Influence of Eucommia ulmoides Extract on the Growth, Glucose Metabolism, and Antioxidant Capacity of Largemouth Bass (Micropterus salmoides)
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Diets
2.2. Experimental Fish and Management
2.3. Sample Collection
2.4. Growth Index Test
2.5. Body Composition and Biochemical Analysis
2.6. Real-Time PCR Analysis
2.7. Statistical Analysis
3. Results
3.1. Whole-Fish Growth Index and Body Composition Analysis
3.2. Analysis of Antioxidant Indexes in Intestine
3.3. Plasma Biochemical Analysis
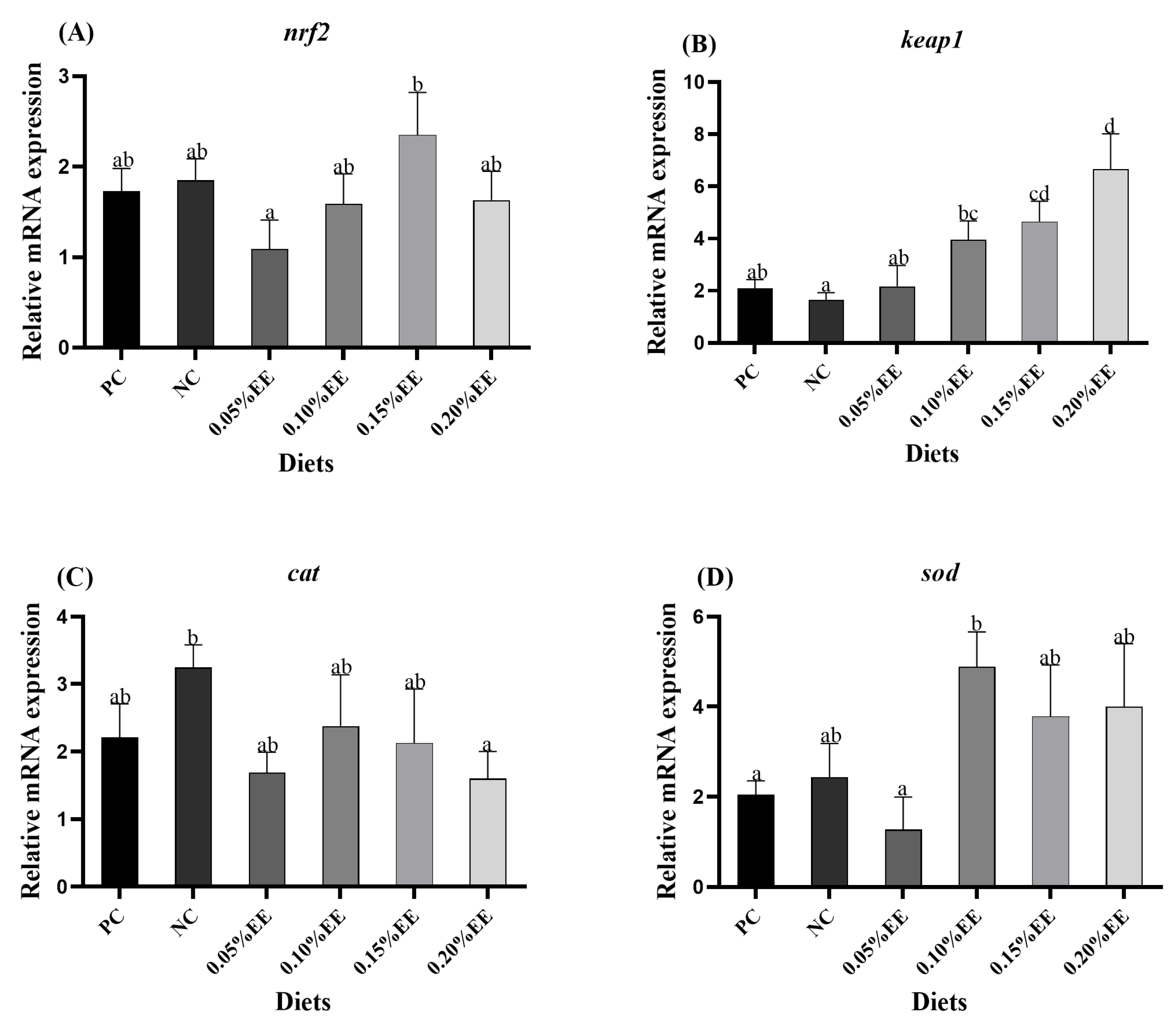
3.4. Analysis of Antioxidant-Related Pathways
3.5. Expression Analysis of Glucose Metabolism-Related Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, X.; Steele, J.C.; Meng, X.Z. Usage, residue, and human health risk of antibiotics in Chinese aquaculture: A review. Environ. Pollut. 2017, 223, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Shi, Y.; Gao, L.; Liu, J.; Cai, Y. Occurrence of antibiotics in water, sediments, aquatic plants, and animals from Baiyangdian Lake in North China. Chemosphere 2012, 89, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Nakamichi, S.; Habibullah-Al-Mamun, M.; Tani, K.; Masunaga, S.; Matsuda, H. Occurrence, distribution, ecological and resistance risks of antibiotics in surface water of finfish and shellfish aquaculture in Bangladesh. Chemosphere 2017, 188, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.R.; Davoodi, H. Herbal plants and their derivatives as growth and health promoters in animal nutrition. Vet. Res. Commun. 2011, 35, 169–180. [Google Scholar] [CrossRef]
- Gadde, U.; Kim, W.H.; Oh, S.T.; Lillehoj, H.S. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: A review. Anim. Health Res. Rev. 2017, 18, 26–45. [Google Scholar] [CrossRef]
- Tan, X.; Sun, Z.; Liu, Q.; Ye, H.; Zou, C.; Ye, C.; Lin, H.; Wang, A. Effects of dietary ginkgo biloba leaf extract on growth performance, plasma biochemical parameters, fish composition, immune responses, liver histology, and immune and apoptosis-related genes expression of hybrid grouper (Epinephelus lanceolatus♂× Epinephelus fuscoguttatus♀) fed high lipid diets. Fish Shellfish Immunol. 2018, 72, 399–409. [Google Scholar] [PubMed]
- Shabana, M.S.; Karthika, M.; Ramasubramanian, V. Effect of dietary Citrus sinensis peel extract on growth performance, digestive enzyme activity, muscle biochemical composition, and metabolic enzyme status of the freshwater fish, Catla catla. J. Basic Appl. Zool. 2019, 80, 51. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Khodadadian Zou, H.; Paknejad, H.; Ahmadifar, E.; Van Doan, H. Non-specific immune responses and intestinal immunity of common carp (Cyprinus carpio) fed Jujube (Ziziphus jujube) fruit extract. Aquac. Res. 2018, 49, 2995–3003. [Google Scholar] [CrossRef]
- He, X.; Wang, J.; Li, M.; Hao, D.; Yang, Y.; Zhang, C.; He, R.; Tao, R. Eucommia ulmoides Oliv.: Ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 2014, 151, 78–92. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.E.; Liu, G.; Oladele, O.A.; Rahu, N.; Tossou, M.C.; Yin, Y. Health-promoting properties of Eucommia ulmoides: A review. Evid.-Based Complement. Altern. Med. 2016, 2016, 5202908. [Google Scholar] [CrossRef]
- Huang, D.; Zhu, J.; Zhang, L.; Ge, X.; Ren, M.; Liang, H. Dietary supplementation with Eucommia ulmoides leaf extract improved the intestinal antioxidant capacity, immune response, and disease resistance against Streptococcus agalactiae in genetically improved farmed tilapia (GIFT; Oreochromis niloticus). Antioxidants 2022, 11, 1800. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, C.; Wang, X.; Zhou, H.; Mai, K.; He, G. The effects of dietary Eucommia ulmoides Oliver on growth, feed utilization, antioxidant activity and immune responses of turbot (Scophthalmus maximus L.). Aquac. Nutr. 2019, 25, 367–376. [Google Scholar] [CrossRef]
- Lu, Y.-P.; Zheng, P.-H.; Xu, J.-R.; Cao, Y.-L.; Li, J.-T.; Hao, C.-G.; Zhang, Z.-L.; Xian, J.-A.; Zhang, X.-X.; Wang, A.-L. Effects of Dietary Eucommia ulmoides Leaf Extract on Growth, Muscle Composition, Hepatopancreas Histology, Immune Responses and Microcystin-LR Resistance of Juvenile Red Claw Crayfish (Cherax quadricarinatus). Fishes 2023, 8, 20. [Google Scholar] [CrossRef]
- Yang, H.; Xu, Z.; Li, X.Q.; Tan, S.M.; Cheng, Z.; Leng, X.J. Influences of dietary Eucommia ulmoides extract on growth, flesh quality, antioxidant capacity and collagen-related genes expression in grass carp (Ctenopharyngodon idellus). Anim. Feed Sci. Technol. 2021, 277, 114965. [Google Scholar] [CrossRef]
- Yang, H.; Xu, Z.; Tan, S.; Zhang, C.; Li, X.; Leng, X. In vitro effects of Eucommia ulmoides and its active components on the growth, lipid metabolism and collagen metabolism of grass carp (Ctenopharyngodon idellus) hepatocyte and intramuscular fibroblast. J. Fish Biol. 2022, 101, 597–612. [Google Scholar] [CrossRef]
- Yang, H.; Xu, Z.; Xu, X.; Rahman, M.M.; Li, X.; Leng, X. Transcriptomic and biochemical analyses revealed the improved growth, lipid metabolism, and flesh quality of grass carp (Ctenopharyngodon idellus) by dietary Eucommia ulmoides bark and leaf supplementation. J. Anim. Sci. 2022, 100, skac250. [Google Scholar] [CrossRef]
- Bai, J.; Li, S. Development of largemouth bass (Micropterus salmoides) culture. In Aquaculture in China: Success Stories and Modern Trends; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; pp. 421–429. [Google Scholar]
- Zhang, B.Y.; Yang, H.L.; Yuan, Z.H.; Sun, X.O.; Yin, J.H.; Xu, J.J.; Cai, G.H.; Sun, Y.Z. Commensal microorganisms ameliorate the adverse effects of high wheat starch diet on the growth performance, glucose and lipid metabolisms in juvenile largemouth bass, Micropterus salmoides. Aquac. Rep. 2024, 39, 102452. [Google Scholar] [CrossRef]
- Ren, M.; Liao, Y.; Xie, J.; Liu, B.; Zhou, Q.; Ge, X.; Cui, H.; Pan, L.; Chen, R. Dietary arginine requirement of juvenile blunt snout bream, Megalobrama amblycephala. Aquaculture 2013, 414, 229–234. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed.; Association of Official Analytical Chemists, Inc.: Arlington, VA, USA, 2003. [Google Scholar]
- Stone, D.A.; Gaylord, T.G.; Johansen, K.A.; Overturf, K.; Sealey, W.M.; Hardy, R.W. Evaluation of the effects of repeated fecal collection by manual stripping on the plasma cortisol levels, TNF-α gene expression, and digestibility and availability of nutrients from hydrolyzed poultry and egg meal by rainbow trout, Oncorhynchus mykiss (Walbaum). Aquaculture 2008, 275, 250–259. [Google Scholar]
- Sun, W.T.; Li, X.Q.; Xu, H.B.; Chen, J.N.; Xu, X.Y.; Leng, X.J. Effects of dietary chlorogenic acid on growth, flesh quality and serum biochemical indices of grass carp (Ctenopharyngodon idella). Aquac. Nutr. 2017, 23, 1254–1263. [Google Scholar] [CrossRef]
- Huang, W.; Yao, C.; Liu, Y.; Xu, N.; Yin, Z.; Xu, W.; Miao, Y.; Mai, K.; Ai, Q. Effects of dietary Eucommia ulmoides leaf extract on growth performance, expression of feeding-related genes, activities of digestive enzymes, antioxidant capacity, immunity and cytokines expression of large yellow croaker (Larimichthys crocea) larvae. Br. J. Nutr. 2022, 128, 1711–1719. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.W.; Mendis, W.R.H.; Jeong, B.C.; Lim, T.J.; Ahn, J.C.; Jung, S.J.; Kang, S.Y. Optimization of Eucommia ulmoides Oliver bark extracts by Box-Behnken design and their effects on growth performance, plasma biochemical and immunological parameters, and antioxidant enzyme-and inflammation-related genes expression in Japanese eel, Anguilla japonica. Aquac. Rep. 2022, 26, 101320. [Google Scholar]
- Sun, W.T.; He, M.; Xu, X.Y.; Li, X.Q.; Pan, W.Q.; Leng, X.J. Comparison study of three compounds in Eucommia ulmoides on growth, flesh quality of grass carp (Ctenopharyngodon idella). Aquac. Nutr. 2019, 25, 906–916. [Google Scholar] [CrossRef]
- Li, J.; Xu, W.; Lai, W.; Kong, A.; Ai, Q. Effect of dietary methionine on growth performance, lipid metabolism and antioxidant capacity of large yellow croaker (Larimichthys crocea) fed with high lipid diets. Aquaculture 2021, 536, 736388. [Google Scholar] [CrossRef]
- Luo, A.; Song, C.; Wu, X.; Li, M.; Shi, C.; Wu, S.; Wei, L.; Peng, F.; Mo, P. Effects of compound plant extracts on growth performance, antioxidant capacity, and histomorphology of liver and intestine of rice field eel (Monopterus albus). J. World Aquac. Soc. 2024, 55, e13065. [Google Scholar] [CrossRef]
- Yilmaz, S.; Ergün, S.; Yıgıt, M. Effects of dietary FARMARIN® XP supplement on immunological responses and disease resistance of rainbow trout (Oncorhynchus mykiss). Aquaculture 2018, 496, 211–220. [Google Scholar] [CrossRef]
- Mensinger, A.F.; Walsh, P.J.; Hanlon, R.T. Blood biochemistry of the oyster toadfish. J. Aquat. Anim. Health 2005, 17, 170–176. [Google Scholar] [CrossRef]
- Wagner, T.; Congleton, J.L. Blood chemistry correlates of nutritional condition, tissue damage, and stress in migrating juvenile chinook salmon (Oncorhynchus tshawytscha). Can. J. Fish. Aquat. Sci. 2004, 61, 1066–1074. [Google Scholar] [CrossRef]
- Coz-Rakovac, R.; Strunjak-Perovic1, I.; Hacmanjek, M.; Popovic, N.T.; Lipej, Z.; Sostaric, B. Blood chemistry and histological properties of wild and cultured sea bass (Dicentrarchus labrax) in the North Adriatic Sea. Vet. Res. Commun. 2005, 29, 677–687. [Google Scholar] [CrossRef]
- Metón, I.; Mediavilla, D.; Caseras, A.; Cantó, E.; Fernández, F.; Baanante, I.V. Effect of diet composition and ration size on key enzyme activities of glycolysis–gluconeogenesis, the pentose phosphate pathway and amino acid metabolism in liver of gilthead sea bream (Sparus aurata). Br. J. Nutr. 1999, 82, 223–232. [Google Scholar] [CrossRef]
- Kumar, S.; Sahu, N.P.; Pal, A.K.; Choudhury, D.; Yengkokpam, S.; Mukherjee, S.C. Effect of dietary carbohydrate on haematology, respiratory burst activity and histological changes in L. rohita juveniles. Fish Shellfish Immunol. 2005, 19, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wang, D.; Zhang, H.; Zhang, Y.; Wang, J.; Qi, R.; Yang, J.; Sheng, H.; Xu, Y.; Li, M. Rapid responses of adipocytes to iron overload increase serum TG level by decreasing adiponectin. J. Cell. Physiol. 2021, 236, 7544–7553. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, F.; Guo, Q.; Wang, W.; Zhang, L.; Yin, Y.; Gong, S.; Han, M.; Yin, Y. Effects of Different Supplemental Levels of Eucommia ulmoides Leaf Extract in the Diet on Carcass Traits and Lipid Metabolism in Growing–Finishing Pigs. Front. Vet. Sci. 2022, 8, 828165. [Google Scholar] [CrossRef]
- Hirata, T.; Ikeda, T.; Fujikawa, T.; Nishibe, S. The chemistry and bioactivity of Eucommia ulmoides Oliver leaves. Stud. Nat. Prod. Chem. 2014, 41, 225–260. [Google Scholar]
- Hirata, T.; Kobayashi, T.; Wada, A.; Ueda, T.; Fujikawa, T.; Miyashita, H.; Ikeda, T.; Tsukamoto, S.; Nohara, T. Anti-obesity compounds in green leaves of Eucommia ulmoides. Bioorganic Med. Chem. Lett. 2011, 21, 1786–1791. [Google Scholar] [CrossRef]
- Gharaei, A.; Ghaffari, M.; Keyvanshokooh, S.; Akrami, R. Changes in metabolic enzymes, cortisol and glucose concentrations of Beluga (Huso huso) exposed to dietary methylmercury. Fish Physiol. Biochem. 2011, 37, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Wang, G.; Zhao, H.; Wang, Y.; Mo, W.; Wu, H.; Huang, Y. Effect of high level of carbohydrate and supplementation of condensed tannins on growth performance, serum metabolites, antioxidant and immune response, and hepatic glycometabolism gene expression of Lateolabrax japonicus. Aquac. Rep. 2020, 18, 100515. [Google Scholar] [CrossRef]
- Enes, P.; Sanchez-Gurmaches, J.; Navarro, I.; Gutiérrez, J.; Oliva-Teles, A. Role of insulin and IGF-I on the regulation of glucose metabolism in European sea bass (Dicentrarchus labrax) fed with different dietary carbohydrate levels. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2010, 157, 346–353. [Google Scholar] [CrossRef]
- Borrebaek, B.; Waagbø, R.; Christophersen, B.; Tranulis, M.A.; Hemre, G.I. Adaptable hexokinase with low affinity for glucose in the liver of Atlantic salmon (Salmo salar). Comp. Biochem. Physiol. Part B Comp. Biochem. 1993, 106, 833–836. [Google Scholar] [CrossRef]
- Enes, P.; Peres, H.; Couto, A.; Oliva-Teles, A. Growth performance and metabolic utilization of diets including starch, dextrin, maltose or glucose as carbohydrate source by gilthead sea bream (Sparus aurata) juveniles. Fish Physiol. Biochem. 2010, 36, 903–910. [Google Scholar] [CrossRef]
- Jin, J.; Médale, F.; Kamalam, B.S.; Aguirre, P.; Véron, V.; Panserat, S. Comparison of glucose and lipid metabolic gene expressions between fat and lean lines of rainbow trout after a glucose load. PLoS ONE 2014, 9, e105548. [Google Scholar] [CrossRef] [PubMed]
- Hemre, G.I.; Mommsen, T.P.; Krogdahl, Å. Carbohydrates in fish nutrition: Effects on growth, glucose metabolism and hepatic enzymes. Aquac. Nutr. 2002, 8, 175–194. [Google Scholar] [CrossRef]
- Zhao, L.; Liao, L.; Tang, X.; Liang, J.; Liu, Q.; Luo, W.; Adan, A.; Luo, J.; Li, Z.; Yang, S.; et al. High-carbohydrate diet altered conversion of metabolites, and deteriorated health in juvenile largemouth bass. Aquaculture 2022, 549, 737816. [Google Scholar] [CrossRef]
- de Sotillo, D.V.R.; Hadley, M. Chlorogenic acid modifies plasma and liver concentrations of: Cholesterol, triacylglycerol, and minerals in (fa/fa) Zucker rats. J. Nutr. Biochem. 2002, 13, 717–726. [Google Scholar] [CrossRef]
- Kim, H.Y.; Moon, B.H.; Lee, H.J.; Choi, D.H. Flavonol glycosides from the leaves of Eucommia ulmoides O. with glycation inhibitory activity. J. Ethnopharmacol. 2004, 93, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Xiao, W.W.; Liu, Y.; Jiang, J.; Hu, K.; Jiang, W.D.; Li, S.H.; Zhao, X.Q. Methionine hydroxy analogue prevents oxidative damage and improves antioxidant status of intestine and hepatopancreas for juvenile Jian carp (Cyprinus carpio var. Jian). Aquac. Nutr. 2011, 17, 595–604. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Zhang, F.L.; Hao, Q.; Zhang, Q.S.; Lv, H.Y.; Yang, Y.L.; Zhang, Z.; Zhou, Z.G. Influences of dietary Eucommia ulmoides leaf extract on the hepatic lipid metabolism, inflammation response, intestinal antioxidant capacity, intestinal microbiota, and disease resistance of the channel catfish (Ictalurus punctatus). Fish Shellfish Immunol. 2022, 123, 75–84. [Google Scholar] [CrossRef]
- Graça-Souza, A.V.; Maya-Monteiro, C.; Paiva-Silva, G.O.; Braz, G.R.; Paes, M.C.; Sorgine, M.H.; Oliveira, M.F.; Oliveira, P.L. Adaptations against heme toxicity in blood-feeding arthropods. Insect Biochem. Mol. Biol. 2006, 36, 322–335. [Google Scholar] [CrossRef]
- Parihar, M.S.; Javeri, T.; Hemnani, T.; Dubey, A.K.; Prakash, P. Responses of superoxide dismutase, glutathione peroxidase and reduced glutathione antioxidant defenses in gills of the freshwater catfish (Heteropneustes fossilis) to short-term elevated temperature. J. Therm. Biol. 1997, 22, 151–156. [Google Scholar] [CrossRef]
- Takamura, C.; Hirata, T.; Ueda, T.; Ono, M.; Miyashita, H.; Ikeda, T.; Nohara, T. Iridoids from the green leaves of Eucommia ulmoides. J. Nat. Prod. 2007, 70, 1312–1316. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef] [PubMed]
- Dragland, S.; Senoo, H.; Wake, K.; Holte, K.; Blomhoff, R. Several culinary and medicinal herbs are important sources of dietary antioxidants. J. Nutr. 2003, 133, 1286–1290. [Google Scholar] [CrossRef] [PubMed]
- Akinmoladun, A.C.; Obuotor, E.M.; Farombi, E.O. Evaluation of antioxidant and free radical scavenging capacities of some Nigerian indigenous medicinal plants. J. Med. Food 2010, 13, 444–451. [Google Scholar] [CrossRef]
- Hendra, R.; Ahmad, S.; Oskoueian, E.; Sukari, A.; Shukor, M.Y. Antioxidant, anti-inflammatory and cytotoxicity of Phaleria macrocarpa (Boerl.) Scheff fruit. BMC Complement. Altern. Med. 2011, 11, 110. [Google Scholar] [CrossRef]
- Park, S.A.; Choi, M.S.; Jung, U.J.; Kim, M.J.; Kim, D.J.; Park, H.M.; Park, Y.B.; Lee, M.K. Eucommia ulmoides Oliver leaf extract increases endogenous antioxidant activity in type 2 diabetic mice. J. Med. Food 2006, 9, 474–479. [Google Scholar] [CrossRef]


| Ingredients | PC | NC | 0.05%EE | 0.10%EE | 0.15%EE | 0.20%EE |
|---|---|---|---|---|---|---|
| Fish meal 1 | 47.00 | 47.00 | 47.00 | 47.00 | 47.00 | 47.00 |
| Blood meal 1 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 |
| Soybean meal 1 | 14.00 | 14.00 | 14.00 | 14.00 | 14.00 | 14.00 |
| Wheat flour 1 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 |
| Tapioca starch | 6.00 | 4.00 | 6.00 | 6.00 | 6.00 | 6.00 |
| Rice bran | 7.00 | 7.00 | 7.00 | 7.00 | 7.00 | 7.00 |
| Microcrystalline cellulose | 3.48 | 5.48 | 3.43 | 3.38 | 3.33 | 3.28 |
| Shrimp paste | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Fish oil | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Vitamin premix 2 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Mineral premix 2 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Calcium dihydrogen phosphate | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Soybean oil | 4.52 | 4.52 | 4.52 | 4.52 | 4.52 | 4.52 |
| Choline chloride | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| EE(%) 3 | 0.00 | 0.00 | 0.05 | 0.10 | 0.15 | 0.20 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Analyzed proximate composition | ||||||
| Dry matter (%) | 90.40 | 90.44 | 90.02 | 90.20 | 90.24 | 90.15 |
| Crude protein (%) | 47.10 | 47.00 | 47.08 | 47.15 | 47.09 | 47.12 |
| Crude lipid (%) | 12.10 | 12.13 | 12.15 | 12.18 | 12.09 | 12.05 |
| Crude ash (%) | 10.03 | 10.11 | 10.06 | 10.14 | 10.10 | 10.05 |
| Crude fibre (%) NFE (%) 4 | 6.53 14.64 | 8.42 12.78 | 6.48 14.25 | 6.42 14.31 | 6.38 14.58 | 6.33 14.60 |
| Genes | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Accession No. |
|---|---|---|---|
| nrf2 | AGAGACATTCGCCGTAGA | TCGCAGTAGAGCAATCCT | NM_212855.2 |
| cat | CTATGGCTCTCACACCTTC | TCCTCTACTGGCAGATTCT | MK614708.1 |
| sod | GGTGTTTAAAGCCGTTTGTGTT | CCTCTGATTTCTCCTGTCACCT | XM_038708943.1 |
| pk | CACGCAACACTGGCATCATC | TCGAAGCTCTCACATGCCTC | MT431526.1 |
| gk | CCCTTGTGGGCAGGAGAAAA | ACAACTGAGTCCTCCTTGCG | XP_023260296.1 |
| pepck | GGCAAAACCTGGAAGCAAGG | ATAATGGCGTCGATGGGGAC | MT431525.1 |
| keap1 | CGTACGTCCAGGCCTTACTC | TGACGGAAATAACCCCCTGC | XP_018520553.1 |
| gapdh | ACTGTCACTCCTCCATCTT | CACGGTTGCTGTATCCAA | AZA04761.1 |
| Groups | IBW (g) | FBW (g) | FCR | WGR (%) | SGR (%/day) | SR (%) |
|---|---|---|---|---|---|---|
| PC | 36.95 ± 0.06 | 131.30 ± 2.79 ab | 1.52 ± 0.02 d | 255.4 ± 7.99 ab | 2.11 ± 0.04 ab | 96.7 ± 1.67 |
| NC | 37.07 ± 0.07 | 129.23 ± 1.24 a | 1.50 ± 0.03 cd | 248.7 ± 4.00 a | 2.08 ± 0.02 a | 98.3 ± 1.67 |
| 0.05%EE | 37.05 ± 0.10 | 128.66 ± 0.68 a | 1.53 ± 0.03 d | 247.3 ± 2.38 a | 2.07 ± 0.01 a | 96.7 ± 1.67 |
| 0.10%EE | 36.92 ± 0.09 | 135.26 ± 0.56 b | 1.45 ± 0.01 bc | 266.4 ± 1.48 b | 2.16 ± 0.01 b | 95.0 ± 0.00 |
| 0.15%EE | 37.02 ± 0.10 | 146.89 ± 1.45 c | 1.37 ± 0.01 a | 296.8 ± 3.05 c | 2.30 ± 0.01 c | 98.3 ± 1.67 |
| 0.20%EE | 37.02 ± 0.04 | 145.10 ± 1.18 c | 1.43 ± 0.01 ab | 292.0 ± 2.71 c | 2.28 ± 0.01 c | 96.7 ± 1.67 |
| Groups | Moisture (%) | Lipid (%) | Ash (%) | Protein (%) |
|---|---|---|---|---|
| PC | 69.73 ± 0.16 ab | 7.80 ± 0.72 | 4.46 ± 0.14 | 19.00 ± 0.18 |
| NC | 70.06 ± 0.49 ab | 6.97 ± 0.70 | 4.63 ± 0.16 | 17.69 ± 0.09 |
| 0.05%EE | 69.27 ± 0.47 ab | 7.39 ± 0.51 | 4.89 ± 0.16 | 18.14 ± 0.32 |
| 0.10%EE | 69.33 ± 0.11 ab | 7.24 ± 0.86 | 4.59 ± 0.14 | 18.57 ± 0.08 |
| 0.15%EE | 70.76 ± 0.11 a | 6.35 ± 0.08 | 4.70 ± 0.22 | 18.85 ± 0.36 |
| 0.20%EE | 68.46 ± 0.31 b | 6.78 ± 0.99 | 4.65 ± 0.15 | 18.26 ± 0.11 |
| Groups | CAT (U/mgprot) | SOD (U/mgprot) | GSH (umol/mL) |
|---|---|---|---|
| PC | 328.87 ± 77.64 a | 4.27 ± 0.25 | 30.74 ± 17.44 |
| NC | 292.64 ± 55.02 a | 4.66 ± 0.22 | 25.98 ± 7.58 |
| 0.05%EE | 424.24 ± 65.03 a | 4.84 ± 0.31 | 20.43 ± 10.79 |
| 0.10%EE | 239.95 ± 29.40 a | 4.64 ± 0.28 | 27.60 ± 13.10 |
| 0.15%EE | 1347.94 ± 72.10 b | 5.00 ± 0.12 | 23.22 ± 17.72 |
| 0.20%EE | 380.69 ± 78.55 a | 4.89 ± 0.34 | 25.25 ± 11.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, S.; Huang, D.; Ren, M.; Gu, J.; Liang, H. Influence of Eucommia ulmoides Extract on the Growth, Glucose Metabolism, and Antioxidant Capacity of Largemouth Bass (Micropterus salmoides). Fishes 2025, 10, 269. https://doi.org/10.3390/fishes10060269
Zhao S, Huang D, Ren M, Gu J, Liang H. Influence of Eucommia ulmoides Extract on the Growth, Glucose Metabolism, and Antioxidant Capacity of Largemouth Bass (Micropterus salmoides). Fishes. 2025; 10(6):269. https://doi.org/10.3390/fishes10060269
Chicago/Turabian StyleZhao, Shengqi, Dongyu Huang, Mingchun Ren, Jiaze Gu, and Hualiang Liang. 2025. "Influence of Eucommia ulmoides Extract on the Growth, Glucose Metabolism, and Antioxidant Capacity of Largemouth Bass (Micropterus salmoides)" Fishes 10, no. 6: 269. https://doi.org/10.3390/fishes10060269
APA StyleZhao, S., Huang, D., Ren, M., Gu, J., & Liang, H. (2025). Influence of Eucommia ulmoides Extract on the Growth, Glucose Metabolism, and Antioxidant Capacity of Largemouth Bass (Micropterus salmoides). Fishes, 10(6), 269. https://doi.org/10.3390/fishes10060269








