A Hydroacoustic Assessment of the Density, Size, and Biomass of Fish in a Freshwater Reservoir After Non-Classical Biomanipulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Hydroacoustic Survey

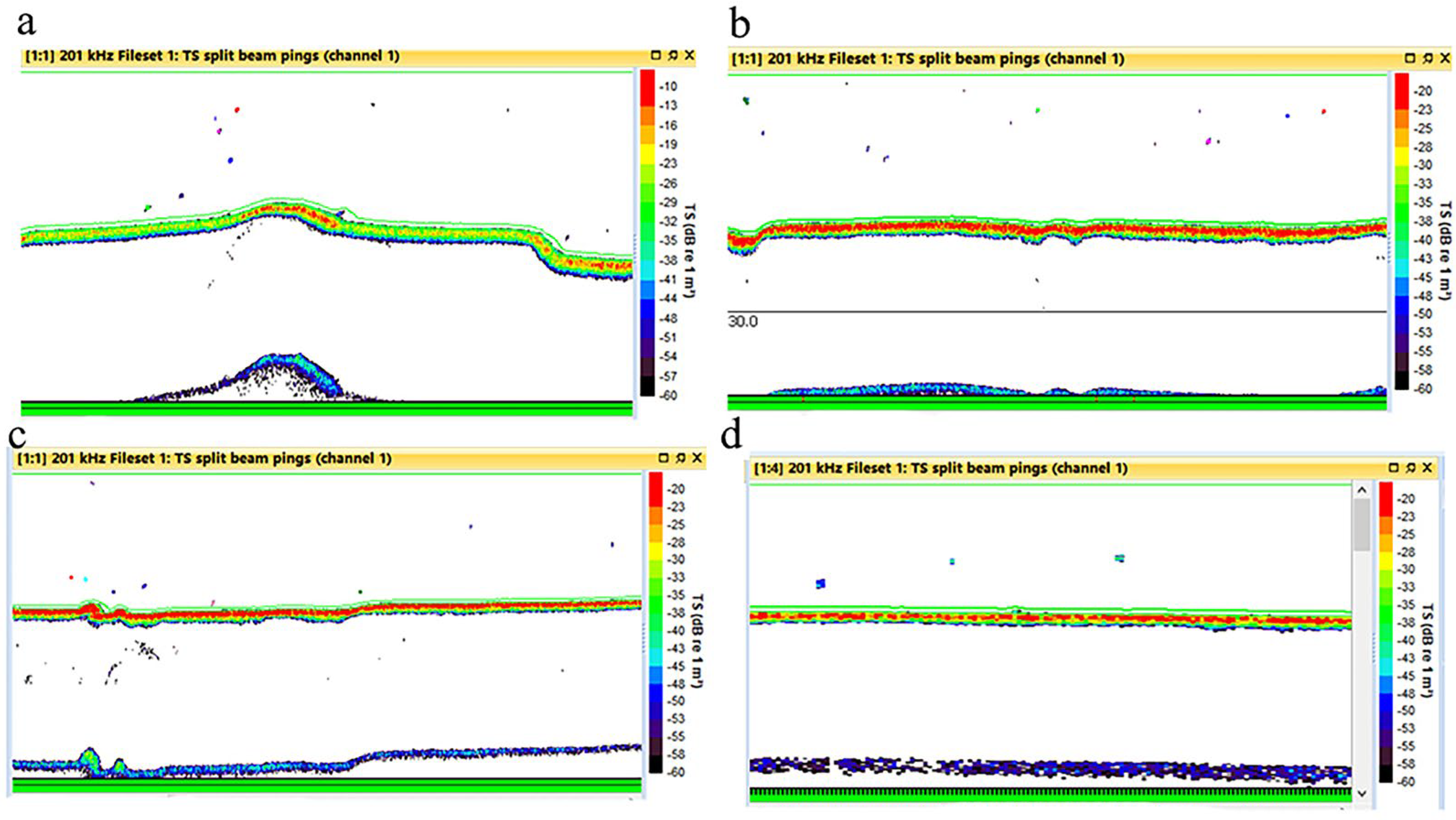
2.3. Acoustic Data Processing
2.4. Fish Sampling and Age Determination
2.5. Acoustic Biomass
2.6. Statistical Analysis
3. Results
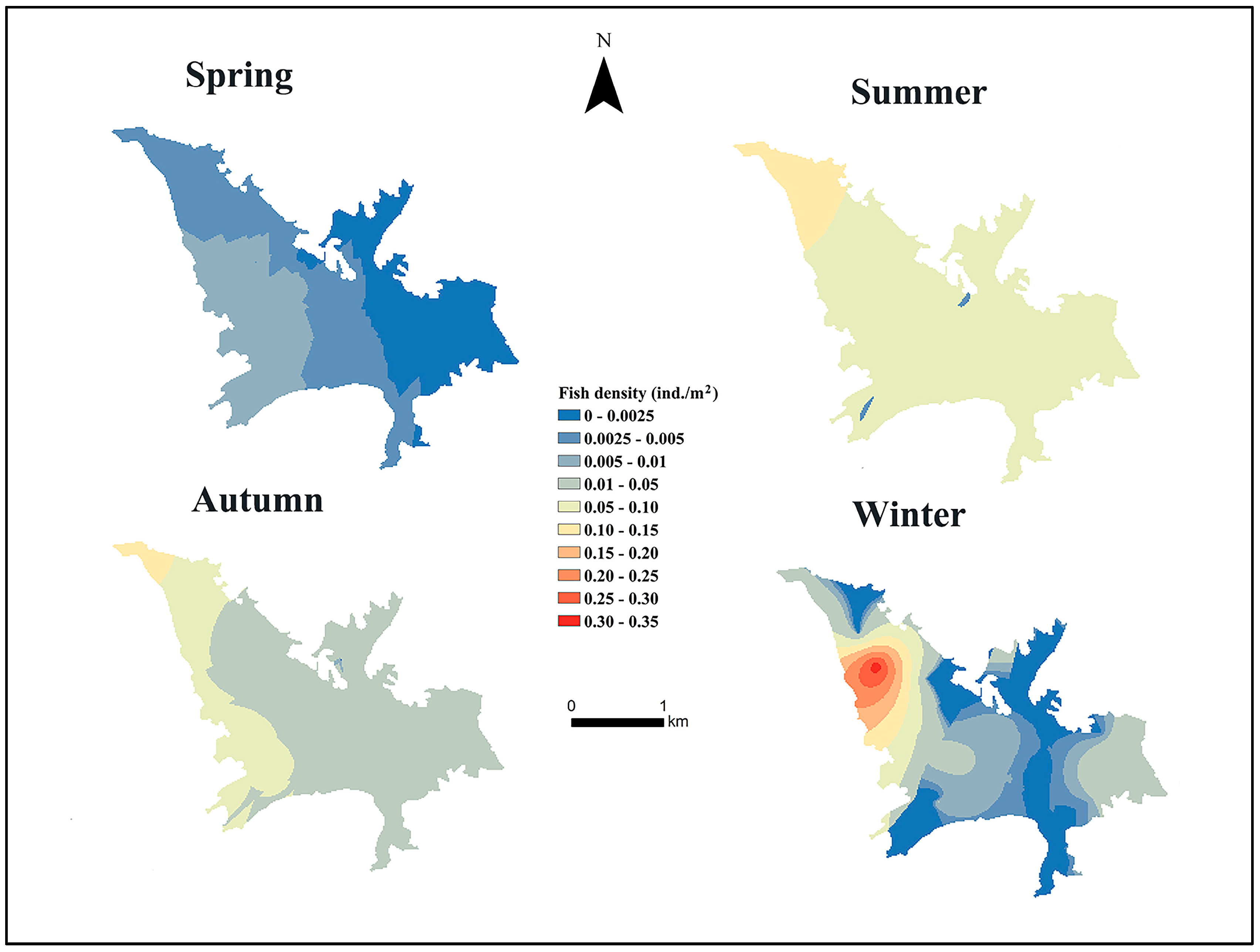
3.1. Spatial and Temporal Variation in Fish Density
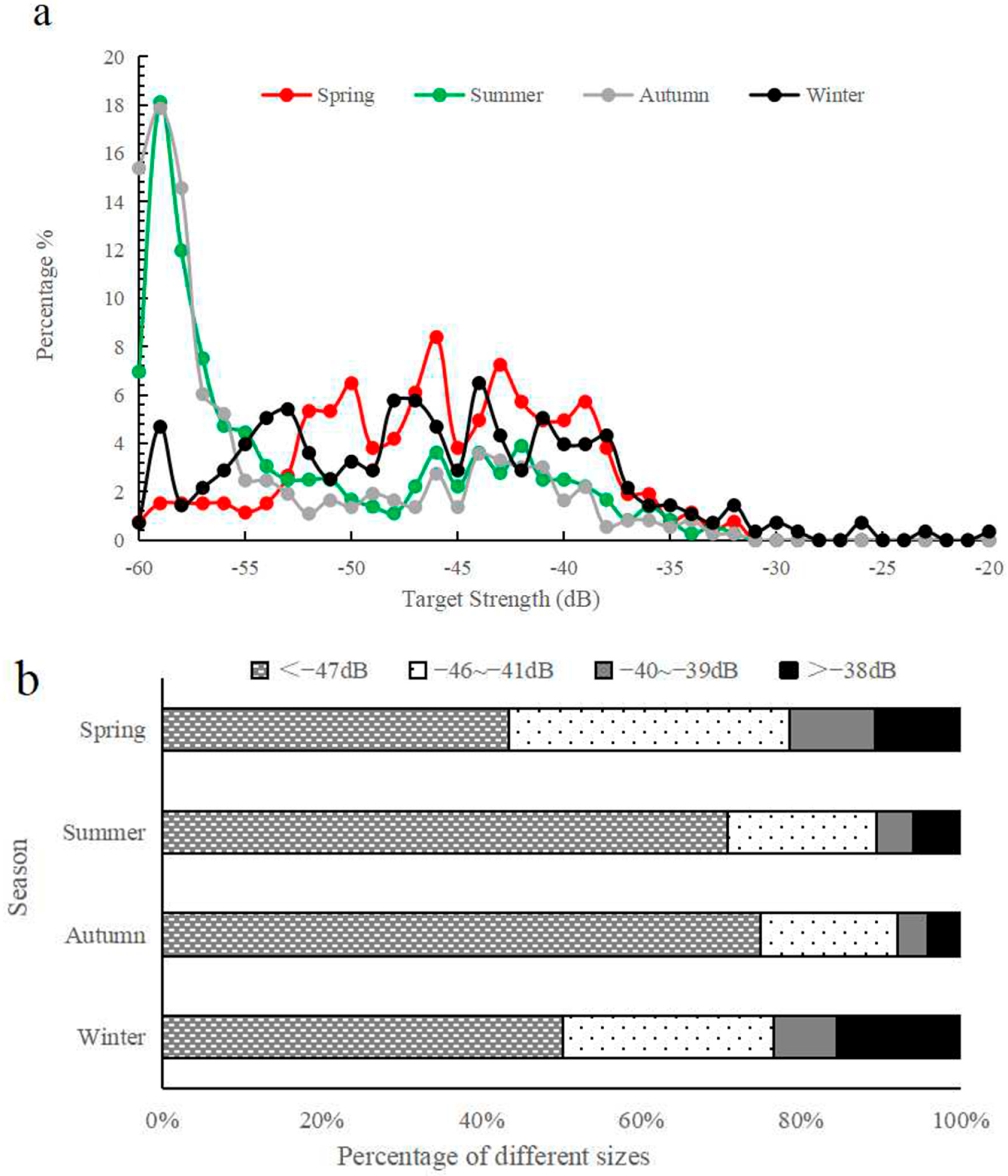
3.2. Comparison of Fish Size Distributions
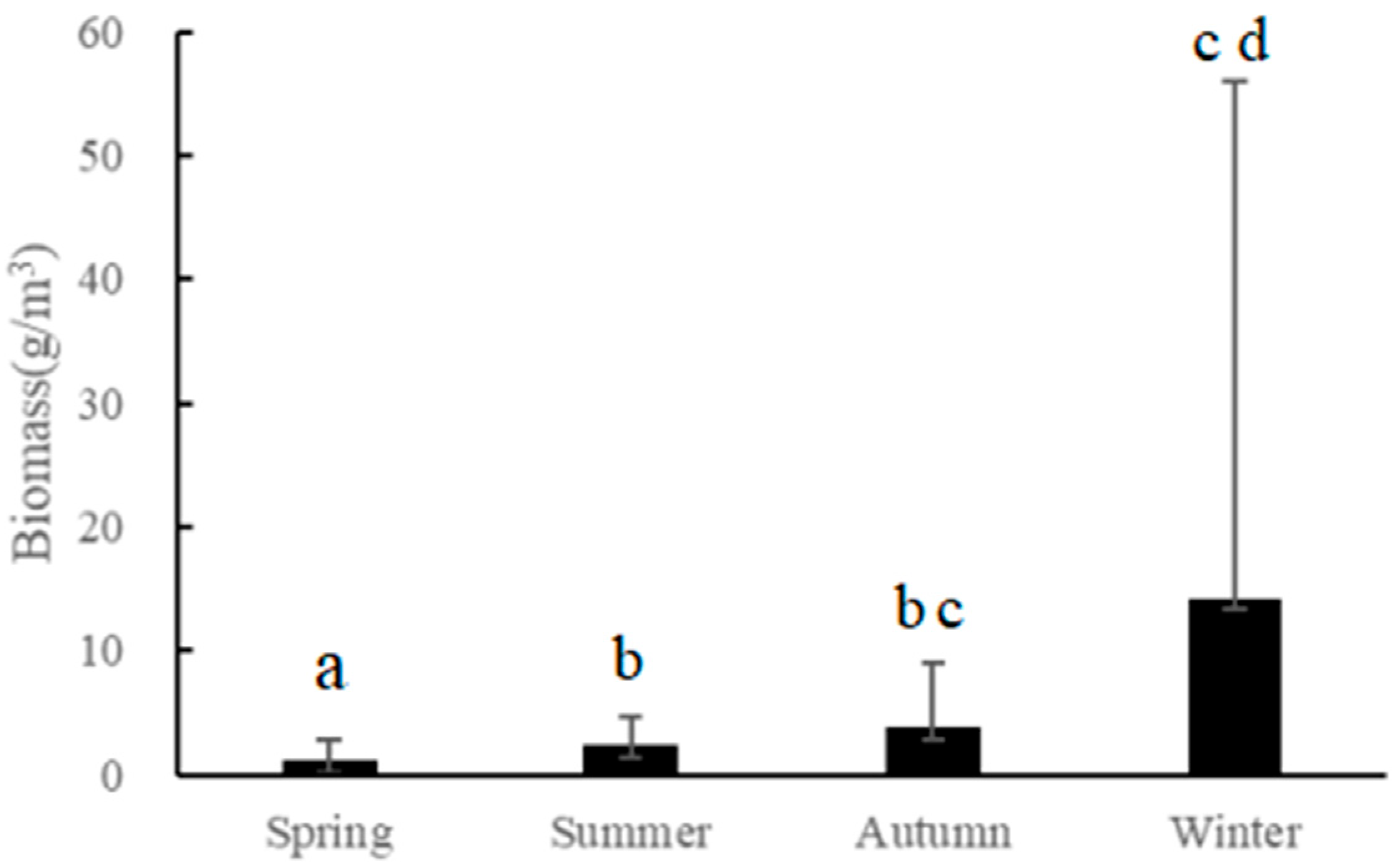
3.3. Acoustic Biomass
3.4. Catch Composition
4. Discussion
4.1. Comparison of Fish Density
4.2. Comparison of Fish Sizes
4.3. Comparison of Acoustic Biomass
4.4. Age Composition of Silver and Bighead Carp
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonilla, S.; Aguilera, A.; Aubriot, L.; Huszar, V.; Almanza, V.; Haakonsson, S.; Izaguirre, I.; Inés O’Farrell; Salazar, A.; Becker, V.; et al. Nutrients and not temperature are the key drivers for cyanobacterial biomass in the Americas. Harmful Algae 2023, 121, 102367. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Q.; Wang, J.; Wei, H.; Qian, J.; Zhang, Y.; Feng, K.; Chen, Q.; Yuan, J.; Liu, J.; et al. Evaluation of the control effect of bighead carp and silver carp on cyanobacterial blooms based on the analysis of differences in algal digestion processes. J. Clean. Prod. 2022, 375, 134106. [Google Scholar] [CrossRef]
- Yu, J.; Li, Y.; Liu, X.; Li, K.; Chen, F.; Gulati, R. The fate of cyanobacterial detritus in the food web of Lake Taihu: A mesocosm study using 13 C and 15 N labeling. Hydrobiologia 2013, 710, 39–46. [Google Scholar] [CrossRef]
- Xie, P.; Liu, J. Practical success of biomanipulation using filter-feeding fish to control cyanobacteria blooms: A synthesis of decades of research and application in a subtropical hypereutrophic lake. Sci. World. J. 2001, 1, 337–356. [Google Scholar] [CrossRef]
- Liu, J.; Xie, P. Unraveling the enigma of the disappearance of water bloom from the East lake (Lake Donghu) of Wuhan. Resour. Environ. Yangtze Basin. 1999, 8, 312–319. (In Chinese) [Google Scholar]
- Guo, L.G.; Wang, Q.; Xie, P.; Tao, M.; Zhang, J.; Niu, Y.; Ma, Z. A non-classical biomanipulation experiment in Gonghu Bay of Lake Taihu: Control of Microcystis blooms using silver and bighead carp. Aquac. Res. 2015, 46, 2211–2224. [Google Scholar] [CrossRef]
- Liu, Q.G.; Chen, Y.; Li, J.L.; Chen, L.Q. The food web structure and ecosystem properties of a filter-feeding carps dominated deep reservoir ecosystem. Ecol. Model. 2007, 203, 279–289. [Google Scholar] [CrossRef]
- Lin, Q.; Jiang, X.; Han, B.P.; Jeppesen, E. Does stocking of filter-feeding fish for production have a cascading effect on zooplankton and ecological state? A study of fourteen (sub) tropical Chinese reservoirs with contrasting nutrient concentrations. Hydrobiologia 2014, 736, 115–125. [Google Scholar] [CrossRef]
- Mao, Z.; Gu, X.; Cao, Y.; Zhang, M.; Zeng, Q.; Chen, H.; Shen, R.; Jeppesen, E. The role of top-down and bottom-up control for phytoplankton in a subtropical shallow eutrophic lake: Evidence based on long-term monitoring and modeling. Ecosystems 2020, 23, 1449–1463. [Google Scholar] [CrossRef]
- Yin, C.; He, W.; Guo, L.; Gong, L.; Yang, Y.; Yang, J.; Ni, L.; Chen, Y.; Jeppesen, E. Can top-down effects of planktivorous fish removal be used to mitigate cyanobacterial blooms in large subtropical highland lakes? Water Res. 2022, 218, 118483. [Google Scholar] [CrossRef]
- Koslow, J.A. The role of acoustics in ecosystem-based fishery management. ICES J. Mar. Sci. 2009, 66, 966–973. [Google Scholar] [CrossRef]
- Burwen, D.L.; Fleischman, S.J. Evaluation of side-aspect target strength and pulse width as potential hydroacoustic discriminators of fish species in rivers. Can. J. Fish. Aquat. Sci. 1998, 55, 2492–2502. [Google Scholar] [CrossRef]
- Lilja, J.; Keskinen, T.; Marjomäki, T.J.; Valkeajärvi, P.; Karjalainen, J. Upstream migration activity of cyprinids and percids in a channel, monitored by a horizontal split-beam echosounder. Aquat. Living. Resour. 2003, 16, 185–190. [Google Scholar] [CrossRef]
- Guo, A.; Yuan, J.; Chu, T.; Lian, Q. Hydroacoustic assessment of fish resources in three reservoirs: The effects of different management strategies on fish density, biomass and size. Fish. Res. 2019, 215, 90–96. [Google Scholar] [CrossRef]
- Rudstam, L.G.; Hansson, S.; Lindem, T.; Einhouse, D.W. Comparison of target strength distributions and fish densities obtained with split and single beam echo sounders. Fish. Res. 1999, 42, 207–214. [Google Scholar] [CrossRef]
- Lima, I.D.; Csatello, J.P. Distribution and abundance of South-west Atlantic anchovy spawners (Engraulis anchoita) in relation to oceanographic processes in the southern Brazilian shelf. Fish. Oceanogr. 1995, 4, 1–16. [Google Scholar] [CrossRef]
- Argent, D.G.; Kimmel, W.G. Efficiency and selectivity of gill nets for assessing fish community composition of large rivers. N. Am. J. Fish. Manag. 2005, 25, 1315–1320. [Google Scholar] [CrossRef]
- Simmonds, J.E.; Maclennan, D.N. Fisheries Acoustics: Theory and Practice, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Egerton, J.P.; Johnson, A.F.; Turner, J.; LeVay, L.; Mascareñas-Osorio, I.; Aburto-Oropeza, O. Hydroacoustics as a tool to examine the effects of Marine Protected Areas and habitat type on marine fish communities. Sci. Rep. 2018, 8, 47. [Google Scholar] [CrossRef]
- Wheeland, L.J.; Rose, G.A.; Shuter, B. Acoustic measures of lake community size spectra. Can. J. Fish. Aquat. Sci. 2016, 73, 557–564. [Google Scholar] [CrossRef]
- Godlewska, M.; Swierzowski, A. Hydroacoustical parameters of fish in reservoirs with contrasting levels of eutrophication. Aquat. Living. Resour. 2003, 16, 167–173. [Google Scholar] [CrossRef]
- Ona, E.; Mitson, R.B. Acoustic sampling and signal processing near the seabed: The deadzone revisited. ICES J. Mar. Sci. 1996, 4, 677–690. [Google Scholar] [CrossRef]
- Djemali, I.; Guillard, J.; Yule, D.L. Seasonal and diel effects on acoustic fish biomass estimates: Application to a shallow reservoir with untargeted common carp (Cyprinus carpio). Mar. Freshwater. Res. 2016, 68, 528–537. [Google Scholar] [CrossRef]
- Foote, K.G.; Knudsen, H.P.; Vestnes, G.; Maclennan, D.N.; Simmonds, E.J.N. Calibration of Acoustic Instruments for Fish Density Estimation: A Practical Guide. J. Acoust. Soc. Am. 1988, 83, 831–832. [Google Scholar]
- Aglen, A.J. Random errors of acoustic fish abundance estimates in relation to the survey grid density applied. Fao Fish. Rep. 1983, 300, 293–298. [Google Scholar]
- Godlewska, M.G.; Dlugoszewski, B.A.; Doroszczyk, L.; Jóźwik, A. The relationship between sampling intensity and sampling error—Empirical results from acoustic surveys in Polish vendace lakes. Fish. Res. 2009, 96, 17–22. [Google Scholar] [CrossRef]
- Higginbottom, I.; Woon, S.; Schneider, P. Hydroacoustic Data Processing for Standard Stock Assessment Using Echoview: Technical Manual; Myriax Software Pty Ltd. Publication: Hobart, Australia, 2008; p. 7085. [Google Scholar]
- Zhou, L.; Zeng, L.; Fu, D.; Xu, P.; Zeng, S.; Tang, Q.; Chen, Q.; Chen, L.; Li, G. Fish density increases from the upper to lower parts of the Pearl River Delta, China, and is influenced by tide, chlorophyll-a, water transparency, and water depth. Aquat. Ecol. 2016, 50, 59–74. [Google Scholar] [CrossRef]
- Mann, R.H.K. Observations on the age, growth, reproduction and food of the dace, Leuciscus leuciscus (L.), in two rivers in southern England. J. Fish. Biol. 1973, 5, 707–736. [Google Scholar] [CrossRef]
- Frouzova, J.; Kubecka, J.; Balk, H.; Frouz, J. Target strength of some European fish species and its dependence on fish body parameters. Fish. Res. 2005, 75, 86–96. [Google Scholar] [CrossRef]
- Ye, S.; Li, Z.; Feng, G.; Cao, W. Length-Weight Relationships for Thirty Fish Species in Lake Niushan, a Shallow Macrophytic Yangtze Lake in China. Asian. Fish. Sci. 2007, 20, 217–226. [Google Scholar]
- Zhang, H.; Wang, C.Y.; Du, H.; Wu, J.M.; Shen, L.; Wei, Q.W. Integrating Hydroacoustic and Optical Video Technologies to Identify the Riverbed Substrate at the Spawning Reach of Chinese Sturgeon. Adv. Mater. Res. 2014, 955–959, 1235–1240. [Google Scholar] [CrossRef]
- Pinkas, L.; Oliphant, M.S.; Iverson, I.L.K. Fish bulletion 152. Food habits of albacore, bluefin tuna, and bonito in California waters. Calif. Fish. Game 1971, 152, 1–105. [Google Scholar]
- Wang, Y.; Rhoads, B.L.; Wang, D. Assessment of the flow regime alterations in the middle reach of the Yangtze River associated with dam construction: Potential ecological implications. Hydrol. Process. 2016, 30, 3949–3966. [Google Scholar] [CrossRef]
- Lian, Y.; Ye, S.; Godlewska, M.; Huang, G.; Wang, J.; Chen, S.; Zhao, X.; Du, X.; Liu, J.; Li, Z. Diurnal, seasonal and inter-annual variability of fish density and distribution in the Three Gorges Reservoir (China) assessed with hydroacoustics. Limnol.—Ecol. Manag. Inland Waters 2017, 63, 97–106. [Google Scholar] [CrossRef]
- Hessler, T.M.; Chapman, D.C.; Paukert, C.P.; Jolley, J.C.; Byrne, M.E. Winter habitat selection and efficacy of telemetry to aid grass carp removal efforts in a large reservoir. N. Am. J. Fish. Manag. 2023, 43, 189–202. [Google Scholar] [CrossRef]
- Bartolini, T.; Butail, S.; Porfiri, M. Temperature influences sociality and activity of freshwater fish. Environ. Biol. Fish. 2015, 98, 825–832. [Google Scholar] [CrossRef]
- Fernando, C.H.; Holík, J. Fish in Reservoirs. Int. Rev. Der Gesamten Hydrobiol. Hydrogr. 1991, 76, 149–167. [Google Scholar] [CrossRef]
- Prchalová, M.; Kubečka, J.; Čech, M.; Frouzová, J.; Draštík, V.; Hohausová, E.; Jůza, T.; Kratochvíl, M.; Matěna, J.; Peterka, J.; et al. The effect of depth, distance from dam and habitat on spatial distribution of fish in an artificial reservoir. Ecol. Freshw. Fish. 2009, 18, 247–260. [Google Scholar] [CrossRef]
- Jonsson, N. Influence of water flow, water temperature and light on fish migration in rivers. Nordic. J. Freshw. Res. 1991, 66, 20–35. [Google Scholar]
- Luo, B.; Zhou, X.; Zhang, C.; Bao, J.; Mei, F.; Lian, Y.; Zhang, D.; Hu, S.; Guo, L.; Duan, M. Hydroacoustic survey on fish spatial distribution in the early impoundment stage of Yuwanghe Reservoir in southwest China. Front. Mar. Sci. 2023, 10, 1119411. [Google Scholar] [CrossRef]
- Yi, C.L.; Guo, L.; Ni, L.; Luo, C. Silver carp exhibited an enhanced ability of biomanipulation to control cyanobacteria bloom compared to bighead carp in hypereutrophic Lake Taihu mesocosms. Eco. Eng. 2016, 89, 7–13. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, P.; Huang, X. A review of nontraditional biomanipulation. Sci. World J. 2008, 8, 1184–1196. [Google Scholar] [CrossRef] [PubMed]
- Boswell, K.M.; Wilson, M.P.; Wilson, C.A. Hydroacoustics as a tool for assessing fish biomass and size distribution associated with discrete shallow water estuarine habitats in Louisiana. Estuar. Coast. 2007, 30, 607–617. [Google Scholar] [CrossRef]
- Rasmus, N.J.; Bo, L. Hydroacoustic ex situ target strength measurements on juvenile cod (Gadus morhua L.). ICES J. Mar. Sci. 1999, 56, 627–639. [Google Scholar]
- Kracker, L. Hydroacoustic Surveys: A Non-Destructive Approach to Monitoring Fish Distributions at National Marine Sanctuaries; Noaa Technical Memorandum Nos Nccos; National Ocean Service of the National Oceanic and Atmospheric Administration (NOAA): Washington, DC, USA, 2007. [Google Scholar]
- Lian, Y.; Huang, G.; Godlewska, M.; Cai, X.; Li, C.; Ye, S.; Liu, J.; Li, Z. Hydroacoustic estimates of fish biomass and spatial distributions in shallow lakes. J. Ocean. Limnol. 2018, 36, 587–597. [Google Scholar] [CrossRef]
- Punt, A.E.; Smith, D.C. Assessments of species in the Australian South East Fishery can be sensitive to the method used to convert from size-to age-composition data. Mar. Freshwater. Res. 2001, 52, 683–690. [Google Scholar] [CrossRef]
| Year | Released Species | Release Size (g/ind.) | Release Weight (×104 kg) | Released Number (×104 ind.) | Fish Catch (×104 kg) |
|---|---|---|---|---|---|
| 2017 | Bighead carp | 500–1500 | 1.75 | 2.3 | 4 |
| Bighead carp | 250–300 | 0.99 | 3.7 | ||
| Silver carp | 250–300 | 0.59 | 2.2 | ||
| 2018 | Bighead carp | 500–1000 | 1.76 | 2.3 | 4 |
| Silver carp | 500–1000 | 0.76 | 1.0 | ||
| 2019 | Bighead carp | 500–1000 | 1.96 | 2.6 | 9 |
| Bighead carp | 150–250 | 0.56 | 2.8 | ||
| Silver carp | 500–750 | 1.84 | 3.1 | ||
| Silver carp | 150–250 | 0.405 | 2.0 | ||
| 2017–2019 | Total | - | 10.615 | 22 | 17 |
| 2020 | Silver carp | 250–500 | 6.15 | 16.38 | 0 |
| Bighead carp | 250–500 | 1.22 | 3.25 | ||
| Silver carp | 100 | 2.44 | 24.4 | ||
| Total | - | 9.81 | 44.03 |
| Season | Fish Density (ind./1000 m3) | ||
|---|---|---|---|
| Mean (Mean ± S.E.) | Min | Max | |
| Spring | 3.33 ± 4.46 a | 0 | 27.2 |
| Summer | 75.24 ± 72.45 b | 6 | 285.4 |
| Autumn | 56.22 ± 63.26 bc | 7 | 264.3 |
| Winter | 20.37 ± 68.42 c | 0 | 331.4 |
| Survey Season | Target Strength (dB) | |||
|---|---|---|---|---|
| Minimum | Maximum | Mean | N | |
| Spring | −59.92 | −29.92 | −41.05 a | 262 |
| Summer | −59.93 | −31.04 | −44.66 b | 359 |
| Autumn | −59.98 | −29.58 | −45.55 b | 607 |
| Winter | −59.66 | −20.19 | −38.12 c | 277 |
| Order | Family | Species | Number of Samples | IRI |
|---|---|---|---|---|
| Cypriniformes | Cyprinidae | Hypophthalmichthys molitrix | 29 | 10,396 |
| Aristichthys nobilis | 6 | 6219 | ||
| Xenocypris davidi | 2 | 487 | ||
| Culter dabryi dabryi | 1 | 145 | ||
| Megalobrama amblycephala | 2 | 292 | ||
| Acheilognathus macropterus | 1 | 113 | ||
| Siluriformes | Eleotridae | Odontobutis potamophilus | 1 | 119 |
| Serranidae | Siniperca chuatsi | 2 | 318 | |
| Decapoda | Palaemonidae | Macrobrachium nipponense | 2 | 218 |
| Age | Number | Mean Body Length/cm | Mean Body Mass/g | |
|---|---|---|---|---|
| Silver carp | 1 | 28 | 25.89 ± 2.41 | 306.74 ± 80.18 |
| 2 | 1 | 41.50 | 974.20 | |
| Bighead carp | 1 | 5 | 22.10 ± 2.74 | 204.26 ± 89.10 |
| 6 | 1 | 69.00 | 10,400.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, A.; Lian, Q.; Sheng, P.; Zhang, A.; Yuan, J.; Iida, K. A Hydroacoustic Assessment of the Density, Size, and Biomass of Fish in a Freshwater Reservoir After Non-Classical Biomanipulation. Fishes 2025, 10, 274. https://doi.org/10.3390/fishes10060274
Guo A, Lian Q, Sheng P, Zhang A, Yuan J, Iida K. A Hydroacoustic Assessment of the Density, Size, and Biomass of Fish in a Freshwater Reservoir After Non-Classical Biomanipulation. Fishes. 2025; 10(6):274. https://doi.org/10.3390/fishes10060274
Chicago/Turabian StyleGuo, Aihuan, Qingping Lian, Pengcheng Sheng, Aiju Zhang, Julin Yuan, and Kohji Iida. 2025. "A Hydroacoustic Assessment of the Density, Size, and Biomass of Fish in a Freshwater Reservoir After Non-Classical Biomanipulation" Fishes 10, no. 6: 274. https://doi.org/10.3390/fishes10060274
APA StyleGuo, A., Lian, Q., Sheng, P., Zhang, A., Yuan, J., & Iida, K. (2025). A Hydroacoustic Assessment of the Density, Size, and Biomass of Fish in a Freshwater Reservoir After Non-Classical Biomanipulation. Fishes, 10(6), 274. https://doi.org/10.3390/fishes10060274






