Expanding Host Range: First Record of Eustrongylides excisus in Padogobius bonelli (Gobiidae) from the Po River (Northwest Italy)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Fish Sampling
2.3. Fish Identification and Parasite Investigation
2.4. Molecular Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bouwknegt, M.; Devleesschauwer, B.; Graham, H.; Robertson, L.J.; van der Giessen, J.W.; The Euro-FBP Workshop Participants. Prioritisation of Food-Borne Parasites in Europe, 2016. Euro. Surveill. 2018, 23, 17–00161. [Google Scholar] [CrossRef] [PubMed]
- Menconi, V.; Lazzaro, E.; Bertola, M.; Guardone, L.; Mazzucato, M.; Prearo, M.; Bilska-Zajac, E.; Cortinovis, L.; Manfrin, A.; Arcangeli, G.; et al. The Occurrence of Freshwater Fish-Borne Zoonotic Helminths in Italy and Neighbouring Countries: A Systematic Review. Animals 2023, 13, 3793. [Google Scholar] [CrossRef] [PubMed]
- Spalding, M.G.; Forrester, D.J. Pathogenesis of Eustrongylides ignotus (Nematoda: Dioctophymatoidea) in Ciconiiformes. J. Wildl. Dis. 1993, 29, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Coyner, D.F.; Spalding, M.G.; Forrester, D.J. Epizootiology of Eustrongylides ignotus in Florida: Distribution, Density, and Natural Infections in Intermediate Hosts. J. Wildl. Dis. 2002, 38, 483–499. [Google Scholar] [CrossRef]
- Menconi, V.; Tedesco, P.; Pastorino, P.; Confortini, I.; Esposito, G.; Tomasoni, M.; Mugetti, D.; Gustinelli, A.; Dondo, A.; Pizzul, E.; et al. Could Fish Feeding Behaviour and Size Explain Prevalence Differences of the Nematode Eustrongylides excisus among Species? The Case Study of Lake Garda. Water 2021, 13, 3581. [Google Scholar] [CrossRef]
- Melo, F.T.D.V.; Melo, C.D.S.B.; Nascimento, L.d.C.S.D.; Giese, E.G.; Furtado, A.P.; dos Santos, J.N. Morphological Characterization of Eustrongylides Sp. Larvae (Nematoda, Dioctophymatoidea) Parasite of Rhinella marina (Amphibia: Bufonidae) from Eastern Amazonia. Rev. Bras. Parasitol. Vet. 2016, 25, 235–239. [Google Scholar] [CrossRef]
- Goncharov, S.L.; Soroka, N.M.; Pashkevich, I.Y.; Dubovyi, A.I.; Bondar, A.O. Infection of Predatory Fish with Larvae of Eustrongylides excisus (Nematoda, Dioctophymatidae) in the Delta of the Dnipro River and the Dnipro-Buh Estuary in Southern Ukraine. Vestn. Zool. 2018, 52, 137–144. [Google Scholar] [CrossRef]
- Dezfuli, B.S.; Manera, M.; Lorenzoni, M.; Pironi, F.; Shinn, A.P.; Giari, L. Histopathology and the inflammatory response of European perch, Perca fluviatilis muscle infected with Eustrongylides sp. (Nematoda). Parasit. Vectors 2015, 8, 227. [Google Scholar] [CrossRef]
- Eberhard, M.L.; Hurwitz, H.; Sun, A.M.; Coletta, D. Intestinal perforation caused by larval Eustrongylides (Nematoda: Dioctophymatoidae) in New Jersey. Am. J. Trop. Med. Hyg. 1989, 40, 648–650. [Google Scholar] [CrossRef]
- Caudill, G.; Wolf, D.; Caudill, D.; Brown, J.; Shearn-Bochsler, V.A. Juvenile wading-bird mortality event in urban Jacksonville, Florida, associated with the parasite Eustrongylides. Florida Field Nat. 2014, 42, 108–113. [Google Scholar]
- Cole, R.A. Eustrongylidosis. In Field Manual of Wildlife Diseases: General Field Procedures and Diseases of Birds; Friend, M., Franson, J.C., Eds.; USA Department of the Interior Fish and Wildlife Service: Washington, DC, USA, 1999; pp. 223–228. [Google Scholar]
- Moravec, F. Nematodes parasitic in fishes as larvae. In Parasitic Nematodes of Freshwater Fishes of Europe; Moravec, F., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1994; pp. 374–421. [Google Scholar]
- Bjelić-Čabrilo, O.; Novakov, N.; Ćirković, M.; Kostić, D.; Popović, E.; Aleksić, N.; Lujić, J. The first determination of Eustrongylides excisus Jägerskiöld, 1909—Larvae (Nematoda: Dioctophymatidae) in the pike-perch Sander lucioperca in Vojvodina (Serbia). Helminthologia 2013, 50, 291–294. [Google Scholar] [CrossRef]
- Honcharov, S.L.; Soroka, N.M.; Galat, M.V.; Zhurenko, O.V.; Dubovyi, A.I.; Dzhmil, V.I. Eustrongylides (Nematoda: Dioctophymatidae): Epizootology and special characteristics of the development biology. Helminthologia 2022, 59, 127. [Google Scholar] [CrossRef]
- Okon, E.M.; Okocha, R.C.; Taiwo, A.B.; Michael, F.B.; Bolanle, A.M. Dynamics of co-infection in fish: A review of pathogen-host interaction and clinical outcome. Fish Shellfish. Immunol. Rep. 2023, 4, 100096. [Google Scholar] [CrossRef] [PubMed]
- Wittner, M.; Turner, J.W.; Jacquette, G.; Ash, L.R.; Salgo, M.P.; Tanowitz, H.B. Eustrongylidiasis: A parasitic infection acquired by eating sushi. N. Engl. J. Med. 1989, 320, 1124–1126. [Google Scholar] [CrossRef]
- Guerin, P.F.; Marapendi, S.; MC Grail, L. Intestinal perforation caused by larval Eustrongylides. Morb. Mort. Week Rep. 1982, 31, 383–389. [Google Scholar]
- Gunby, P. One worm in the minnow equals too many in the gut. JAMA 1982, 248, 163. [Google Scholar] [CrossRef]
- Narr, L.L.; O’Donnell, J.G.; Libster, B.; Alessi, P.; Abraham, D. Eustrongylidiasis a parasitic infection acquired by eating live minnow. J. Am. Osteopath. Assoc. 1996, 96, 400–402. [Google Scholar] [CrossRef]
- Eberhard, M.L.; Ruiz-Tiben, E. Cutaneous emergence of Eustrongylides in two persons from South Sudan. Am. J. Trop. Med. Hyg. 2014, 90, 315–317. [Google Scholar] [CrossRef]
- Shamsi, S.; Francis, N.; Masiga, J.; Barton, D.P.; Zhu, X.; Pearce, L.; McLellan, M. Occurrence and Characterisation of Eustrongylides Species in Australian Native Birds and Fish. Food Waterborne Parasitol. 2023, 30, e00189. [Google Scholar] [CrossRef]
- Honcharov, S.L.; Soroka, N.M.; Halat, M.V.; Dubovyi, A.I.; Zhurenko, V.V.; Halushko, I.A. Distribution of the Nematodes of the Genus Eustrongylides (Nematoda, Dioctophymatidae) in the World. Regul. Mech. Biosyst. 2022, 13, 73–79. [Google Scholar] [CrossRef]
- Jakob, E.; Hanel, R.; Klimpel, S.; Zumholz, K. Salinity Dependence of Parasite Infestation in the European Eel Anguilla anguilla in Northern Germany. ICES J. Mar. Sci. 2009, 66, 358–366. [Google Scholar] [CrossRef]
- Gagliardi, A.; Preatoni, D.G.; Wauters, L.A.; Martinoli, A. Selective Predators or Choosy Fishermen? Relation between Fish Harvest, Prey Availability and Great Cormorant (Phalacrocorax carbo sinensis) Diet. Ital. J. Zool. 2015, 82, 544–555. [Google Scholar] [CrossRef]
- Branciari, R.; Ranucci, D.; Miraglia, D.; Valiani, A.; Veronesi, F.; Urbani, E.; Lo Vaglio, G.; Pascucci, L.; Franceschini, R. Occurrence of parasites of the genus Eustrongylides spp.(Nematoda: Dioctophymatidae) in fish caught in Trasimeno lake, Italy. Ital. J. Food Saf. 2016, 5, 6130. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, R.; Valiani, A.; Ranucci, D.; Roila, R.; Palma, G.; Agnetti, F.; Di Giacinto, G.; Branciari, R. Eustrongylides spp. parasite risk management in Atherina boyeri from Lake Trasimeno. Ital. J. Food Saf. 2023, 12, 11338. [Google Scholar] [CrossRef]
- Pastorino, P.; Squadrone, S.; Berti, G.; Esposito, G.; Bondavalli, F.; Renzi, M.; Pizzul, E.; Kazmi, S.S.U.H.; Barceló, D.; Abete, M.C.; et al. Occurrence of rare earth elements in water, sediment, and freshwater fish of diverse trophic levels and feeding ecology: Insights from the Po river (northwest Italy). Environ. Res. 2024, 240, 117455. [Google Scholar] [CrossRef]
- Sollazzo, C.; Scanu, G.; Aste, F.; Pineschi, G.; Belli, M.; Balzamo, S.; Martone, C.; Cadoni, F.; Bernabei, S.; D’Antoni, S.; et al. Protocollo di campionamento e analisi della fauna ittica nei sistemi lotici guadabili. In Metodi Biologici per le Acque Superficiali Interne, 111/2014; Manuali e Linee Guida; ISPRA: Rome, Italy, 2014; pp. 1–15. Available online: https://www.isprambiente.gov.it/files/pubblicazioni/manuali–lineeguida/MLG__111_2014_Metodi_Biologici_acque.pdf (accessed on 23 May 2025).
- AIIAD. Check List Ittiofauna Italiana_GdL3.0_05032021.xlsx. Check List v.3.0.0 del 5 March 2021. Available online: http://www.aiiad.it (accessed on 23 May 2025).
- Menconi, V.; Riina, M.V.; Pastorino, P.; Mugetti, D.; Canola, S.; Pizzul, E.; Bona, M.C.; Dondo, A.; Acutis, P.L.; Prearo, M. First occurrence of Eustrongylides spp. (Nematoda: Dioctophymatidae) in a subalpine lake in Northwest Italy: New data on distribution and host range. Int. J. Environ. Res. Public Health 2020, 17, 4171. [Google Scholar] [CrossRef]
- Mazzone, A.; Caffara, M.; Gustinelli, A.; Agnetti, F.; Sgariglia, E.; Lo Vaglio, G.; Fioravanti, M.L. Morphological and molecular characterization of larval and adult stages of Eustrongylides excisus (Nematoda: Dioctophymatoidea) with histopathological observations. J. Parasitol. 2019, 105, 882–889. [Google Scholar] [CrossRef]
- Cribb, T.H.; Anderson, G.R.; Adlard, R.D.; Bray, R.A. A DNA-based demonstration of a three-host lifecycle for the Bivesiculidae (Platyhelminthes: Digenea). Int. J. Parasitol. 1998, 28, 1791–1795. [Google Scholar] [CrossRef]
- Gustinelli, A.; Caffara, M.; Florio, D.; Otachi, E.O.; Wathuta, E.M.; Fioravanti, M.L. First description of the adult stage of Clinostomum cutaneum Paperna, 1964 (Digenea: Clinostomidae) from grey herons Ardea cinerea L. and a redescription of the metacercaria from the Nile tilapia Oreochromis niloticus niloticus (L.) in Kenya. Syst. Parasitol. 2010, 76, 39–51. [Google Scholar] [CrossRef]
- Gandolfi, G.; Tongiorgi, P. Taxonomic position, distribution and biology of the gobies present in the Italian fresh-waters, Padogobius martensi (Giinther) and Gobius niigricans Canestrini (Osteichthyes, Gobiidae). Ann. Mus. Civ. St. Nat. Genova 1974, 80, 92–118. [Google Scholar]
- Zaharieva, R.G.; Zaharieva, P.G.; Kirin, D.A. Ecological study on helminths of three species of Gobiidae from the Danube River, Bulgaria. Helminthologia 2023, 60, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, R.; Guardone, L.; Armani, A.; Ranucci, D.; Roila, R.; Valiani, A.; Susini, F.; Branciari, R. Five-years management of an emerging parasite risk (Eustrongylides sp., Nematoda) in a fishery supply chain located on Trasimeno Lake (Italy). Food Control 2022, 136, 108858. [Google Scholar] [CrossRef]
- Castiglione, D.; Di Maggio, M.; Guardone, L.; Ricci, E.; Tinacci, L.; Guglielmone, G.; Coltraro, M.; Susini, F.; Armani, A. Eustrongylides excisus in fish species caught in the Massaciuccoli Lake (Northwest Tuscany, Italy): Implications for freshwater fish quality and public health. Food Control 2023, 153, 109894. [Google Scholar] [CrossRef]
- Prearo, M.; Menconi, V.; Bondavalli, F.; Gabetti, A.; Manganza, A.; Abbà, M.; Bezzo Llufrio, T.; Zanoli, A.; Esposito, G.; Pastorino, P. Distribuzione e prevalenza di Eustrongylides excisus nella fauna ittica lacustre del Piemonte. Ittiopatologia 2023, 20, 27–53. [Google Scholar]
- Maganza, A.; Mossotto, C.; Rizzioli, B.; Esposito, G.; Gabetti, A.; Bondavalli, F.; Aimone, B.; Bovero, S.; Pastorino, P.; Prearo, M. Primo ritrovamento di Eustrongylides excisus nel Parco Naturale dei Laghi di Avigliana (TO). In Proceedings of the XXVIII Convegno Nazionale Società Italiana di Patologia Ittica, Cesenatico, Italy, 27–28 June 2024; Available online: https://www.sipi-online.it/wp-content/uploads/2024/03/Atti_XXVIII-Convegno-SIPI_Cesenatico.pdf (accessed on 23 May 2025).
- Guardone, L.; Ricci, E.; Susini, F.; Polsinelli, E.; Guglielmone, G.; Armani, A. First detection of Eustrongylides excisus (Nematoda: Dioctophymatidae) in big-scale sand smelt (Atherina boyeri) from the lake Massaciuccoli (Northwest Tuscany, Italy): Implications for public health and seafood quality. Food Control 2021, 120, 107517. [Google Scholar] [CrossRef]
- Švažas, S.; Chukalova, N.; Grishanov, G.; Pūtys, Ž.; Sruoga, A.; Butkauskas, D.; Raudonikis, L.; Prakas, P. The role of great cormorant (Phalacrocorax carbo sinensis) for fish stock and dispersal of helminthes parasites in the Curonian Lagoon area. Vet. Med. Zoot. 2011, 55, 79–85. [Google Scholar]
- Delmastro, G.B.; Boano, G.; Conte, P.L.; Fenoglio, S. Great cormorant predation on Cisalpine pike: A conservation conflict. Eur. J. Wildl. Res. 2015, 61, 743–748. [Google Scholar] [CrossRef]
- Root, T.L.; Price, J.T.; Hall, K.R.; Schneider, S.H.; Rosenzweig, C.; Pounds, J.A. Fingerprints of global warming on wild animals and plants. Nature 2003, 421, 57–60. [Google Scholar] [CrossRef]
- Tøttrup, A.P.; Thorup, K.; Rainio, K.; Yosef, R.; Lehikoinen, E.; Rahbek, C. Avian migrants adjust migration in response to environmental conditions en route. Biol. Lett. 2008, 4, 685–688. [Google Scholar] [CrossRef]
- Polley, L.; Thompson, R.A. Parasite zoonoses and climate change: Molecular tools for tracking shifting boundaries. Trends Parasitol. 2009, 25, 285–291. [Google Scholar] [CrossRef]
- Soana, E.; Gervasio, M.P.; Granata, T.; Colombo, D.; Castaldelli, G. Climate change impacts on eutrophication in the Po River (Italy): Temperature-mediated reduction in nitrogen export but no effect on phosphorus. J. Environ. Sci. 2024, 143, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Verdonschot, P.F. Oligochaetes and eutrophication; an experiment over four years in outdoor mesocosms. Hydrobiologia 1996, 334, 169–183. [Google Scholar] [CrossRef]
- Bo, T.; López-Rodríguez, M.J.; Fenoglio, S.; Cammarata, M.; de Figueroa, J.M.T. Feeding habits of Padogobius bonelli (osteichthyes: Gobiidae) in the curone creek (northwest Italy): Territoriality influences diet? J. Freshw. Ecol. 2010, 25, 367–371. [Google Scholar] [CrossRef]
- Timi, J.T.; Poulin, R. Why ignoring parasites in fish ecology is a mistake. Int. J. Parasitol. 2020, 50, 755–761. [Google Scholar] [CrossRef]

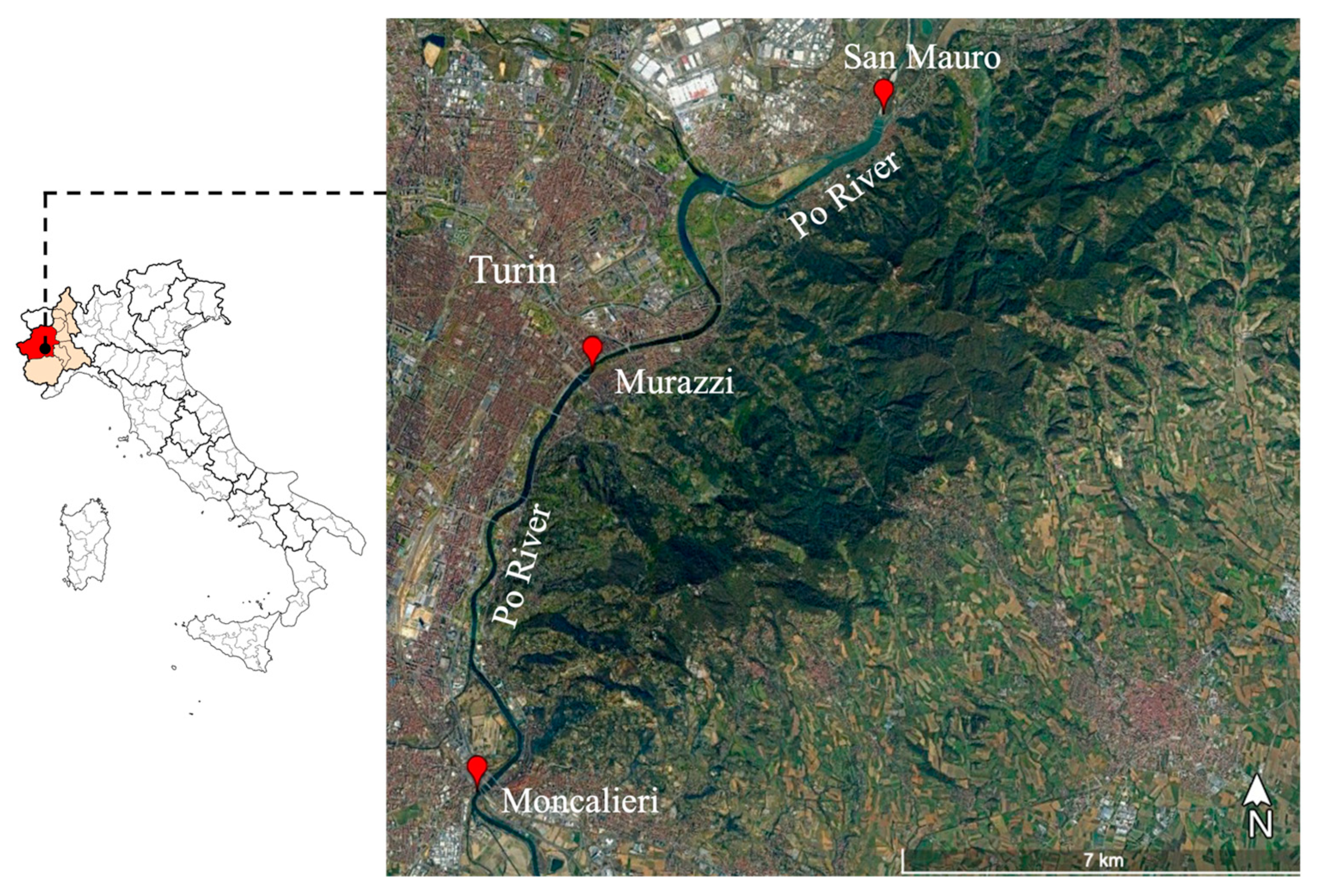


| Sampling Sites | Species | Origin | Mean Weight (g) | Mean Length (cm) | No. |
|---|---|---|---|---|---|
| Moncalieri | Barbus barbus | NN | 26.67 ± 28.32 | 12.58 ± 4.38 | 26 |
| Telestes muticellus | N | 11.16 | 10 | 1 | |
| Squalius squalus | N | 1.03 ± 0.27 | 4.5 ± 0.71 | 2 | |
| Rhodeus amarus | NN | 2.61 ± 0.56 | 6.75 ± 0.96 | 4 | |
| Padogobius bonelli | N | 2.49 ± 1.16 | 6.31 ± 1.44 | 8 | |
| Salmo trutta | NN | 90.39 | 21 | 1 | |
| Murazzi | Leuciscus sp. | NN | 1.74 ± 2.39 | 5.05 ± 1.86 | 22 |
| Barbus barbus | NN | 9.16 ± 5.97 | 9.56 ± 3.11 | 9 | |
| Romanogobio benacensis | N | 8.95 | 11 | 1 | |
| Padogobius bonelli | N | 1.90 ± 0.93 | 4.92 ± 1.02 | 6 | |
| Carassius carassius | NN | 2.14 ± 1.64 | 5 ± 1.41 | 2 | |
| Lepomis gibbosus | NN | 7.48 | 8.5 | 1 | |
| Pseudorasbora parva | NN | 2.31 ± 0.25 | 7.25 ± 0.35 | 2 | |
| Cobitis bilineata | N | 1.69 ± 0.86 | 6.75 ± 2.07 | 6 | |
| Rhodeus amarus | NN | 2.51 ± 0.38 | 5.69 ± 0.37 | 8 | |
| San Mauro | Padogobius bonelli | N | 3.43 ± 3.44 | 6.13 ± 2.29 | 4 |
| Silurus glanis | NN | 56.52 | 21 | 1 | |
| Rhodeus amarus | NN | 1.46 | 5 | 1 | |
| Cobitis bilineata | N | 1.99 ± 0.88 | 7.13 ± 1.03 | 8 | |
| Telestes muticellus | N | 7.21 ± 2.25 | 8.17 ± 0.76 | 3 | |
| Perca fluviatilis | NN | 63.44 | 18.5 | 1 | |
| Pseudorasbora parva | NN | 2.68 ± 0.34 | 6.58 ± 0.10 | 4 | |
| Barbus barbus | NN | 12.05 ± 5.43 | 9.73 ± 1.45 | 28 | |
| Squalius squalus | N | 0.64 ± 0.21 | 4.02 ± 0.80 | 21 |
| Region | Water Basin | Year of Sampling | Fish Species | Parasitological Index * | References | ||
|---|---|---|---|---|---|---|---|
| P% | MI | MA | |||||
| Umbria | Lake Trasimeno | 2015 | P. fluviatilis | 6.84 | 1 | NA | [25] |
| M. salmoides | 1.89 | 1 | NA | ||||
| A. boyeri | 0.13 | 1 | NA | ||||
| Lake Trasimeno | 2021 | P. fluviatilis | 67.99 | 8.28 | 6 | [36] | |
| M. salmoides | 0.26 | 1 | 0.003 | ||||
| T. tinca | 0.13 | 1 | 0.0013 | ||||
| A. melas | 2.72 | 1 | 0.0273 | ||||
| C. carpio | 0.02 | 1 | 0.0002 | ||||
| Tuscany | Lake Massacciuccoli | 2019 | A. boyeri | 2.3 | 1 | 0.2 | [40] |
| Lake Massacciuccoli | 2022 | A. melas | 28.6 | 1.7 | 0.49 | [37] | |
| S. glanis | 75 | 5.7 | 4.3 | ||||
| C. ramada | NA | NA | 0.07 | ||||
| L. gibbosus | 4.3 | 1 | 0.04 | ||||
| M. salmoides | 25 | 1 | 0.25 | ||||
| A. boyeri | 5.1 | 1 | 0.05 | ||||
| Lombardy–Veneto | Lake Garda | 2020 | P. fluviatilis | 14.41 | 1 | 0.014 | [5] |
| M. salmoides | 30 | 1.33 | 0.4 | ||||
| L. gibbosus | 3.10 | 1 | 0.023 | ||||
| Piedmont–Lombardy–Swiss | Lake Maggiore | 2019–2022 | P. fluviatilis | 8.20–20 | 1.54 | 0.15 | [38] |
| Piedmont | Lake Viverone | 2019–2022 | P. fluviatilis | 25.33 | 1.11 | 0.28 | |
| L. gibbosus | 40.83 | 1.01 | 0.44 | ||||
| Lake Candia | 2019–2022 | L. gibbosus | 92.04 | 2.86 | 2.63 | ||
| M. salmoides | 83.82 | 1 | 0.84 | ||||
| Lake Sirio | 2019–2022 | L. gibbosus | 2.86 | 1 | 0.03 | ||
| Lake Pistono | 2019–2022 | L. gibbosus | 2.67 | 1.58 | 0.04 | ||
| Lake Nero | 2019–2022 | P. fluviatilis | 5.41 | 1.25 | 0.07 | ||
| L. gibbosus | 10.32 | 1.23 | 0.13 | ||||
| Lake San Michele | 2019–2022 | P. fluviatilis | 10 | 1.67 | 0.17 | ||
| L. gibbosus | 18.29 | 1.53 | 0.28 | ||||
| M. salmoides | 17.86 | 1 | 0.179 | ||||
| Lake Alice Superiore | 2019–2022 | P. fluviatilis | 4.29 | 1 | 0.04 | ||
| L. gibbosus | 2.57 | 1.12 | 0.03 | ||||
| Great Lake of Avigliana | 2023 | L. gibbosus | NA | NA | NA | [39] | |
| A. melas | NA | NA | NA | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maganza, A.; Mossotto, C.; Gabetti, A.; Gozlan, R.E.; Combe, M.; Esposito, G.; Bondavalli, F.; Bertoli, M.; Pizzul, E.; Pastorino, P.; et al. Expanding Host Range: First Record of Eustrongylides excisus in Padogobius bonelli (Gobiidae) from the Po River (Northwest Italy). Fishes 2025, 10, 254. https://doi.org/10.3390/fishes10060254
Maganza A, Mossotto C, Gabetti A, Gozlan RE, Combe M, Esposito G, Bondavalli F, Bertoli M, Pizzul E, Pastorino P, et al. Expanding Host Range: First Record of Eustrongylides excisus in Padogobius bonelli (Gobiidae) from the Po River (Northwest Italy). Fishes. 2025; 10(6):254. https://doi.org/10.3390/fishes10060254
Chicago/Turabian StyleMaganza, Alessandra, Camilla Mossotto, Alice Gabetti, Rodolphe Elie Gozlan, Marine Combe, Giuseppe Esposito, Fabio Bondavalli, Marco Bertoli, Elisabetta Pizzul, Paolo Pastorino, and et al. 2025. "Expanding Host Range: First Record of Eustrongylides excisus in Padogobius bonelli (Gobiidae) from the Po River (Northwest Italy)" Fishes 10, no. 6: 254. https://doi.org/10.3390/fishes10060254
APA StyleMaganza, A., Mossotto, C., Gabetti, A., Gozlan, R. E., Combe, M., Esposito, G., Bondavalli, F., Bertoli, M., Pizzul, E., Pastorino, P., & Prearo, M. (2025). Expanding Host Range: First Record of Eustrongylides excisus in Padogobius bonelli (Gobiidae) from the Po River (Northwest Italy). Fishes, 10(6), 254. https://doi.org/10.3390/fishes10060254









