Characterization of Immune Response Against Mycobacterium marinum Infection in Coho Salmon (Oncorhynchus kisutch)
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Challenge and Sample Collection
2.2. RNA Extraction, Library Construction, and Sequencing
2.3. Basic Bioinformatics Analysis
2.4. GO and Pathway Enrichment and Analysis
2.5. qPCR Analysis of Immune-Related Genes
2.6. Quantitative PCR for Determining Mycobacterial Loads
3. Results
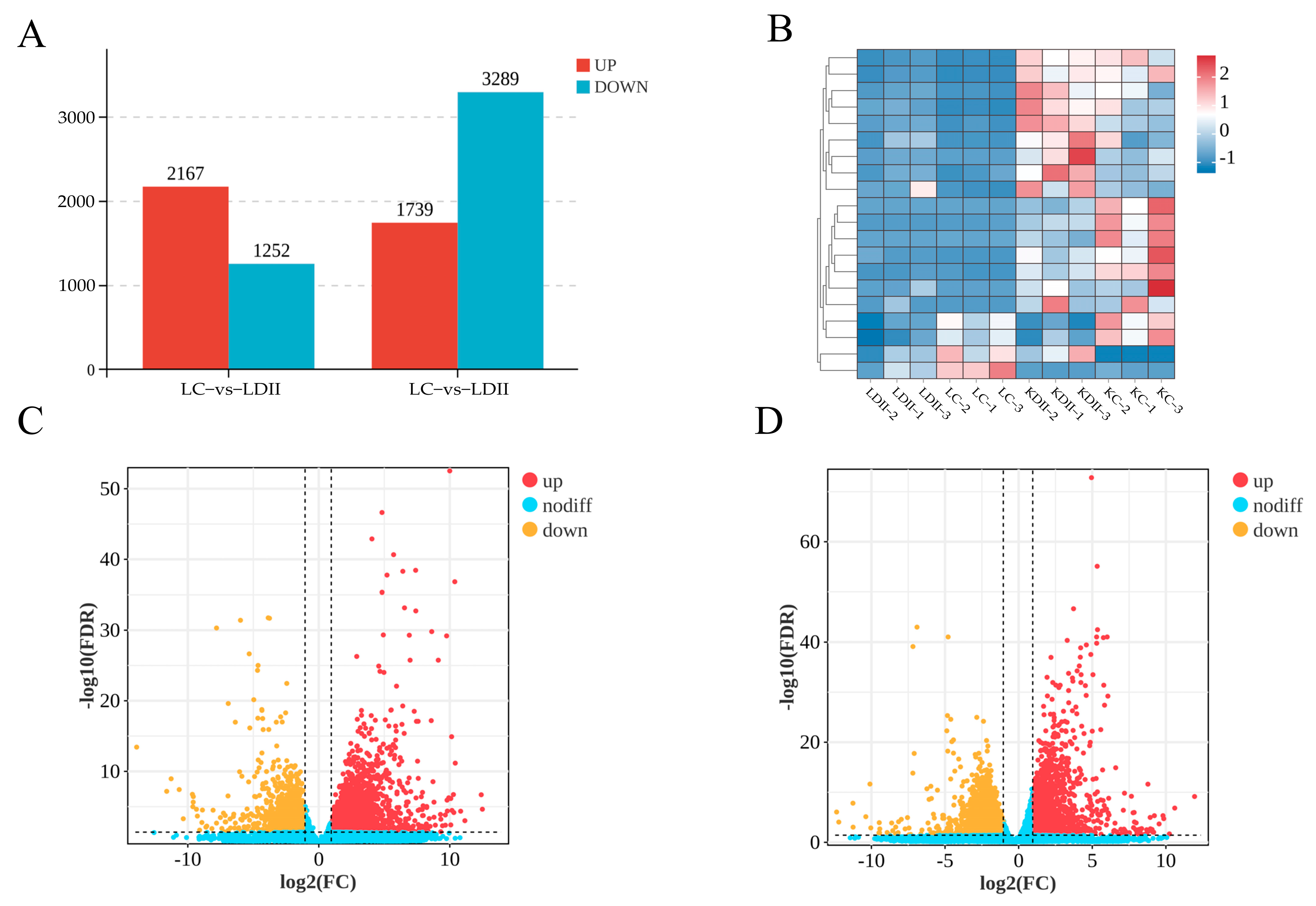
3.1. Analysis of Sample Relationships and Differentially Expressed Genes
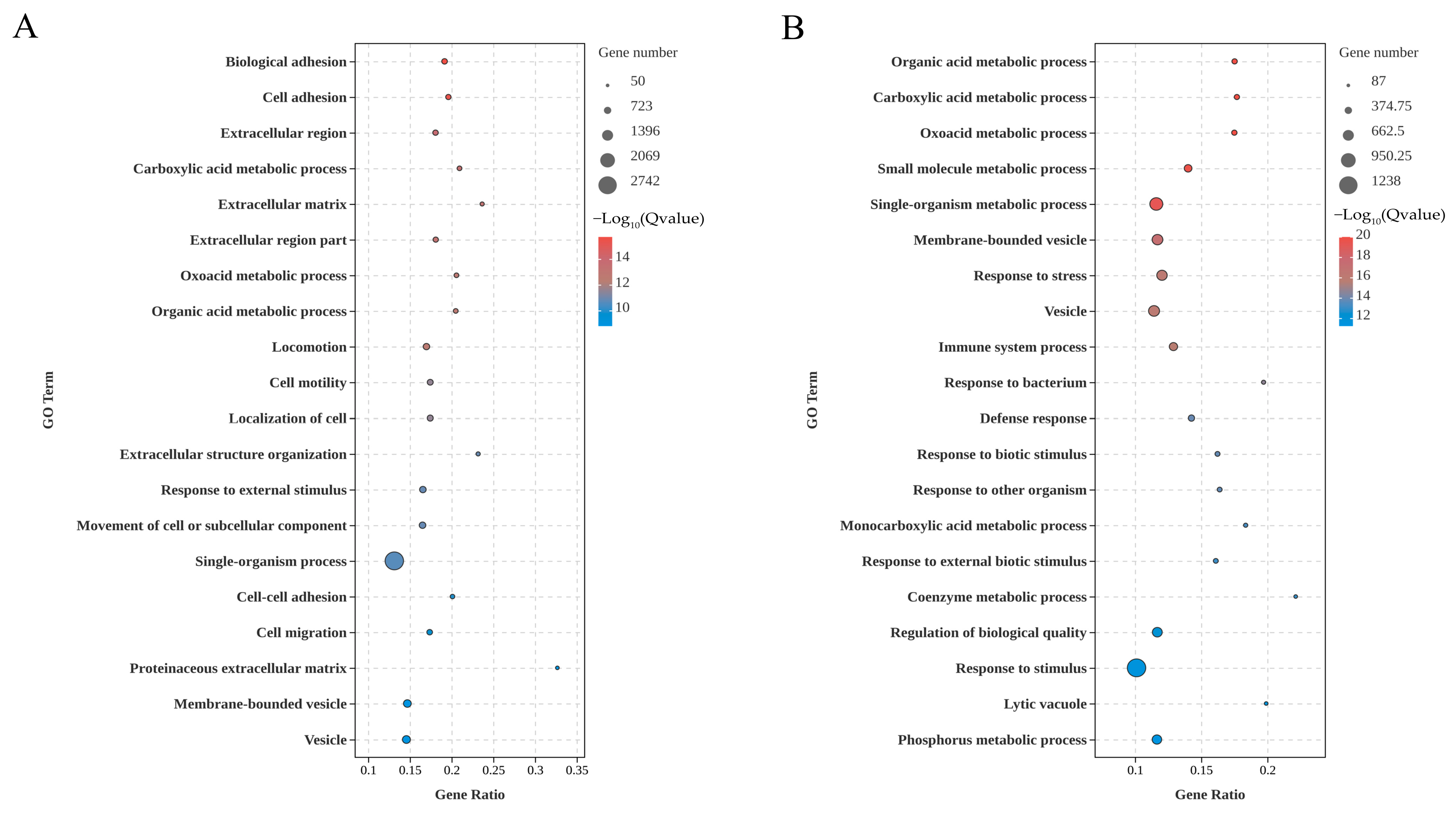
3.2. GO Enrichment of the DEGs in Kidney and Liver
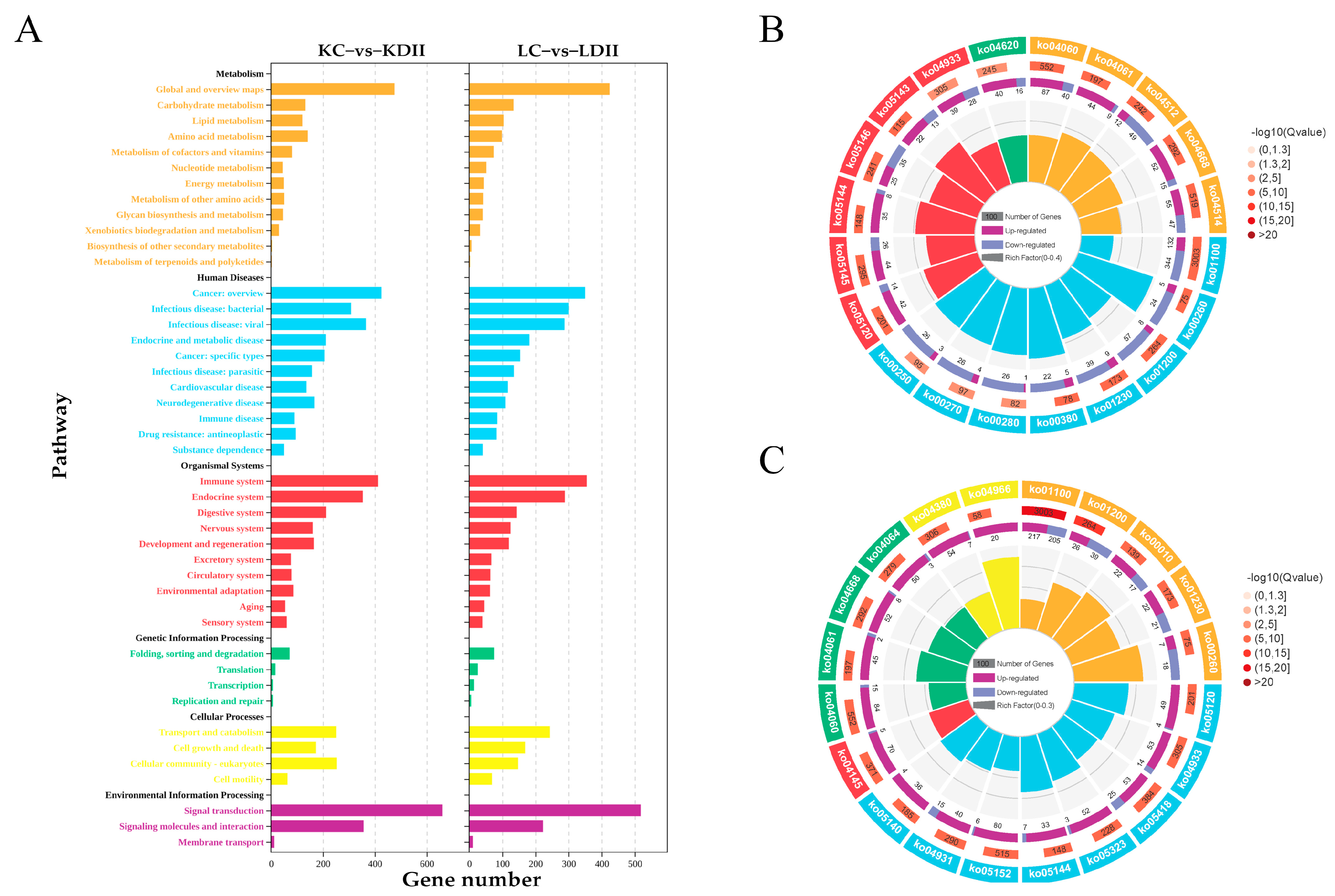
3.3. KEGG Enrichment of the DEGs in the Kidney and Liver
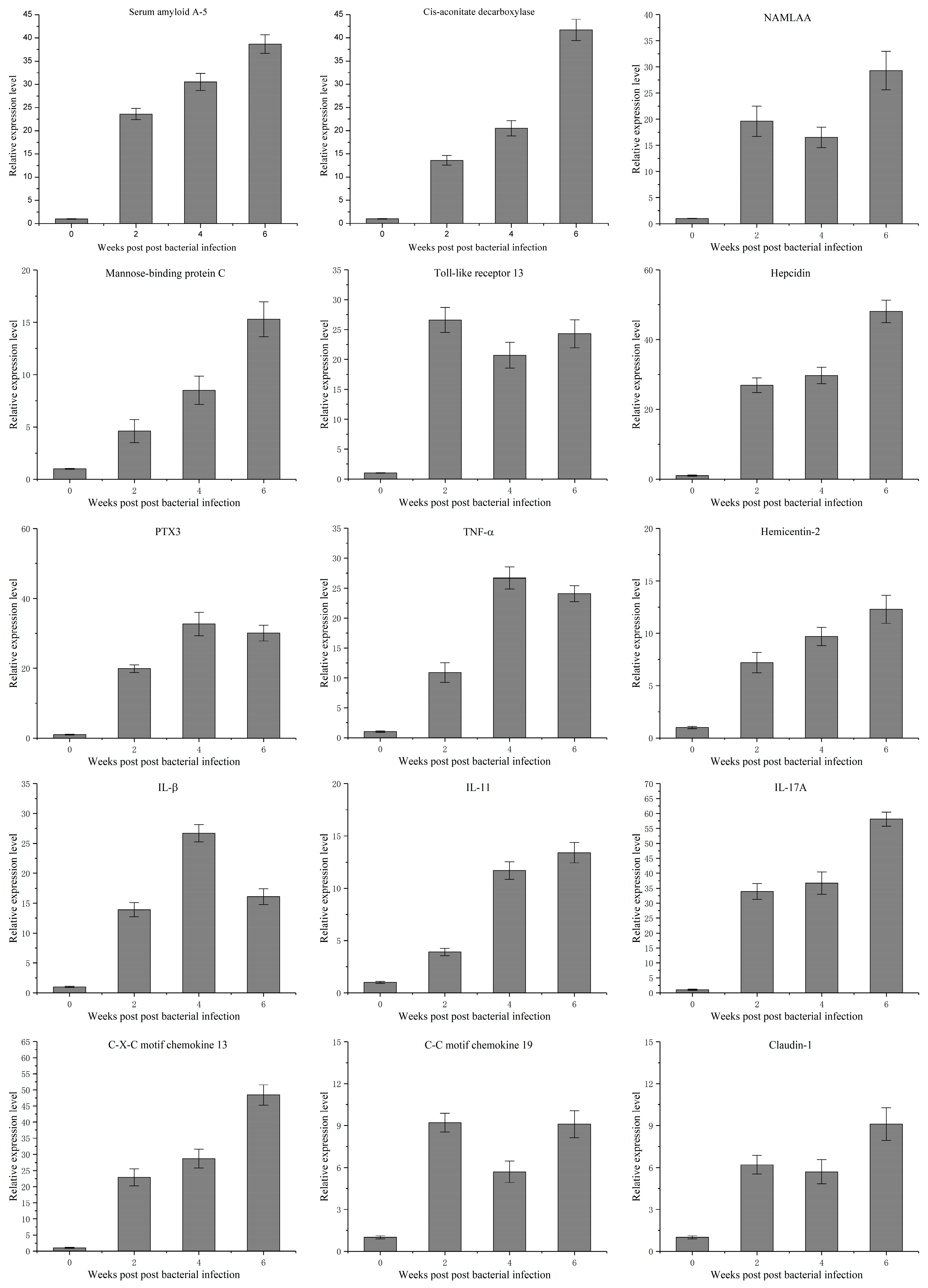
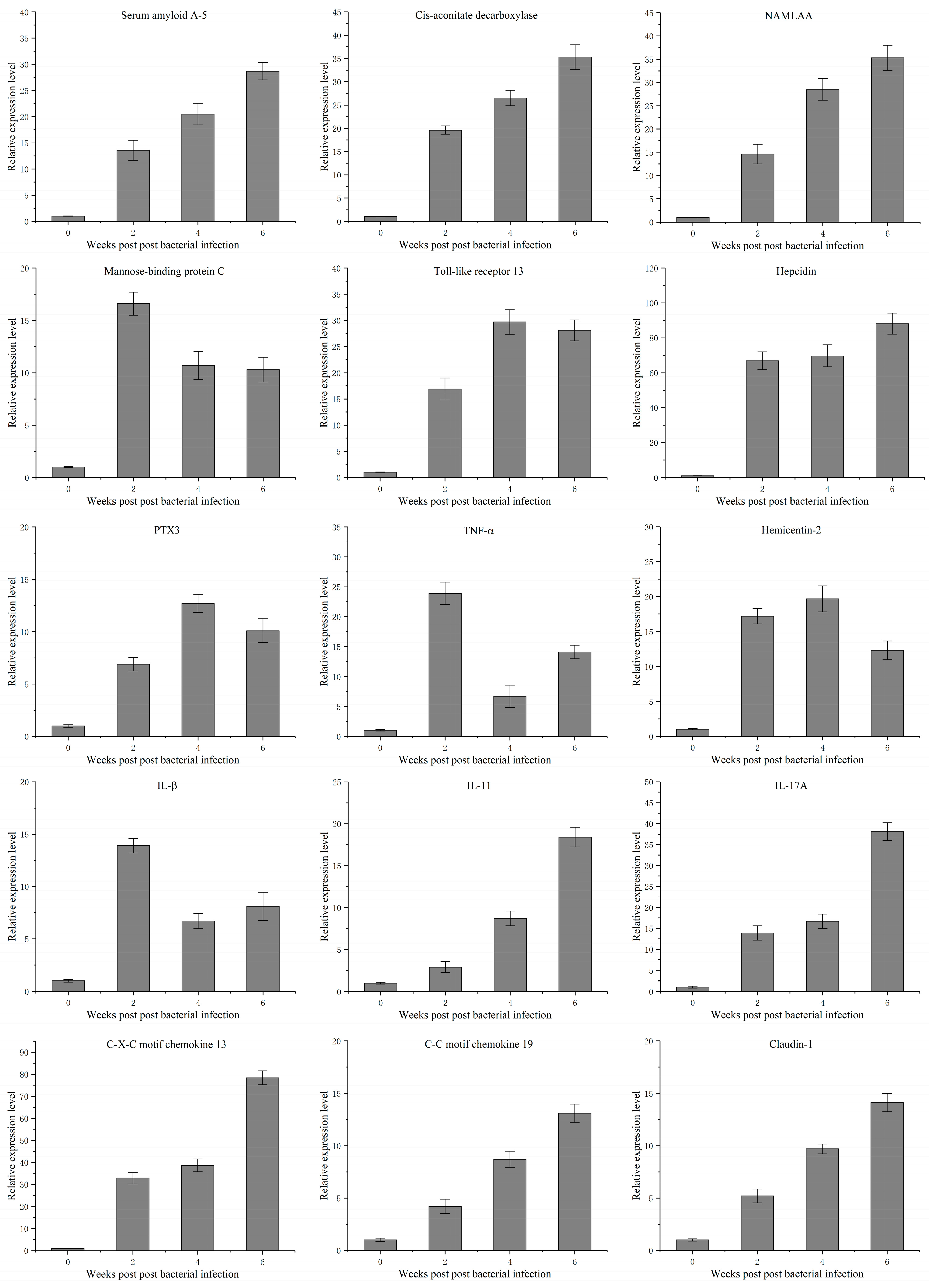
3.4. Temporal Expression Characteristics of 15 Genes Related to Mycobacterium marinum Infection
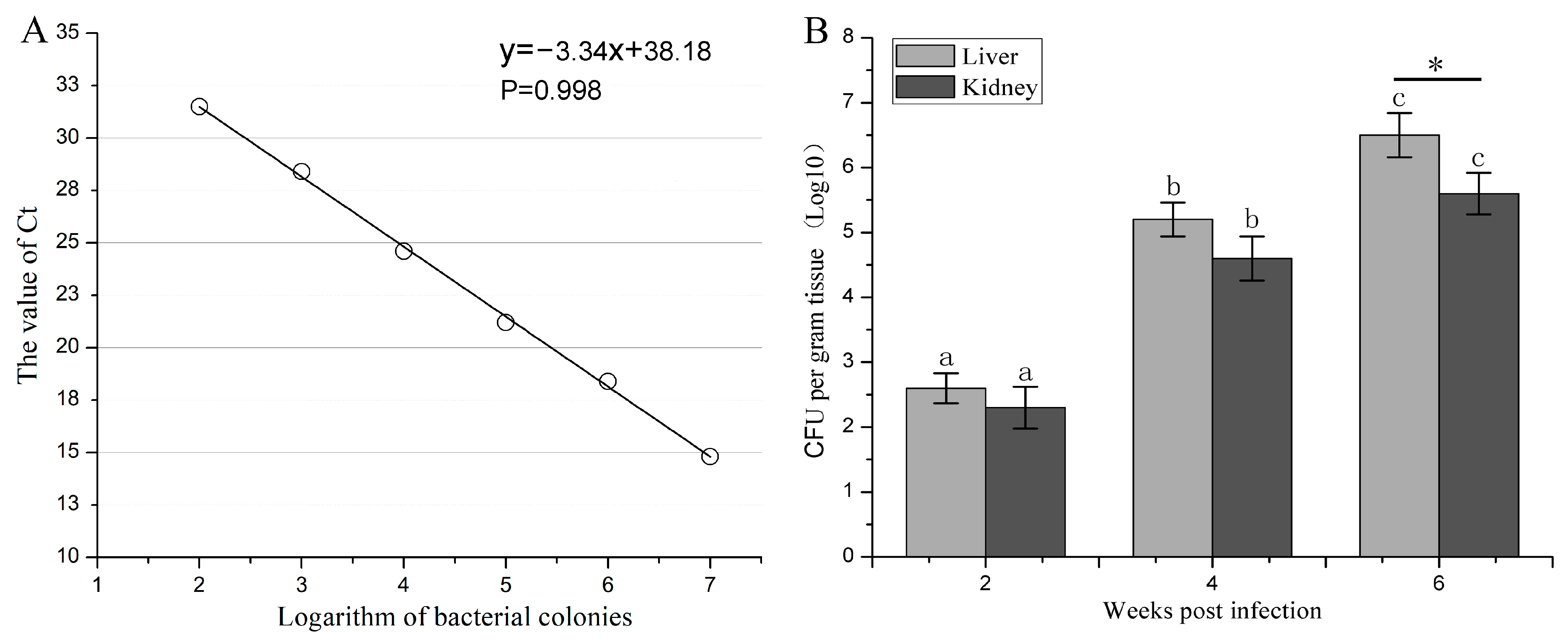
3.5. The Proliferation Dynamic of M. marinum in Liver and Kidney
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Canetti, D.; Riccardi, N.; Antonello, R.M.; Di Bella, S.; Farinazzo, A.; Matteelli, A.; Codecasa, L.R.; Tacconelli, E.; Alagna, R. Mycobacterium marinum: A brief update for clinical purposes. Eur. J. Intern. Med. 2022, 105, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.G.; Stout, J.E. Twenty-eight cases of Mycobacterium marinum infection: Retrospective case series and literature review. Infection 2015, 43, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Aubry, A.; Chosidow, O.; Caumes, E.; Robert, J.; Cambau, E. Sixty-three cases of Mycobacterium marinum infection: Clinical features, treatment, and antibiotic susceptibility of causative isolates. Arch. Intern. Med. 2002, 162, 1746–1752. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.S.; Chiu, C.H.; Yang, C.H.; Leu, H.S.; Huang, C.T.; Chen, Y.C.; Wu, T.L.; Chang, P.Y.; Su, L.H.; Kuo, A.J.; et al. Fish tank granuloma caused by Mycobacterium marinum. PLoS ONE 2012, 7, e41296. [Google Scholar] [CrossRef]
- Cosma, C.L.; Sherman, D.R.; Ramakrishnan, L. The secret lives of the pathogenic mycobacteria. Annu. Rev. Microbiol. 2003, 57, 641–676. [Google Scholar] [CrossRef]
- Hashish, E.; Merwad, A.; Elgaml, S.; Amer, A.; Kamal, H.; Elsadek, A.; Marei, A.; Sitohy, M. Mycobacterium marinum infection in fish and man: Epidemiology, pathophysiology and management; a review. Vet. Quart. 2018, 38, 35–46. [Google Scholar] [CrossRef]
- Decostere, A.; Hermans, K.; Haesebrouck, F. Piscine mycobacteriosis: A literature review covering the agent and the disease it causes in fish and humans. Vet. Microbiol. 2004, 99, 159–166. [Google Scholar] [CrossRef]
- Ramsay, J.M.; Watral, V.; Schreck, C.B.; Kent, M.L. Husbandry stress exacerbates mycobacterial infections in adult zebrafish, Danio rerio (Hamilton). J. Fish Dis. 2009, 32, 931–941. [Google Scholar] [CrossRef]
- Chen, W.; Gao, S. Current status of industrialized aquaculture in China: A review. Environ. Sci. Pollut. Res. 2023, 30, 32278–32287. [Google Scholar] [CrossRef]
- Duan, Z.; Zhou, Y.; Liu, W.; Zhang, J.; Ren, H.; Li, H.; Zhu, X.; Zhao, Y.; Chen, P.; Li, C. Variations in flavor according to fish size in rainbow trout (Oncorhynchus mykiss). Aquaculture 2020, 526, 735398. [Google Scholar] [CrossRef]
- Noble, A.C.; Summerfelt, S.T. Diseases encountered in rainbow trout cultured in recirculating systems. Annu. Rev. Fish Dis. 1996, 6, 65–92. [Google Scholar] [CrossRef]
- Rurangwa, E.; Verdegem, M.C.J. Microorganisms in recirculating aquaculture systems and their management. Rev. Aquac. 2015, 7, 117–130. [Google Scholar] [CrossRef]
- Aich, N.; Nama, S.; Biswal, A.; Paul, T.; Biswas, P. A review on recirculating aquaculture systems: Challenges and opportunities for sustainable aquaculture. Innov. Farm. 2020, 5, 17–24. [Google Scholar]
- Ford, J.S.; Myers, R.A. A global assessment of salmon aquaculture impacts on wild salmonids. PLoS Biol. 2008, 6, e33. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, X.; Wang, K.; Su, F.; Chen, J.; Shi, S.; Zhao, L.; Yu, X.; Zeng, L. Outbreak of infectious pancreatic necrosis virus (IPNV) in farmed rainbow trout in China. Acta Trop. 2017, 170, 63–69. [Google Scholar] [CrossRef]
- Jia, P.; Breyta, R.B.; Li, Q.; Qian, X.; Wu, B.; Zheng, W.; Wen, Z.; Liu, Y.; Kurath, G.; Liu, H.; et al. Insight into infectious hematopoietic necrosis virus (IHNV) in Chinese rainbow trout aquaculture from virus isolated from 7 provinces in 2010–2014. Aquaculture 2018, 496, 239–246. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, S.H.; Di, J.; Dong, X.; Wang, Y.; Yao, Y.; Liu, X.; Liu, Y.; Qin, Q.; Wang, X.; et al. Inactivated Mycobacterium vaccine induces innate immunity against M. ulcerans ecovar Liflandii (MuLiflandii ASM001) infection in Dabry’s sturgeon (Acipenser dabryanus). Aquaculture 2019, 512, 734312. [Google Scholar] [CrossRef]
- Conesa, A.; Götz, S.; García-Gómez, J.M.; Terol, J.; Talón, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef]
- Ye, J.; Fang, L.; Zheng, H.; Zhang, Y.; Chen, J.; Zhang, Z.; Wang, J.; Li, S.; Li, R.; Bolund, L.; et al. WEGO: A web tool for plotting GO annotations. Nucleic Acids Res. 2006, 34, W293–W297. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, M.; Li, F.; Qin, M.; Yang, Q.; Yu, H.; Xu, J.; Liu, Y.; Tong, T. Evaluation of fermented soybean meal to replace a portion fish meal on growth performance, antioxidant capacity, immunity, and mTOR signaling pathway of Coho Salmon (Oncorhynchus kisutch). Aquac. Nutr. 2023, 2558173. [Google Scholar] [CrossRef]
- Luukinen, H.; Hammarén, M.M.; Vanha-Aho, L.M.; Parikka, M. Modeling tuberculosis in Mycobacterium marinum infected adult zebrafish. J. Vis. Exp. 2018, 140, e58299. [Google Scholar]
- Slany, M.; Makovcova, J.; Jezek, P.; Bodnarova, M.; Pavlik, I. Relative prevalence of Mycobacterium marinum in fish collected from aquaria and natural freshwaters in central Europe. J. Fish Dis. 2014, 37, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, D.T.; Rhodes, M.W. Mycobacteriosis in fishes: A review. Vet. J. 2009, 180, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Dinh-Hung, N.; Dong, H.T.; Senapin, S.; Pimsannil, K.; Thompson, K.D.; Shinn, A.P.; Soontara, C.; Sirimanapong, W.; Chatchaiphan, S.; Rodkhum, C. Insight into characteristics and pathogenicity of five rapidly growing non-tuberculous Mycobacterium species isolated from the Siamese fighting fish, Betta splendens. Aquaculture 2023, 575, 739822. [Google Scholar] [CrossRef]
- Dinh-Hung, N.; Dong, H.T.; Senapin, S.; Linh, N.V.; Shinn, A.P.; Pirarat, N.; Hirono, I.; Chatchaiphan, S.; Rodkhum, C. Infection and histopathological consequences in Siamese fighting fish (Betta splendens) due to exposure to a pathogenic Mycobacterium chelonae via different routes. Aquaculture 2024, 579, 740191. [Google Scholar] [CrossRef]
- Davidovich, N.; Pretto, T.; Sharon, G.; Zilberg, D.; Blum, S.E.; Baider, Z.; Edery, N.; Klement, E.; Kaidar-Shwartz, I.; Dveyrin, Z.; et al. Cutaneous appearance of mycobacteriosis caused by Mycobacterium marinum, affecting gilthead seabream (Sparus aurata) cultured in recirculating aquaculture systems. Aquaculture 2020, 528, 735507. [Google Scholar] [CrossRef]
- Yanong, R.P.E.; Pouder, D.B.; Falkinham, J.O., III. Association of mycobacteria in recirculating aquaculture systems and mycobacterial disease in fish. J. Aquat. Anim. Health 2010, 22, 219–223. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, B.; Wang, T.; Bonni, A.; Zhao, G. Robust principal component analysis for accurate outlier sample detection in RNA-Seq data. BMC Bioinform. 2020, 21, 269. [Google Scholar] [CrossRef]
- Eslamloo, K.; Caballero-Solares, A.; Inkpen, S.M.; Emam, M.; Kumar, S.; Bouniot, C.; Avendaño-Herrera, R.; Jakob, E.; Rise, M.L. Transcriptomic profiling of the adaptive and innate immune responses of Atlantic salmon to Renibacterium salmoninarum infection. Front. Immunol. 2020, 11, 567838. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Parsons, J.T.; Horwitz, A.R.; Schwartz, M.A. Cell adhesion: Integrating cytoskeletal dynamics and cellular tension. Nat. Rev. Mol. Cell Biol. 2010, 11, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.; O’Neill, L.A. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015, 25, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, Y.; Niu, W.; Wang, X.; He, H.; Zeng, Q.; Lin, Y.; Jia, Y.; Zhou, J. Liver transcriptome analysis reveals important factors involved in the response to Newcastle disease virus infection. Funct. Integr. Genom. 2015, 15, 671–682. [Google Scholar]
- Robinson, M.W.; Harmon, C.; O’Farrelly, C. Liver immunology and its role in inflammation and homeostasis. Cell. Mol. Immunol. 2016, 13, 267–276. [Google Scholar] [CrossRef]
- Zhu, L.Y.; Nie, L.; Zhu, G.; Xiang, L.X.; Shao, J.Z. Advances in research of fish immune-relevant genes: A comparative overview of innate and adaptive immunity in teleosts. Dev. Comp. Immunol. 2013, 39, 39–62. [Google Scholar] [CrossRef]
- Tort, L.; Balasch, J.C.; Mackenzie, S. Fish immune system. A crossroads between innate and adaptive responses. Immunologia 2003, 22, 277–286. [Google Scholar]
- Mokhtar, D.M.; Zaccone, G.; Alesci, A.; Cavestro, T.; Marino, F. Main components of fish immunity: An overview of the fish immune system. Fishes 2023, 8, 93. [Google Scholar] [CrossRef]
- Magnadóttir, B. Innate immunity of fish (overview). Fish Shellfish Immunol. 2006, 20, 137–151. [Google Scholar] [CrossRef]
- Zou, J.; Secombes, C.J. The function of fish cytokines. Biology 2016, 5, 23. [Google Scholar] [CrossRef]
- Doherty, D.G. Immunity, tolerance and autoimmunity in the liver: A comprehensive review. J. Autoimmun. 2016, 66, 60–75. [Google Scholar] [CrossRef]
- Bayne, C.J.; Gerwick, L. The acute phase response and innate immunity of fish. Dev. Comp. Immunol. 2001, 25, 725–743. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, P.N.; Vázquez-Dorado, S.; Neves, J.V.; Wilson, J.M. Dual function of fish hepcidin: Response to experimental iron overload and bacterial infection in sea bass (Dicentrarchus labrax). Dev. Comp. Immunol. 2006, 30, 1156–1167. [Google Scholar] [CrossRef] [PubMed]
- Villarroel, F.; Bastías, A.; Casado, A.; Amthauer, R.; Concha, M.I. Serum amyloid A: A typical acute-phase reactant in rainbow trout? Dev. Comp. Immunol. 2008, 32, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Cray, C.; Zaias, J.; Altman, N.H. Acute phase response in animals: A review. Comp. Med. 2009, 59, 517–526. [Google Scholar]
- Dang, Y.; Meng, X.; Wang, S.; Li, H.; Zhang, X.; Xu, X.; Xiong, Y.; Wang, X. Mannose-binding lectin and its roles in immune responses in grass carp (Ctenopharyngodon idella) against Aeromonas hydrophila. Fish Shellfish Immunol. 2018, 72, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Bergman, I.M. Toll-like receptors (TLRs) and mannan-binding lectin (MBL): On constant alert in a hostile environment. Ups. J. Med. Sci. 2011, 116, 90–99. [Google Scholar] [CrossRef]
- Boltaña, S.; Reyes-Lopez, F.; Morera, D.; Goetz, F.; MacKenzie, S.A. Divergent responses to peptidoglycans derived from different E. coli serotypes influence inflammatory outcome in trout, Oncorhynchus mykiss, macrophages. BMC Genom. 2011, 12, 34. [Google Scholar] [CrossRef]
- Rajme-Manzur, D.; Gollas-Galván, T.; Vargas-Albores, F.; Martínez-Porchas, M.; Hernández-Oñate, M.Á.; Hernández-López, J. Granulomatous bacterial diseases in fish: An overview of the host’s immune response. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2021, 261, 111058. [Google Scholar] [CrossRef] [PubMed]
- Huising, M.O.; Kruiswijk, C.P.; van Schijndel, J.E.; Savelkoul, H.F.; Flik, G.; Verburg-van Kemenade, B.M. Multiple and highly divergent IL-11 genes in teleost fish. Immunogenetics 2005, 57, 432–443. [Google Scholar] [CrossRef]
- Cronan, M.R.; Tobin, D.M. Fit for consumption: Zebrafish as a model for tuberculosis. Dis. Model. Mech. 2014, 7, 777–784. [Google Scholar] [CrossRef]
- Torraca, V.; Masud, S.; Spaink, H.P.; Meijer, A.H. Macrophage-pathogen interactions in infectious diseases: New therapeutic insights from the zebrafish host model. Dis. Model. Mech. 2014, 7, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Bottazzi, B.; Doni, A.; Garlanda, C.; Mantovani, A. An integrated view of humoral innate immunity: Pentraxins as a paradigm. Annu. Rev. Immunol. 2009, 28, 157–183. [Google Scholar] [CrossRef] [PubMed]
- Lund, V.; Olafsen, J.A. A comparative study of pentraxin-like proteins in different fish species. Dev. Comp. Immunol. 1998, 22, 185–194. [Google Scholar] [CrossRef]
- Günzel, D.; Yu, A.S. Claudins and the modulation of tight junction permeability. Physiol. Rev. 2013, 93, 525–569. [Google Scholar] [CrossRef]
- Sun, L.; Liu, S.; Bao, L.; Li, Y.; Feng, J.; Liu, Z. Claudin multigene family in channel catfish and their expression profiles in response to bacterial infection and hypoxia as revealed by meta-analysis of RNA-Seq datasets. Comp. Biochem. Physiol. Part D Genom. Proteom. 2015, 13, 60–69. [Google Scholar] [CrossRef]
- Deng, F.; Wang, D.; Chen, F.; Lu, T.; Li, S. Molecular characterization and expression analysis of claudin-4-like in rainbow trout involved in Flavobacterium psychrophilum infection. Fish Shellfish Immunol. 2022, 130, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Swaim, L.E.; Connolly, L.E.; Volkman, H.E.; Humbert, O.; Born, D.E.; Ramakrishnan, L. Mycobacterium marinum infection of adult zebrafish causes caseating granulomatous tuberculosis and is moderated by adaptive immunity. Infec. Immun. 2006, 74, 6108–6117. [Google Scholar] [CrossRef]
- Talaat, A.M.; Reimschuessel, R.; Wasserman, S.S.; Trucksis, M. Goldfish, Carassius auratus, a novel animal model for the study of Mycobacterium marinum pathogenesis. Infec. Immun. 1998, 66, 2938–2942. [Google Scholar] [CrossRef]
- Flynn, J.L.; Chan, J. Tuberculosis: Latency and reactivation. Infec. Immun. 2001, 69, 4195–4201. [Google Scholar] [CrossRef]

| Gene Name | Sequences (5′-3′) | Primer Source (Genbank No.) |
|---|---|---|
| Serum amyloid A-5 | F:GCGACATGAAGGATGCCAAC | XM_020478634 |
| R:TCCTCATGTCCTCGACCACT | ||
| Cis-aconitate decarboxylase | F:CTCCAACGAGGCTCAGAATATC | XM_031789418 |
| R: TGAGAGCGTTCCAGAGAGA | ||
| N-acetylmuramoyl-L-alanine amidase | F:CTGTGGTGAGGGAAGGAATAAA | XM_020486665 |
| R:CACTGACACCGTGGGATAATAG | ||
| Mannose-binding protein C | F:CTTTGACGATGCTGTTCAGTTC | XM_020469364 |
| R:CCTTCAAAGACCTGAGTGAGAG | ||
| Toll-like receptor 13 | F:AGGGACTGTGTCATCTACCTATC | XM_031792536 |
| R:GTTTGGGTGCAGGTCCTTAATA | ||
| Hepcidin | F:TTCAGGTTCAAGCGTCAGAG | XM_031790947 |
| R:CAGGCAGGTCCTCAGAATTT | ||
| Pentraxin-3 | F:GGACACAGGCTATCCAACTTATC | XM_020471418 |
| R:CATCTCTGCTGATGCCACTT | ||
| Tumor necrosis factor α | F:GGCGAGCATACCACTCCTCT | Zhang et al., 2023 [20] |
| R:TCGGACTCAGCATCACCGTA | ||
| Hemicentin-2 | F:GTCAGTCCGTCTGGTGAAATAG | XM_020469630 |
| R:CCCGTCCGTTCTTCATTCTT | ||
| Interleukin-1β | F:GCGACATGGTGCGTTTCCTTTT | Zhang et al., 2023 [20] |
| R:TGTCTACCGGTTTGGTGTAGTCCT | ||
| Interleukin-17A | F:GGTCTTTGGACGGAGAGTAATG | XM_020477819 |
| R:GAGAGACCTGGTGGCTTTATG | ||
| C-X-C motif chemokine 13 | F:ACACTACTTGTTGGTCGTACTG | XM_020477664 |
| R:GAAACCTGGTGGGAGAGATAAA | ||
| C-C motif chemokine 19 | F:CCACAGAGTGGTGAAGTCATAC | XM_020458446 |
| R:GCACACAGTCTAAGGTTCTTCT | ||
| Claudin-1 | F:CTTGCTTTGATCGGTCTTGTTG | XM_020480182 |
| R:GTTACCGTGCTCTCTGTGTAAG | ||
| β-actin | F:CCAAAGCCAACAGGGAGAA | Zhang et al., 2023 [20] |
| R:AGGGACAACACCGCCTGGAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Xu, D.; Yu, X.; Gai, C.; Ye, H.; Diao, J. Characterization of Immune Response Against Mycobacterium marinum Infection in Coho Salmon (Oncorhynchus kisutch). Fishes 2025, 10, 268. https://doi.org/10.3390/fishes10060268
Li L, Xu D, Yu X, Gai C, Ye H, Diao J. Characterization of Immune Response Against Mycobacterium marinum Infection in Coho Salmon (Oncorhynchus kisutch). Fishes. 2025; 10(6):268. https://doi.org/10.3390/fishes10060268
Chicago/Turabian StyleLi, Le, Danlei Xu, Xiaoqing Yu, Chunlei Gai, Haibin Ye, and Jing Diao. 2025. "Characterization of Immune Response Against Mycobacterium marinum Infection in Coho Salmon (Oncorhynchus kisutch)" Fishes 10, no. 6: 268. https://doi.org/10.3390/fishes10060268
APA StyleLi, L., Xu, D., Yu, X., Gai, C., Ye, H., & Diao, J. (2025). Characterization of Immune Response Against Mycobacterium marinum Infection in Coho Salmon (Oncorhynchus kisutch). Fishes, 10(6), 268. https://doi.org/10.3390/fishes10060268





