1. Introduction
Fish maternal germplasm resources carry abundant genetic information, which helps to maintain the genetic diversity of species and enhance their ability to adapt to environmental changes. The individual females usually pass on good traits such as disease resistance and high yield to their offspring, which are crucial in breeding practices aimed at assisting in the formation of new lines. Therefore, as an important part of biobanking, the cryopreservation of maternal germplasm resources is of great significance, especially for endangered species. The eggs, oocytes, embryos and ovarian tissue are important maternal germplasm resources [
1,
2]. However, compared with sperm, it is still difficult to cryopreserve eggs, oocytes and embryos due to the large volume of cells, high intracellular water and yolk compartment [
1].
In recent years, ovarian tissue from several teleosts, including the rainbow trout (
Oncorhynchus mykiss) [
3], brown trout (
Salmo trutta) [
4], cyprinid honmoroko (
Gnathopogon caerulescens) [
5], common carp (
Cyprinus carpio) [
6], Piracanjunba (
Brycon orbignyanus) [
7], Murray River Rainbowfish (
Melanotaenia fluviatilis) [
8], sturgeon [
9,
10,
11] and zebrafish [
12], has been successfully cryopreserved. In previous research, the ovarian tissues were mostly isolated from immature individuals for cryopreservation, when the tissue mainly contained a large number of oogonia (i.e., germ stem cells), which could be used as donor cells for transplantation after thawing. This method provides a new approach for the conservation and utilization of precious, endangered and elite germplasm resources. In rainbow trout, common carp and Siberian sturgeon (
Acipenser baerii), the ovaries were cooled at −1 °C/min for 90 min using specific equipment (e.g., a Bicell plastic freezing container) before immersion in liquid nitrogen, and the oogonia after cryopreservation were successfully used for transplantation [
3,
6,
9]. Different from the slow freezing method, in brown trout, Piracanjunba and cyprinid honmoroko, ovaries with high concentrations of cryoprotectants have been rapidly cryopreserved via vitrification, resulting in high cell integrity and viability [
4,
5,
7]. Whether slow freezing or vitrification was performed, the cryoprotectants played a vital role in the freezing process. Cryoprotectants can be categorized into two types: permeating and non-permeating [
13]. Permeating cryoprotectants permeate cell membranes, which include dimethyl sulfoxide (DMSO), methanol (MeOH), propylene glycol (PG), ethylene glycol (EG) and glycerol (Gly). Meanwhile, non-permeating cryoprotectants include trehalose, fetal bovine serum (FBS), bovine serum albumin (BSA), sucrose, egg yolk powder and skim milk powder. A combination of cryoprotectants at appropriate concentrations can minimize the damage caused by the formation of ice crystals to cells or tissues during freezing. The cryoprotectants DMSO, PG, EG, MeOH, trehalose and FBS have been widely applied for the cryopreservation of ovarian tissues. It has been shown that DMSO and PG have a good preservation effect when used in the vitrification of salmon and sturgeon ovaries [
4,
5,
10]. Meanwhile, trehalose and FBS have mostly been used for slow freezing in some species [
3,
11].
Although a small number of endangered or commercially valuable fish have been cryopreserved with relatively high cell survival rates, there remain some problems to be solved. Slow freezing and vitrification, as two common cooling procedures, have certain limitations [
14]. Slow freezing methods require complex and expensive equipment to control the temperature precisely and are not suitable for the timely preservation of ovary materials in the sea, field, laboratory or farm without relevant equipment [
15,
16]. Meanwhile, vitrification—unlike slow cooling—only requires high concentrations of cryoprotectants (especially permeating agents such as DMSO) [
17]. However, at high concentrations, although effectively reducing the formation of ice crystals, these cryoprotectants may be highly toxic to cells [
18]. In addition, ovarian cryopreservation approaches are mainly applied to immature ovaries, which are limited by the developmental period and the small size of the tissue. Compared to immature ovaries, the ovaries at the stage of vitellogenesis are generally larger and contain various types of germ cells. If they can be cryopreserved successfully, not only can the oogonia be separated for transplantation and other operations, but the vitellogenic oocytes can also be induced for artificial insemination. This is expected to expand the scope of preservation and application of frozen germplasm.
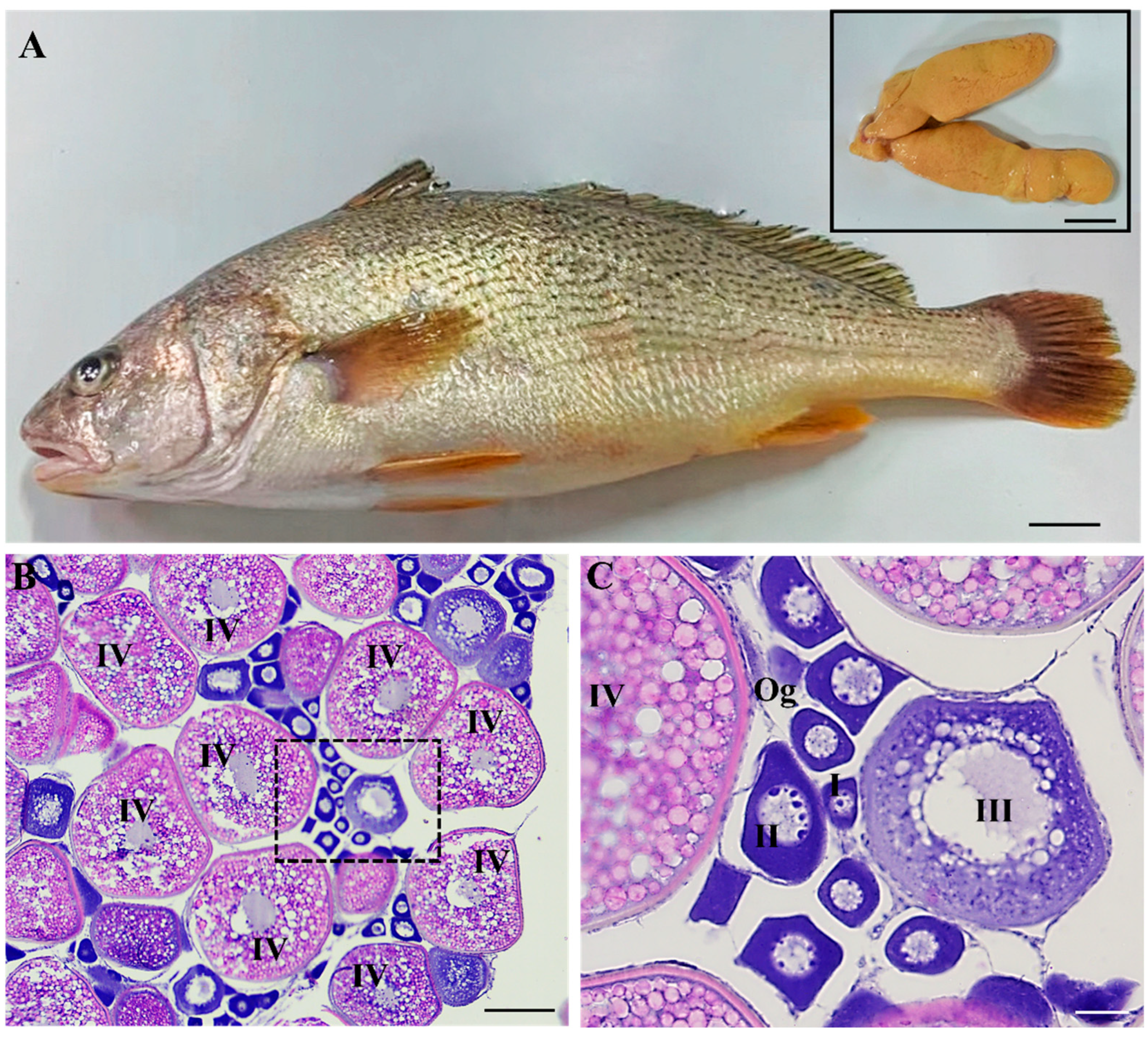
The yellow drum (
Nibea albiflora) is an important fish species in the Sciaenidae family. It displays asynchronous oocyte development, with ovaries containing oocytes at multiple developmental stages simultaneously. This allows for batch spawning during the reproductive season (May–July). As a marine culture fish, it is of great economic value in China. In recent years, with the rapid development of marine aquaculture, yellow drums have suffered from higher rates of disease, leading to significant economic losses [
19]. Furthermore, overwinter mortalities limit the aquaculture of this species [
20]. Therefore, it is very important to cultivate new strains that are resistant to disease and stress and keep them frozen in time for the healthy and sustainable development of yellow drum culture. However, to date, the cryopreservation of yellow drum ovaries has not been reported. In this study, we aimed to establish a simple and efficient preservation system for ovaries at the stage of vitellogenesis from yellow drum. The ovaries were isolated from female individuals in May and cut into tissue blocks of approximately 1 cm
3, then immersed in 15% PG, 15% FBS and 2M trehalose as cryoprotectants for 1 h on ice. Subsequently, the tissues were removed and cryopreserved using three different freezing procedures. After 7 days, the tissues were thawed and digested, and the morphology, structure and survival rate of cells were detected using the AO-EB Double Fluorescence Staining Kit. Furthermore, the expression levels of genes related to female reproductive development were analyzed via RT-qPCR in order to estimate cell viability. This study provides a new method for the preservation of yellow drum germplasm, thus promoting the development of artificial culture while providing a reference for similar preservation in other fish.
2. Materials and Methods
2.1. Collection of Fish and Ovaries
Five female yellow drum in May were obtained from Ningde Yiye Marine Industry Development Co., Ltd. The basic indicators of body weight, total length and body length were measured after anesthetization by MS-222 (Sigma-Aldrich, St. Louis, MI, USA). The total length was approximately 31 ± 2.0 cm, body length was 28 ± 1.5 cm, and body weight was 450 ± 35 g. Subsequently, the ovaries of the fish were removed and weighed, of which one portion was fixed in Bouin’s reagent for histology and the other was placed in L-15 medium on ice for cryopreservation. Based on the weight of the ovaries and body, the gonadosomatic index (GSI) was obtained as 8.72 ± 1.2, suggesting that these female individuals were at the stage of vitellogenesis.
The cryopreservation of ovarian tissue from yellow drum was approved by the Ethics Committee of Ningde Normal University (NDNU-LL-202508).
2.2. Histology
The tissue sections and H&E staining of the ovary were performed according to Zhao et al. [
21]. Briefly, samples were fixed, dehydrated, clarified and embedded in paraffin, and then 5 μm thick sections were cut by a manual microtome (Leica RM2125, Wetzlar, Germany). Finally, the images were photographed using a fluorescence microscope (Leica DM7500, Wetzlar, Germany).
2.3. Experimental Design
Referring to previous studies [
3,
10,
11], PG (SINOPHARM), FBS (AusgeneX, Shanghai, China) and trehalose (Bomei, Maanshan, China) were selected for use as cryoprotectants in this study, which were diluted into a cryoprotectant comprising 15% (
v/
v) PG, 15% (
v/
v) FBS and 0.2 M trehalose with 50% extender. The 100% extender was based on the study of Lee et al. [
3], which contained 55.27 mM Hepes, 375.48 mM NaCl, 7.28 mM KCl, 23.10 mM KH
2PO
4, 3.82 mM Na
2HPO
4, 3.64 mM sodium pyruvate, 2.6 mM CaCl
2·2H
2O and 1.4 mM MgCl
2·6H
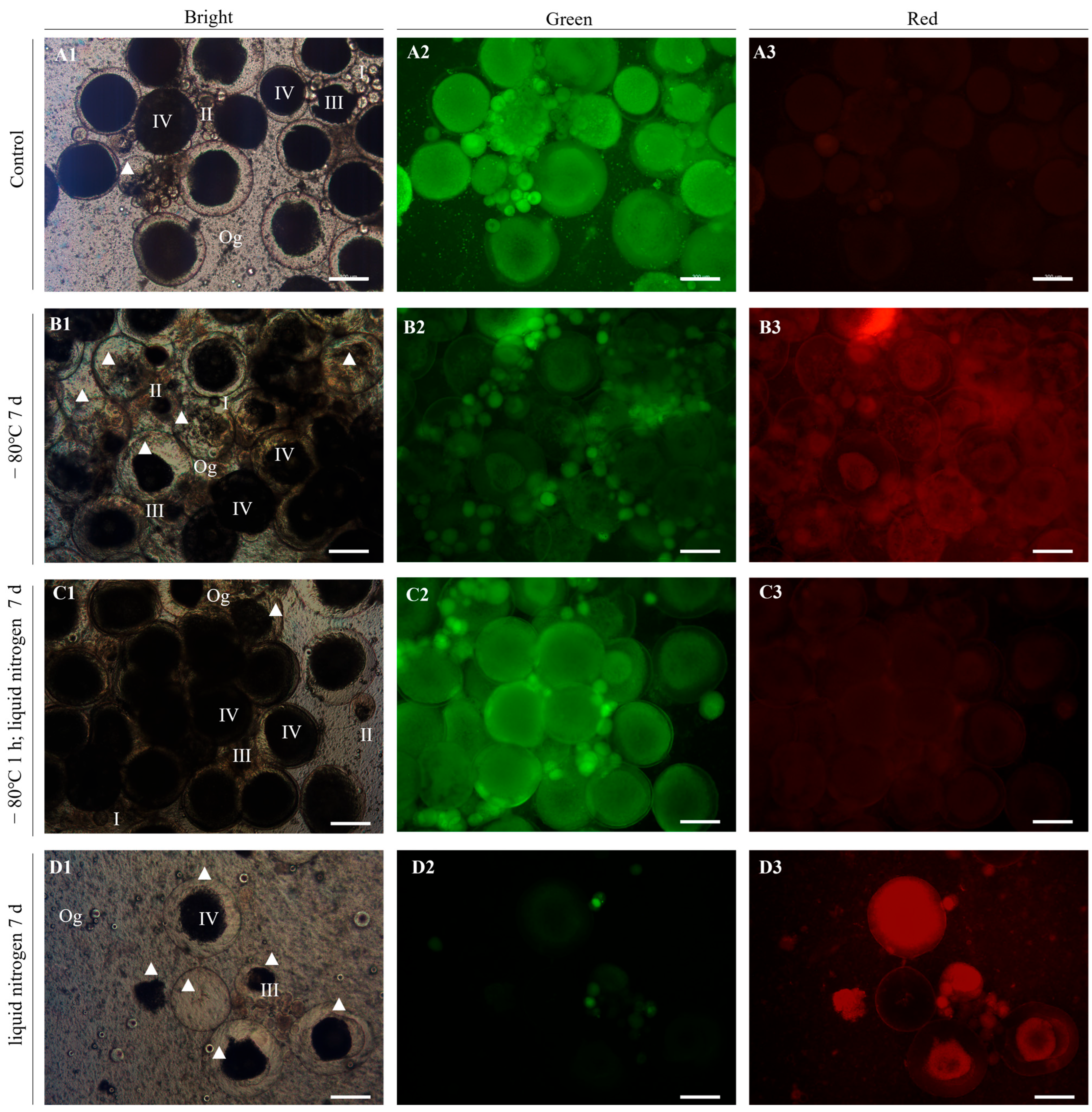
2O. In order to explore the cryopreservation effect of yellow drum ovaries under different freezing procedures. According to preliminary laboratory research, three procedures were adopted with −80 °C refrigerator, liquid nitrogen, and their combination (−80 °C refrigerator for 1 h; liquid nitrogen).
2.4. Cryopreservation Protocols
The isolated ovaries were cut into tissue blocks of approximately 1 cm
3, then covered with about 1 mL of cryoprotectant mixture in a 2 mL cryopreservation tube. Three parallel samples were used. Subsequently, the ovary tissues were equilibrated on ice for 60 min, and the cryoprotectants were removed from the tissues after equilibrium had been reached. Then, the three parallel samples were cryopreserved via one of the three freezing procedures, respectively. Upon cryopreservation for 7 days, the tissues were thawed by a water bath (10 °C; 8 min), according to the previous studies of Lee et al. [
3] and Lujić et al. [
10] with minor modifications. Finally, the ovaries were rehydrated and restored to room temperature in L-15 for about 10 min. Untreated fresh ovarian tissue served as a control.
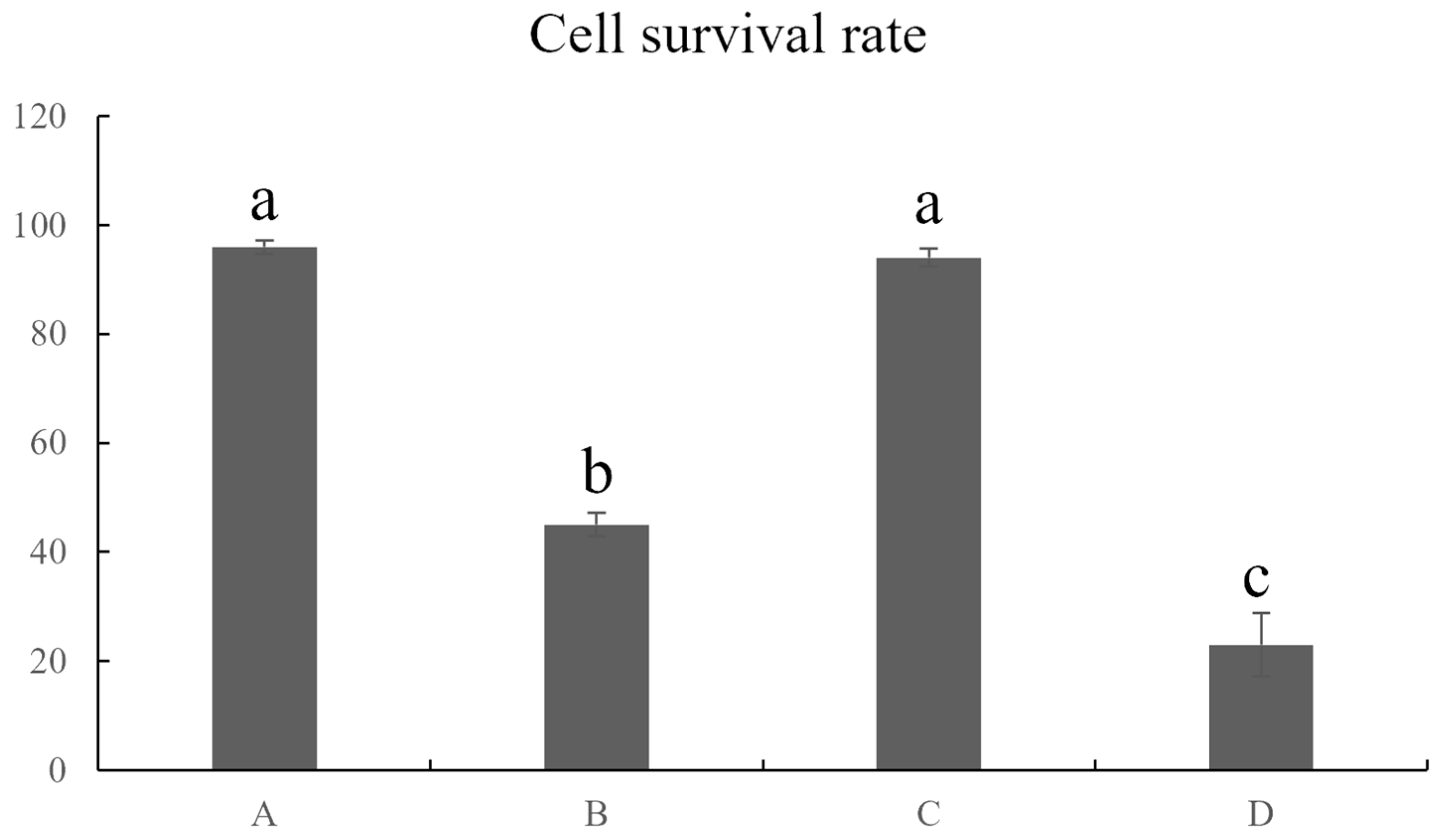
2.5. Detection of Cell Survival Rate
The AO-EB Double Fluorescence Staining Kit (Phygene, Fuzhou, China) was used to detect the survival rate of germ cells. The thawed tissue blocks were processed into dispersed cells with 0.25% Trypsin–EDTA solution (Pricella, Wuhan, China) for 2 h at 28 °C after being washed with L-15. Then, according to the manuals of the kit, the working solution of AO/EB was configured for cell staining. Based on the kit’s instructions, under a fluorescence microscope (Leica DMI8, Wetzlar, Germany), the live and dead cells could show green and red fluorescence after treatment with the working solution, respectively. Therefore, the cell survival rate can be calculated by the following formula. Cell viability = green cells/total cells × 100%.
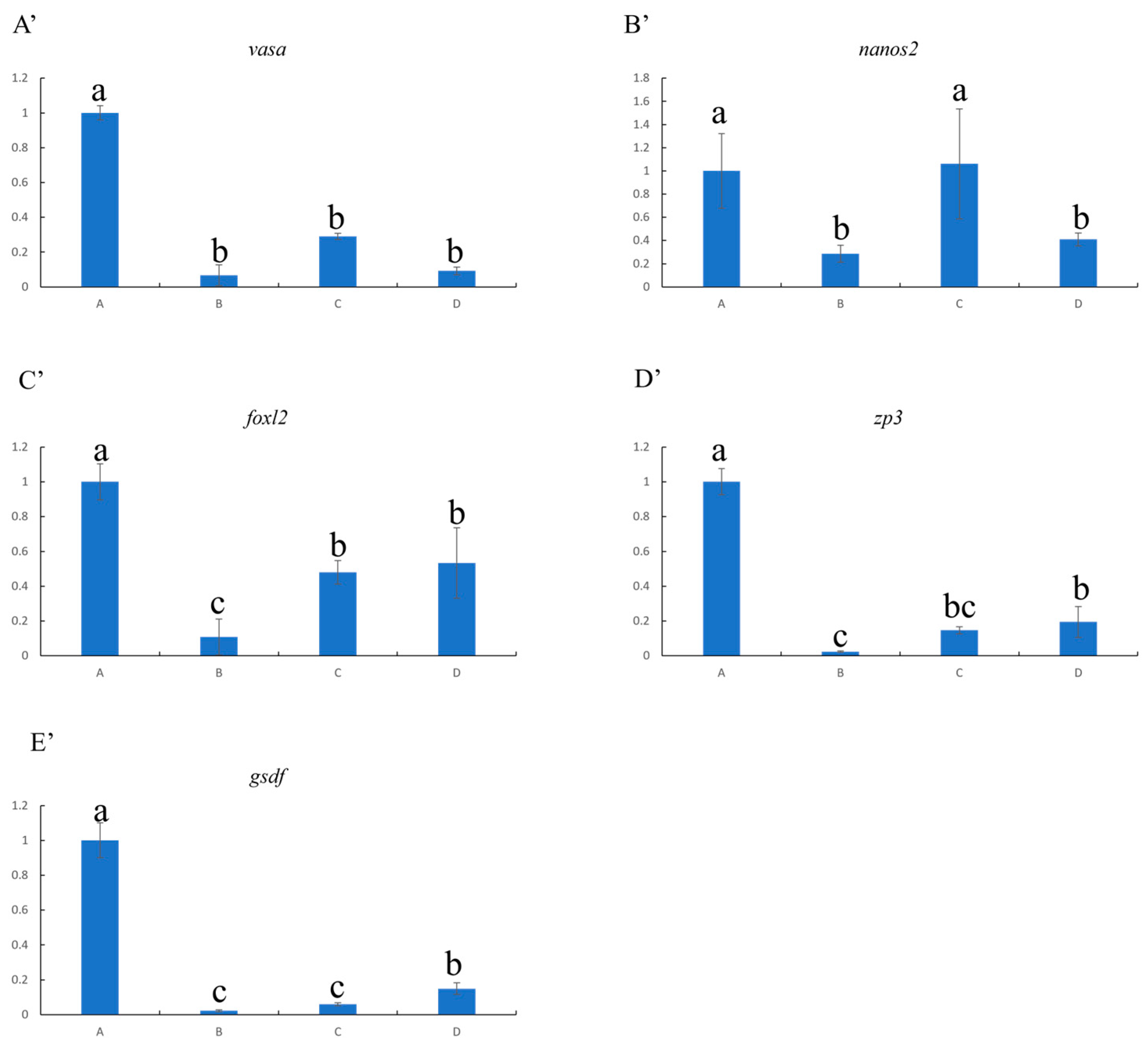
2.6. Analysis of Gene Expression Related to Reproductive Development
After thawing and digestion, some of the isolated cells were also subjected to quantitative real-time polymerase chain reaction (qRT-PCR).
β-actin and
18s were used as internal controls, and genes related to reproductive development, such as
vasa,
nanos2,
foxl2,
zp3 and
gsdf, were detected to assess the effect of cryopreservation. The qRT-PCR primers for these genes were designed using the Primer 5 software and are shown in
Table 1. According to the manufacturer’s instructions, total RNA of isolated cells from the control and experiment groups was extracted using the SPARK easy Tissue/Cell RNA kit (Sparkjade, Jinan, China), and cDNA synthesis was performed using the PrimeScriptTM RT reagent kit with gDNA Eraser (Takara, Osaka, Japan). qRT-PCR was performed with TB Green
® Premix Ex TaqTM II (Tli RNaseH Plus) (Takara, Osaka, Japan) using a QuantStudio 3 system (Applied Biosystems, Foster City, CA, USA). Briefly, a total volume of 20 μL, including 4 μL of 2× TB Green
® Premix Ex Taq II, 0.4 μL of 50 × ROX Reference Dye II, 0.8 μL of forward and reverse primers (10 μM), 6 μL of RNase-free water and 2 μL (<100 ng) of template DNA, was used, and the program was set as follows: 95 °C for 30 s, 35 cycles of 95 °C for 5 s, and 60 °C for 34 s. The results were analyzed according to the comparative Ct method. In this method,
vasa,
nanos2,
foxl2,
zp3 and
gsdf expression levels were normalized against
β-actin and
18s expression, generating a ΔCt. Relative expression was calculated according to the 2
−ΔΔCt equation.
2.7. Statistical Analysis
The data of cell survival rate and gene expression was analyzed by software SPSS 26. Each group was presented as mean ± standard error (SE). And the different groups were carried out to one-way ANOVA, then performed Tukey’s test. The p < 0.05 presented significant differences between the different groups.
4. Discussion
The present study performed the optimal cryopreservation of ovaries at the stage of vitellogenesis from yellow drum for the first time (
Figure 5). The ovaries were isolated from female individuals during the reproduction period and cryopreserved with a cryoprotectant combination using three different freezing procedures. After thawing, the ovaries refrigerated at −80 °C for 1 h and then transferred to liquid nitrogen for 7 d showed the highest cell survival rate (>90%), as determined using the AO-EB Double Fluorescence Staining kit. Furthermore, the qRT-PCR results revealed that genes related to reproductive development, including
vasa,
foxl2,
zp3 and
gsdf, were all down-regulated, while the expression of the nanos2 gene—which is specifically distributed in oogonia—was maintained at a higher level, similar to that in the control group. These results indicate that the viability of germ stem cells (oogonia) was not weakened after freezing, and the oogonia could be isolated from the cryopreserved ovaries for germ cell transplantation. These results are similar to those relating to the cryopreservation of ovarian tissues at early stages from Siberian sturgeon [
9] and Murray River Rainbowfish [
8]. As such, this study established a simple, rapid, efficient and less toxic cryopreservation system for yellow drum, which is of great significance for the preservation of the germplasm resources of Sciaenidae family members.
Regarding the cryopreservation of fish ovaries, previous studies have mainly focused on the preservation of immature ovaries [
7,
9,
11]. In this study, we attempted to cryopreserve ovaries at the stage of vitellogenesis obtained from yellow drum. According to the results of the cell morphology and structure, cell viability and gene expression analyses after freezing, it can be concluded that the effect of the optimal cryopreservation protocol proposed in this study after freezing was good for germ cells, especially oogonia. It can be speculated that, in addition to cryoprotectant agents and procedures, the ovarian support structure may also promote the efficiency of this cryopreservation protocol. In contrast to previous studies, the cryopreservation of ovaries at the stage of vitellogenesis allows for not only the preservation of isolated oogonia (as is the case for cryopreserved immature ovaries) but also vitellogenic oocytes, enabling the possibility of subsequent artificial insemination. This could be considered as an alternative route for eggs and embryos that are traditionally hard to preserve. Moreover, considering the large volume of ovaries at the stage of vitellogenesis, they cannot be frozen whole, as is generally the case for immature ovary cryopreservation. Therefore, in this study, the ovaries were first cut into tissue blocks of approximately 1 cm
3 before preservation.
In previous studies, slow cooling and vitrification methods have been mainly used for the cryopreservation of fish ovaries [
3,
4,
5,
6]. In rainbow trout, Siberian sturgeon and Murray River Rainbowfish, slow cooling methods showed a good preservation effect, yielding cell viabilities above 70% [
3,
8,
9]. However, slow cooling requires special temperature control equipment (e.g., freezer containers) to cool the ovaries at a controlled rate of −1 °C/minute over the course of more than 1 h before their transfer into liquid nitrogen, which greatly increases the cost associated with preservation. Furthermore, this approach is not suitable in cases where the timely preservation of ovaries is necessary, such as sea, field, laboratory or farm conditions without the required equipment. In addition, vitrification usually involves the use of high concentrations of cryoprotectants—especially permeating agents—to rapidly cool the ovaries. For example, in cyprinid honmoroko, the cryopreservation of whole ovaries required a vitrification medium including 5M PG and 5M DMSO [
5]. It should be noted that high concentrations of permeating cryoprotectants, such as DMSO, exhibit good preservation ability but increase germ cell toxicity. Compared to the previous studies, the present study achieved the cryopreservation of yellow drum ovaries using a low-toxicity cryoprotectant without requiring any special temperature control equipment.
5. Conclusions
This study is the first to explore the cryopreservation of ovaries at the stage of vitellogenesis from an economically important marine fish species. The cryoprotectant, comprising 15% PG, 15% FBS and 0.2 M trehalose, demonstrated a high cell survival rate (>90%) when used for the cryopreservation of yellow drum ovaries (especially for oogonia), with good cell viability confirmed through morphological and molecular identification. In the process of cryopreservation, the large volumes of ovaries were cut into tissue blocks, then refrigerated at −80 °C for 1 h before being transferred to liquid nitrogen for 7 d. Compared with the commonly used methods of slow cooling and vitrification, the preservation of ovarian tissues in this study did not require special equipment and the smaller tissue blocks facilitated the penetration of cryoprotectants, thus reducing the cytotoxicity associated with high concentrations of cryoprotectants. The present study provided a convenient, rapid, efficient and less toxic method for the cryopreservation of ovaries at the stage of vitellogenesis, which is important for germplasm resource preservation and fish breeding.