1. Introduction
The large yellow croaker (
Larimichthys crocea) is one of the most economically significant marine aquaculture species in China. It is predominantly distributed along the coastal waters of the East China Sea, with the highest production volumes observed in the Fujian and Zhejiang provinces. Historical overfishing had severely depleted wild stocks of
L. crocea, bringing them to the verge of collapse. Advancements in artificial breeding technologies and large-scale aquaculture practices over recent years have spurred a gradual recovery and rapid growth of the industry [
1,
2,
3]. The proportion of
L. crocea in China’s total marine fish aquaculture production has been steadily increasing, positioning it as a cornerstone a of China’s marine aquaculture sector. Concurrently, the expansion of aquaculture has heightened technological requirements, particularly regarding the modernization of farming methodologies and the improvement of production efficiency.
Over recent decades, the adaptation of large-scale offshore deep-water cages (hereafter referred to as “large sea cages”) for the aquaculture of
L. crocea has increased significantly. This trend is driven by the need to expand farming space and improve rearing environments. In contrast to industrialized aquaculture systems and small-scale inshore cage setups, large sea cages are typically deployed in high-current environments with elevated water exchange rates, which facilitate improved water quality parameters. Among the hydrodynamic variables, current velocity emerges as a critical determinant of fish vitality and health in large sea cages, serving as a key indicator of adaptive capacity. Fluctuations in current velocity can stimulate fish sensory mechanisms, thereby triggering behavioral and locomotory adjustments that ultimately impact feeding efficiency, growth performance, and immune function [
4,
5,
6]. The behavioral responses of fish to current changes are complex. For example, in cage systems, Atlantic salmon (
Salmo salar) exhibit transitions from circumferential swimming patterns to rheotactic orientations and aggregated schooling behavior as current velocity increases [
7]. Currents also affect swimming stability and threat-response rates [
8], reflecting species-specific adaptations to distinct hydrodynamic environments. Empirical evidence further demonstrates that current velocity modulates growth performance, stress responses, and immune parameters in farmed fish [
5], suggesting that hydrodynamic conditions act as environmental regulators of the physiological state, thereby governing behavioral phenotypes and adaptive strategies. Surveys with local aquaculturists have documented frequent occurrences of external lesions in cage-cultured
L. crocea, particularly on the caudal fins and oral regions, although etiological mechanisms remain undefined. A plausible hypothesis posits that the large current velocity in offshore cage environments may disrupt natural swimming mechanics, leading to excessive crowding or increased physical friction against netting structures—pathways that could precipitate tissue damage. Despite substantial advancements in offshore aquaculture infrastructure, the mechanistic links between current dynamics, fish vitality, and spatial behavior remain poorly characterized—a critical knowledge gap impeding the optimization of cage placement and production sustainability. Previous investigations have predominantly relied upon indoor flume tanks or small-scale cage trials, but these confined systems impose behavioral restrictions and fail to recapitulate the complex hydrodynamic environments of offshore cages, thereby limiting the ecological relevance of observed fish responses. Consequently, there is an imperative for in situ investigations to characterize current-induced effects on
L. crocea within large-scale commercial cage systems, providing foundational data for the evidence-based management of open-ocean aquaculture operations.
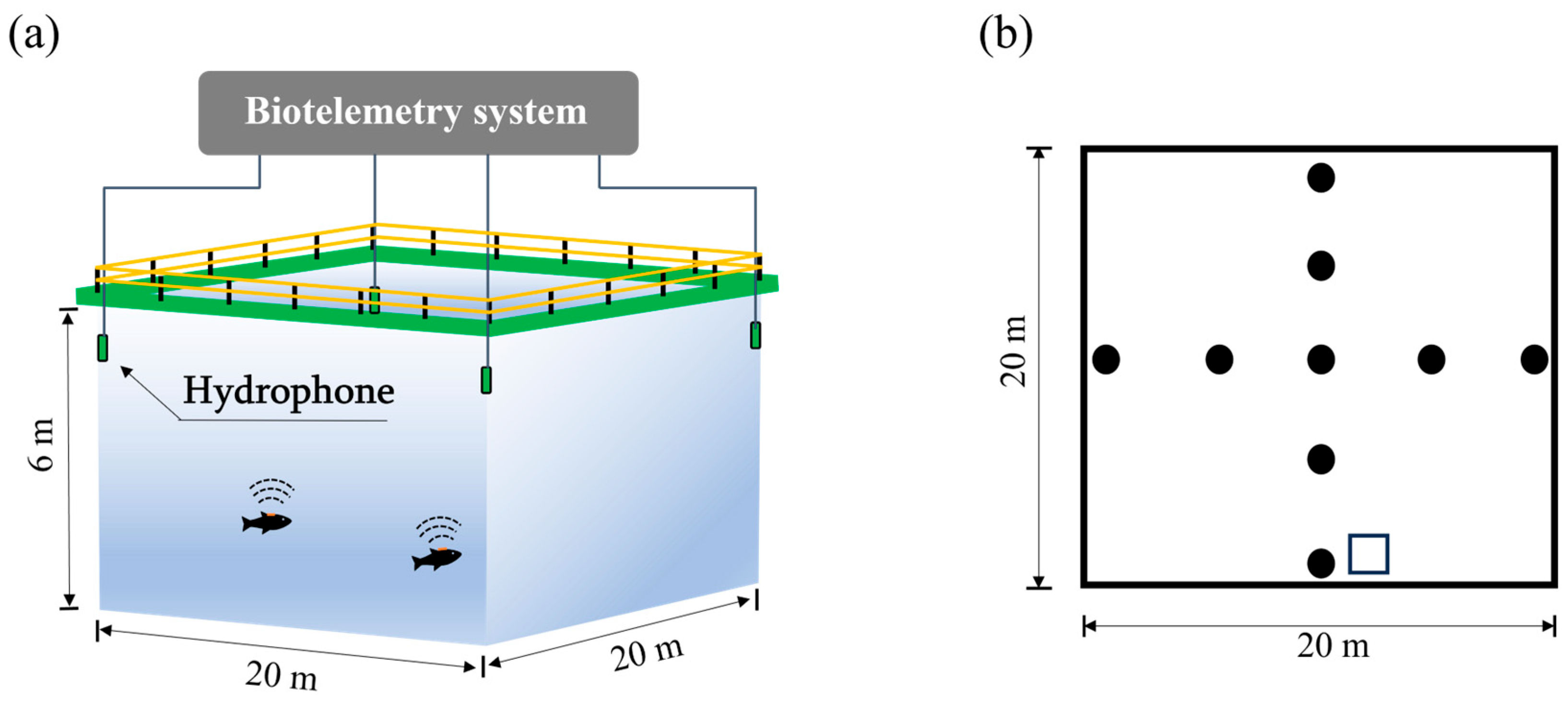
Compared to land-based recirculating aquaculture systems (RASs) and nearshore small-scale cages, large offshore cages offer significantly expanded rearing volumes. However, conventional monitoring approaches, such as direct visual observation and underwater camera-based techniques face inherent limitations due to restricted visibility ranges, rendering them ineffective for the comprehensive behavioral monitoring of fish swimming dynamics across these vast aquatic environments. Acoustic methodologies have emerged as a robust solution for fish behavior monitoring, leveraging the low attenuation of sound waves in water and their independence from turbidity or photic conditions. Key acoustic methodologies frequently employed in the assessment of fish behavior encompass multibeam sonar, scientific echosounders, and ultrasonic biotelemetry [
9,
10]. Multibeam sonar enables the detection of large-scale fish school distributions. However, in aquaculture settings characterized by elevated fish densities, signal overlap frequently compromises the acquisition of detailed and valuable data, such as individual swimming velocities and school density. Scientific echosounders enable the monitoring of fish schools within the beam’s purview, thereby supporting the investigation of behavioral traits like vertical migration patterns, spatial distribution, and density fluctuations. Yet, the narrow beam width of scientific echosounders employed for fish tracking imposes significant constraints on the observable volume, presenting challenges for characterizing behavior within large sea cages. Ultrasonic biotelemetry technology utilizes acoustic signal transmitters (pingers), which can be either surgically implanted or attached to study fish [
10,
11]. This method employs hydrophone arrays to receive transmitted signals and a hyperbolic positioning algorithm to locate tagged fish, thereby enabling precise reconstruction of swimming trajectories [
10,
12,
13]. The ultrasonic biotelemetry system offers distinct advantages over multibeam and echosounder systems by facilitating large-scale, individual-level tracking with high-resolution spatial–temporal data. Contemporary ultrasonic biotelemetry systems are typically configured for multi-target monitoring, using unique coding to distinguish signals from multiple tagged individuals. Additionally, miniaturized systems now incorporate auxiliary sensors (e.g., for temperature and depth measurements) to acquire concurrent environmental data, enhancing the adoption of ultrasonic biotelemetry in fish behavior research. Originally employed for tracking migratory behavior in species like Atlantic salmon (
Salmo salar), this technology has recently been increasingly applied to monitor the behavioral patterns of farmed fish within large-scale aquaculture enclosures. Representative applications include evaluating locomotor performance and spatial utilization patterns of Atlantic cod (
Gadus morhua) in commercial-scale circular sea cages [
14], and the behavioral characterization of Northern whitefish (
Coregonus peled) in set nets within Lake Sayram conducted by Liu et al. [
12]. Collectively, these studies demonstrate biotelemetry’s preeminence for large-scale aquatic behavior monitoring, combining individual-level resolution with scalable spatial coverage.
To investigate the effects of water flow on the behavior of L. crocea, this study utilized ultrasonic biotelemetry to tag and track L. crocea in a commercial-scale offshore sea-cage system maintained at a low stocking density. The reduced stocking density was intentionally implemented to prevent stress responses associated with overcrowding, thereby allowing for a focused investigation into the effects of water flow on fish behavior. Through in situ three-dimensional tracking experiments, this study characterized the depth distribution and spatial behavioral patterns of the tagged fish, with a specific focus on their behavioral responses to dynamic current conditions. The findings are expected to provide evidence-based insights for optimizing the design and siting of large sea cages for L. crocea production systems.
4. Discussion
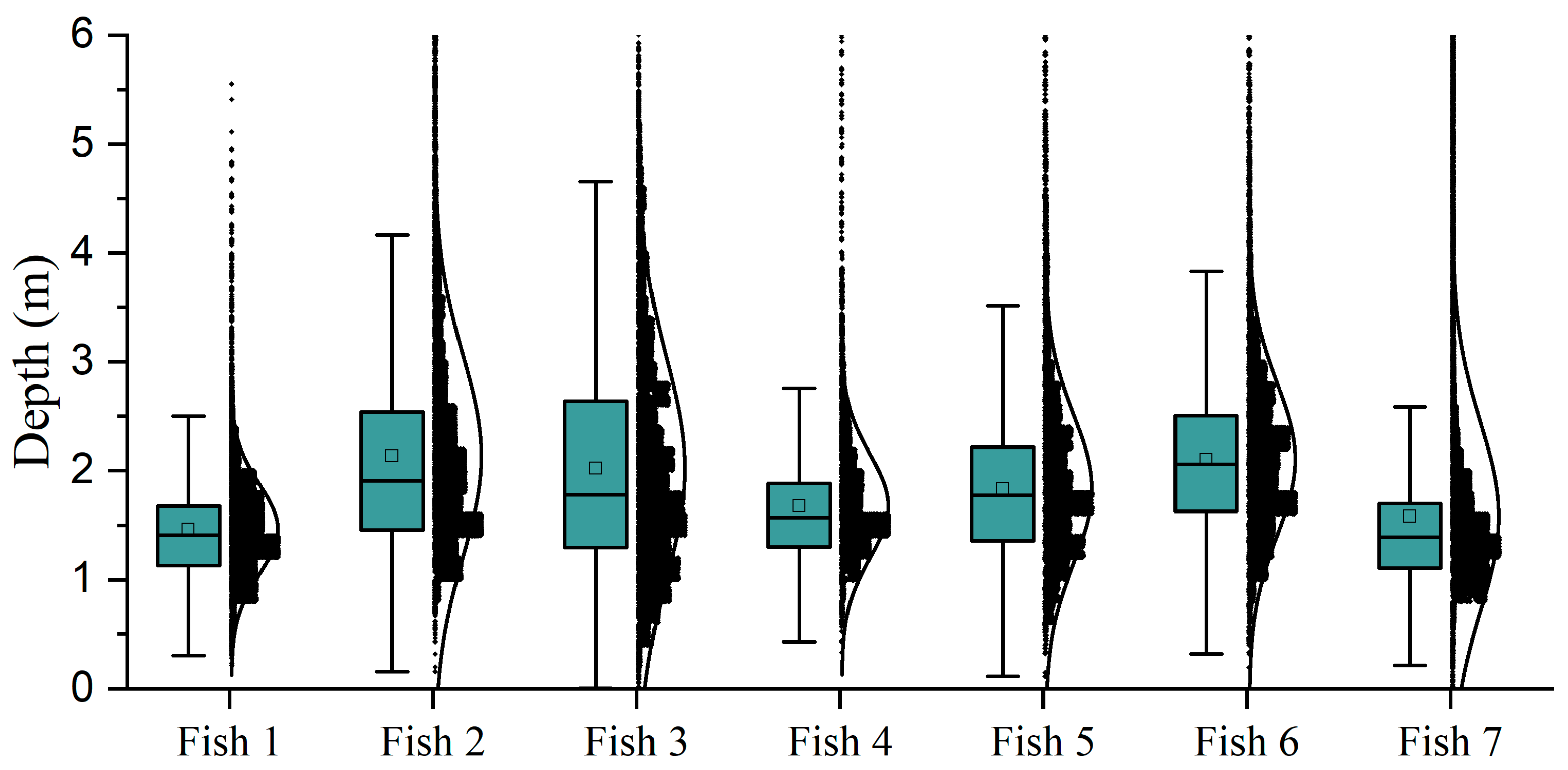
In this study, the spatial distribution behavior of cage-cultured
L. crocea was systematically monitored. With regarding to swimming depth, the fish predominantly occupied the upper water volume of the cage (1–2.6 m) throughout the experimental period. Temporal variation in swimming depth revealed no distinct diel vertical migration pattern among the tagged fish (
Figure S1). This depth distribution pattern differed from that reported by Song et al. [
17]. This discrepancy may stem from two key factors: (1) the smaller body length of the
L. crocea in their study (about 31.8 cm) compared to those in this experiment, and (2) seasonal variations in water temperature between the studies, as thermal conditions are known to influence fish vertical distribution. However, the study by Song et al. [
17] did not specify water temperature information. According to aquaculture technicians, daily water temperature fluctuations within the same season typically remain within 1 °C. Therefore, future studies should conduct seasonal experiments to further investigate the effects of water temperature on the behavior of
L. crocea. Notably, while previous studies have documented diel vertical migration (DVM) in cage-cultured fish such as nighttime deepening behavior observed in gilthead seabream (
Sparus aurata) by Muñoz et al. and diurnal vertical shuttling behavior observed in Rockfish (
Sebastes ruberrimus) by Sthapit et al. [
18]. These patterns contrast with the depth distribution of
L. crocea in this study, which may be attributed to light intensity—a known factor influencing fish vertical distribution. The high turbidity and low light penetration in the experimental waters, caused by elevated sediment levels, could explain the observed discrepancies. However, since light intensity was not measured in this trial, this hypothesis remains speculative. Future studies should incorporate concurrent measurements of light intensity and their influence on the depth distribution of
L. crocea. Notably, the depth distribution of fish is closely associated with sea-cage utilization efficiency, a critical factor in determining optimal cage depth configuration. The swimming depth distribution data of
L. crocea obtained in this study may offer valuable insights for optimizing depth configuration in future sea-cage designs for this species.
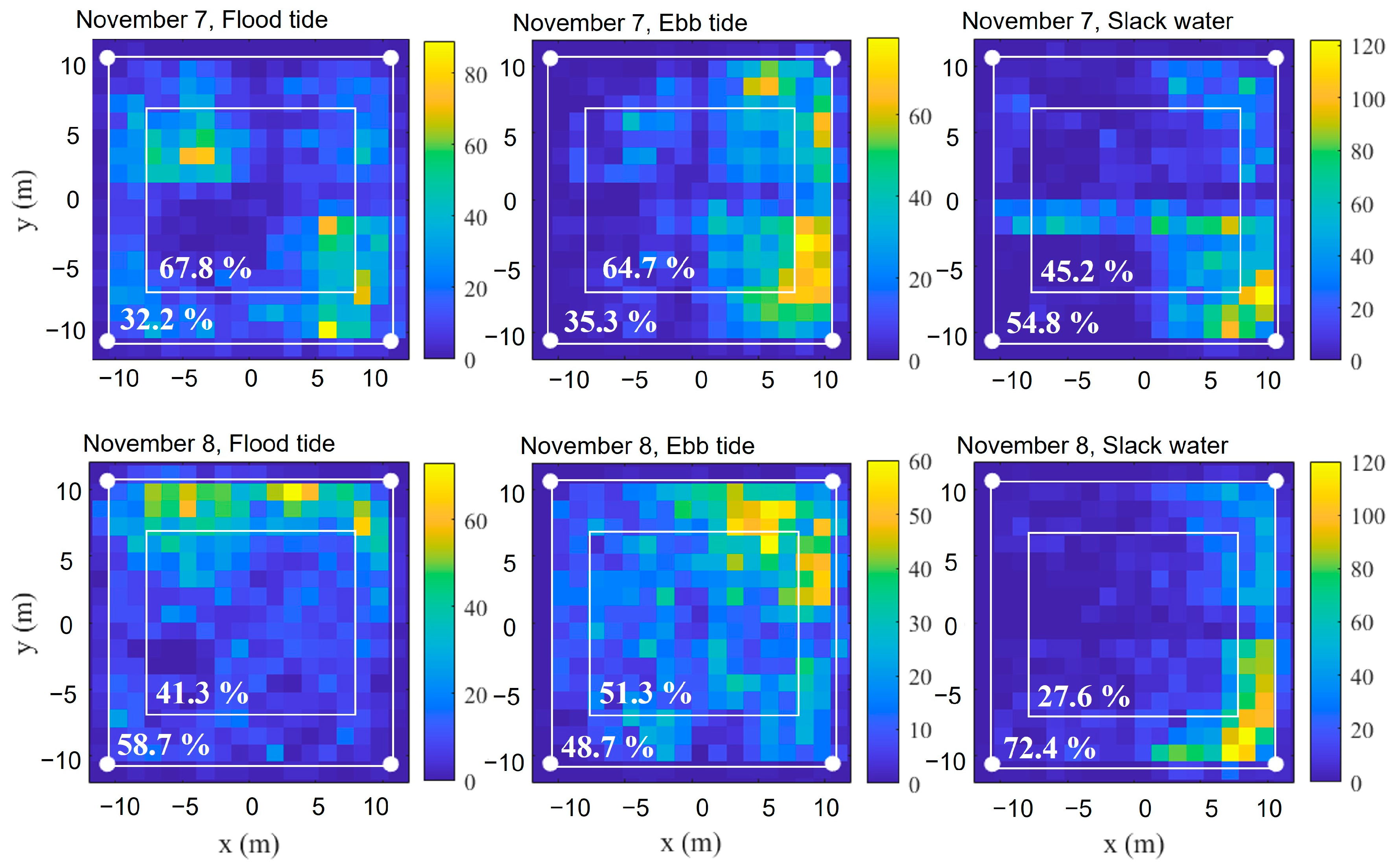
In terms of horizontal distribution,
L. crocea exhibited predominantly stochastic spatial distributions within the large sea cages. While occasional localized aggregations were observed, such as the lower-right quadrant clustering between 15:30 and 16:30 on 8 November, as illustrated in
Figure 3, individuals also displayed whole-cage movement patterns, as seen in their swimming behavior throughout the entire cage space between 09:03 and 10:30 on 7 November (
Figure 3). This lack of consistent directional movement contrasts with the circular swimming patterns documented in other cage-cultured species. For instance, Hamano et al. demonstrated that bluefin tuna (
Thunnus orientalis) in circular cages typically exhibit persistent perimeter swimming [
19], while Anras reported similar edge-circling behavior in cage-cultured rainbow trout (
Oncorhynchus mykiss) [
20]. Notably, no significant circular swimming pattern was observed in the
L. crocea during this study. This behavioral difference may be attributed to cage geometry, as both Hamano et al. and Anras and Lagardère employed circular cages, whereas this study utilized a square configuration. Additionally, the number of
L. crocea reared in the large sea cage during this study was approximately 500 individuals, resulting in a relatively low stocking density of 0.19 individuals per cubic meter (ind/m
3) compared to commercial systems. The low density was selected to minimize stress responses associated with overcrowding, thereby facilitating unobstructed observation of current-induced behavioral responses in
L. crocea. However, the low density may likely contribute to the absence of circular swimming behavior. Previous studies have demonstrated the behavior change of cage-cultured fish with stocking density [
21,
22]. Consequently, while a lower density reduces stress-induced behavioral responses in
L. crocea caused by crowding, it may also potentially affect their natural schooling behavior. Given the influence of stocking density on fish behavior [
21,
22], future research should systematically examine its effects by establishing controlled density gradients. Such investigations would yield valuable insights to optimize cage aquaculture practices for this species.
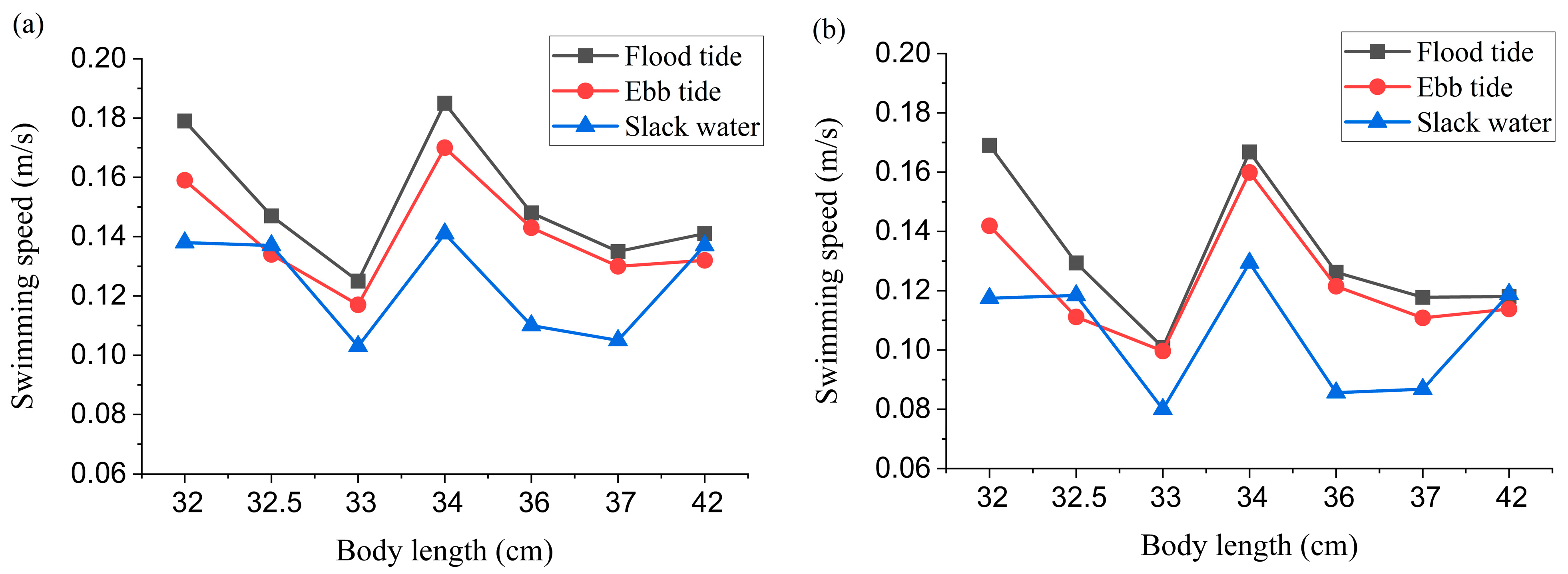
To characterize the influence of tidal conditions on the spatial distribution of
L. crocea, distribution patterns were analyzed across flood, ebb, and slack tide phases. Notably, the occurrence frequency of
L. crocea appearances near the cage edges was significantly higher during slack tides compared to both the flood and ebb phases (
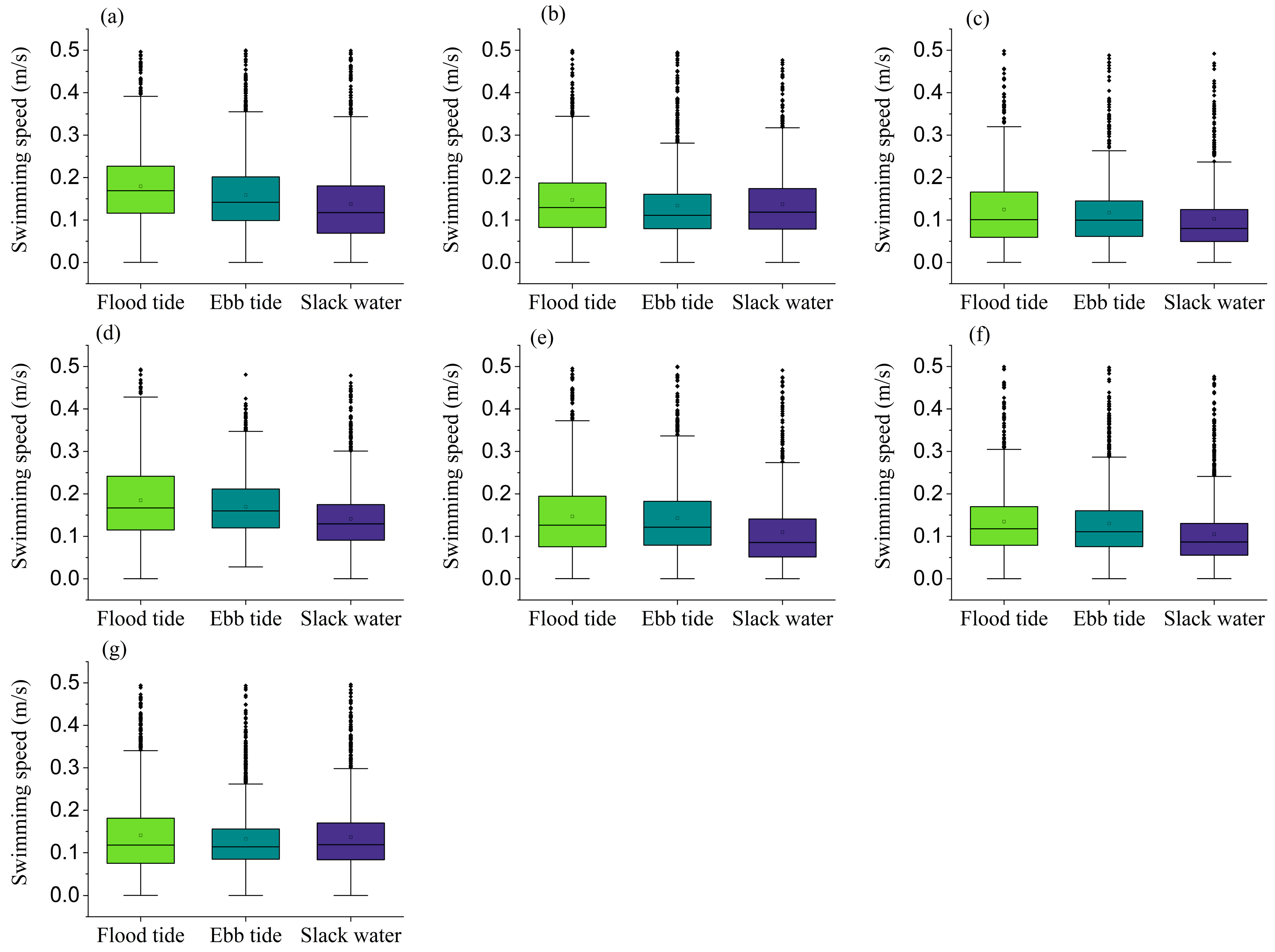
Figure 4). Concurrently, swimming speed distributions exhibited higher velocities during both flood and ebb tides than during slack water (
Figure 5), indicating that
L. crocea exhibit increased activity during tidal currents (flood and ebb tides). The higher current velocities during these periods did not negatively impact their free-swimming behavior. The observed behavioral plasticity has direct implications for optimal site selection in cage aquaculture, particularly concerning hydrodynamic environmental factors. Furthermore, considering that studies quantifying the in situ swimming speeds of
L. crocea are remarkably scarce, this study provides the first empirical measurements of free-ranging velocity dynamics in large sea cages. The present study provides pioneering measurements of swimming speed variations in free-ranging individuals under large-sea-cage conditions. These original data represent valuable contributions to the understanding of the species’ locomotor behavior, offering new baseline parameters for future research in aquaculture, ethology, and hydrodynamic modeling. Notably, the present study demonstrates that
L. crocea can maintain free-swimming activity even under these high-flow conditions. Considering that current velocities during flood and ebb tides are generally significantly higher than during slack water, the findings in this study suggest that this species is unlikely to cluster densely near cage nets or along enclosure boundaries due to hydrodynamic stress in their natural habitat, thereby reducing the risks of physical injury caused by high-flow conditions. From an ethological perspective, the current hydrodynamic regime in the study area appears to pose no adverse effects on this species. Conversely, existing research indicates that high-flow environments may enhance physiological fitness and improve flesh quality [
23,
24]. However, the limited velocity gradient resolution in this study precludes the identification of optimal flow conditions for aquaculture applications, warranting further systematic investigation.
It is important to note that this study was conducted with only seven specimens of L. crocea. Although the behavioral characteristics observed in these individuals were comparable, future studies that incorporate a larger number of tagged fish would undoubtedly yield more robust and detailed conclusions.
As a preliminary investigation into the effects of water flow on the swimming behavior of
L. crocea in a large-sea-cage environment, this study has delineated its spatial distribution and variations in swimming speed during flood, ebb, and slack tides. However, several knowledge gaps remain: (1) the behavioral responses of the
L. crocea to specific flow velocity gradients remain unclear, particularly regarding the critical swimming speed that may influence its free movement within cage environments; (2) the aquaculture cage setting presents a complex interplay of environmental factors; in addition to hydrodynamics, variables such as temperature and stocking density may also modulate swimming behavior [
25,
26,
27,
28]. Therefore, future studies should employ more refined experimental designs to disentangle the intricate relationships between the behavior of
L. crocea and multiple environmental parameters. Such research will enhance our understanding of optimal aquaculture conditions and species-specific welfare in aquaculture cages [
29].
5. Conclusions
The study successfully employed ultrasonic biotelemetry to tag and track L. crocea in a large sea cage, obtaining high-resolution behavioral datasets including swimming depths and movement trajectories. The spatial distribution and swimming characteristics of L. crocea were systematically compared across flood, ebb, and slack tide phases, demonstrating that the tidal flow regimes did not adversely affect free-swimming behavior in this species. These findings provide critical empirical reference information for the design, construction, and site selection of large-scale L. crocea aquaculture systems. Moreover, the study demonstrated a robust capability for monitoring fine-scale movement patterns of L. crocea in large-sea-cage environments using an acoustic biotelemetry system. These findings provide a methodological reference for future applications of this technology to address challenges in large-scale marine sea-cage aquaculture systems and optimize farming efficiency.
Previous studies have established that fish behavior serves as the most direct indicator of welfare status and environmental stress responses, representing a critical metric for evaluating aquaculture environment suitability. However, the conditions within net pens are highly complex, with multiple environmental factors (e.g., light intensity, stocking density, and water temperature) potentially influencing L. crocea behavior. Therefore, more comprehensive studies are needed to elucidate the specific effects of different environmental variables on behavioral patterns.
Prospectively, integrating multi-sensor observation systems represents a promising avenue for enhancing behavioral monitoring capabilities. For instance, combined multibeam sonar with ultrasonic biotelemetry enables the simultaneous tracking and analysis of both individual and school behavioral responses, thereby providing more robust evidence for understanding fish–environment interactions. Such technological integration would significantly advance our ability to optimize aquaculture practices based on species-specific behavioral ecology.