Abstract
Aquaponics is an integrated method of aquatic animal and plant cultivation in a closed recycling system where the wastewater from aquatic animals is purified by microbes, which transform pollutants into nutrients for plants at the end of the chain. This technology allows to the efficiency of the area to be increased by a combination of cultivated plants and aquatic animals. Aquaponics produces environmentally friendly products by reducing fertilizer use and wastewater volume, increasing the extent of reuse by up to >90%. A promising way to increase efficiency in aquaponics is to use bacterial preparations (probiotics). This will allow control of the development of pathogens in the growing system, improving water quality and the growth rate of aquatic organisms. This paper overviews the experience of using probiotic preparations in aquaponic systems. It is shown that probiotics are able to increase the survival rate of aquatic organisms, improve the hydrochemical regime in recirculating aquaculture systems, and mitigate the risk of pathogenic contamination. There are a number of problems in aquaponics that prevent it from becoming more widespread and achieving maximum productivity, including problems with optimal pH and temperature, problems with nutrient and oxygen depletion, as well as diseases caused by phytopathogens and fish pathogens. The probiotics used do not take into account the biological needs of all components of the aquaponic system. The development of probiotic preparations from soil bacteria of the genus Bacillus will allow us to create a new class of probiotics specifically for aquaponics. Such preparations will work in a wide pH range, which will allow us to achieve maximum productivity for all components of aquaponics: animals, plants and bacteria.
Keywords:
aquaculture; bacterial component; Bacillus; phytostimulation; water quality; disease resistance Key Contribution:
The main objective of the review is to analyze and systematize the scientific knowledge of the influence of probiotic bacteria on the functioning of aquaponic systems. The authors present materials on the role of bacteria in aquaponics as well as the effect of probiotics on plants and aquatic animals. Current problems in aquaponics and possible ways of solving them through the development of new probiotics are shown.
1. Introduction
Aquaculture today is a dynamically developing agricultural sector with a rich history of development [1,2].
The world’s population growth makes the intensification of every agricultural sector important, especially in the case of clean fresh water. However, the production intensification in aquaculture leads to the deterioration of aquatic organisms’ habitat quality due to the accumulation of waste products and feed residues [3]. Water purification technologies with various types of filtration have been developed to solve this problem. There are various chemicals widely used for maintaining the smooth operation of farms [4].
The development of cultivation biotechnologies that take into account modern environmental problems, such as soil depletion, environmental pollution, limited water resources, and increasing fertilizer costs, is necessary. One such technology is aquaponics [5].
Aquaponics is a method of integrated cultivation of aquatic animals and plants in which wastewater (generated during the cultivation of aquatic animals) is purified by the microbial community of the system, converting pollutants into nutrients for plants [6]. Aquaponics allows the amount of agricultural products obtained to be significantly increased due to the combined cultivation of biological objects and increased efficiency per unit of area used [7], using water polluted as a result of production for growing additional objects in a hydroponic system [8,9,10,11,12]. As a result of the transformation of organic matter, water is purified, which can be reused in the fish-growing cycle. The transition to environmentally friendly production of aquatic animals and plants is made possible with aquaponics [13]. There is also a reduction in the amount of fertilizers used for plant growth and the volume of wastewater discharged [14,15]. This technology allows for the reuse of up to >90% of wastewater [16,17].
Aquaponic technology has been successfully used to grow rainbow trout (Oncorhynchus mykiss) (Walbaum, 1792) [18,19], African catfish (Clarias gariepinus Burchell, 1822) and other catfish [20,21], Eurasian perch (Perca fluviatilis Linnaeus, 1758) [22], goldfish [23], whiteleg shrimp (Litopenaeus vannamei Boone, 1931) [24], Nile tilapia (Oreochromis niloticus Linnaeus, 1758) [25], and other aquatic organisms.
Most often, lettuce is used as a plant component in aquaponic systems, but there have been attempts to grow tomatoes [26], cucumbers [22] and strawberries [27]. It has been suggested that peppermint is the most promising ammonium utilizer for aquaponics.
The bacterial component in aquaponics is fundamental for its efficient and sustainable functioning [12,28] since bacteria are involved in the processes of nitrification, decomposition of organic matter, denitrification, phosphorus mineralization, and iron cycling, and they also inhibit the emergence of phytopathogens [29].
Probiotics are bacteria that have the ability to have a positive effect on a macroorganism [30], which can be used to prevent or treat diseases. The term “probiotic” was first used in 1974 [31]. The use of such bacteria in aquaponic systems will increase the efficiency of this method of aquaculture. In addition, probiotics allow the cultivation of aquatic organisms without the use of hormones, chemicals, and antibiotics [32]. Increased productivity in aquaponics is achieved through various mechanisms, including nitrogen fixation, solubilization of mineral nutrients, and mineralization of organic matter. The use of probiotics in aquaponics is a strategy to mitigate environmental impacts and promote sustainable agriculture [33]. Nasser Kasozi et al. noted that available reports on the effects of probiotics in aquaculture focused on information on their effects on fish growth, survival, and immunity [33]. The peculiarity of aquaponics, consisting of multiple biological components, requires an integrated approach in studying the effects of probiotics: assessing the effects on fish, plants, and microorganisms together. There are not many studies evaluating the effectiveness of probiotics in aquaponic systems. Further development in this area requires the attention of researchers on the problem of selecting effective probiotics that will have a positive effect on all components of the system. The available scientific works devoted to the study of the influence of probiotics on aquaponic systems are focused on the specific case of growing a certain species of fish or crustaceans together with plants. Review articles in this area are sporadic. However, review papers make a significant contribution to development in this area, as they summarize and analyze a large amount of scientific data. In this regard, the purpose of this article was to summarize and analyze the data on the results of the use of various probiotic additives in aquaponics. In this case, we proceeded from the concept of the mutual influence of all three components in the system—plants, animals, and bacteria.
2. Materials and Methods
In this paper, the authors used the comparative-analytical method of research, as well as synthesis and generalization. Published scientific articles in peer-reviewed publications or chapters in monographs served as the material for this work. Suitable sources were searched in publicly available reference databases: Science Direct, Research Gate, Google Scholar, National MedLine, Wiley online library and others. Sources were searched using the keywords: “aquaculture”, “probiotics”, “aquaponics”, “fish”, “plants”, and “bacteria”. Keywords were used in various combinations and some (“aquaculture”, “probiotics”, and “aquaponics”) were also used alone. Time range was not used in the literature search, which allowed more scientific papers to be examined. Both review papers and articles with the results of original research on the topic were considered. In total, the authors analyzed more than 500 scientific papers related to aquaponics and probiotics, of which more than 100 are included in the list of sources used in this paper.
3. The Role of the Bacterial Component in Aquaponics
An aquaponic system consists of two main components: a tank for growing fish or other aquatic organisms and a hydroponic component for growing plants. Every aquaponic system contains a filter for coarse mechanical water purification in addition to the main components, and a filter for biological purification, which allows the purification of water from chemical contaminants for the use of bacteria.
Nitrifying microorganisms of the genera Nitrosomonas, Nitrobacter, and Nitrospira, which live in water, are added as a bacterial component in aquaponic systems [34]. They represent three key genera of bacteria that play crucial roles in the nitrogen cycle of aquaponic systems.
Bacteria in an aquaponic system convert ammonium into nitrate [35], purifying water from toxic substances [36]. Due to this, the nitrate level in aquaponic systems is significantly lower compared to other technologies for growing aquaculture objects. Thus, during the integrated cultivation of largemouth bass and lemongrass with onions for two months in a recirculating aquaculture system (RAS) and in aquaponics, the average nitrate content was 60.98% lower in aquaponics than in an RAS. A decrease in nitrate levels was recorded on the 48th day of the experiment.
It has been shown that the deterioration of habitat quality reduces the resistance of cultured aquatic organisms to diseases of various etiologies [37]. Ammonia is a toxic pollutant for aquatic organisms because, even at low concentrations of ammonia (e.g., 0.05 mg L−1), negative effects such as stress in fish can be observed, leading to deterioration of their health and decreased resistance to diseases. High concentrations of ammonia can cause acute and chronic toxic effects, including impaired growth and reduced feed efficiency; damage to gills, which makes breathing difficult; and increased susceptibility to infections and diseases [38,39,40].
Previously, the most commonly used two-step system was Nitrosomonas to convert toxic ammonia (NH3) produced from fish waste and uneaten fish food to nitrite (NO2−) in the first step of nitrification, and Nitrobacter to further oxidize the nitrite produced by Nitrosomonas into nitrate (NO3−) that plants can readily absorb and utilize as a nutrient for growth [41,42].
Recent research has shown that Nitrospira, specifically comammox (complete ammonia oxidizing) Nitrospira, can perform the entire nitrification process of converting ammonia to nitrate in a single organism. Comammox Nitrospira were found to be abundant and play a major role in nitrification even in highly efficient aquaponic systems with low steady-state ammonia concentrations. Moreover, Nitrospira can outcompete canonical ammonia and nitrite oxidizers under certain conditions in aquaponics [34,43,44]. The process is shown schematically in Figure 1.
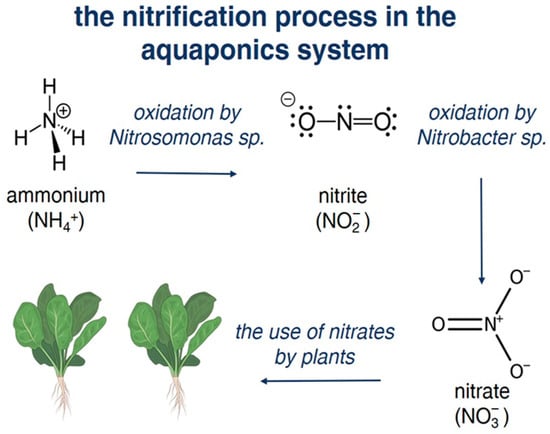
Figure 1.
Nitrification process in an aquaponic system.
The potential of using Nitrobacter and Nitrosomonas bacteria as an inoculum for the purification of wastewater from ammonia has been shown. A significant decrease in ammonia levels (p < 0.05) occurs with the addition of nitrifying bacteria Nitrosomonas and Nitrobacter to the system. The ammonia concentrations in the case of the bacteria being present in the system were 0.178 ± 0.0673 mg L−1 and 0.172 ± 0.0673 mg L−1 [25]. Ammonia reduction in the system was detected without spare doses of nitrificators but it was significantly slower due to the negligible concentration of primary bacteria.
As a probiotic supplement, which also has the ability to utilize ammonia, it is also possible to use bacteria of the genus Bacillus. The absorption of ammonia is achieved through diffusion, which then plays a role in the metabolism of nitrogen for glutamate synthesis [45].
Aquaponic systems are not sterile—in addition to the bacteria that are intentionally inoculated to improve the efficiency of the system, a community of microorganisms inevitably forms in the units from the gastrointestinal tracts of animals, the rhizosphere of plants, and from the air. Metagenomic analysis of aquaponic communities shows that the most common microbial groups in these systems are Archaea, Chlorobi, Armatimonadetes, Firmicutes, Latescibacteria, Gemmatimonadetes, Planctomycetes, Nitrospirae, Cyanobacteria, Saccharibacteria, Chloroflexi, Actinobacteria, Acidobacteria, Verrucomcrobia, Bacteroidetes, and Proteobacteria [34].
Fischer et al. demonstrated significant differences between the bacterial community in an RAS and an aquaponic system. The bacterial diversity index in the aquaponic system in this study was 44.8% higher than in an RAS. This was partly due to the detection of bacteria associated with plant roots, which was supported by other studies [35]. Interestingly, the aquaponic system was not an appropriate environment for the common Streptomyces bacteria. Fischer et al. suggested that this was due to the low phosphorus content in the aquaponic system due to its constant uptake by plants. This fact may be an important argument for the use of aquaponics in aquaculture, since geosmin, one of the metabolites of Streptomyces, is the cause of the unpleasant odor in fish after growing in an RAS.
Other bacteria also play a role in the functioning of aquaponic communities. Equally important to nitrification is solubilization, the breakdown of complex organic molecules that make up fish waste and uneaten food into nutrients that can be absorbed by plants. An example of the important role of bacteria in aquaponics is the conversion of insoluble phytates into phosphorus, which is available for uptake by plants [46].
It is known that genera like Flavobacterium and Sphingobacterium help to decompose organic matter and break down complex molecules like proteins. Some Comamonadaceae species produce siderophores that protect against fungal pathogens like Fusarium and Rhizoctonia, acting as biocontrol agents. Lysobacter are plant growth-promoting bacteria (PGPB) that enhance plant growth and produce antibiotics to fight plant diseases [47].
The complex microbiome in aquaponics is essential for biological filtration and mineralization of the nutrients required for plant growth [48]. Therefore, establishing a diverse and balanced bacterial ecosystem is key for the optimal functioning of aquaponic systems. Ongoing research aims to elucidate the microbial interactions to further improve the productivity and sustainability of aquaponic systems [12].
4. Experience of Using Probiotics in Aquaponics
Probiotics are “live microorganisms which, when administered in adequate amounts, confer a health benefit on the host”, as defined by the World Health Organization and the Food and Agriculture Organization of the United Nations [49,50] and by the International Scientific Association for Probiotics and Prebiotics (ISAPP) in 2014 [51].
There are strict criteria for the selection of probiotic bacteria, including safety, functionality, and technological utility, as established by the World Health Organization (WHO), the Food and Drug Administration (FAO) and the European Food Safety Authority (EFSA) [52].
The effect of probiotic preparations on various living objects has been studied for a long time. Numerous studies on the use of probiotics in aquaculture are devoted to the study of various fish-breeding characteristics of cultured objects, including sturgeon [53], trout [54], carp [55], catfish [56], Australian red-claw crayfish [57], shrimp [58], and others. Probiotics are introduced in aquaculture through feed and by adding strains into water [59] (Figure 2).
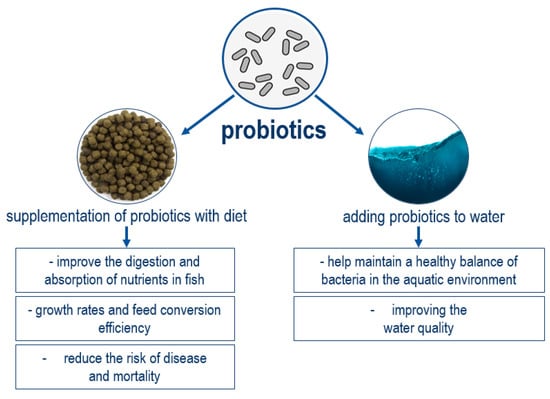
Figure 2.
Probiotic application methods in aquaculture [59,60,61,62].
Probiotics have repeatedly demonstrated their potential for use in classical aquaculture [63,64]. Aquaponic technologies have just begun their development and there is no so much information about probiotic use in these systems compared to “classical” aquaculture. Nevertheless, it is possible to note the growing interest of researchers and producers in studying the effects of probiotics on aquaponic system functioning (Table 1).

Table 1.
Experience with the use of probiotic supplements in aquaponics.
In summarizing the data, it can be noted that bacteria of the genus Bacillus are mentioned in such works most often. They are capable of having a beneficial effect not only on fish but also on other components of the aquaponic system, both living and non-living.
In our opinion, bacteria of the genera Bacillus and Paenibacillus are promising for use in aquaponic systems since they affect all three of their components. The introduction of probiotics into aquaponic systems should certainly begin with those bacteria that have already shown their usefulness when used in aquaculture. Firstly, species of these genera are known for their antagonistic activity against fish pathogens in aquaculture. For example, in experiments infecting Nile tilapia with a pathogenic bacterium Aeromonas hydrophila under the influence of bacteria Bacillus (B. velezensis TPS3N, B. subtilis TPS4 и B. amyloliquefaciens TPS17), fish had increased titers of immunoglobulin M, lysozyme, and alkaline phosphatase [74]. This fact may indicate an immunomodulatory effect of probiotics. It has been repeatedly proven that bacteria of the genus Bacillus are able to increase the survival of aquatic organisms [75] and increase the resistance to pathogens, even to such aggressive ones as Yersinia ruckeri and Vibrio harveyi [76,77].
Secondly, bacilli promote plant growth [78,79,80,81]. Bacilli are capable of producing volatile organic compounds (VOCs), which stimulate plant growth and inhibit the development of phytopathogens. For instance, the strain B. amyloliquefaciens FZB42 is a producer of microcin B17, streptolysin S, and amylocycline. These metabolites are able to inhibit the growth of pathogenic organisms (fungi and bacteria). Bacilli are able to affect the level of activity of plant genes responsible for the body’s defense mechanisms. Exposure to VOCs increased the expression of the gene R RRS1 (bacterial wilt resistance gene) along with increased expression of NPR1 and EDS1 [78].
B. subtilis bacteria, widely known in agriculture, help plants to assimilate nitrogen, phosphorus, and iron, which are not available in the environment in a plant-available form. For example, B. subtilis produces organic acids that facilitate the conversion of phosphorus into soluble form. In addition, B. subtilis produces 2 VOCs: 3-hydroxy-2-butanone (acetoin), and 2,3-butanediol. These metabolites stimulate plant growth by affecting cytokinitin and ethylene. Also, the effect of bacilli is recorded on auxin activity. In addition to influencing plant hormone homeostasis, B. subtilis is able to produce phytohormones such as cytokinitin on its own. In addition to influencing plant growth factors, B. subtilis is able to increase plant resistance to unfavorable environmental factors (e.g., drought). One such mechanism is the regulation of genes encoding the biosynthesis of abscisic acid, a hormone responsible for regulating stress in plants. Under drought stress, bacilli can regulate the synthesis of osmoprotectants [79]. It was found that some bacilli (e.g., B. velezensis BS89) are capable of producing a mixture of auxins, hydrolytic enzymes, and vitamins [80].
Third, Bacillus have the ability to oxidize ammonium under heterotrophic conditions (heterotrophic nitrification), and studies have previously been published on the characterization of B. subtilis strains with nitrifying–denitrifying activity that remove ammonium in marine aquaculture tanks [82]. Some bacilli species are known to have a process called sulfammox (ammonium oxidation under sulfate-reducing conditions) [83]. This process removes ammonium from the system through a reaction that reduces sulfate dissolved in water. Unlike conventional sulfate reduction, this reaction produces molecular sulfur instead of the toxic sulfide anion. These types of bacilli are typical in wastewater treatment systems [84] but have never been used in aquaponic systems. The use of microorganisms with such metabolic properties in addition to ammonium removal may serve as an additional biological control for sulfidogenic sulfate reduction, which is undesirable in aquaponics due to competition for electron acceptors.
In addition, bacilli are also capable of improving the hydrochemical regime in closed water supply systems [85]. Researchers have observed that probiotic bacteria of the genus Bacillus help to increase the availability of nitrate to aquaponic plants [33]. This helps to maintain the level of nitrogen compounds that is toxic to aquatic animals at a safe level [85].
In addition to bacilli, bacteria of the genus Lactobacillus deserve attention as probiotics for aquaponics. Some members of this genus (e.g., L. acidophilus) are able to suppress the development of phytopathogens such as Alternaria spp. and Fusarium oxysporium. This effect is achieved through the synthesis of organic acids, hydrogen peroxide, and bacteriocins by bacteria [86].
Thus, it can be concluded that probiotics are used in aquaponics. Probiotics can enhance the performance of aquaponic systems by improving fish growth, decreasing the feed conversion ratio (FCR), and increasing plant yields. They can achieve these benefits through various mechanisms, such as nitrogen fixation, mineral nutrient solubilization, organic matter mineralization, plant hormone modulation, and biocontrol. In aquaponics, probiotics can also be added directly into water or mixed with feed. They have shown the ability to improve water quality parameters, microbial communities, and overall system productivity. Probiotics like Bacillus spp. are particularly effective due to their ability to survive in harsh environmental conditions and produce antimicrobial substances that help fight pathogens and fish diseases [33].
5. Effect of Probiotics on Growth of Aquatic Animals in Aquaponic Systems
Modern aquaculture, including aquaponic systems, has significantly expanded the pool of cultivated species. In particular, invertebrates—shrimp—are becoming quite popular objects of cultivation. The existing problems in the industry, such as various etiologies of shrimp diseases, large amounts of discharged wastewater, and others [65], require an integrated environmental approach to their solution. A possible way to solve these problems is to inoculate the system with probiotic preparations. It has been shown that the introduction of probiotics increases the resistance of shrimp and other aquatic organisms to diseases [65], which significantly increases the efficiency of aquaculture.
The addition of probiotics to shrimp–plant aquaponic systems contributes to high shrimp survival even at high stocking densities [87]. This is due to the ability of probiotics to increase the body’s resistance to adverse external factors (stress) and the resistance of aquatic organisms to pathogens such as Vibrio spp. [65,88,89]. With the spread of antibiotic resistance around the world, the ability of probiotics to inhibit pathogenic bacteria is an encouraging strategy for selecting new effective therapeutic agents for disease control in aquaculture. The antibacterial effect of probiotics is achieved through several mechanisms: (1) synthesis of antibiotics and bacteriocins, (2) production of siderophores, (3) release of enzymes or hydrogen peroxide, and (4) release of organic acids that alter the intestinal pH and create unfavorable conditions for pathogens. In addition, probiotics affect the degree of activity of the nonspecific immune response of fish by increasing lysozyme activity and neutrophil migration. When growing fish in aquaculture, animals experience stress, which is associated with the intensification of production. Bacteria of the genus Lactobacillus are able to reduce the level of cortisol (stress marker) in fish tissues, and bacteria of the genus Alteromonas are able to increase glycogen and triglyceride stores in the fish liver, which also allows the organism to fight against stress [65]. In addition to survival, probiotics induce a decrease in the feed conversion ratio, which reduces the cost of the resulting commercial products [87]. At the same time, researchers have expressed several opinions about the reasons for the increased growth rate of aquatic animals: (1) increased appetite and (2) increased digestibility of consumed feed [65]. The second opinion is supported by the fact that probiotic bacteria produce exoenzymes (proteases, amylases, and lipases). This ability is also found in probiotic yeast (Debaryomyces hansenii HF1), which produces amylase and trypsin [65].
Growing fish in aquaponic systems with probiotic supplements also shows advantages. The specific growth rate of fish and their survival rate increase [69].
From the data in Table 1, it is obvious that Bacillus-based preparations are used as probiotic additives most often in aquaponic systems and they have many positive effects on fish and aquatic organisms (Figure 3).

Figure 3.
Effect of bacteria of the genus Bacillus on fish [65].
The method of serving probiotics (in feed or in water) affects the dose but not the quality because microorganisms in closed systems circulate with water and the probiotics pass through aquatic animals’ gastrointestinal systems, returning to the water again.
6. Effect of Probiotics on Plant Growth in Aquaponic Systems
The plant component of the aquaponic system is an important link in the multi-stage wastewater purification process, and it is also a source of additional agricultural product. The component can be called a “phytofilter”, which works in addition to the main biological filter, but allows additional products to be obtained.
It is also possible to intensify the processes of toxic substance utilization by introducing probiotic preparations into the system. It increases the growth rate of plants in aquaponics. For example, the weight of fresh lettuce shoots in a plant component of an aquaponic systems with the addition of probiotics was 15.3% higher than in the control system (without the addition of probiotics) [67]. Other studies have shown that the wet weight of the final harvest with the addition of probiotics was 23.8% higher than in the control experiment (24.84 ± 0.18 and 20.07 ± 0.02 g, respectively).
Better development of the root system of plants was also observed in experimental groups treated with probiotics [29]. Inoculation of the aquaponic system with Bacillus bacteria stimulates plant growth and increases phosphorus availability. When treated with probiotics, plants receive a greater amount of the elements necessary for their growth. As a result, the average dry weight of plants when adding probiotics increased more than 3 times compared to the control group (1.30 ± 0.92 and 4.09 ± 0.87 g, respectively). The chlorophyll index of plants grown in an aquaponic system with the addition of probiotics was almost 1.5 times higher than that in the control system [45].
Phytostimulation in an aquaponic system can be attributed to the fact that bacteria considered probiotic can also be classified as PGPB. Such bacteria can improve the efficiency of mineral uptake by plants by solubilizing nutrients such as phosphorus and improving nitrogen fixation. This is critical in aquaponics, where nutrient availability is directly linked to fish waste, which serves as the main source of nutrients for plants [89]. PGPB can also help to reduce plant stress through various mechanisms, including the production of phytohormones and the modulation of stress-related responses. This is particularly important in aquaponics, where environmental conditions can fluctuate due to the dual nature of the system (fish and plants) [90].
They can also act as biocontrol agents, helping to suppress plant diseases that might otherwise thrive in the nutrient-rich aquaponic environment, potentially reducing the need for chemical pesticides [48].
These stimulation mechanisms have been successfully applied in both hydroponics [89] and aquaponics [90]. Species of the genera Agrobacterium, Pseudomonas, Azospirillum, and Bacillus have been considered as suitable targets. The use of PGPB can reduce the dependence on synthetic fertilizers and improve the natural recycling of nutrients in the system, promoting a more environmentally friendly approach to food production.
7. Addressing Existing Aquaponic Problems with Probiotics and Modulating the Overall Microbial Community
In modern aquaponics, there are a number of pressing issues that prevent it from becoming more widespread and reaching maximum productivity and efficiency:
- The problem of optimum pH;
- The problem of optimum temperature;
- The problem of water pollution and turbidity, including algae;
- The problem of depletion of nutrients and oxygen;
- Diseases caused by phytopathogens and fish pathogens [6,28,91].
Using new non-traditional bacteria for aquaponics can solve these problems.
7.1. The Problem of Optimum pH
In particular, some new bacterial species can significantly aid in stabilizing pH levels in aquaponic systems. Nitrifying bacteria perform optimally at pH levels above 7.5. When pH levels drop below 6.0, their activity is inhibited, leading to ammonia accumulation, which is toxic to fish [29].
At the same time, the optimal pH range for fish in aquaponic systems is typically between 6.5 and 8.5, with most fish species performing best in the 7.0 to 8.0 range. For example, trout and other cold-water fish species prefer a slightly lower pH range of 6.5 to 7.5. Significant fluctuations or pH levels outside the tolerable range can stress fish and make them more susceptible to disease. When it comes to plants, the generally recommended pH for growing plants is slightly acidic (5.5–5.8). In a typical aquaponic system, a compromise pH of 6.8 to 7.2 is suitable for most fish species while still allowing for adequate nitrification [92,93]. However, this “compromise” system clearly does not work at its maximum potential. Thus, the availability of phosphorus, Fe, Cu, zinc (Zn), boron (B), and Mn to plants is significantly reduced when the pH is above 6.5–7.0 [94].
Introducing new bacterial species that can thrive at slightly lower pH levels could help to maintain nitrification rates, thus stabilizing pH. In addition, improving microbial diversity can increase the resilience of the system against pH fluctuations, as different bacteria may have varying tolerances and functionalities that can adapt to changing conditions. Some bacterial species can produce alkaline byproducts during metabolic processes, which can help to counteract the acidity caused by nitrification [95]. For instance, denitrifying bacteria can convert nitrates back into nitrogen gas, a process that can increase pH levels in weakly buffered systems [29]. The use of biofloc technology, which relies on heterotrophic bacteria, can also contribute to pH stabilization. These bacteria can utilize organic matter and enhance nutrient cycling while producing compounds that buffer pH levels [12].
7.2. Optimization of Temperature Resistance
Although probiotics do not directly control water temperature, they increase system stability under suboptimal conditions. Bacillus probiotics remain functional over a wide temperature range (17–34 °C/63–93 °F), providing efficient ammonia conversion even with minor fluctuations. By accelerating organic matter decomposition, probiotics reduce the metabolic load on nitrifying bacteria, indirectly keeping them active at lower temperatures. In trials, Bacillus-treated systems maintained stable nitrate levels despite temperature fluctuations, preventing ammonia toxicity [48].
7.3. Reducing Water Pollution and Turbidity
When Bacillus breaks down ammonia (NH3) and nitrite (NO2−) into less harmful nitrate (NO3−), the concentration of nitrogen compounds that promotes algal growth is reduced. Probiotics convert fish waste and uneaten food into CO2 instead of sludge, minimizing turbidity and organic pollution. Although turbidity may temporarily increase due to bacterial activity, long-term water clarity improves as solids are broken down. Bacillus strains also mineralize organic phosphorus, reducing soluble phosphate levels that contribute to eutrophication. A 2-week trial showed that the addition of Bacillus bacteria reduced NH3 levels by 25% and NO2− levels by 10% while increasing plant biomass by 18% [48,96].
7.4. Prevention of Nutrient and Oxygen Deficiencies
Probiotics improve nutrient recycling and oxygen utilization efficiency. By converting fish waste into bioavailable nitrate and phosphate, probiotics ensure a constant supply of nutrients to plants. For example, lettuce grown in systems supplemented with Bacillus showed 15–20% higher phosphorus and zinc uptake. Lettuce grown with Bacillus had 20% higher shoot dry weight and an improved nutrient profile [48].
7.5. Pathogen and Disease Control
Probiotics suppress phytopathogens and fish pathogens through a number of known mechanisms common to all systems where probiotics are used.
Competitive exclusion—Bacillus colonizes surfaces in the water and intestines of fish, displacing such pathogens such as Aeromonas and Vibrio [96].
Production of antimicrobial substances such as bacteriocins, lysozymes, and siderophores that directly inhibit pathogens [96,97].
Immunostimulation—Probiotics enhance fish immunity by increasing phagocytic activity and antibody production, which reduces mortality during disease outbreaks [98].
7.6. Systemic Approach in Aquaponics
Although it is rarely discussed in the literature as a separate issue, in aquaponics, as in any technology, there is a question of the limit of maximum efficiency, in this case, in the speed and volume of conversion of nutrients into biomass. In order to if not reach the maximum then at least significantly increase this indicator, it is necessary to approach the issue and look for ways to intensify metabolic processes in the aquaponic ecosystem.
The effects of probiotics on all components of this ecosystem can be summarized in the form of a diagram, as shown in Figure 4.

Figure 4.
Effect of probiotics on three main components of aquaponics. 1–5—interactions in the aquaponic system (explanation in the text). The * sign indicates those interactions that are enhanced by the introduction of probiotics. The ** sign indicates interactions that may be affected indirectly.
In Figure 4, the number 1 indicates the interaction of animals with bacteria. What is most important here is that bacteria are able to process ammonia and organic residues. Unlike autotrophic bacteria, often used in aquaponic systems, heterotrophic nitrifiers can decompose the remains of dead animals themselves and not just the resulting ammonia.
The number 2 represents the impact of bacteria on animals in aquaculture. In a traditional aquaponic microbial community, the production of metabolites that affect animals is minimal or poorly understood, so this relationship is limited to the removal of toxic ammonia. Adding probiotics to the system will enhance this interaction, as described above in Section 4 and Section 5.
The number 3 denotes the effects of bacteria on plants in this system. The intensification of nitrate production processes and phytostimulation by bacteria are described in Section 6.
The number 4 represents the effect that aquaponic plants have on bacteria. It is known that in aquaponics plant roots play a significant role in shaping the surrounding microbial community through the release of root exudates into the rhizosphere. These include sugars, amino acids, organic acids, and phenolic compounds. Some exudates act as chemoattractants, selectively promoting the colonization of beneficial bacteria around the roots. Studies have shown that plants can exert a strong selective pressure on the microbial community, leading to a distinct rhizobiome that differs from the surrounding water column [99]. Stimulation of plant growth by PGPB, described above, will also cause an intensification of this process, so that connections 3 and 4 in this scheme strengthen each other according to the principle of positive feedback, achieving maximum efficiency.
The number 5 indicates the influence of plants on aquaculture objects. Plants in the aquaponic system utilize fish waste and fish feed residues as nutrients, improving water quality by reducing the levels of these waste products. Plants produce oxygen that is released into the water, helping to oxygenate the fish’s environment and help stabilize the pH in the aquaponic system [100,101,102]. In addition, antioxidant components in plant roots can reduce oxidative stress in fish [103]. These processes can also be intensified through the stimulation of plant growth (indirect effect).
The number 6 represents the impact of animals on plants in aquaponics. There is little evidence of direct effects, but in terms of animal waste as a source of organic matter, the connection is clear. Fish processing byproducts, such as hydrolysates, are rich in proteins and amino acids that can act as biostimulants. These compounds enhance nutrient uptake efficiency in plants and can improve root architecture, leading to better growth and yield [104,105]. The nutrients derived from fish waste not only support plant growth but also enhance the efficiency of nutrient utilization within the system [106,107].
It is also interesting to note that animal feed residues introduced into the system can also have an impact on plants [46], with phosphorus being particularly important for plant growth and often being a limiting factor in aquaponic systems [106,107].
Finally, we have designated interactions within the microbial community with the number 7. Naturally, the introduction of probiotics will change the entire structure of the microbiome, and the exact effects of each probiotic bacteria have yet to be assessed using modern metatranscriptomic methods. However, it can be assumed that the influence of probiotic bacteria of the genus Bacillus and those close to them will be beneficial. Given the diversity of bacilli functions, they can be called a kind of stabilizers in the artificial aquaponic ecosystem. Given their diversity in soil consortia [108], we can continue the thought and assume that they play a similar role in nature. Thus, it is known that Bacillus can modulate the activity of other microbes [109] and increase the richness of bacterial and fungal communities [110].
While probiotic supplementation offers numerous benefits, its application in aquaponics requires careful consideration of microbial community dynamics. Heterotrophic bacteria, including Bacillus spp., can compete with autotrophic nitrifiers (Nitrosomonas and Nitrospira) for dissolved oxygen (DO) and inorganic carbon [111]. In systems with high organic loads from fish feed or plant root exudates, heterotrophic overgrowth can reduce DO levels below the 5–8 mg L−1 threshold critical for fish respiration and nitrification [112]. For example, biofilm-clogged substrates in nutrient film technique (NFT) systems exhibited 60–90% reductions in hydraulic conductivity due to heterotrophic biomass accumulation, potentially destabilizing water flow and gas exchange [113].
The carbon-to-nitrogen (C:N) ratio is a crucial control parameter. At C:N > 10:1, heterotrophs dominate nitrogen cycling, diverting ammonium toward biomass production rather than nitrification. This creates conflicting requirements: plants thrive at C:N ratios of 20–30:1 for optimal growth, while nitrifiers require C:N < 4:1 [114,115]. This means Bacillus-derived proteases may accelerate organic matter mineralization, inadvertently fueling heterotrophic blooms that depress pH through CO2 production—a direct threat to nitrifier activity below pH 6.5. Balancing plant productivity with microbial stability therefore requires precision in probiotic dosing and maintaining the C:N ratio at 8–12:1 through controlled feed inputs and carbon supplementation.
Bacteria of the genus Bacillus are known as beneficial microorganisms for plants and animals, but only in an aquaponic systems is it possible to evaluate their impact on both groups simultaneously, as well as on the entire artificial ecosystem as a whole.
Studying the effects of individual microorganisms in aquaponic systems, in addition to the obvious practical benefits, is also of fundamental importance because it allows us to evaluate the role of these microorganisms in natural communities in a controlled environment, i.e., an aquaponic installation.
8. Future Directions
Considering all of the above, several directions for future research can be identified. We believe that integrating new technologies in aquaponics workflow can improve this technology’s efficiency and upgrade aquaponics from artisanal practice to precision agriculture.
Obviously, metagenomic profiling is a go-to technology to explore any multispecies bacterial community. But metatranscriptomic profiling of biofilms could identify the subtle regulatory mechanisms and communications in these communities, for example, the quorum-sensing trigger signals for undesirable heterotrophic dominance. Time-resolved metabolomics may reveal interspecies cross-feeding networks—for instance, Bacillus siderophores could enhance iron uptake in other bacteria in a consortium [116]. Quorum quenching is also one of the underestimated mechanisms of action in aquaculture probiotics that could be explored via omics technologies [117]. Also, portable nanopore sequencers now enable on-site monitoring of functional genes (amoA and nxrB) to monitor equilibrium in the system and preempt nitrification collapse [118].
Another promising direction is developing bacterial consortia including new and rare species that are usually not considered as probiotics. Developments in synthetic biology can provide precision microbiome engineering, combining CRISPR editing with synthetic ecology principles to create self-regulating aquaponic systems. There are gene-modified strains of, for instance, ammonia-oxidizing bacteria [119] or aquaculture probiotics. The next step can be developing multifunctional strains or more effective consortia.
Some existing aquaculture problems can be solved only by implementing engineering techniques. For example, to establish the optimal dosage and prevent negative effects, we should quantify the threshold effects of probiotic inoculation density on system performance. Automated dosing systems synchronized with real-time ammonia sensors (e.g., ion-selective electrodes) could maintain bacterial populations up to 106–107 CFU mL−1, the therapeutic range observed in tilapia trials [102].
The integration of biofloc technology with aquaponics also represents a sustainable innovation that combines microbial-driven aquaculture with plant cultivation. In this system, the biofloc, as an aggregation of heterotrophic bacteria, algae, and organic matter, serves dual roles. It maintains water quality by converting toxic ammonia into microbial protein and provides supplemental nutrition for fish, while nutrient-rich water from the aquaculture component is recirculated to fertilize plants. Studies demonstrate that adding probiotics such Bacillus spp. and Streptomyces spp. to biofloc-aquaponic systems enhances fish growth, feed efficiency, and survival rates by stabilizing microbial communities and suppressing pathogens. Simultaneously, plants benefit from bioavailable nutrients like nitrate and phosphate, with their root systems further filtering water and reducing ammonia levels. This synergy not only boosts productivity but also aligns with circular economy principles, offering a scalable solution for resource-limited environments [62,120,121].
9. Conclusions
Thus, it can be concluded that probiotic bacteria introduced into aquaponic systems can contribute to the intensification of growing plant and animal crops as well as reduce the environmental impact of their production. Among potential probiotics, representatives of the genus Bacillus stand out in particular. They have positive effects on the animal and plant components of the system and complement the microbial community, increasing its diversity.
Author Contributions
Conceptualization, D.R. and E.P.; methodology, A.O.; software, M.O.; validation, V.S., M.O. and S.T.; formal analysis, D.R.; investigation, A.O.; resources, V.S.; data curation, M.O.; writing—original draft preparation, V.S. and S.T.; writing—review and editing, E.P.; visualization, V.S.; supervision, A.O.; project administration, D.R.; funding acquisition, D.R. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by the Russian Science Foundation, grant no. 23-76-30006 “Molecular aquaculture strategy in the design of novel synbiotic preparations for improvement of health and quality in fishery”.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| RAS | Recirculating aquaculture system |
| PGPB | Plant growth-promoting bacteria |
| FCR | Feed conversion ratio |
| VOCs | Volatile organic compounds |
| DO | Dissolved oxygen |
| NFT | Nutrient film technique |
| C:N | Carbon-to-nitrogen |
References
- Nash, C. The History of Aquaculture; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Boyd, C.E.; Tucker, C.S. Pond Aquaculture Water Quality Management; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Sukardi, P.; Soedibya, P.H.T.; Pramono, T.B. Produksi budidaya ikan nila (Oreochromis niloticus) sistem bioflok dengan sumber karbohidrat berbeda. J. AJIE-Asian J. Innov. Entrep. 2018, 3, 198–203. [Google Scholar]
- Dauda, A.B.; Ajadi, A.; Tola-Fabunmi, A.S.; Akinwole, A.O. Waste production in aquaculture: Sources, components and managements in different culture systems. Aquac. Fish. 2019, 4, 81–88. [Google Scholar] [CrossRef]
- Bartelme, R.P.; Oyserman, B.O.; Blom, J.E.; Sepulveda-Villet, O.J.; Newton, R.J. Stripping away the soil: Plant growth promoting microbiology opportunities in aquaponics. Front. Microbiol. 2018, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Yep, B.; Zheng, Y. Aquaponic trends and challenges—A review. J. Clean. Prod. 2019, 228, 1586–1599. [Google Scholar] [CrossRef]
- Goddek, S.; Joyce, A.; Kotzen, B.; Dos-Santos, M. Aquaponics and global food challenges. In Aquaponics Food Production Systems: Combined Aquaculture and Hydroponic Production Technologies for the Future; Springer: Cham, Switzerland, 2019; pp. 3–17. [Google Scholar] [CrossRef]
- Rakocy, J.E. Aquaponics—Integrating fish and plant culture. In Aquaculture Production Systems; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 344–386. [Google Scholar]
- Tidwell, J.H. On the drawing board. Aquac. Prod. Syst. 2012, 80, 395. [Google Scholar] [CrossRef]
- Somerville, C.; Cohen, M.; Pantanella, E.; Stankus, A.; Lovatelli, A. Small-Scale Aquaponic Food Production: Integrated Fish and Plant Farming; FAO Fisheries and Aquaculture Technical Paper; FAO: Rome, Italy, 2014. [Google Scholar]
- Nugroho, R.A.; Pambudi, L.T.; Chilmawati, D.; Haditomo, A.H.C. Aplikasi teknologi aquaponic pada budidaya ikan air tawar untuk optimalisasi kapasitas produksi. J. Saintek Perikan. 2012, 8, 46–51. [Google Scholar] [CrossRef]
- Joyce, A.; Timmons, M.; Goddek, S.; Pentz, T. Bacterial relationships in aquaponics: New research directions. In Aquaponics Food Production Systems: Combined Aquaculture and Hydroponic Production Technologies for the Future; Springer: Cham, Switzerland, 2019; pp. 145–161. [Google Scholar] [CrossRef]
- Rakocy, J. Aquaculture engineering—The status of aquaponics, part 1. Aquac. Mag. 1999, 25, 83–88. [Google Scholar]
- Adler, P.R.; Harper, J.K.; Takeda, F.; Summerfelt, S.T. Economic analysis of an aquaponic system for the integrated production of rainbow trout and plants. Int. J. Recirc. Aquac. 2000, 1, 15–34. [Google Scholar] [CrossRef]
- Cerozi, B.D.S.; Fitzsimmons, K. Phosphorus dynamics modeling and mass balance in an aquaponics system. Agric. Syst. 2017, 153, 94–100. [Google Scholar] [CrossRef]
- Tyson, R.V.; Treadwell, D.D.; Simonne, E.H. Opportunities and challenges to sustainability in aquaponic systems. HortTechnology 2011, 21, 6–13. [Google Scholar] [CrossRef]
- Dalsgaard, J.; Lund, I.; Thorarinsdottir, R.; Drengstig, A.; Arvonen, K.; Pedersen, P.B. Farming different species in RAS in Nordic countries: Current status and future perspectives. Aquac. Eng. 2013, 53, 2–13. [Google Scholar] [CrossRef]
- Forchino, A.A.; Lourguioui, H.; Brigolin, D.; Pastres, R. Aquaponics and sustainability: The comparison of two different aquaponic techniques using the Life Cycle Assessment (LCA). Aquac. Eng. 2017, 77, 80–88. [Google Scholar] [CrossRef]
- Birolo, M.; Bordignon, F.; Trocino, A.; Fasolato, L.; Pascual, A.; Carlo, N.; Carmelo, M.; Xiccato, G. Effects of stocking density on the growth and flesh quality of rainbow trout (Oncorhynchus mykiss) reared in a low-tech aquaponic system. Aquaculture 2020, 529, 735653. [Google Scholar] [CrossRef]
- Endut, A.; Jusoh, A.; Ali, N.; Nik, W.W.; Hassan, A. A study on the optimal hydraulic loading rate and plant ratios in recirculation aquaponic system. Bioresour. Technol. 2010, 101, 1511–1517. [Google Scholar] [CrossRef]
- Handayani, R.; Dinoto, A. Effect of fermented feed supplementation in circulated aquaponic system with catfish (Clarias sp.) on growth of lettuce (Lactuca sativa L.). IOP Conf. Ser. Earth Environ. Sci. 2020, 572, 012009. [Google Scholar] [CrossRef]
- Graber, A.; Junge, R. Aquaponic Systems: Nutrient recycling from fish wastewater by vegetable production. Desalination 2009, 246, 147–156. [Google Scholar] [CrossRef]
- Nuwansi, K.K.T.; Verma, A.K.; Prakash, C.; Tiwari, V.K.; Chandrakant, M.H.; Shete, A.P.; Prabhath, G.P.W.A. Effect of water flow rate on polyculture of koi carp (Cyprinus carpio var. koi) and goldfish (Carassius auratus) with water spinach (Ipomoea aquatica) in recirculating aquaponic system. Aquac. Int. 2016, 24, 385–393. [Google Scholar] [CrossRef]
- Fierro-Sañudo, J.F.; Rodríguez-Montes de Oca, G.A.; León-Cañedo, J.A.; Alarcón-Silvas, S.G.; Mariscal-Lagarda, M.M.; Díaz-Valdés, T.; Páez-Osuna, F. Production and management of shrimp (Penaeus vannamei) in co-culture with basil (Ocimum basilicum) using two sources of low-salinity water. Lat. Am. J. Aquat. Res. 2018, 46, 63–71. [Google Scholar] [CrossRef]
- Dwiardani, K.H.; Rahardja, B.S. Utilization of Nitrosomonas sp and Nitrobacter sp probiotic towards total suspended solid and ammonia level in nile tilapia culturing using aquaponic system. IOP Conf. Ser. Earth Environ. Sci. 2021, 679, 012067. [Google Scholar] [CrossRef]
- Suhl, J.; Dannehl, D.; Kloas, W.; Baganz, D.; Jobs, S.; Scheibe, G.; Schmidt, U. Advanced aquaponics: Evaluation of intensive tomato production in aquaponics vs. conventional hydroponics. Agric. Water Manag. 2016, 178, 335–344. [Google Scholar] [CrossRef]
- Abbey, M.; Anderson, N.O.; Yue, C.; Short, G.; Schermann, M.; Phelps, N.; Venturell, P.; Vickers, Z. An Analysis of Strawberry (Fragaria χ ananassa) Productivity in Northern Latitudinal Aquaponic Growing Conditions. J. Am. Pomol. Soc. 2019, 73, 22–37. [Google Scholar] [CrossRef]
- Eck, M.; Körner, O.; Jijakli, M.H. Nutrient cycling in aquaponics systems. In Aquaponics Food Production Systems: Combined Aquaculture and Hydroponic Production Technologies for the Future; Springer: Cham, Switzerland, 2019; pp. 231–246. [Google Scholar] [CrossRef]
- Kasozi, N.; Abraham, B.; Kaiser, H.; Wilhelmi, B. The complex microbiome in aquaponics: Significance of the bacterial ecosystem. Ann. Microbiol. 2021, 71, 1. [Google Scholar] [CrossRef]
- Gatesoupe, F.J. The use of probiotics in aquaculture. Aquaculture 1999, 180, 147–165. [Google Scholar] [CrossRef]
- Parker, R.B. Probiotics, the other half of the antibiotic story. Anim. Nutr. Health 1974, 29, 4–8. [Google Scholar]
- Priyadarshini, S.; Gopinath, V.; Priyadharsshini, N.M.; MubarakAli, D.; Velusamy, P. Synthesis of anisotropic silver nanoparticles using novel strain, Bacillus flexus and its biomedical application. Colloids Surf. B Biointerfaces 2013, 102, 232–237. [Google Scholar] [CrossRef]
- Kasozi, N.; Iwe, G.D.; Walakira, J.; Langi, S. Integration of probiotics in aquaponic systems: An emerging alternative approach. Aquac. Int. 2024, 32, 2131–2150. [Google Scholar] [CrossRef]
- Heise, J.; Müller, H.; Probst, A.J.; Meckenstock, R.U. Ammonium removal in aquaponics indicates participation of comammox Nitrospira. Curr. Microbiol. 2021, 78, 894–903. [Google Scholar] [CrossRef]
- Schmautz, Z.; Graber, A.; Jaenicke, S.; Goesmann, A.; Junge, R.; Smits, T.H. Microbial diversity in different compartments of an aquaponics system. Arch. Microbiol. 2017, 199, 613–620. [Google Scholar] [CrossRef]
- Hasan, Z.; Andriani, Y.; Hamdani, H.; Sahidin, A.; Surbakti, S.B. Effect of probiotics addition with different dosage on water quality performance of Sangkuriang Catfish (Clarias gariepinus) farming in the aquaponic system. In Proceedings of the 1st International Conference on Islam, Science and Technology 2021, ICONISTECH 2019, Bandung, Indonesia, 11–12 July 2019. [Google Scholar] [CrossRef]
- Gjedrem, T. Disease resistant fish and shellfish are within reach: A review. J. Mar. Sci. Eng. 2015, 3, 146–153. [Google Scholar] [CrossRef]
- Ip, Y.K.; Chew, S.F. Ammonia production, excretion, toxicity, and defense in fish: A review. Front. Physiol. 2010, 1, 134. [Google Scholar] [CrossRef]
- Boyd, C.E. Ammonia toxicity degrades animal health, growth. Glob. Aquac. Advocate Dec. 2013. Available online: https://aquafishcrsp.oregonstate.edu/sites/aquafishcrsp.oregonstate.edu/files/gaa_boyd_nov13_1.pdf (accessed on 27 February 2025).
- Lin, W.; Luo, H.; Wu, J.; Hung, T.C.; Cao, B.; Liu, X.; Yang, P. A review of the emerging risks of acute ammonia nitrogen toxicity to aquatic decapod crustaceans. Water 2022, 15, 27. [Google Scholar] [CrossRef]
- Putra, I.; Pamukas, N.A.; Rusliadi, R. Peningkatan kapasitas produksi akuakultur pada pemeliharaan ikan selais (Ompok sp) sistem aquaponik. J. Perikan. Dan Kelaut. 2013, 18, 1–10. [Google Scholar]
- Taragusti, A.S.; Santanumurti, M.B.; Rahardja, B.S. Effectiveness of Nitrobacter on the specific growth rate, survival rate and feed conversion ratio of dumbo catfish Clarias sp. with density differences in the aquaponic system. IOP Conf. Ser. Earth Environ. Sci. 2019, 236, 012088. [Google Scholar] [CrossRef]
- Mehrani, M.J.; Sobotka, D.; Kowal, P.; Ciesielski, S.; Makinia, J. The occurrence and role of Nitrospira in nitrogen removal systems. Bioresour. Technol. 2020, 303, 122936. [Google Scholar] [CrossRef]
- Yang, X.; Wu, Y.; Shu, L.; Gu, H.; Liu, F.; Ding, J.; Zeng, J.; Wang, C.; He, Z.; Xu, M.; et al. Unraveling the important role of comammox Nitrospira to nitrification in the coastal aquaculture system. Front. Microbiol. 2024, 15, 1355859. [Google Scholar] [CrossRef] [PubMed]
- Cerozi, B.; Fitzsimmons, K. Use of Bacillus spp. to enhance phosphorus availability and serve as a plant growth promoter in aquaponics systems. Sci. Hortic. 2016, 211, 277–282. [Google Scholar] [CrossRef]
- Eck, M.; Sare, A.R.; Massart, S.; Schmautz, Z.; Junge, R.; Smits, T.H.; Jijakli, M.H. Exploring bacterial communities in aquaponic systems. Water 2019, 11, 260. [Google Scholar] [CrossRef]
- Krastanova, M.; Sirakov, I.; Ivanova-Kirilova, S.; Yarkov, D.; Orozova, P. Aquaponic systems: Biological and technological parameters. Biotechnol. Biotechnol. Equip. 2022, 36, 305–316. [Google Scholar] [CrossRef]
- Kasozi, N.; Kaiser, H.; Wilhelmi, B. Effect of Bacillus spp. on lettuce growth and root associated bacterial community in a small-scale aquaponics system. Agronomy 2021, 11, 947. [Google Scholar] [CrossRef]
- FAO; WHO. Health and Nutritional Properties of Probiotics in Food including Powder Milk with Live Lactic Acid Bacteria; Food and Agriculture Organization of the United Nations: Rome, Italy; World Health Organization: Cordoba, Argentina, 2001. [Google Scholar]
- FAO; WHO. Guidelines for the Evaluation of Probiotics in Food; Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food; Food and Agriculture Organization of the United Nations: Rome, Italy; World Health Organization: London, ON, Canada, 2002. [Google Scholar]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Zommiti, M.; Feuilloley, M.G.; Connil, N. Update of probiotics in human world: A nonstop source of benefactions till the end of time. Microorganisms 2020, 8, 1907. [Google Scholar] [CrossRef] [PubMed]
- Askarian, F.; Kousha, A.; Salma, W.; Ringø, E. The effect of lactic acid bacteria administration on growth, digestive enzyme activity and gut microbiota in Persian sturgeon (Acipenser persicus) and beluga (Huso huso) fry. Aquac. Nutr. 2011, 17, 488–497. [Google Scholar] [CrossRef]
- Merrifield, D.L.; Bradley, G.; Baker, R.T.M.; Davies, S.J. Probiotic applications for rainbow trout (Oncorhynchus mykiss Walbaum) II. Effects on growth performance, feed utilization, intestinal microbiota and related health criteria postantibiotic treatment. Aquac. Nutr. 2010, 16, 496–503. [Google Scholar] [CrossRef]
- Yanbo, W.; Zirong, X. Effect of probiotics for common carp (Cyprinus carpio) based on growth performance and digestive enzyme activities. Anim. Feed Sci. Technol. 2006, 127, 283–292. [Google Scholar] [CrossRef]
- Al-Dohail, M.A.; Hashim, R.; Aliyu-Paiko, M. Effects of the probiotic, Lactobacillus acidophilus, on the growth performance, haematology parameters and immunoglobulin concentration in African Catfish (Clarias gariepinus, Burchell 1822) fingerling. Aquac. Res. 2009, 40, 1642–1652. [Google Scholar] [CrossRef]
- Méndez-Martínez, Y.; Torres-Navarrete, Y.G.; Cortés-Jacinto, E.; García-Guerrero, M.U.; Hernández-Hernández, L.H.; Verdecia, D.M. Biological, nutritional, and hematoimmune response in juvenile Cherax quadricarinatus (Decapoda: Parastacidae) fed with probiotic mixture. Rev. MVZ Cordoba 2022, 27, e2578. [Google Scholar] [CrossRef]
- Ziaei-Nejad, S.; Rezaei, M.H.; Takami, G.A.; Lovett, D.L.; Mirvaghefi, A.R.; Shakouri, M. The effect of Bacillus spp. bacteria used as probiotics on digestive enzyme activity, survival and growth in the Indian white shrimp Fenneropenaeus indicus. Aquaculture 2006, 252, 516–524. [Google Scholar] [CrossRef]
- Zhou, X.; Tian, Z.; Wang, Y.; Li, W. Effect of treatment with probiotics as water additives on tilapia (Oreochromis niloticus) growth performance and immune response. Fish Physiol. Biochem. 2010, 36, 501–509. [Google Scholar] [CrossRef]
- Sahu, M.K.; Swarnakumar, N.S.; Sivakumar, K.; Thangaradjou, T.; Kannan, L. Probiotics in aquaculture: Importance and future perspectives. Indian J. Microbiol. 2008, 48, 299–308. [Google Scholar] [CrossRef]
- Tabassum, T.; Mahamud, A.S.U.; Acharjee, T.K.; Hassan, R.; Snigdha, T.A.; Islam, T.; Alam, R.; Khoiam, M.U.; Akter, F.; Azad, R.; et al. Probiotic supplementations improve growth, water quality, hematology, gut microbiota and intestinal morphology of Nile tilapia. Aquac. Rep. 2021, 21, 100972. [Google Scholar] [CrossRef]
- Nadia, Z.M.; Akhi, A.R.; Roy, P.; Farhad, F.B.; Hossain, M.M.; Salam, M.A. Yielding of aquaponics using probiotics to grow tomatoes with tilapia. Aquac. Rep. 2023, 33, 101799. [Google Scholar] [CrossRef]
- Irianto, A.; Austin, B. Probiotics in aquaculture. J. Fish Dis. 2002, 25, 633–642. [Google Scholar] [CrossRef]
- Hai, N.V. The use of probiotics in aquaculture. J. Appl. Microbiol. 2015, 119, 917–935. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.T.; Brown, P.B. Sustainable marine aquaponics: Effects of shrimp to plant ratios and C/N ratios. Front. Mar. Sci. 2021, 8, 771630. [Google Scholar] [CrossRef]
- Huitzitl-López, M.G.; Monroy-Dosta, M.D.C.; Castro-Mejía, J.; Vázquez-Silva, G.; Chávez Serrano, E.M. Growth evaluation of Ambystoma mexicanum and Ocimum basilicum with application of Bacillus subtilis probiotic in aquaponic system. Int. J. Aquat. Sci. 2018, 9, 93–98. [Google Scholar]
- Kasozi, N.; Kaiser, H.; Wilhelmi, B. Determination of phylloplane associated bacteria of lettuce from a small-scale aquaponic system via 16S rRNA gene amplicon sequence analysis. Horticulturae 2022, 8, 151. [Google Scholar] [CrossRef]
- Said, M.M.; Zaki, F.M.; Ahmed, O.M. Effect of the Probiotic (Bacillus spp.) on Water Quality, Production Performance, Microbial Profile, and Food Safety of the Nile Tilapia and Mint in Recirculating Aquaponic System. Egypt. J. Aquat. Biol. Fish. 2022, 26, 351–372. [Google Scholar] [CrossRef]
- Taufik, I.; Setijaningsih, L.; Widyastuti, Y.R. Performance growth and survival of Hemibagrus nemurus with probiotic application on aquaponics. IOP Conf. Ser. Earth Environ. Sci. 2022, 1119, 012070. [Google Scholar] [CrossRef]
- Sirakov, I.; Velichkova, K.; Slavcheva-Sirakova, D. The ProViotic® supplemented diet on growth performance, biochemical blood parameters and meat quality of common carp (Cyprinus carpio L.) and growth of lettuce (Lactuca sativa) cultivated in aquaponics. J. Hyg. Eng. Des. 2021, 34, 26–30. [Google Scholar]
- Setiawan, D.; Rahardja, B.S. Utilization of Nitrosomonas sp. and Nitrobacter sp. probiotic towards nitrite and nitrate level in nile tilapia (Oreochromis niloticus) using aquaponic system. IOP Conf. Ser. Earth Environ. Sci. 2021, 718, 012098. [Google Scholar] [CrossRef]
- Dhahiyat, Y.; Andriani, Y.; Sahidin, A.; Farizi, I. Impact of Red Water System (RWS) application on water quality of catfish culture using aquaponics. IOP Conf. Ser. Earth Environ. Sci. 2018, 139, 012009. [Google Scholar] [CrossRef]
- Sirakov, I.; Velichkova, K.; Stoyanova, S.; Kaymakanova, M.; Slavcheva-Sirakova, D.; Atanasova, R.; Staykov, Y. Effect of synbiotic dietary supplementation on growth, physiological and immunological parameters in common carp (Cyprinus carpio L.) fingerlings and on yield and physiological parameters in lettuce (Lactuca sativa L.), cultivated in mesocosmos aquaponic system. Bulg. J. Agric. Sci. 2018, 24 (Suppl. S1), 140–149. [Google Scholar]
- Kuebutornye, F.K.; Wang, Z.; Lu, Y.; Abarike, E.D.; Sakyi, M.E.; Li, Y.; Hlordzi, V. Effects of three host-associated Bacillus species on mucosal immunity and gut health of Nile tilapia, Oreochromis niloticus and its resistance against Aeromonas hydrophila infection. Fish Shellfish Immunol. 2020, 97, 83–95. [Google Scholar] [CrossRef]
- Rengpipat, S.; Rukpratanporn, S.; Piyatiratitivorakul, S.; Menasaveta, P. Immunity enhancement in black tiger shrimp (Penaeus monodon) by a probiont bacterium (Bacillus S11). Aquaculture 2000, 191, 271–288. [Google Scholar] [CrossRef]
- Raida, M.K.; Larsen, J.L.; Nielsen, M.E.; Buchmann, K. Enhanced resistance of rainbow trout, Oncorhynchus mykiss (Walbaum), against Yersinia ruckeri challenge following oral administration of Bacillus subtilis and B. licheniformis (BioPlus2B). J. Fish Dis. 2003, 26, 495–498. [Google Scholar] [CrossRef]
- Vaseeharan, B.; Ramasamy, P. Control of pathogenic Vibrio spp. by Bacillus subtilis BT23, a possible probiotic treatment for black tiger shrimp Penaeus monodon. Lett. Appl. Microbiol. 2003, 36, 83–87. [Google Scholar] [CrossRef]
- Tahir, H.A.S.; Gu, Q.; Wu, H.; Niu, Y.; Huo, R.; Gao, X. Bacillus volatiles adversely affect the physiology and ultra-structure of Ralstonia solanacearum and induce systemic resistance in tobacco against bacterial wilt. Sci. Rep. 2017, 7, 40481. [Google Scholar] [CrossRef] [PubMed]
- Blake, C.; Christensen, M.N.; Kovács, Á.T. Molecular aspects of plant growth promotion and protection by Bacillus subtilis. Mol. Plant-Microbe Interact. 2021, 34, 15–25. [Google Scholar] [CrossRef]
- Chebotar, V.K.; Chizhevskaya, E.P.; Vorobyov, N.I.; Bobkova, V.V.; Pomyaksheva, L.V.; Khomyakov, Y.V.; Konovalov, S.N. The quality and productivity of strawberry (Fragaria× ananassa Duch.) improved by the inoculation of PGPR Bacillus velezensis BS89 in field experiments. Agronomy 2022, 12, 2600. [Google Scholar] [CrossRef]
- Sretenović, M.; Tamaš, N.; Zec, G.; Stojanoski, M.; Tešić, N.; Miletić, N.; Djordjević, B. Productivity, biocontrol and postharvest fruit quality of strawberry cultivar ‘Clery’ using plant growth promoting microorganisms. Cogent Food Agric. 2024, 10, 2310896. [Google Scholar] [CrossRef]
- Huang, F.; Pan, L.; Lv, N.; Tang, X. Characterization of novel Bacillus strain N31 from mariculture water capable of halophilic heterotrophic nitrification–aerobic denitrification. J. Biosci. Bioeng. 2017, 124, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Dominika, G.; Joanna, M.; Jacek, M. Sulfate reducing ammonium oxidation (SULFAMMOX) process under anaerobic conditions. Environ. Technol. Innov. 2021, 22, 101416. [Google Scholar] [CrossRef]
- Cai, L.S.; Wang, L.; Song, K.; Lu, K.L.; Zhang, C.X.; Rahimnejad, S. Evaluation of protein requirement of spotted seabass (Lateolabrax maculatus) under two temperatures, and the liver transcriptome response to thermal stress. Aquaculture 2020, 516, 734615. [Google Scholar] [CrossRef]
- Chen, C.C.; Chen, S.N. Water quality management with Bacillus spp. in the high-density culture of red-parrot fish Cichlasoma citrinellum × C. synspilum. N. Am. J. Aquac. 2001, 63, 66–73. [Google Scholar] [CrossRef]
- Panetto, L.D.; Doria, J.; Santos, C.H.B.; Frezarin, E.T.; Sales, L.R.; de Andrade, L.A.; Rigobelo, E.C. Lactic Bacteria with Plant-Growth-Promoting Properties in Potato. Microbiol. Res. 2023, 14, 279–288. [Google Scholar] [CrossRef]
- Dierberg, F.E.; Kiattisimkul, W. Issues, impacts, and implications of shrimp aquaculture in Thailand. Environ. Manag. 1996, 20, 649–666. [Google Scholar] [CrossRef]
- Martínez Cruz, P.; Ibáñez, A.L.; Monroy Hermosillo, O.A.; Ramírez Saad, H.C. Use of probiotics in aquaculture. Int. Sch. Res. Not. 2012, 2012, 916845. [Google Scholar] [CrossRef]
- Nemutanzhela, M.E.; Roets, Y.; Gardiner, N.; Lalloo, R. The use and benefits of Bacillus based biological agents in aquaculture. Sustain. Aquac. Tech. 2014, 19, 1–34. [Google Scholar] [CrossRef]
- Olmos, J.; Acosta, M.; Mendoza, G.; Pitones, V. Bacillus subtilis, an ideal probiotic bacterium to shrimp and fish aquaculture that increase feed digestibility, prevent microbial diseases, and avoid water pollution. Arch. Microbiol. 2020, 202, 427–435. [Google Scholar] [CrossRef]
- Kuebutornye, F.K.; Abarike, E.D.; Lu, Y. A review on the application of Bacillus as probiotics in aquaculture. Fish Shellfish Immunol. 2019, 87, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Stegelmeier, A.A.; Rose, D.M.; Joris, B.R.; Glick, B.R. The use of PGPB to promote plant hydroponic growth. Plants 2022, 11, 2783. [Google Scholar] [CrossRef]
- Piñero, M.C.; Collado-González, J.; Otálora, G.; López-Marín, J.; Del Amor, F.M. Plant Growth-Promoting Rhizobacteria as Tools to Improve the Growth of Kohlrabi (Brassica oleracea var. gongylodes) Plants in an Aquaponics System. Plants 2024, 13, 595. [Google Scholar] [CrossRef] [PubMed]
- Aleksic, N.; Sustersic, V. Analysis of application of aquaponic system as a model of the circular economy: A review. Reciklaza I Odrziv. Razvoj. 2020, 13, 73–86. [Google Scholar] [CrossRef]
- Wang, Y.J.; Yang, T.; Kim, H.J. pH dynamics in aquaponic systems: Implications for plant and fish crop productivity and yield. Sustainability 2023, 15, 7137. [Google Scholar] [CrossRef]
- Rahayu, S.; Amoah, K.; Huang, Y.; Cai, J.; Wang, B.; Shija, V.M.; Jin, X.; Anokyewaa, M.A.; Jiang, M. Probiotics application in aquaculture: Its potential effects, current status in China and future prospects. Front. Mar. Sci. 2024, 11, 1455905. [Google Scholar] [CrossRef]
- Torres-Maravilla, E.; Parra, M.; Maisey, K.; Vargas, R.A.; Cabezas-Cruz, A.; Gonzalez, A.; Tello, M.; Bermúdez-Humarán, L.G. Importance of Probiotics in Fish Aquaculture: Towards the Identification and Design of Novel Probiotics. Microorganisms 2024, 12, 626. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Sun, Y.Z.; Wang, A.; Zhou, Z. Probiotics as means of diseases control in aquaculture, a review of current knowledge and future perspectives. Front. Microbiol. 2018, 9, 2429. [Google Scholar] [CrossRef]
- Zou, Y.; Hu, Z.; Zhang, J.; Xie, H.; Guimbaud, C.; Fang, Y. Effects of pH on nitrogen transformations in media-based aquaponics. Bioresour. Technol. 2016, 210, 81–87. [Google Scholar] [CrossRef]
- Lobanov, V.; Keesman, K.J.; Joyce, A. Plants dictate root microbial composition in hydroponics and aquaponics. Front. Microbiol. 2022, 13, 848057. [Google Scholar] [CrossRef]
- Hu, Z.; Lee, J.W.; Chandran, K.; Kim, S.; Brotto, A.C.; Khanal, S.K. Effect of plant species on nitrogen recovery in aquaponics. Bioresour. Technol. 2015, 188, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Suhl, J.; Oppedijk, B.; Baganz, D.; Kloas, W.; Schmidt, U.; van Duijn, B. Oxygen consumption in recirculating nutrient film technique in aquaponics. Sci. Hortic. 2019, 255, 281–291. [Google Scholar] [CrossRef]
- Mao, H.; Wang, B.; Zhao, J.; Wang, Y.; Du, X.; Shi, Q. Influences of Aquaponics System on Growth Performance, Antioxidant Parameters, Stress Parameters and Gene Expression of Carassius auratus. Fishes 2023, 8, 360. [Google Scholar] [CrossRef]
- Senavirathna, M.D.H.J.; Zhaozhi, L.; Fujino, T. Root adsorption of microplastic particles affects the submerged freshwater macrophyte Egeria densa. Water Air Soil Pollut. 2022, 233, 80. [Google Scholar] [CrossRef]
- Madende, M.; Hayes, M. Fish by-product use as biostimulants: An overview of the current state of the art, including relevant legislation and regulations within the EU and USA. Molecules 2020, 25, 1122. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Chen, Q.F.; Wang, J.N.; Liu, T.; Zhao, W.Y. Substrates, plants, and their combinations for water purification of urban household aquaponics systems. Int. J. Environ. Res. Public Health 2022, 19, 10276. [Google Scholar] [CrossRef]
- Ulaş, A.; Yücel, Y.C.; Ulaş, F. The application of fish wastewater to improve the plant growth, development and yield of lettuce (Lactuca sativa L.). Int. J. Agric. Environ. Food Sci. 2022, 6, 100–107. [Google Scholar] [CrossRef]
- Fruscella, L.; Kotzen, B.; Paradelo, M.; Milliken, S. Investigating the effects of fish effluents as organic fertilisers on onion (Allium cepa) yield, soil nutrients, and soil microbiome. Sci. Hortic. 2023, 321, 112297. [Google Scholar] [CrossRef]
- Yahya, G.; Ebada, A.; Khalaf, E.M.; Mansour, B.; Nouh, N.A.; Mosbah, R.A.; Saber, S.; Moustafa, M.; Negm, S.; El-Sokkary, M.M.A.; et al. Soil-associated Bacillus species: A reservoir of bioactive compounds with potential therapeutic activity against human pathogens. Microorganisms 2021, 9, 1131. [Google Scholar] [CrossRef]
- Andrić, S.; Meyer, T.; Ongena, M. Bacillus Responses to Plant-Associated Fungal and Bacterial Communities. Front. Microbiol. 2020, 11, 1350. [Google Scholar] [CrossRef]
- Eck, M.; Szekely, I.; Massart, S.; Jijakli, M.H. Ecological Study of Aquaponics Bacterial Microbiota over the Course of a Lettuce Growth Cycle. Water 2021, 13, 2089. [Google Scholar] [CrossRef]
- Thakur, K.; Kuthiala, T.; Singh, G.; Arya, S.K.; Iwai, C.B.; Ravindran, B.; Khoo, K.S.; Chang, S.W.; Awasthi, M.K. An alternative approach towards nitrification and bioremediation of wastewater from aquaponics using biofilm-based bioreactors: A review. Chemosphere 2023, 316, 137849. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Li, Y.; Wang, X.; Zhang, H.; Liu, Y. Maximizing nutrient recovery from aquaponics wastewater with autotrophic or heterotrophic management strategies. Bioresour. Technol. Rep. 2023, 21, 101360. [Google Scholar] [CrossRef]
- Peng, Z.; Chen, P.; Li, Y.; Wang, X.; Zhang, H. C/N ratio shifted autotrophic partial nitrification to heterotrophic nitrification and aerobic denitrification in high-strength ammonium wastewater treatment. SSRN Electron. J. 2024. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, Y.; Li, J.; Wang, L.; Chen, H. Responses of various carbon to nitrogen ratios to microbial communities, kinetics, and nitrogen metabolic pathways in aerobic granular sludge reactor. Bioresour. Technol. 2023, 367, 128225. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.L.; Kanoh, K.; Kamino, K. Effect of exogenous siderophores on iron uptake activity of marine bacteria under iron-limited conditions. Appl. Environ. Microbiol. 2001, 67, 1710–1717. [Google Scholar] [CrossRef]
- Rwezawula, P.; Mwanja, W.W.; Vereecke, N.; Bossier, P.; Vanrompay, D. Advancing aquaculture probiotic discovery via an innovative protocol for isolation of indigenous, heat and salt tolerant, quorum quenching probiotic candidates. Front. Microbiol. 2025, 16, 1558238. [Google Scholar] [CrossRef]
- Ruiz, A.; Scicchitano, D.; Palladino, G.; Nanetti, E.; Candela, M.; Furones, D.; Sanahuja, I.; Carbó, R.; Gisbert, E.; Andree, K.B. Microbiome study of a coupled aquaponic system: Unveiling the independency of bacterial communities and their beneficial influences among different compartments. Sci. Rep. 2023, 13, 19704. [Google Scholar] [CrossRef]
- Sayavedra-Soto, L.A.; Stein, L.Y. Genetic transformation of ammonia-oxidizing bacteria. Methods Enzymol. 2021, 486, 389–402. [Google Scholar] [CrossRef]
- Yu, Y.-B.; Choi, J.-H.; Lee, J.-H.; Jo, A.-H.; Han, S.W.; Han, S.-H.; Choi, H.J.; Choi, C.Y.; Kang, J.-C.; Min, E.; et al. Biofloc Application Using Aquaponics and Vertical Aquaculture Technology in Aquaculture: Review. Fishes 2023, 8, 543. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Sharifinia, M.; Akhavan-Bahabadi, M.; Emerenciano, M.G.C. Probiotics and Phytobiotics as Dietary and Water Supplements in Biofloc Aquaculture Systems. Aquac. Nutr. 2024, 1, 3089887. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).