Isolation, Characterization, and Assessment of Probiotic Lactococcus lactis from the Intestinal Tract of Largemouth Bass (Micropterus salmoides)
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Strains from Intestine
2.2. Characterization of the Bacterial Isolates
2.2.1. Determination of Acid Production Capacity
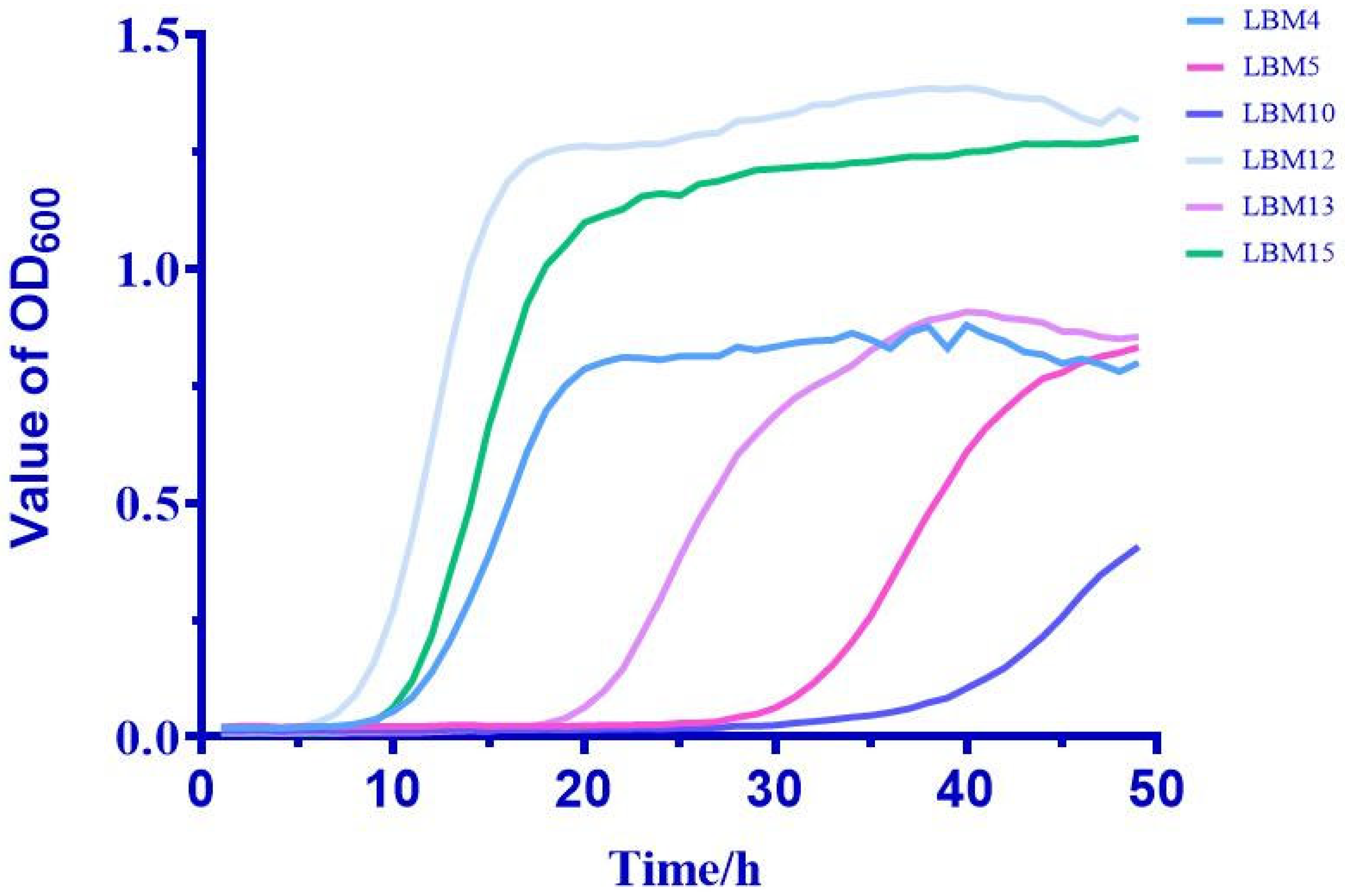
2.2.2. Growth Curve Determination
2.2.3. Determination of Enzymatic Activities
2.3. Determination of Antagonistic Activity to Aquatic Pathogens
2.4. Identification of Isolate
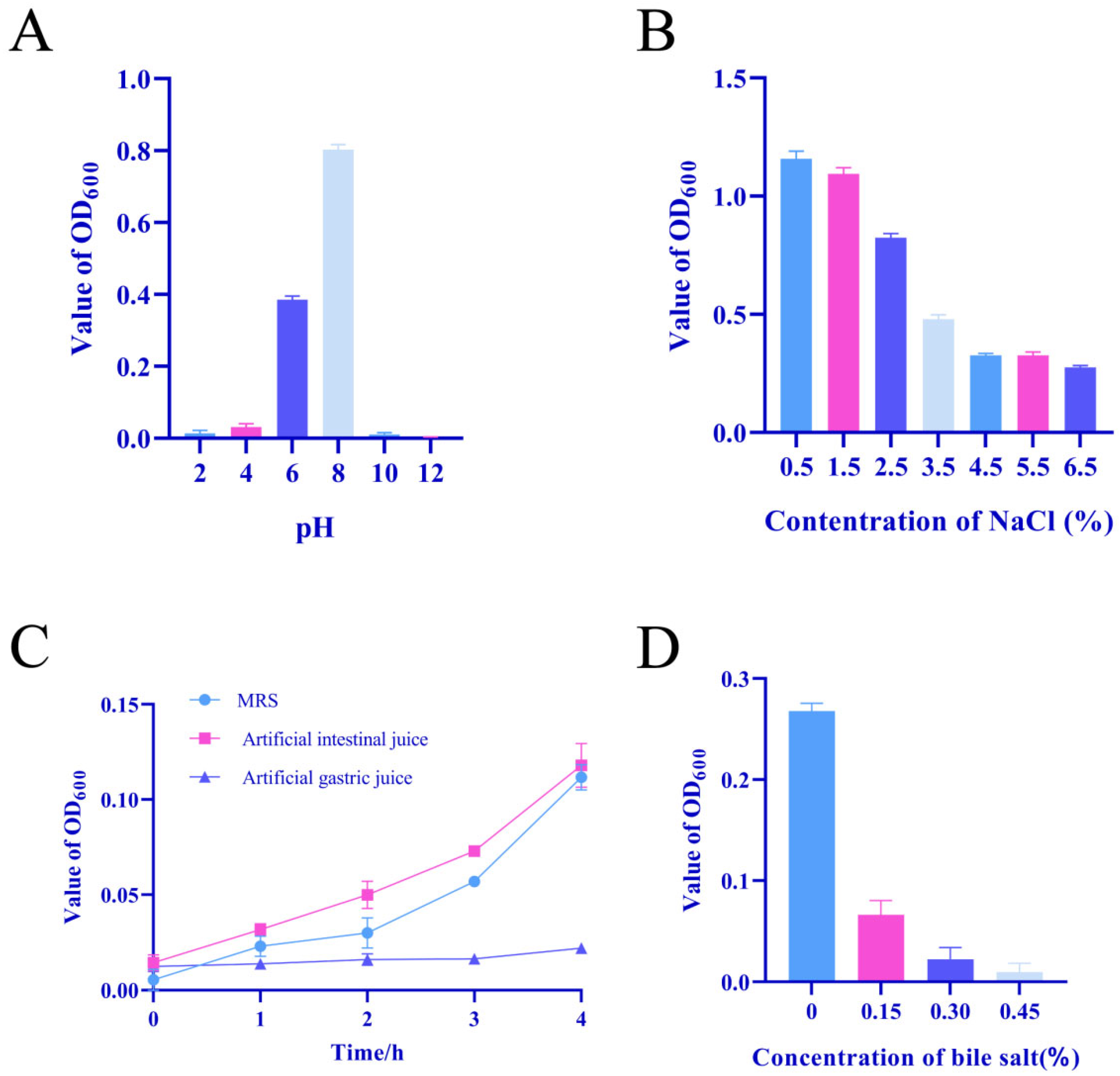
2.5. Tolerance Assay
2.6. Adhesion Capability Determination
2.6.1. Preparation of Fish Mucus
2.6.2. Glass Slide-Based Comprehensive Adhesion Measurement
2.6.3. Fluorescence-Based Comprehensive Adhesion Measurement
2.7. Safety Tests
2.7.1. Hemolytic Assay
2.7.2. Antibiotic Sensitivity Test
2.7.3. In Vivo Experiment
3. Results
3.1. Isolation and Physiological Properties of Isolates
3.2. Antagonistic Activity
3.3. Identification of LBM15
3.4. Probiotic Potential of LAB Isolates
3.5. Adherence Properties of LAB Strains
3.6. Safety Aspects of LBM15
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guillen, J.; Natale, F.; Carvalho, N.; Casey, J.; Hofherr, J.; Druon, J.-N.; Fiore, G.; Gibin, M.; Zanzi, A.; Martinsohn, J.T. Global seafood consumption footprint. Ambio 2019, 48, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Sagada, G.; Chen, J.M.; Shen, B.Q.; Huang, A.X.; Sun, L.H.; Jiang, J.H.; Jin, C.H. Optimizing protein and lipid levels in practical diet for juvenile northern snakehead fish (Channa argus). Anim. Nutr. 2017, 3, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, G.; Ray, A.K. The advancement of probiotics research and its application in fish farming industries. Res. Vet. Sci. 2017, 115, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Etyemez, M.; Balcazar, J.L. Isolation and characterization of bacteria with antibacterial properties from Nile tilapia (Oreochromis niloticus). Res. Vet. Sci. 2016, 105, 62–64. [Google Scholar] [CrossRef]
- Quiñones, R.A.; Fuentes, M.; Montes, R.M.; Soto, D.; León-Muñoz, J. Environmental issues in Chilean salmon farming: A review. Rev. Aquac. 2019, 11, 375–402. [Google Scholar] [CrossRef]
- Ahmad, A.; Abdullah, S.R.S.; Abu Hasan, H.; Othman, A.R.; Ismail, N.I. Aquaculture industry: Supply and demand, best practices, effluent and its current issues and treatment technology. J. Environ. Manag. 2021, 287, 112271. [Google Scholar] [CrossRef]
- Ray, A.K.; Ghosh, K.; Ringo, E. Enzyme-producing bacteria isolated from fish gut: A review. Aquac. Nutr. 2012, 18, 465–492. [Google Scholar] [CrossRef]
- Martinez Cruz, P.; Ibanez, A.L.; Monroy Hermosillo, O.A.; Ramirez Saad, H.C. Use of probiotics in aquaculture. ISRN Microbiol. 2012, 2012, 916845. [Google Scholar] [CrossRef]
- Wang, X.; Yao, Y.X.; Ge, H.; Zhang, J.N.; Zhang, J.L.; Yan, Q.P. Isolation and identification of probiotic Bacillus subtilis AJQ03 from the intestinal tract of Anguilla japonica (Japanese eel). Front. Microbiol. 2024, 15, 1446299. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, X.; Lin, X.; Wu, J.; Wang, P.; Zhu, C.; Yan, Q. Isolation and characterization of probiotic Lysinibacillus species from the gastrointestinal tract of large yellow croaker (Larimichthys crocea). Front. Mar. Sci. 2024, 11, 1408979. [Google Scholar] [CrossRef]
- Wang, P.; Wang, S.; Zhu, C.; Sun, Y.; Yan, Q.; Yi, G. Monascus purpureus M-32 fermented soybean meal improves the growth, immunity parameters, intestinal morphology, disease resistance, intestinal microbiota and metabolome in Pacific white shrimp (Litopenaeus vannamei). Anim. Nutr. 2024, 17, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Wanka, K.M.; Damerau, T.; Costas, B.; Krueger, A.; Schulz, C.; Wuertz, S. Isolation and characterization of native probiotics for fish farming. BMC Microbiol. 2018, 18, 119. [Google Scholar] [CrossRef] [PubMed]
- Jose, N.M.; Bunt, C.R.; Hussain, M.A. Comparison of Microbiological and Probiotic Characteristics of Lactobacilli Isolates from Dairy Food Products and Animal Rumen Contents. Microorganisms 2015, 3, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Ringo, E.; Gatesoupe, F.J. Lactic acid bacteria in fish: A review. Aquaculture 1998, 160, 177–203. [Google Scholar] [CrossRef]
- Balcazar, J.L.; Vendrell, D.; de Blas, I.; Ruiz-Zarzuela, I.; Muzquiz, J.L.; Girones, O. Characterization of probiotic properties of lactic acid bacteria isolated from intestinal microbiota of fish. Aquaculture 2008, 278, 188–191. [Google Scholar] [CrossRef]
- Llewellyn, M.S.; Boutin, S.; Hoseinifar, S.H.; Derome, N. Teleost microbiomes: The state of the art in their characterization, manipulation and importance in aquaculture and fisheries. Front. Microbiol. 2014, 5, 207. [Google Scholar] [CrossRef]
- Ibrahem, M.D. Evolution of probiotics in aquatic world: Potential effects, the current status in Egypt and recent prospectives. J. Adv. Res. 2015, 6, 765–791. [Google Scholar] [CrossRef]
- Butt, R.L.; Volkoff, H. Gut Microbiota and Energy Homeostasis in Fish. Front. Endocrinol. 2019, 10, 9. [Google Scholar] [CrossRef]
- Lazado, C.C.; Caipang, C.M.A.; Rajan, B.; Brinchmann, M.F.; Kiron, V. Characterization of GP21 and GP12: Two Potential Probiotic Bacteria Isolated from the Gastrointestinal Tract of Atlantic Cod. Probiotics Antimicrob. Proteins 2010, 2, 126. [Google Scholar] [CrossRef]
- Hua, X.J.; Li, C.; Xiao, Y.C.; Lu, Y.N.; Liu, X.Q. Oral administration of recombinant Lactococcus lactis expressing largemouth bass (Micropterus salmoides) IFNa3 protein enhances immune response against largemouth bass virus (LMBV) infection. Fish Shellfish Immunol. 2024, 154, 109875. [Google Scholar] [CrossRef]
- Todorov, S.D.; Lima, J.M.S.; Bucheli, J.E.V.; Popov, I.V.; Tiwari, S.K.; Chikindas, M.L. Probiotics for Aquaculture: Hope, Truth, and Reality. Probiotics Antimicrob. Proteins 2024, 16, 2007–2020. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; He, M.W.; Cao, Z.J.; Xie, Z.Y.; Liu, C.S.; Wang, S.F.; Guo, W.L.; Zhang, X.; Zhou, Y.C. Effects of dietary administration of Lactococcus lactis HNL12 on growth, innate immune response, and disease resistance of humpback grouper (Cromileptes altivelis). Fish Shellfish Immunol. 2018, 82, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Ringo, E.; Hoseinifar, S.H.; Ghosh, K.; Van Doan, H.; Becks, B.R.; Song, S.K. Lactic Acid Bacteria in Finfish-An Update. Front. Microbiol. 2018, 9, 1818. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhao, L.; Li, Q.; Huang, L.; Qin, Y.; Zhuang, Z.; Wang, X.; Huang, H.; Zhang, J.; Zhang, J.; et al. Pseudomonas plecoglossicida fliP gene affects the immune response of Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂ to infection. Fish Shellfish Immunol. 2023, 140, 108971. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, B.; Zhang, Z.; Huang, Y.; Xu, Z.; Chen, X.; Huang, Y.; Jian, J.; Yan, Q. Involvement and characterization of NLRCs and pyroptosis-related genes in Nile tilapia (Oreochromis niloticus) immune response. Fish Shellfish Immunol. 2022, 130, 602–611. [Google Scholar] [CrossRef]
- Ayyash, M.M.; Abdalla, A.K.; AlKalbani, N.S.; Baig, M.A.; Turner, M.S.; Liu, S.Q.; Shah, N.P. Invited review: Characterization of new probiotics from dairy and nondairy products-Insights into acid tolerance, bile metabolism and tolerance, and adhesion capability. J. Dairy Sci. 2021, 104, 8363–8379. [Google Scholar] [CrossRef]
- Alakomi, H.L.; Skyttä, E.; Saarela, M.; Mattila-Sandholm, T.; Latva-Kala, K.; Helander, I.M. Lactic acid permeabilizes gram-negative bacteria by disrupting the outer membrane. Appl. Environ. Microbiol. 2000, 66, 2001–2005. [Google Scholar] [CrossRef]
- Xia, L.; Cheng, G.; Wang, P.; Wang, X.; Dong, Z.; Mu, Q.; Yu, J.; Jiang, Z.; Xiao, J.; Feng, H.; et al. Screening and identification of probiotics from the intestinal tract of largemouth bass (Micropterus salmoides) for use as a feed additive and bacterial infection control. Aquaculture 2024, 584, 740661. [Google Scholar] [CrossRef]
- León, J.; Pellón, F.J.; Unda, V.; David, J.; Anaya, C.A.M.; Mendoza, V.Í. Producción de enzimas extracelulares por bacterias aisladas de invertebrados marinos. Rev. Peru. Biol. 2014, 7, 202–210. [Google Scholar] [CrossRef][Green Version]
- Vinderola, G.; Capellini, B.; Villarreal, F.; Suárez, V.; Quiberoni, A.; Reinheimer, J. Usefulness of a set of simple in vitro tests for the screening and identification of probiotic candidate strains for dairy use. LWT-Food Sci. Technol. 2008, 41, 1678–1688. [Google Scholar] [CrossRef]
- Aarti, C.; Khusro, A.; Varghese, R.; Arasu, M.V.; Agastian, P.; Al-Dhabi, N.A.; Ilavenil, S.; Choi, K.C. In vitro investigation on probiotic, anti-Candida, and antibiofilm properties of Lactobacillus pentosus strain LAP1. Arch. Oral Biol. 2018, 89, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Charteris, W.P.; Kelly, P.M.; Morelli, L.; Collins, J.K. Development and application of an in vitro methodology to determine the transit tolerance of potentially probiotic Lactobacillus and Bifidobacterium species in the upper human gastrointestinal tract. J. Appl. Microbiol. 1998, 84, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Ouwehand, A.C.; Kirjavainen, P.V.; Grönlund, M.M.; Isolauri, E.; Salminen, S.J. Adhesion of probiotic micro-organisms to intestinal mucus. Int. Dairy J. 1999, 9, 623–630. [Google Scholar] [CrossRef]
- He, L.; Mao, M.; Zhao, L.; Li, Q.; Zhuang, Z.; Wang, X.; Huang, H.; Wang, Q.; Yan, Q. A novel small non-coding RNA 562 mediates the virulence of Pseudomonas plecoglossicida by regulating the expression of fliP, a key component of flagella T3SS. Fish Shellfish Immunol. 2024, 151, 109752. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Z.; Wang, S.; Wang, X.; Zhang, D.; Wang, Q.; Lin, L.; Wang, G.; Guo, Z.; Chen, Y. Inhibitory effect of probiotic Bacillus spp. isolated from the digestive tract of Rhynchocypris Lagowskii on the adhesion of common pathogenic bacteria in the intestinal model. Microb. Pathog. 2022, 169, 105623. [Google Scholar] [CrossRef]
- Rahayu, S.; Amoah, K.; Huang, Y.; Cai, J.; Wang, B.; Shija, V.M.; Jin, X.; Anokyewaa, M.A.; Jiang, M.Y. Probiotics application in aquaculture: Its potential effects, current status in China and future prospects. Front. Mar. Sci. 2024, 11, 1455905. [Google Scholar] [CrossRef]
- Govindaraj, K.; Samayanpaulraj, V.; Narayanadoss, V.; Uthandakalaipandian, R. Isolation of Lactic Acid Bacteria from Intestine of Freshwater Fishes and Elucidation of Probiotic Potential for Aquaculture Application. Probiotics Antimicrob. Proteins 2021, 13, 1598–1610. [Google Scholar] [CrossRef]
- Nemcova, R. Selection criteria of lactobacilli for probiotic use. Vet. Med. 1997, 42, 19–27. [Google Scholar]
- Reuben, R.C.; Roy, P.C.; Sarkar, S.L.; Alam, R.-U.; Jahid, I.K. Isolation, characterization, and assessment of lactic acid bacteria toward their selection as poultry probiotics. BMC Microbiol. 2019, 19, 253. [Google Scholar] [CrossRef]
- Carvalho, I.T.; Santos, L. Antibiotics in the aquatic environments: A review of the European scenario. Environ. Int. 2016, 94, 736–757. [Google Scholar] [CrossRef]
- O’Flaherty, E.; Cummins, E. Antibiotic resistance in surface water ecosystems: Presence in the aquatic environment, prevention strategies, and risk assessment. Hum. Ecol. Risk Assess. 2017, 23, 299–322. [Google Scholar] [CrossRef]
- Cabello, F.C.; Godfrey, H.P.; Tomova, A.; Ivanova, L.; Doelz, H.; Millanao, A.; Buschmann, A.H. Antimicrobial use in aquaculture re-examined: Its relevance to antimicrobial resistance and to animal and human health. Environ. Microbiol. 2013, 15, 1917–1942. [Google Scholar] [CrossRef] [PubMed]
- Smith, P. Antimicrobial resistance in aquaculture. Rev. Sci. Tech.-Off. Int. Epizoot. 2008, 27, 243–264. [Google Scholar] [CrossRef]
- De Vuyst, L.; Leroy, F. Bacteriocins from lactic acid bacteria: Production, purification, and food applications. J. Mol. Microbiol. Biotechnol. 2007, 13, 194–199. [Google Scholar] [CrossRef]
- Li, H.; Meiqin, M.; Lingmin, Z.; Qi, L.; Hui, G.; Jiao-nan, Z.; Jiaolin, Z.; Qingpi, Y. sRNA113 regulates the motility of Pseudomonas plecoglossicida to affect the immune response against infection in pearl gentian grouper. Zool. Res. 2025, 46, 152. [Google Scholar]
- Luis Balcazar, J.; De Blas, I.; Ruiz-Zarzuela, I.; Vendrell, D.; Calvo, A.C.; Marquez, I.; Girones, O.; Luis Muzquiz, J. Changes in intestinal microbiota and humoral immune response following probiotic administration in brown trout (Salmo trutta). Br. J. Nutr. 2007, 97, 522–552. [Google Scholar] [CrossRef]
- Yoshiyama, M.; Wu, M.; Sugimura, Y.; Takaya, N.; Kimoto-Nira, H.; Suzuki, C. Inhibition of Paenibacillus larvae by lactic acid bacteria isolated from fermented materials. J. Invertebr. Pathol. 2013, 112, 62–67. [Google Scholar] [CrossRef]
- Hwanhlem, N.; Buradaleng, S.; Wattanachant, S.; Benjakul, S.; Tani, A.; Maneerat, S. Isolation and screening of lactic acid bacteria from Thai traditional fermented fish (Plasom) and production of Plasom from selected strains. Food Control. 2011, 22, 401–407. [Google Scholar] [CrossRef]
- Tang, Y.; Jiao, J.; Zhao, L.; Zhuang, Z.; Wang, X.; Fu, Q.; Huang, H.; Huang, L.; Qin, Y.; Zhang, J.; et al. The contribution of exbB gene to pathogenicity of Pseudomonas plecoglossicida and its interactions with Epinephelus coioides. Fish Shellfish Immunol. 2022, 120, 610–619. [Google Scholar] [CrossRef]
- Byun, J.W.; Park, S.C.; Benno, Y.; Oh, T.K. Probiotic effect of Lactobacillus sp. DS-12 in flounder (Paralichthys olivaceus). J. Gen. Appl. Microbiol. 1997, 43, 305–308. [Google Scholar] [CrossRef]
- Harzevili, A.R.S.; Van Duffel, H.; Dhert, P.; Swings, J.; Sorgeloos, P. Use of a potential probiotic Lactococcus lactis AR21 strain for the enhancement of growth in the rotifer Brachionus plicatilis (Muller). Aquac. Res. 1998, 29, 411–417. [Google Scholar] [CrossRef]
- Gatesoupe, F.J. The use of probiotics in aquaculture. Aquaculture 1999, 180, 147–165. [Google Scholar] [CrossRef]
- García-Hernández, Y.; Pérez-Sánchez, T.; Boucourt, R.; Balcázar, J.L.; Nicoli, J.R.; Moreira-Silva, J.; Rodríguez, Z.; Fuertes, H.; Nuñez, O.; Albelo, N.; et al. Isolation, characterization and evaluation of probiotic lactic acid bacteria for potential use in animal production. Res. Vet. Sci. 2016, 108, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, T.C.; Costa, W.K.A.d.; Barão, C.E.; Rosset, M.; Magnani, M. Vegan probiotic products: A modern tendency or the newest challenge in functional foods. Food Res. Int. 2021, 140, 110033. [Google Scholar] [CrossRef]
- Wang, W.; Gänzle, M. Chapter Three—Toward rational selection criteria for selection of probiotics in pigs. In Advances in Applied Microbiology; Gadd, G.M., Sariaslani, S., Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 107, pp. 83–112. [Google Scholar]
- Verschuere, L.; Rombaut, G.; Sorgeloos, P.; Verstraete, W. Probiotic Bacteria as Biological Control Agents in Aquaculture. Microbiol. Mol. Biol. Rev. 2000, 64, 655–671. [Google Scholar] [CrossRef]
- Vaughan, E.E.; Heilig, H.; Ben-Amor, K.; de Vos, W.M. Diversity, vitality and activities of intestinal lactic acid bacteria and bifidobacteria assessed by molecular approaches. Fems Microbiol. Rev. 2005, 29, 477–490. [Google Scholar] [CrossRef]
- Li, X.; Ringo, E.; Hoseinifar, S.H.; Lauzon, H.L.; Birkbeck, H.; Yang, D. The adherence and colonization of microorganisms in fish gastrointestinal tract. Rev. Aquac. 2019, 11, 603–618. [Google Scholar] [CrossRef]
- Kos, B.; Suskovic, J.; Vukovic, S.; Simpraga, M.; Frece, J.; Matosic, S. Adhesion and aggregation ability of probiotic strain Lactobacillus acidophilus M92. J. Appl. Microbiol. 2003, 94, 981–987. [Google Scholar] [CrossRef]
- Chantanawilas, P.; Pahumunto, N.; Teanpaisan, R. Aggregation and adhesion ability of various probiotic strains and Candida species: An in vitro study. J. Dent. Sci. 2024, 19, 2163–2171. [Google Scholar] [CrossRef]
- Xin, G.; Zhao, L.; Zhuang, Z.; Wang, X.; Fu, Q.; Huang, H.; Huang, L.; Qin, Y.; Zhang, J.; Zhang, J.; et al. Function of the rpoD gene in Pseudomonas plecoglossicida pathogenicity and Epinephelus coioides immune response. Fish Shellfish Immunol. 2022, 127, 427–436. [Google Scholar] [CrossRef]
- Mack, D.R.; Michail, S.; Wei, S.; McDougall, L.; Hollingsworth, M.A. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am. J. Physiol.-Gastrointest. Liver Physiol. 1999, 276, G941–G950. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Zhao, L.; Li, Q.; Huang, L.; Qin, Y.; Wang, P.; Zhu, C.; Yan, Q. flgC gene is involved in the virulence regulation of Pseudomonas plecoglossicida and affects the immune response of Epinephelus coioides. Fish Shellfish Immunol. 2023, 132, 108512. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.; Xu, L.; Yang, Y.; He, S.; Liu, W.; Ringø, E.; Zhou, Z. Lactobacillus planarum subsp. plantarum JCM 1149 vs. Aeromonas hydrophila NJ-1 in the anterior intestine and posterior intestine of hybrid tilapia Oreochromis niloticus ♀ × Oreochromis aureus ♂: An ex vivo study. Fish Shellfish Immunol. 2013, 35, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Ehrmann, M.A.; Kurzak, P.; Bauer, J.; Vogel, R.F. Characterization of lactobacilli towards their use as probiotic adjuncts in poultry. J. Appl. Microbiol. 2002, 92, 966–975. [Google Scholar] [CrossRef]
- Jawan, R.; Abbasiliasi, S.; Tan, J.S.; Mustafa, S.; Halim, M.; Ariff, A.B. Influence of Culture Conditions and Medium Compositions on the Production of Bacteriocin-Like Inhibitory Substances by Lactococcus lactis Gh1. Microorganisms 2020, 8, 1454. [Google Scholar] [CrossRef]
- Guo, X.-H.; Kim, J.-M.; Nam, H.-M.; Park, S.-Y.; Kim, J.-M. Screening lactic acid bacteria from swine origins for multistrain probiotics based on in vitro functional properties. Anaerobe 2010, 16, 321–326. [Google Scholar] [CrossRef]
- Angmo, K.; Kumari, A.; Savitri; Bhalla, T.C. Probiotic characterization of lactic acid bacteria isolated from fermented foods and beverage of Ladakh. Lwt-Food Sci. Technol. 2016, 66, 428–443. [Google Scholar] [CrossRef]
- Mathara, J.M.; Schillinger, U.; Kutima, P.M.; Mbugua, S.K.; Guigas, C.; Franz, C.; Holzapfel, W.H. Functional properties of Lactobacillus plantarum strains isolated from Maasai traditional fermented milk products in Kenya. Curr. Microbiol. 2008, 56, 315–321. [Google Scholar] [CrossRef]
- Salminen, S.; von Wright, A.; Morelli, L.; Marteau, P.; Brassart, D.; de Vos, W.M.; Fonden, R.; Saxelin, M.; Collins, K.; Mogensen, G.; et al. Demonstration of safety of probiotics—A review. Int. J. Food Microbiol. 1998, 44, 93–106. [Google Scholar] [CrossRef]
- Yanina Bustos, A.; Font de Valdez, G.; Raya, R.; de Almeida, A.M.; Fadda, S.; Pia Taranto, M. Proteomic analysis of the probiotic Lactobacillus reuteri CRL1098 reveals novel tolerance biomarkers to bile acid-induced stress. Food Res. Int. 2015, 77, 599–607. [Google Scholar] [CrossRef]
- Arya, M.; Shahi, N.; Bisht, I.; Pandey, N.; Mallik, S.K. Probiotic potential of Bacillus velezensis STPB10 sourced from the gut microbiota of a hillstream fish Schizothorax richardsonii (Gray, 1832) for aquaculture applications. Sci. Rep. 2025, 15, 17580. [Google Scholar] [CrossRef]



| Strian | Zone Diameter/mm |
|---|---|
| LBM4 | 27.56 ± 0.49 |
| LBM5 | 30.43 ± 0.51 |
| LBM10 | 27.88 ± 0.47 |
| LBM12 | 24.17 ± 0.71 |
| LBM13 | 27.63 ± 0.58 |
| LBM15 | 26.99 ± 0.44 |
| Pathogens | Inhibition Zone Diameter/mm |
|---|---|
| V. alginolyticus | 18.22 ± 0.32 |
| V. anguillarum | 11.97 ± 0.60 |
| V. harveyi | 11.71 ± 0.32 |
| A. hydrophila | 12.35 ± 0.50 |
| E. tarda | 10.15 ± 0.33 |
| P. plecoglossicida | / |
| Antibiotic | Drug Doses (μg) | Inhibition Zone Diameters (mm) | Sensitivity |
|---|---|---|---|
| Penicillin G | 10 | 32.61 ± 0.40 | S |
| Oxacillin | 1 | 17.33 ± 0.52 | S |
| Ampicillin | 10 | 32.71 ± 0.44 | S |
| Carbenicillin | 100 | 33.63 ± 0.46 | S |
| Piperacillin | 100 | 31.02 ± 0.54 | S |
| Cefalexin | 30 | 23.10 ± 0.20 | S |
| Cefazolin | 30 | 26.16 ± 0.36 | S |
| Cephradine | 30 | 25.36 ± 0.25 | S |
| Cefuroxime | 30 | 34.70 ± 0.53 | S |
| Ceftazidime | 30 | 23.96 ± 0.35 | S |
| Ceftriaxone | 30 | 32.72 ± 0.38 | S |
| Cefoperazone | 75 | 29.61 ± 0.50 | S |
| Amikacin | 30 | 10.52 ± 0.17 | R |
| Gentamicin | 10 | 7.61 ± 0.33 | R |
| Kanamycin | 30 | / | R |
| Neomycin | 30 | / | R |
| Tetracycline | 30 | 15.17 ± 0.69 | I |
| Doxycycline | 30 | 17.54 ± 0.43 | S |
| Minocycline | 30 | 15.65 ± 0.35 | I |
| Erythromycin | 15 | 9.93 ± 0.43 | R |
| Midecamycin | 30 | 19.53 ± 0.54 | S |
| Norfloxacin | 30 | 9.92 ± 0.44 | R |
| Ofloxacin | 5 | 18.14 ± 0.53 | S |
| Ciprofloxacin | 5 | 16.21 ± 0.50 | I |
| Vancomycin | 30 | 17.74 ± 0.12 | S |
| Polymyxin B | 300 | / | R |
| Sulfamethoxazole | 23.75 | 12.95 ± 0.52 | R |
| Furazolidone | 300 | 17.64 ± 0.51 | S |
| Chloramphenicol | 30 | 25.92 ± 0.68 | S |
| Clindamycin | 2 | 17.41 ± 0.29 | I |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Zhang, J.; Zhang, J.; Zou, W.; Yan, Q. Isolation, Characterization, and Assessment of Probiotic Lactococcus lactis from the Intestinal Tract of Largemouth Bass (Micropterus salmoides). Fishes 2025, 10, 291. https://doi.org/10.3390/fishes10060291
Chen X, Zhang J, Zhang J, Zou W, Yan Q. Isolation, Characterization, and Assessment of Probiotic Lactococcus lactis from the Intestinal Tract of Largemouth Bass (Micropterus salmoides). Fishes. 2025; 10(6):291. https://doi.org/10.3390/fishes10060291
Chicago/Turabian StyleChen, Xiaoyu, Jiaonan Zhang, Jiaolin Zhang, Wenzheng Zou, and Qingpi Yan. 2025. "Isolation, Characterization, and Assessment of Probiotic Lactococcus lactis from the Intestinal Tract of Largemouth Bass (Micropterus salmoides)" Fishes 10, no. 6: 291. https://doi.org/10.3390/fishes10060291
APA StyleChen, X., Zhang, J., Zhang, J., Zou, W., & Yan, Q. (2025). Isolation, Characterization, and Assessment of Probiotic Lactococcus lactis from the Intestinal Tract of Largemouth Bass (Micropterus salmoides). Fishes, 10(6), 291. https://doi.org/10.3390/fishes10060291






