microglia and macrophages in metolachlor-treated embryos suggest an activated state, potentially in response to neuronal damage or elevated pro-inflammatory signals. Such activation can lead to the release of cytotoxic factors, further exacerbating neuronal injury and contributing to a cycle of neuroinflammation and neurodegeneration.
5. Conclusions
Collectively, our findings indicate that sub-lethal exposure to metolachlor during early zebrafish development can disrupt neurogenesis, induce neuronal apoptosis, activate glial cells, and trigger neuroinflammatory responses. These molecular and morphological alterations could affect the proper development of the nervous system as well as the behavioral patterns of the animals. Therefore, these results highlight the importance of such studies, as chronic exposure to the herbicide, metolachlor, can have detrimental effects on the life cycle of aquatic organisms, given its widespread presence in environmental contexts of surface waters, like rivers and lakes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes10060292/s1, Figure S1: Brightfield imaging of zebrafish embryos non treated and treated with metolachlor at different concentrations. Figure S2: qPCR analysis of il1b transcript level in non-treated and after metolachlor exposure in zebrafish embryo.
Author Contributions
Conceptualization, P.C.; methodology, M.F., S.I., and P.C.; software, P.C.; validation, P.C.; formal analysis M.F. and P.C.; investigation, P.C.; resources, P.C.; data curation, M.F. and P.C.; writing—original draft preparation, M.F., S.I., and P.C.; writing—review and editing, P.C.; visualization, P.C.; supervision, P.C.; project administration, P.C.; funding acquisition, P.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by national public funds (grant numbers Cacialli-RFO2024/2025) from the Italian Ministry of University and Research (MIUR).
Institutional Review Board Statement
Fish were raised according to FELASA and European guidelines. No authorization was required since all the experiments were performed before 5 days post-fertilization. All efforts were made to comply with the 3R guidelines.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sule, R.O.; Condon, L.; Gomes, A.V. A Common Feature of Pesticides: Oxidative Stress—The Role of Oxidative Stress in Pesticide-Induced Toxicity. Oxid. Med. Cell Longev. 2022, 2022, 5563759. [Google Scholar] [CrossRef] [PubMed]
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide exposure, safety issues, and risk assessment indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Li, W.; Li, Q.; Zhang, D.; Fang, W.; Yan, D.; Li, Y.; Wang, Q.; Jin, X.; Cao, A. Metolachlor metal-organic framework nanoparticles for reducing leaching, ecotoxicity and improving bioactivity. Pest. Manag. Sci. 2022, 78, 5366–5378. [Google Scholar] [CrossRef] [PubMed]
- de Souza, R.M.; Seibert, D.; Quesada, H.B.; de Jesus Bassetti, F.; Fagundes-Klen, M.R.; Bergamasco, R. Occurrence, impacts and general aspects of pesticides in surface water: A review. Process Saf. Environ. Prot. 2020, 135, 22–37. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Hela, D.G.; Albanis, T.A. The status of pesticide pollution in surface waters (rivers and lakes) of Greece. Part I. Review on occurrence and levels. Environ. Pollut. 2006, 141, 555–570. [Google Scholar] [CrossRef]
- Lowry, D.M.; Greiner, D.; Fretheim, M.; Ubben, M.; Dhanwada, K.R. Mechanism of metolachlor action due to alterations in cell cycle progression. Cell Biol. Toxicol. 2013, 29, 283–291. [Google Scholar] [CrossRef]
- Rodrigues, P.; Oliva-Teles, L.; Guimarães, L.; Carvalho, A.P. Occurrence of Pharmaceutical and Pesticide Transformation Products in Freshwater: Update on Environmental Levels, Toxicological Information and Future Challenges. Rev. Environ. Contam. Toxicol. 2022, 260, 14. [Google Scholar] [CrossRef]
- Pillai, P.; Davis, D.E.; Truelove, B. Effects of Metolachlor on Germination, Growth, Leucine Uptake, and Protein Synthesis. Weed Sci. 1979, 27, 634–637. [Google Scholar] [CrossRef]
- Mercurio, P.; Mueller, J.F.; Eaglesham, G.; O’Brien, J.; Flores, F.; Negri, A.P. Degradation of Herbicides in the Tropical Marine Environment: Influence of Light and Sediment. PLoS ONE 2016, 11, e0165890. [Google Scholar] [CrossRef]
- Rozmánková, E.; Pípal, M.; Bláhová, L.; Njattuvetty Chandran, N.; Morin, B.; Gonzalez, P.; Bláha, L. Environmentally relevant mixture of S-metolachlor and its two metabolites affects thyroid metabolism in zebrafish embryos. Aquat. Toxicol. 2020, 221, 105444. [Google Scholar] [CrossRef]
- Rašković, B.; Poleksić, V.; Vuković, G.; Špirović Trifunović, B.; Božić, G.; Ćupić Miladinović, D.; Marković, Z.; Brkić, D. Acute and Subchronic Exposure of the Common Carp (Cyprinus carpio) to Herbicide S-Metolachlor. Water 2023, 15, 4182. [Google Scholar] [CrossRef]
- Goutte, A.; Martin, N.; Alliot, F.; Angelier, F.; Blanchouin, A.; Costantini, D.; Lesimple, M.; Ribout, C.; Traoré, S.; Villalta, R.; et al. From cells to recapture rates: Responses and recovery of a wild fish after an experimental exposure to a widely used herbicide. Environ. Sci. Pollut. Res. Int. 2024, 44, 8. [Google Scholar] [CrossRef] [PubMed]
- Velisek, J.; Stara, A.; Zuskova, E.; Kubec, J.; Buric, M.; Kouba, A. Effects of s-metolachlor on early life stages of marbled crayfish. Pestic. Biochem. Physiol. 2019, 153, 87–94. [Google Scholar] [CrossRef]
- Pereira, S.P.; Fernandes, M.A.; Martins, J.D.; Santos, M.S.; Moreno, A.J.; Vicente, J.A.; Videira, R.A.; Jurado, A.S. Toxicity assessment of the herbicide metolachlor comparative effects on bacterial and mitochondrial model systems. Toxicol. Vitr. 2009, 23, 1585–1590. [Google Scholar] [CrossRef]
- Cacialli, P.; Ricci, S.; Servetto, G.P.; Franceschini, V.; Ruiz-Zepeda, F.; Vigliaturo, R. Altered Morpho-Functional Features of Neurogenesis in Zebrafish Embryos Exposed to Non-Combustion-Derived Magnetite. Int. J. Mol. Sci. 2024, 25, 6459. [Google Scholar] [CrossRef]
- Cacialli, P.; Ricci, S.; Frabetti, F.; Ferrando, S.; Franceschini, V. Exposure of Zebrafish Embryos to Urea Affects NOS1 Gene Expression in Neuronal Cells. Environments 2024, 11, 41. [Google Scholar] [CrossRef]
- Yang, L.; Ivantsova, E.; Souders, C.L.; Martyniuk, C.J. The agrochemical S-metolachlor disrupts molecular mediators and morphology of the swim bladder: Implications for locomotor activity in zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2021, 208, 111641. [Google Scholar] [CrossRef]
- Quintaneiro, C.; Patrício, D.; Novais, S.C.; Soares, A.M.V.M.; Monteiro, M.S. Endocrine and physiological effects of linuron and S-metolachlor in zebrafish developing embryos. Sci. Total Environ. 2017, 586, 390–400. [Google Scholar] [CrossRef]
- Ou-Yang, K.; Feng, T.; Han, Y.; Li, G.; Li, J.; Ma, H. Bioaccumulation, metabolism and endocrine-reproductive effects of metolachlor and its S-enantiomer in adult zebrafish (Danio rerio). Sci. Total Environ. 2022, 802, 149826. [Google Scholar] [CrossRef]
- Park, H.C.; Hong, S.K.; Kim, H.S.; Kim, S.H.; Yoon, E.J.; Kim, C.H.; Miki, N.; Huh, T.L. Structural comparison of zebrafish Elav/Hu and their differential expressions during neurogenesis. Neurosci. Lett. 2000, 279, 81–84. [Google Scholar] [CrossRef]
- Ellett, F.; Pase, L.; Hayman, J.W.; Andrianopoulos, A.; Lieschke, G.J. mpeg1 promoter transgenes direct macrophage-lineage expression in zebrafish. Blood 2011, 117, e49–e56. [Google Scholar] [CrossRef] [PubMed]
- Bernardos, R.L.; Raymond, P.A. GFAP transgenic zebrafish. Gene Expr. Patterns 2006, 6, 1007–1013. [Google Scholar] [CrossRef]
- Mahony, C.B.; Cacialli, P.; Pasche, C.; Monteiro, R.; Savvides, S.N.; Bertrand, J.Y. Hapln1b, a central organizer of the ECM, modulates kit signaling to control developmental hematopoiesis in zebrafish. Blood Adv. 2021, 5, 4935–4948. [Google Scholar] [CrossRef]
- Fan, C.Y.; Cowden, J.; Simmons, S.O.; Padilla, S.; Ramabhadran, R. Gene expression changes in developing zebrafish as potential markers for rapid developmental neurotoxicity screening. Neurotoxicol. Teratol. 2010, 32, 91–98. [Google Scholar] [CrossRef]
- Cacialli, P.; Lucini, C. Analysis of the Expression of Neurotrophins and Their Receptors in Adult Zebrafish Kidney. Vet. Sci. 2022, 9, 296. [Google Scholar] [CrossRef] [PubMed]
- Cacialli, P. Expression of Nerve Growth Factor and Its Receptor TrkA in the Reproductive System of Adult Zebrafish. Vet. Sci. 2022, 9, 225. [Google Scholar] [CrossRef]
- Ricci, S.; Lazzari, M.; Maurizii, M.; Franceschini, V.; Milani, L.; Cacialli, P. Analysis of clasp2 Transcription Pattern in Male Germ Cells during Spermatogenesis: A Comparative Study in Zebrafish (Danio rerio) and Guppy (Poecilia reticulata). Animals 2023, 13, 3617. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Einhorn, Z.; Mercurio, S.; Lee, S.; Lau, B.; Mione, M.; Wilson, S.W.; Guo, S. Neurogenin1 is a determinant of zebrafish basal forebrain dopaminergic neurons and is regulated by the conserved zinc finger protein Tof/Fezl. Proc. Natl. Acad. Sci. USA 2006, 103, 5143–5148. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Ma, Q.; Chen, Z.; del Barco Barrantes, I.; de la Pompa, J.L.; Anderson, D.J. neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron 1998, 20, 469–482. [Google Scholar] [CrossRef]
- Sun, Y.; Nadal-Vicens, M.; Misono, S.; Lin, M.Z.; Zubiaga, A.; Hua, X.; Fan, G.; Greenberg, M.E. Neurogenin promotes neurogenesis and inhibits glial differentiation by independent mechanisms. Cell 2001, 104, 365–376. [Google Scholar] [CrossRef]
- Zhang, D.; Hu, X.; Qian, L.; O’Callaghan, J.P.; Hong, J.S. Astrogliosis in CNS pathologies: Is there a role for microglia? Mol. Neurobiol. 2010, 41, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Andermann, P.; Ungos, J.; Raible, D.W. Neurogenin1 defines zebrafish cranial sensory ganglia precursors. Dev. Biol. 2002, 251, 45–58. [Google Scholar] [CrossRef]
- McGraw, H.F.; Nechiporuk, A.; Raible, D.W. Zebrafish dorsal root ganglia neural precursor cells adopt a glial fate in the absence of neurogenin1. J. Neurosci. 2008, 28, 12558–12569. [Google Scholar] [CrossRef] [PubMed]
- Blader, P.; Fischer, N.; Gradwohl, G.; Guillemot, F.; Strähle, U. The activity of neurogenin1 is controlled by local cues in the zebrafish embryo. Development 1997, 124, 4557–4569. [Google Scholar] [CrossRef]
- Korzh, V.; Sleptsova, I.; Liao, J.; He, J.; Gong, Z. Expression of zebrafish bHLH genes ngn1 and nrd defines distinct stages of neural differentiation. Dev. Dyn. 1998, 213, 92–104. [Google Scholar] [CrossRef]
- Gyimah, E.; Xu, H.; Dong, X.; Qiu, X.; Zhang, Z.; Bu, Y.; Akoto, O. Developmental neurotoxicity of low concentrations of bisphenol A and S exposure in zebrafish. Chemosphere 2021, 262, 128045. [Google Scholar] [CrossRef]
- Prakash, V.; Chauhan, S.S.; Ansari, M.I.; Jagdale, P.; Ayanur, A.; Parthasarathi, R.; Anbumani, S. 4-Methylbenzylidene camphor induced neurobehavioral toxicity in zebrafish (Danio rerio) embryos. Environ. Res. 2024, 242, 117746. [Google Scholar] [CrossRef]
- Wang, H.; Meng, Z.; Zhou, L.; Cao, Z.; Liao, X.; Ye, R.; Lu, H. Effects of acetochlor on neurogenesis and behaviour in zebrafish at early developmental stages. Chemosphere 2019, 220, 954–964. [Google Scholar] [CrossRef]
- Özdemir, S.; Altun, S.; Özkaraca, M.; Ghosi, A.; Toraman, E.; Arslan, H. Cypermethrin, chlorpyrifos, deltamethrin, and imidacloprid exposure up-regulates the mRNA and protein levels of bdnf and c-fos in the brain of adult zebrafish (Danio rerio). Chemosphere 2018, 203, 318–326. [Google Scholar] [CrossRef]
- Yamashita, M. Apoptosis in zebrafish development. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2003, 136, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Li, Y.; Long, L.; Li, D.; Jia, Q.; Wang, Y.; Shen, Q.; Tang, Y.; Wen, L.; Kung, H.F.; et al. Knockdown of FoxO3a induces increased neuronal apoptosis during embryonic development in zebrafish. Neurosci. Lett. 2010, 484, 98–103. [Google Scholar] [CrossRef]
- Blahová, J.; Plhalová, L.; Hostovský, M.; Divišová, L.; Dobšíková, R.; Mikulíková, I.; Stěpánová, S.; Svobodová, Z. Oxidative stress responses in zebrafish Danio rerio after subchronic exposure to atrazine. Food Chem. Toxicol. 2013, 61, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Thiel, N.A.; Sachett, A.; Schneider, S.E.; Garbinato, C.; Decui, L.; Eichwald, T.; Conterato, G.M.M.; Latini, A.; Piato, A.; Siebel, A.M. Exposure to the herbicide 2,4-dichlorophenoxyacetic acid impairs mitochondrial function, oxidative status, and behavior in adult zebrafish. Environ. Sci. Pollut. Res. Int. 2020, 27, 45874–45882. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, K.K. Glial fibrillary acidic protein: From intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci. 2015, 38, 364–374. [Google Scholar] [CrossRef]
- Iribarne, M.; Hyde, D.R. Different inflammation responses modulate Müller glia proliferation in the acute or chronically damaged zebrafish retina. Front. Cell Dev. Biol. 2022, 10, 892271. [Google Scholar] [CrossRef]
- Hewett, S.J.; Jackman, N.A.; Claycomb, R.J. Interleukin-1β in Central Nervous System Injury and Repair. Eur. J. Neurodegener. Dis. 2012, 1, 195–211. [Google Scholar]
- Ogryzko, N.V.; Hoggett, E.E.; Solaymani-Kohal, S.; Tazzyman, S.; Chico, T.J.; Renshaw, S.A.; Wilson, H.L. Zebrafish tissue injury causes upregulation of interleukin-1 and caspase-dependent amplification of the inflammatory response. Dis. Model. Mech. 2014, 7, 259–264. [Google Scholar] [CrossRef]
- Tong, L.; Balazs, R.; Soiampornkul, R.; Thangnipon, W.; Cotman, C.W. Interleukin-1 beta impairs brain derived neurotrophic factor-induced signal transduction. Neurobiol. Aging 2008, 29, 1380–1393. [Google Scholar] [CrossRef]
- Argaw, A.T.; Zhang, Y.; Snyder, B.J.; Zhao, M.L.; Kopp, N.; Lee, S.C.; Raine, C.S.; Brosnan, C.F.; John, G.R. IL-1beta regulates blood-brain barrier permeability via reactivation of the hypoxia-angiogenesis program. J. Immunol. 2006, 177, 5574–5584. [Google Scholar] [CrossRef]
- Xu, J.; Wang, T.; Wu, Y.; Jin, W.; Wen, Z. Microglia Colonization of Developing Zebrafish Midbrain Is Promoted by Apoptotic Neuron and Lysophosphatidylcholine. Dev. Cell 2016, 38, 214–222. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).