A Review of Standardization in Mississippi’s Multidecadal Inland Fisheries Monitoring Program
Abstract
1. Introduction
- A description of the Bureau’s standardized monitoring program.
- Quantitative and qualitative analysis of the monitoring program.
- Identification of the benefits and challenges of monitoring program standardization.
- Outline of opportunities for improvement and further operationalization.
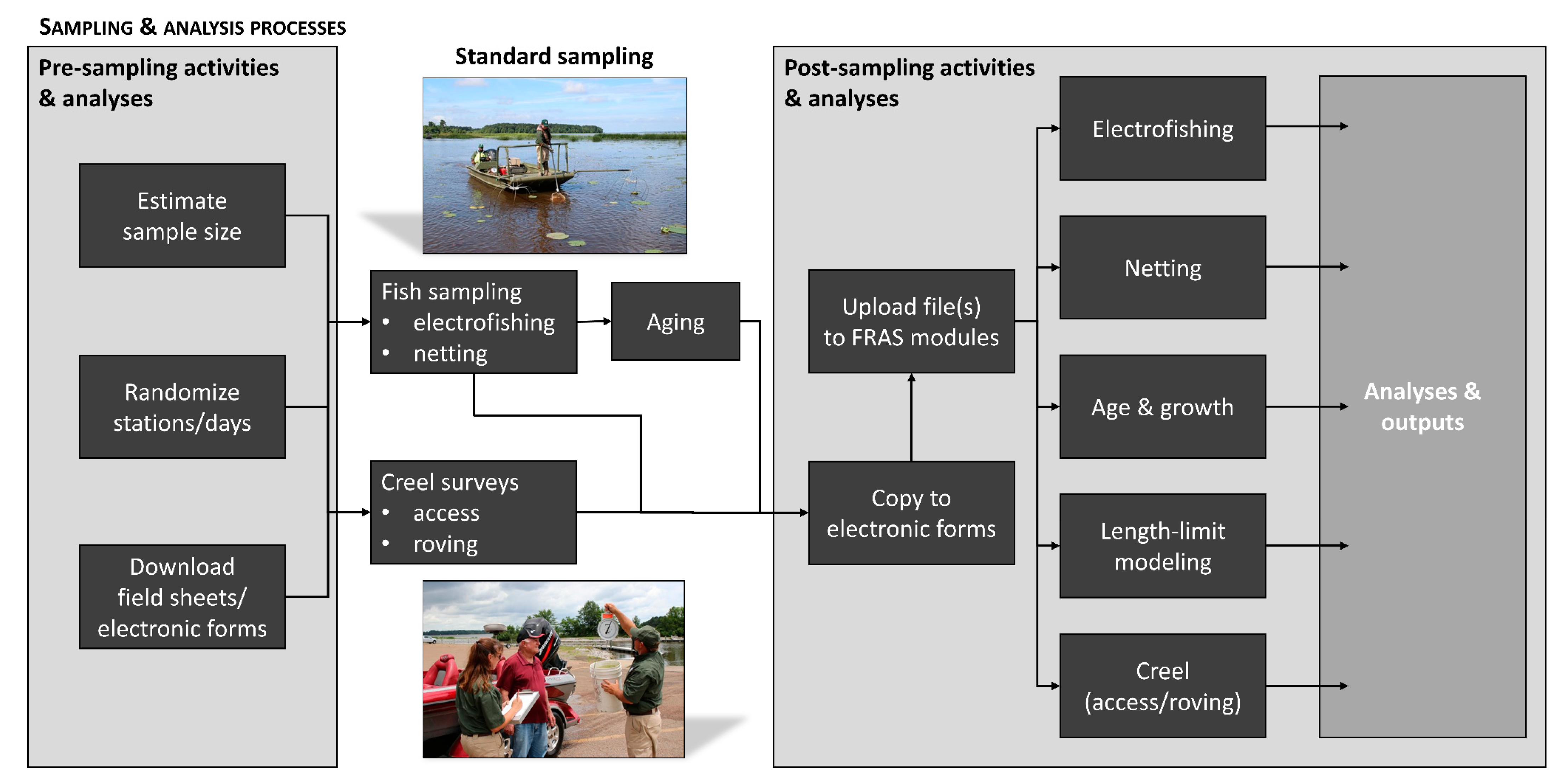
2. Overview of the Mississippi Inland Fisheries Monitoring Program
2.1. Pre-Sampling Activities and Analyses
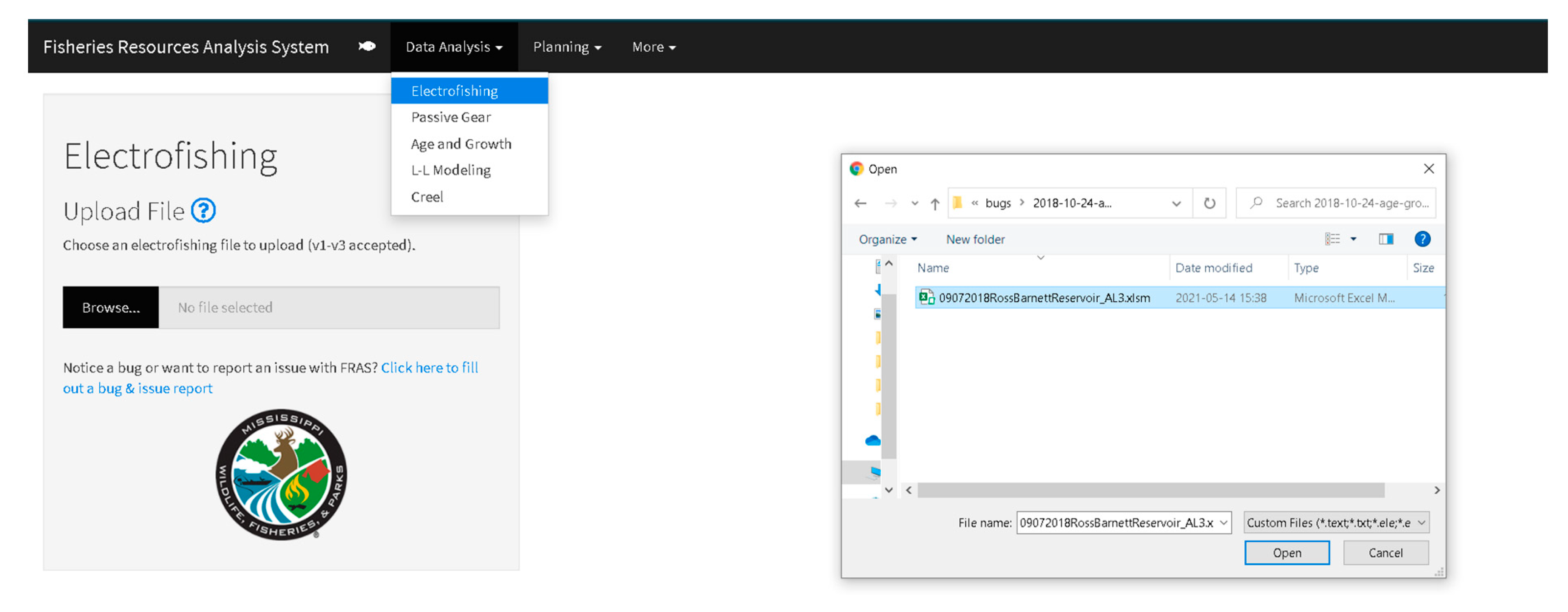
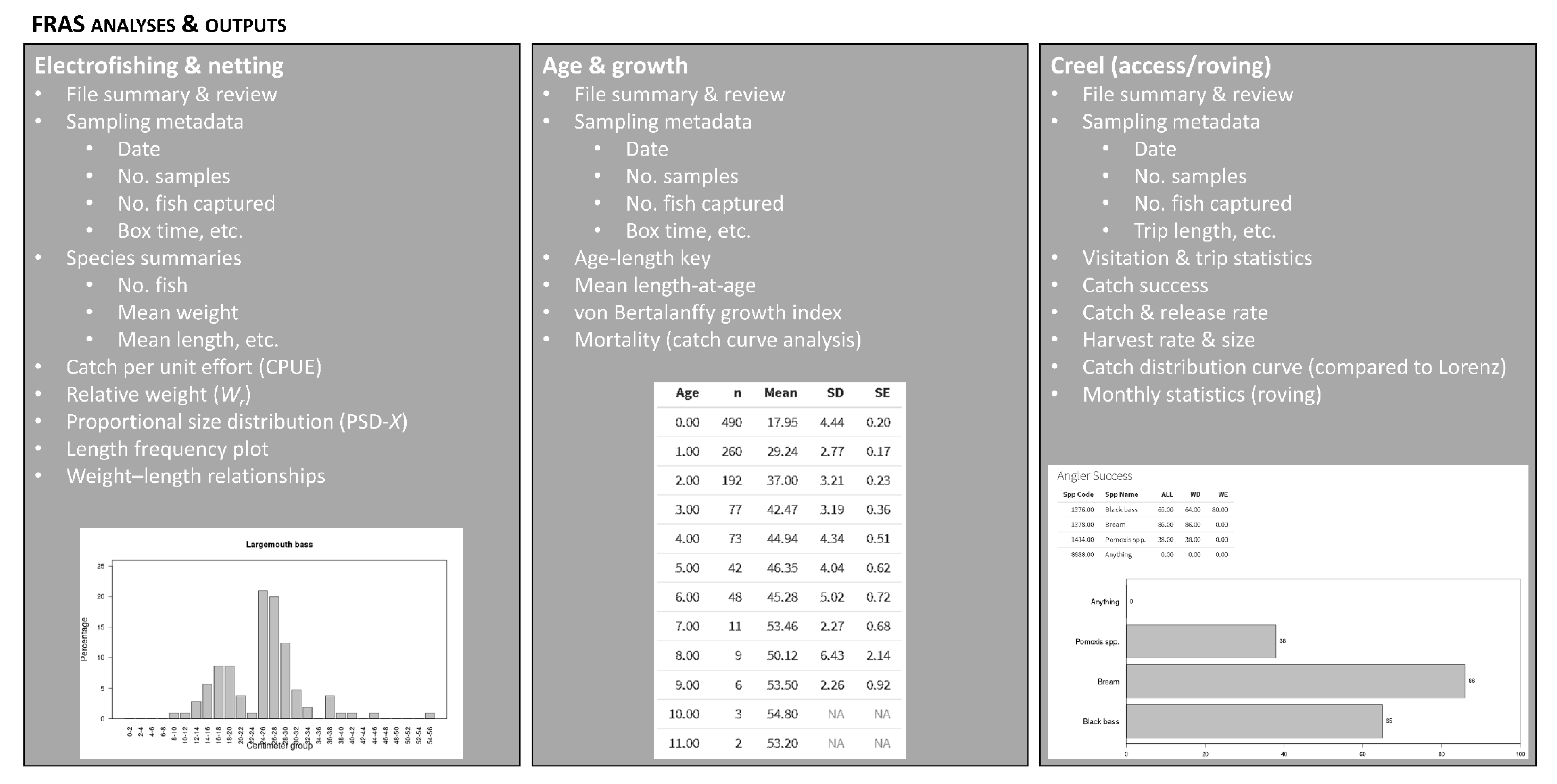
2.2. Post-Sampling Activities and Analyses
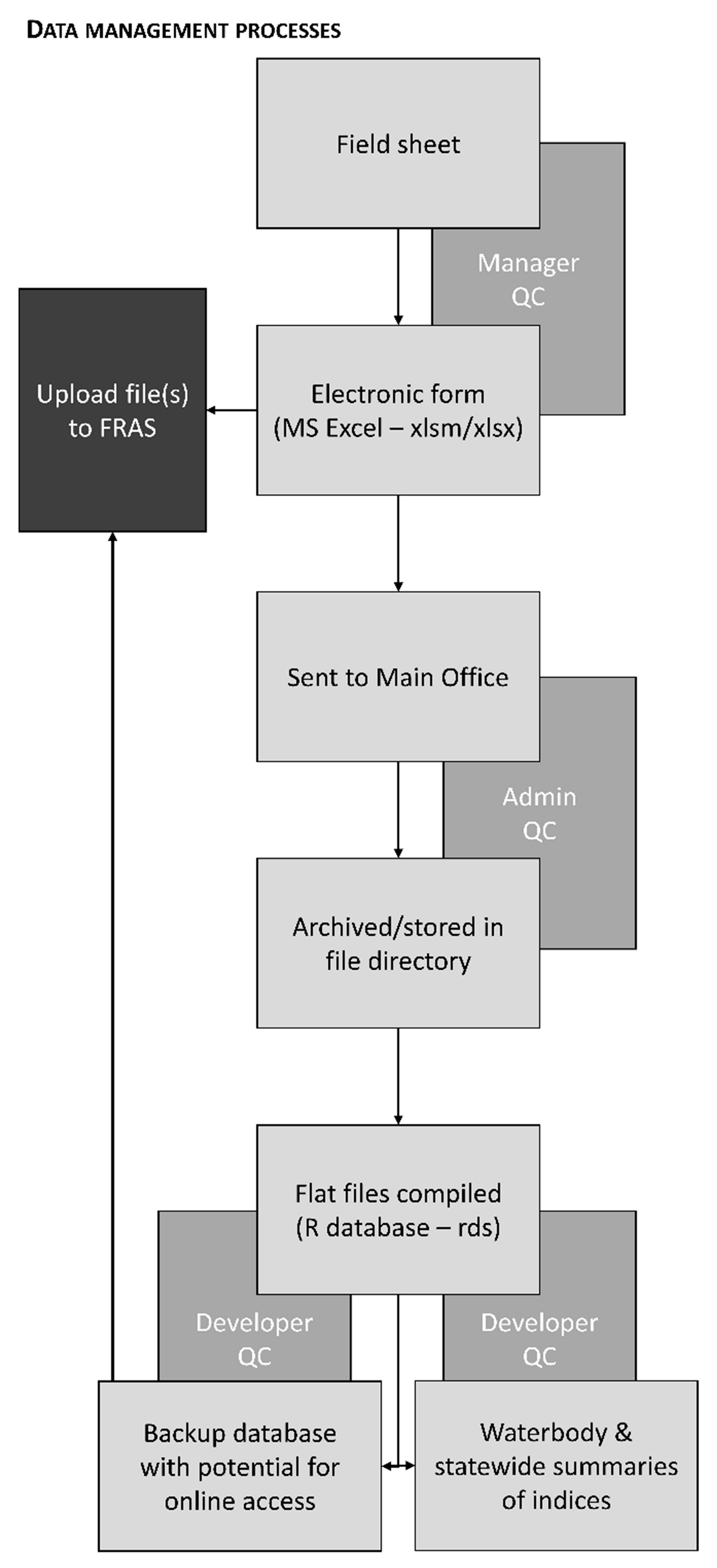
2.3. Central Archiving and Documentation
3. A Retrospective Look at the Monitoring Program
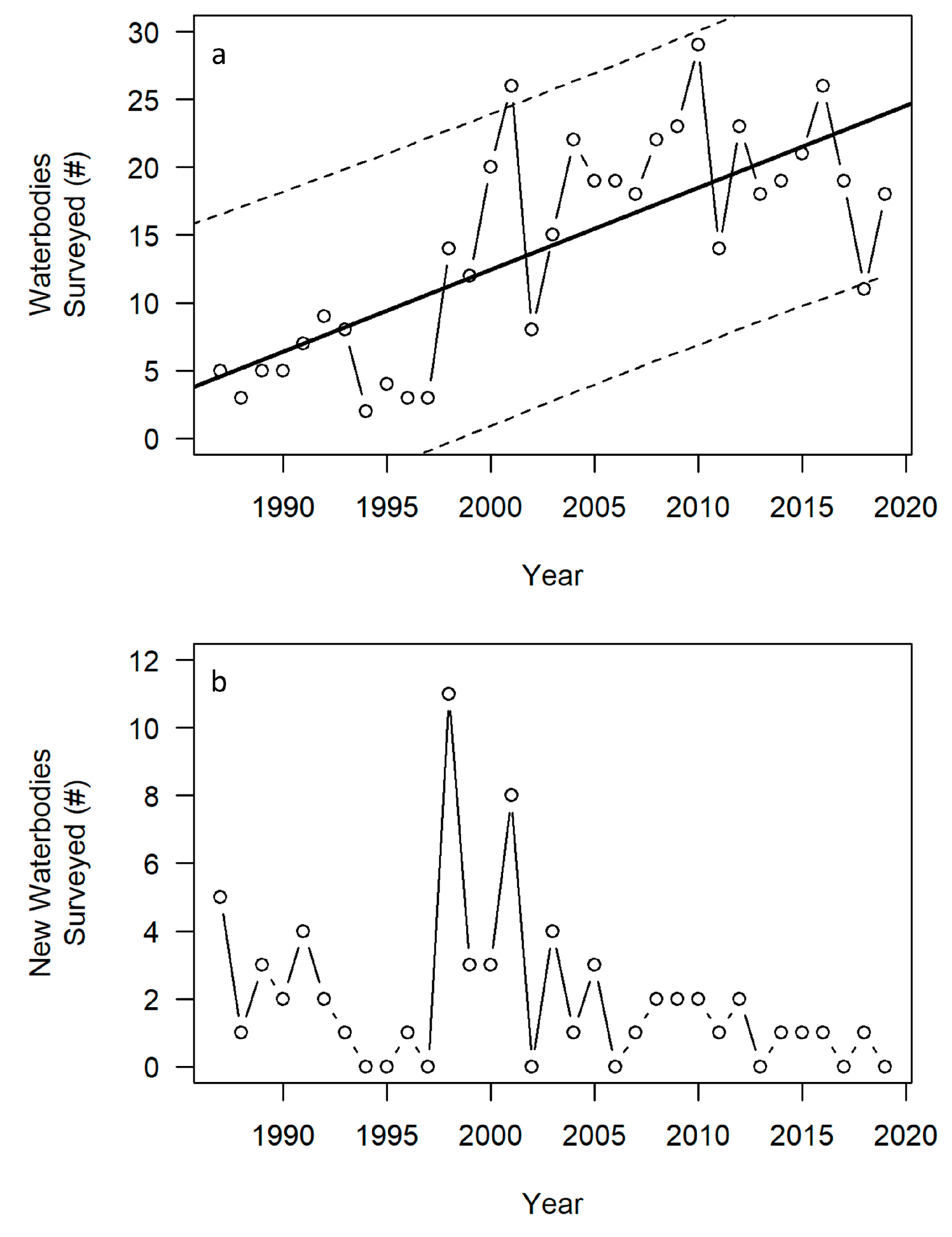
3.1. Quantitative Summary and Trends
3.2. Qualitative Reflections and Perspectives
4. Benefits, Challenges, and Opportunities of Monitoring Program Standardization
5. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COVID-19 | Coronavirus disease 2019 |
| CPE | Catch per effort |
| CPH | Anglers’ catch per hour |
| CSV | Comma-Separated Values |
| CV | Coefficient of variation |
| f | Effort |
| FRAS | Fisheries Resources Analysis System |
| H | Harvest |
| HPH | Anglers’ harvest per hour |
| MDWFP | Mississippi Department of Wildlife, Fisheries, and Parks |
| MS | Microsoft |
| PSD | Proportional size distribution |
| Q# | Question number |
| QC | Quality control |
| SD | Standard deviation |
| SMART | Specific, measurable, achievable, relevant, time-bound |
| USA | United States of America |
| Wr | Relative weight |
| XLSM | Microsoft Excel Macro-Enabled Workbook |
| XLSX | Microsoft Excel Open Extensible Markup Language Spreadsheet |
References
- Pope, K.L.; Lochmann, S.E.; Young, M.K. Methods for Assessing Fish Populations. In Inland Fisheries Management in North America; Hubert, W.A., Quist, M.C., Eds.; American Fisheries Society: Bethesda, MD, USA, 2010; pp. 325–351. ISBN 978-1-934874-16-5. [Google Scholar]
- Noble, R.L.; Austen, D.J.; Pegg, M.A. Fisheries Management Study Design Considerations. In Analysis and Interpretation of Freshwater Fisheries Data; Guy, C.S., Brown, M.L., Eds.; American Fisheries Society: Bethesda, MD, USA, 2007; pp. 31–49. [Google Scholar]
- Bonar, S.A.; Contreras-Balderas, S.; Iles, A.C. An Introduction to Standardized Sampling. In Standard Methods for Sampling North American Freshwater Fishes; Bonar, S.A., Hubert, W.A., Willis, D.W., Eds.; American Fisheries Society: Bethesda, MD, USA, 2009; pp. 1–12. [Google Scholar]
- Lester, N.P.; Sandstrom, S.; de Kerckhove, D.T.; Armstrong, K.; Ball, H.; Amos, J.; Dunkley, T.; Rawson, M.; Addison, P.; Dextrase, A.; et al. Standardized Broad-Scale Management and Monitoring of Inland Lake Recreational Fisheries: An Overview of the Ontario Experience. Fisheries 2020, 46, 107–118. [Google Scholar] [CrossRef]
- Bonar, S.A.; Hubert, W.A. Standard Sampling of Inland Fish: Benefits, Challenges, and a Call for Action. Fisheries 2002, 27, 10–17. [Google Scholar] [CrossRef]
- USFWS Congressional and Legislative Affairs Federal Aid in Sport Fish Restoration Act. Available online: https://www.fws.gov/law/federal-aid-sport-fish-restoration-act-1950 (accessed on 5 December 2019).
- Analysis and Interpretation of Freshwater Fisheries Data; Guy, C.S., Brown, M.L., Eds.; American Fisheries Society: Bethesda, MD, USA, 2007; ISBN 978-1-888569-77-3. [Google Scholar]
- Standard Methods for Sampling North American Freshwater Fishes; Bonar, S.A., Hubert, W.A., Willis, D.W., Eds.; American Fisheries Society: Bethesda, MD, USA, 2009; ISBN 978-1-934874-10-3. [Google Scholar]
- Inland Fisheries Management in North America; Hubert, W.A., Quist, M.C., Eds.; American Fisheries Society: Bethesda, MD, USA, 2010; ISBN 978-1-934874-16-5. [Google Scholar]
- Fisheries Techniques; Zale, A.V., Parrish, D.L., Sutton, T.M., Eds.; American Fisheries Society: Bethesda, MD, USA, 2012; ISBN 978-1-934874-29-5. [Google Scholar]
- Allen, T.; Olds, E.; Southwick, R.; Scuderi, B.; Caputo, L.; Howlett, D. Sportfishing in America: An Economic Force; American Sportfishing Association: Alexandria, VA, USA, 2018. [Google Scholar]
- Recreational Boating; Fishing Foundation; The Outdoor Foundation. 2018 Special Report on Fishing; Recreational Boating & Fishing Foundation and the Outdoor Foundation: Alexandria, VA, USA; Washington, DC, USA, 2018. [Google Scholar]
- Mississippi Department of Wildlife Conservation. Guidelines for Standard Lake and Reservoir Surveys; Mississippi Department of Wildlife Conservation: Jackson, MS, USA, 1986. [Google Scholar]
- Gabelhouse, D.; Anderson, R.; Aggus, L.; Austen, D.; Bruch, R.; Dean, J.; Doherty, F.; Dunning, D.; Green, D.; Hoeft, M.; et al. Fish Sampling and Data Analysis Techniques Used by Conservation Agencies in the U.S. and Canada; Fisheries Techniques Standardization Committee, Fisheries Management Section, American Fisheries Society: Bethesda, MD, USA, 1992; p. 118. [Google Scholar]
- Smith, B.J.; Blackwell, B.G. Adoption of AFS Standard Methods: The South Dakota Experience. Fisheries 2019, 44, 567–575. [Google Scholar] [CrossRef]
- Quist, M.C.; Bonvechio, K.I.; Allen, M.S. Statistical Analysis and Data Management. In Standard Methods for Sampling North American Freshwater Fishes; Bonar, S.A., Hubert, W.A., Willis, D.W., Eds.; American Fisheries Society: Bethesda, MD, USA, 2009; pp. 171–194. [Google Scholar]
- Quist, M.C.; Pegg, M.A.; DeVries, D.R. Age and Growth. In Fisheries Techniques; Zale, A.V., Parrish, D.L., Sutton, T.M., Eds.; American Fisheries Society: Bethesda, MD, USA, 2012; pp. 677–732. [Google Scholar]
- Neumann, R.M.; Guy, C.S.; Willis, D.W. Length, Weight, and Associated Indicies. In Fisheries Techniques; Zale, A.V., Parrish, D.L., Sutton, T.M., Eds.; American Fisheries Society: Bethesda, MD, USA, 2012; pp. 637–676. [Google Scholar]
- Allen, M.S.; Hightower, J.E. Fish Population Dynamics: Mortality, Growth, and Recruitment. In Inland Fisheries Management in North America; American Fisheries Society: Bethesda, MD, USA, 2010; pp. 43–80. [Google Scholar]
- Odum, E.P. Fundamentals of Ecology; W. B. Saunders Company: Philadelphia, PA, USA, 1953. [Google Scholar]
- McMahon, T. Size and Shape in Biology. Science 1973, 179, 1201–1204. [Google Scholar] [CrossRef]
- Nichols, J.D.; Williams, B.K. Monitoring for Conservation. Trends Ecol. Evol. 2006, 21, 668–673. [Google Scholar] [CrossRef]
- Conroy, M.J.; Peterson, J.T. Integrating Management, Research, and Monitoring: Balancing the 3-Legged Stool. Gamebird 2006, 6, 2–10. [Google Scholar]
- Brouder, M.J.; Iles, A.C.; Bonar, S.A. Length Frequency, Condition, Growth, and Catch per Effort Indices for Common North American Fishes. In Standard Methods for Sampling North American Freshwater Fishes; Bonar, S.A., Hubert, W.A., Willis, D.W., Eds.; American Fisheries Society: Bethesda, MD, USA, 2009; pp. 231–282. [Google Scholar]
- Schneider, J.C. Michigan Manual of Fisheries Survey Methods II: With Periodic Updates; Michigan Department of Natural Resources, Fisheries Special Report 25: Ann Arbor, MI, USA, 2000; p. 395. [Google Scholar]
- Bryan, S.D. Standard Fish Sampling Protocol for State of Arizona Waters; Arizona Game and Fish Department: Phoenix, AZ, USA, 2004. [Google Scholar]
- Oakley, C.; Dorsey, L. Data Collection & Analysis Guidelines for the Piedmont Region; North Carolina Wildlife Resources Commission, Federal Aid in Sport Fish Restoration: Raleigh, NC, USA, 2013; pp. 1–6. [Google Scholar]
- Marteney, R.; Aberson, L.; Bever, C.; Lynott, S.; Reinke, J.; Shaw, M. Standard Fish Survey Techniques for Small Lakes and Reservoirs; Kansas Department of Wildlife and Parks: Shawnee, KS, USA, 2010. [Google Scholar]
- Fisher, W.L.; Bozek, M.A.; Vokoun, J.C.; Jacobson, R.B. Freshwater Aquatic Habitat Measurements. In Fisheries Techniques; Zale, A.V., Parrish, D.L., Sutton, T.M., Eds.; American Fisheries Society: Bethesda, MD, USA, 2012; pp. 101–161. [Google Scholar]
- Jones, C.M.; Pollock, K.H. Recreational Survey Methods: Estimation of Effort, Harvest, and Released Catch. In Fisheries Techniques; Zale, A.V., Parrish, D.L., Sutton, T.M., Eds.; American Fisheries Society: Bethesda, MD, USA, 2012; pp. 883–919. [Google Scholar]
- Brown, M.L.; Allen, M.S.; Beard, T.D. Data Management and Statistical Techniques. In Fisheries Techniques; Zale, A.V., Parrish, D.L., Sutton, T.M., Eds.; American Fisheries Society: Bethesda, MD, USA, 2012; pp. 17–28. [Google Scholar]
- Clark, R.D., Jr.; Garling, D.L.; Lackey, R.T. Computer Use in Freshwater Fisheries Management. Fisheries 1977, 2, 21–23. [Google Scholar] [CrossRef]
- Bayley, P.B.; Austen, D.J. Fisheries Analysis System: Data Management and Analysis for Fisheries Management and Research. In Mathematical Analysis of Fish Stock Dynamics; American Fisheries Society: Bethesda, MD, USA, 1989; pp. 199–205. [Google Scholar]
- Seigel, R.A. Applicability of “Tablet” Computers for Use by Field Biologists. Herpetol. Rev. 2013, 44, 82–85. [Google Scholar]
- Bonvechio, K.I.; Paudyal, R.; Crandall, C.; Carlson, A.K. Survey Evaluation of Florida’s Freshwater Fisheries Long-Term Monitoring Program. Fishes 2023, 8, 216. [Google Scholar] [CrossRef]
- Schnute, J.T. A General Framework for Developing Sequential Fisheries Models. Can. J. Fish. Aquat. Sci. 1994, 51, 1676–1688. [Google Scholar] [CrossRef]
- Schnute, J.T.; Richards, L.J. Stock Assessment for the 21st Century. Fisheries 1994, 19, 10–16. [Google Scholar] [CrossRef]
- Miranda, L.E. Fisheries Resource Analysis System; Mississippi Department of Wildlife, Fisheries and Parks: Jackson, MS, USA, 1990. [Google Scholar]
- Holder, P.E.; Jeanson, A.L.; Lennox, R.J.; Brownscombe, J.W.; Arlinghaus, R.; Danylchuk, A.J.; Bower, S.D.; Hyder, K.; Hunt, L.M.; Fenichel, E.P.; et al. Preparing for a Changing Future in Recreational Fisheries: 100 Research Questions for Global Consideration Emerging from a Horizon Scan. Rev. Fish Biol. Fish. 2020, 30, 137–151. [Google Scholar] [CrossRef]
- Miranda, L.E.; Coppola, G.; Boxrucker, J. Reservoir Fish Habitats: A Perspective on Coping with Climate Change. Rev. Fish. Sci. Aquac. 2020, 28, 478–498. [Google Scholar] [CrossRef]
- Jentoft, S. Institutions in Fisheries: What They Are, What They Do, and How They Change. Mar. Policy 2004, 28, 137–149. [Google Scholar] [CrossRef]
- Aldridge, C.A.; Miranda, L.E.; Colvin, M.E. Themes of Contemporary Inland Fisheries Goals. Fisheries 2021, 46, 34–39. [Google Scholar] [CrossRef]
- Miranda, L.E.E.; Bull, L.A.A.; Colvin, M.E.E.; Hubbard, W.D.D.; Pugh, L.L.L. Segmentation of Mississippi’s Natural and Artificial Lakes. Lake Reserv. Manag. 2018, 34, 376–397. [Google Scholar] [CrossRef]
- Miranda, L.E. Monitoring Protocols for Inland Fisheries; Mississippi Wildlife, Fisheries & Parks: Jackson, MS, USA, 2005; p. 314. [Google Scholar]
- Miranda, L.E.; Boxrucker, J. Warmwater Fish in Large Standing Waters. In Standard Methods for Sampling North American Freshwater Fishes; Bonar, S.A., Hubert, W.A., Willis, D.W., Eds.; American Fisheries Society: Bethesda, MD, USA, 2009; pp. 29–42. [Google Scholar]
- Miranda, L.E. Standardizing Electrofishing Power for Boat Electrofishing. In Standard Methods for Sampling North American Freshwater Fishes; American Fisheries Society: Bethesda, MD, USA, 2009; pp. 223–230. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Miranda, L.E.; Colvin, M.E.; Shamaskin, A.; Aldridge, C.A. Review, Revision and Development of New Monitoring Protocols to Facilitate the Meeting of Goals and Objectives of Freshwater Fisheries Management in Mississippi; Mississippi Department of Wildlife, Fisheries and Parks: Jackson, MS, USA, 2019. [Google Scholar]
- Chang, W.; Cheng, J.; Allaire, J.; Sievert, C.; Schloerke, B.; Xie, Y.; Allen, J.; McPherson, J.; Dipert, A.; Borges, B. Shiny: Web Application Framework for R. 2023. Available online: https://CRAN.R-project.org/package=shiny (accessed on 14 May 2025).
- Kim, D.; Taylor, A.T.; Near, T.J. Phylogenomics and Species Delimitation of the Economically Important Black Basses (Micropterus). Sci. Rep. 2022, 12, 9113. [Google Scholar] [CrossRef]
- Miranda, L.E.; Hubbard, W.D.; Sangare, S.; Holman, T. Optimizing Electrofishing Sample Duration for Estimating Relative Abundance of Largemouth Bass in Reservoirs. N. Am. J. Fish. Manag. 1996, 16, 324–331. [Google Scholar] [CrossRef]
- Bonvechio, K.I.; Sawyers, R.E.; Leone, E.; Crawford, S. Increasing the Efficiency of Florida’s Freshwater Fisheries Long-Term Monitoring Program. J. Southeast. Assoc. Fish Wildl. Agencies 2014, 1, 7–14. [Google Scholar]
- Miranda, L.E. Sample Sizes for Estimating and Comparing Proportion-Based Indices. N. Am. J. Fish. Manag. 1993, 13, 383–386. [Google Scholar] [CrossRef]
- Hayes, D.; Baker, E.; Bednarz, R.; Borgeson, D.; Braunscheidel, J.; Breck, J.; Bremigan, M.; Harrington, A.; Hay, R.; Lockwood, R.; et al. Developing a Standardized Sampling Program: The Michigan Experience. Fisheries 2003, 28, 18–25. [Google Scholar] [CrossRef]
- Barclay, K.; Voyer, M.; Mazur, N.; Payne, A.M.; Mauli, S.; Kinch, J.; Fabinyi, M.; Smith, G. The Importance of Qualitative Social Research for Effective Fisheries Management. Fish. Res. 2017, 186, 426–438. [Google Scholar] [CrossRef]
- Morales, N.A.; Martin, A. Evaluating a Stakeholder-Driven Bass Regulation Change in Florida: Agency Staff and Stakeholder Perspectives. Fisheries 2018, 43, 361–367. [Google Scholar] [CrossRef]
- Young, J.C.; Rose, D.C.; Mumby, H.S.; Benitez-Capistros, F.; Derrick, C.J.; Finch, T.; Garcia, C.; Home, C.; Marwaha, E.; Morgans, C.; et al. A Methodological Guide to Using and Reporting on Interviews in Conservation Science Research. Methods Ecol. Evol. 2018, 9, 10–19. [Google Scholar] [CrossRef]
- Born, S.M.; Genskow, K.D.; Filbert, T.L.; Hernandez-Mora, N.; Keeper, M.L.; White, K.A. Socioeconomic and Institutional Dimensions of Dam Removals: The Wisconsin Experience. Environ. Manag. 1998, 22, 359–370. [Google Scholar] [CrossRef]
- Austin, Z.; Sutton, J. Qualitative Research: Getting Started. Can. J. Hosp. Pharm. 2014, 67, 436–440. [Google Scholar] [CrossRef]
- Sutton, J.; Austin, Z. Qualitative Research: Data Collection, Analysis, and Management. Can. J. Hosp. Pharm. 2015, 68, 226–231. [Google Scholar] [CrossRef]
- Jentoft, S. Beyond Fisheries Management: The Phronetic Dimension. Mar. Policy 2006, 30, 671–680. [Google Scholar] [CrossRef]
- Bresee, L.C. An Introduction to Developing Surveys for Pharmacy Practice Research. Can. J. Hosp. Pharm. 2014, 67, 286–291. [Google Scholar] [CrossRef]
- Drexler, J.Z.; Raine, H.; Harrington, C.L.; Winter, K.B.; Fraiola, K.; Browning, J.; Burgett, J.; Burney, D.A.; Falinski, K.; Fisher, S.; et al. The Scientific Benefits of a Statewide, Standardized, Coastal Wetland Monitoring Program in Hawa‘i. Ecol. Evol. 2025, 15, e71293. [Google Scholar] [CrossRef]
- Bonar, S.A.; Mercado-Silva, N.; Hubert, W.A.; Beard, T.D.; Dave, G.; Kubečka, J.; Graeb, B.D.S.S.; Lester, N.P.; Porath, M.; Winfield, I.J.; et al. Standard Methods for Sampling Freshwater Fishes: Opportunities for International Collaboration. Fisheries 2017, 42, 150–156. [Google Scholar] [CrossRef]
- Aldridge, C.A.; Colvin, M.E. Writing SMART Objectives for Natural Resource and Environmental Management. Ecol. Solut. Evid. 2024, 5, e12313. [Google Scholar] [CrossRef]
- Walters, C.J.; Hilborn, R. Ecological Optimization and Adaptive Management. Annu. Rev. Ecol. Syst. 1978, 9, 157–188. [Google Scholar] [CrossRef]
- Conroy, M.; Peterson, J. Decision Making in Natural Resource Management: A Structured, Adaptive Approach; Wiley-Blackwell: Hoboken, NJ, USA, 2013; ISBN 978-1-118-50623-3. [Google Scholar]
- Walters, C.J.; Holling, C.S. Large-Scale Management Experiments and Learning by Doing. Ecology 1990, 71, 2060–2068. [Google Scholar] [CrossRef]
- Anderson, S.C.; Keppel, E.A.; Edwards, A.M. Reproducible Visualization of Raw Fisheries Data for 113 Species Improves Transparency, Assessment Efficiency, and Monitoring. Fisheries 2020, 45, 535–543. [Google Scholar] [CrossRef]
- Xie, Y.H. Dynamic Documents with R and knitr, 2nd ed.; Chapman and Hall/CRC: London, UK, 2015; ISBN 978-1498716963. [Google Scholar]
- Gohel, D.; Moog, S.; Heckermann, M. Officer: Manipulation of Microsoft Word and PowerPoint Documents. 2025. Available online: https://github.com/davidgohel/officer (accessed on 14 May 2025).
- Regular, P.M.; Robertson, G.J.; Rogers, R.; Lewis, K.P. Improving the Communication and Accessibility of Stock Assessment Using Interactive Visualization Tools. Can. J. Fish. Aquat. Sci. 2020, 77, 1592–1600. [Google Scholar] [CrossRef]
- McMullin, S.L.; Pert, E. The Process of Fisheries Management. In Inland Fisheries Management in North America; Hubert, W.A., Quist, M.C., Eds.; American Fisheries Society: Bethesda, MD, USA, 2010; pp. 133–155. [Google Scholar]
- Barber, W.E.; Taylor, J.N. The Importance of Goals, Objectives, and Values in the Fisheries Management Process and Organization: A Review. N. Am. J. Fish. Manag. 1990, 10, 365–373. [Google Scholar] [CrossRef]
- Drucker, P. The Practice of Management; Harper & Row: New York, NY, USA, 1954; ISBN 0-08-094236-9. [Google Scholar]
- Bennett, D.H.; Hampton, E.L.; Lackey, R.T. Current and Future Fisheries Management Goals: Implications for Future Management. Fisheries 1978, 3, 10–14. [Google Scholar] [CrossRef]
- Anderson, S.H. Managing Our Wildlife Resources; Prentice-Hall: Upper Saddle River, NJ, USA, 2002; ISBN 978-0-13-019577-7. [Google Scholar]
- Runge, M.C.; Grand, J.B.; Mitchell, M.S. Structured Decision Making. In Wildlife Management and Conservation: Contemporary Principles and Practices; Krausman, P.R., Cain, J.W., Eds.; The John Hopkins University Press: Baltimore, MD, USA, 2013; pp. 51–72. ISBN 978-1-4214-0987-0. [Google Scholar]
- Gregory, R.; Failing, L.; Harstone, M.; Long, G.; McDaniels, T.; Ohlson, D. Structured Decision Making: A Practical Guide to Environmental Management Choices; John Wiley & Sons: West Sussex, UK, 2012; ISBN 1-4443-9853-9. [Google Scholar]
- Bain, M.B. Structured Decision Making in Fisheries Management: Trout Fishing Regulations on the Au Sable River, Michigan. N. Am. J. Fish. Manag. 1987, 7, 475–481. [Google Scholar] [CrossRef]
- Powers, J.E.; Lackey, R.T.; Zuboy, J.R. Decision-Making in Recreational Fisheries Management: An Analysis. Trans. Am. Fish. Soc. 1975, 104, 630–634. [Google Scholar] [CrossRef]
- Powers, J.E.; Lackey, R.T. A Multiattribute Utility Function for Management of a Recreational Resource. Va. Jouranl Sci. 1976, 27, 191–198. [Google Scholar]
- Lackey, R.T. Fisheries Management: Integrating Societal Preference, Decision Analysis, and Ecological Risk Assessment. Environ. Sci. Policy 1998, 1, 329–335. [Google Scholar] [CrossRef]
- Larkin, P.A. An Epitaph for the Concept of Maximum Sustained Yield. Trans. Am. Fish. Soc. 1977, 106, 1–11. [Google Scholar] [CrossRef]
- Walters, C.J.; Hilborn, R. Adaptive Control of Fishing Systems. J. Fish. Res. Board Can. 1975, 33, 145–159. [Google Scholar] [CrossRef]
- Aldridge, C. Towards Structured Planning and Learning at the State Fisheries Agency Scale. Ph.D. Thesis, Mississippi State University, Stakville, MS, USA, 2022. [Google Scholar]
- Lackey, R.T. Fisheries: History, Science, and Management. In Water Encyclopedia: Surface and Agricultural Water; Lehr, J.H., Keeley, J., Eds.; John Wiley and Sons: New York, NY, USA, 2005; pp. 121–129. [Google Scholar]
- Reynolds, J.B.; Kolz, A.L. Electrofishing. In Fisheries Techniques; Zale, A.V., Parrish, D.L., Sutton, T.M., Eds.; American Fisheries Society: Bethesda, MD, USA, 2012; pp. 305–361. [Google Scholar]
- Dolan, C.R.; Miranda, L.E. Immobilization Thresholds of Electrofishing Relative to Fish Size. Trans. Am. Fish. Soc. 2003, 132, 969–976. [Google Scholar] [CrossRef]
- Dolan, C.R.; Miranda, L.E. Injury and Mortality of Warmwater Fishes Immobilized by Electrofishing. N. Am. J. Fish. Manag. 2004, 24, 118–127. [Google Scholar] [CrossRef]
- Quist, M.C.; Isermann, D.A. Age and Growth of Fishes: Principles and Techniques; American Fisheries Society: Bethesda, MD, USA, 2017. [Google Scholar]
- Bonds, C.C.; Van Zee, B. Pitfalls of Relying Solely on Size Structure Indices and Catch per Unit Effort for Management Decisions. In Inland Fisheries Management in North America; Hubert, W.A., Quist, M.C., Eds.; American Fisheries Society: Bethesda, MD, USA, 2010; pp. 332–335. [Google Scholar]
- Gabelhouse, D.W. A Length-Categorization. North Am. J. Fish. Manag. 1984, 4, 273–285. [Google Scholar] [CrossRef]
- Wege, G.J.; Anderson, R.O. Relative Weight (Wr): A New Index of Condition for Largemouth Bass. In AFS Special Publication 5; Novinger, G., Dillard, J., Eds.; American Fisheries Society: Bethesda, MD, USA, 1978; pp. 79–91. [Google Scholar]
- Arlinghaus, R.; Cooke, S.J.; Lyman, J.; Policansky, D.; Schwab, A.; Suski, C.; Sutton, S.G.; Thorstad, E.B. Understanding the Complexity of Catch-and-Release in Recreational Fishing: An Integrative Synthesis of Global Knowledge from Historical, Ethical, Social, and Biological Perspectives. Rev. Fish. Sci. 2007, 15, 75–167. [Google Scholar] [CrossRef]
- Sass, G.G.; Gaeta, J.W.; Allen, M.S.; Suski, C.D.; Shaw, S.L. Effects of Catch-and-Release Angling on a Largemouth Bass (Micropterus salmoides) Population in a North Temperate Lake, 2001–2005. Fish. Res. 2018, 204, 95–102. [Google Scholar] [CrossRef]



| ID | Question | n | Mean Score (SD) |
|---|---|---|---|
| Q1α | How much change in sampling methods has occurred over your career? | 6 | 2.2 (1.0) |
| Q2 α | How much change in routine fisheries analyses has occurred over your career? | 5 | 2.0 (1.0) |
| Q3 α | How much change in reporting and documentation (i.e., survey reports and fisheries management plans) has occurred over your career? | 4 | 3.3 (2.1) |
| Q4aβ | The current sampling protocols are easy to use. | 6 | 4.5 (0.5) |
| Q4bβ | The current sampling protocols capture reliable data. | 6 | 3.7 (0.8) |
| Q4cβ | The current sampling protocols are transferable from waterbody to waterbody. | 6 | 3.5 (1.2) |
| Q6aβ | FRAS outputs are easy to interpret. | 6 | 4.3 (0.8) |
| Q6bβ | FRAS outputs enable clear communication. | 6 | 4.2 (0.8) |
| Q6cβ | FRAS outputs allow for comparisons through space and time. | 6 | 3.8 (0.8) |
| Q8aβ | FRAS has a high utility in your fisheries management process. | 6 | 4.2 (0.8) |
| Q8bβ | FRAS covers the appropriate or necessary analyses. | 6 | 4.0 (0.6) |
| Q8cβ | FRAS is easy to use or intuitive. | 6 | 4.0 (1.5) |
| Q8dβ | FRAS outputs are clear, understandable, or easy to interpret. | 6 | 4.0 (1.1) |
| Q8eβ | FRAS outputs are reliable or can be trusted. | 6 | 3.5 (0.5) |
| Q8fβ | FRAS provides an adequate scope for spatial and temporal comparisons. | 6 | 3.3 (1.6) |
| Q10aβ | The monitoring program* has increased your management efficiency. | 4 | 3.8 (1.0) |
| Q10bβ | The monitoring program has increased your management effectiveness. | 4 | 3.8 (1.0) |
| Q10cβ | The monitoring program has increased your biological and ecological knowledge of the waterbodies you manage. | 4 | 3.5 (1.7) |
| Q10dβ | The monitoring program has increased your awareness of stakeholder values in the waterbodies you manage. | 3 | 3.0 (1.8) |
| Q10eγ | The monitoring program clearly documents the historical conditions and management of waterbodies. | 5 | 4.8 (0.4) |
| Q10fγ | The monitoring program has allowed for learning of which management actions work to meet objectives. | 5 | 4.0 (1.0) |
| Q10gγ | The monitoring program has improved waterbody-specific management. | 5 | 4.2 (1.1) |
| Q10hγ | The monitoring program supports sustainable recreational fisheries. | 6 | 4.3 (1.0) |
| Q10iβ | The monitoring program makes you feel like you contribute to the higher societal goals of the Bureau. | 4 | 4.3 (1.0) |
| Q10jβ | The monitoring program help maintain a “big picture” perspective. | 4 | 3.8 (1.5) |
| Q11a | Objectives in your fisheries management plans have become more specific through use of the monitoring program. | 5 | 4.2 (0.4) |
| Q11b | Objectives in your fisheries management plans have become more measurable (i.e., linked to metrics) through use of the monitoring program. | 4 | 4.3 (1.0) |
| Q11c | Objectives in your fisheries management plans have become more achievable through use of the monitoring program. | 5 | 4.0 (1.0) |
| Q11d | Objectives in your fisheries management plans have become more realistic through use of the monitoring program. | 3 | 4.0 (1.0) |
| Q11e | Objectives for the waterbodies you manage are consistent (within zone) through use of the monitoring program. | 5 | 5.0 |
| Q11f | Objectives between the waterbodies you manage and waterbodies other manage are consistent (statewide) through use of the monitoring program. | 5 | 3.4 (0.9) |
| ID | Question | N | Verbatim Examples | Synthesis |
|---|---|---|---|---|
| Q5 | Is there anything about the current sampling protocols that are or are not working well for you? | 5 |
| Most sampling methods work well, but validating, updating, or expanding them, especially in ways that accommodate difficult-to-access areas, would increase confidence in reliability. |
| Q7 | Is there anything about the current routine fisheries analyses in FRAS that are or are not working well for you? | 4 |
| FRAS provides a quick and efficient way to conduct routine fisheries analyses, but a “black box” approach to data analysis seeds some skepticism. However, further automation is not completely unwelcome, e.g., the compilation of outputs into a standardized report. |
| Q9 | Do you think the monitoring program—protocols for program components (i.e., sampling, analysis, and documentation)—and integrating new technologies (i.e., sampling methods, metrics, analysis software) has better equipped you to address angler questions, concerns, complaints, and expectations? | 5 |
| Proper data management, analysis, and documentation enhanced by technology, like computer applications, provides managers with trustworthy and communicable information needed to manage both fish and angler populations. |
| Q12 | Do you think the monitoring program—protocols for program components (i.e., sampling, analysis, and documentation)—and integrating new technologies (i.e., sampling methods, metrics, analysis software) has helped you clarify objectives for fisheries management plans? | 4 |
| The monitoring program has provided managers with quantitative ranges of common fisheries metrics. The process for constructing and clarifying objectives may be improved by using SMART criteria (i.e., specific, measurable, achievable, realistic, and time-bound). |
| Q13 | What are the most common objectives included in your fisheries management plans? | 5 |
| Objectives, generally, are similar for respondents and waterbodies, but are often only implicitly tied to metrics and remain one-dimensional (i.e., no objective hierarchy). A common pool of objectives linked to metrics could potentially streamline the selection of and standardize objectives. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldridge, C.A.; Colvin, M.E. A Review of Standardization in Mississippi’s Multidecadal Inland Fisheries Monitoring Program. Fishes 2025, 10, 235. https://doi.org/10.3390/fishes10050235
Aldridge CA, Colvin ME. A Review of Standardization in Mississippi’s Multidecadal Inland Fisheries Monitoring Program. Fishes. 2025; 10(5):235. https://doi.org/10.3390/fishes10050235
Chicago/Turabian StyleAldridge, Caleb A., and Michael E. Colvin. 2025. "A Review of Standardization in Mississippi’s Multidecadal Inland Fisheries Monitoring Program" Fishes 10, no. 5: 235. https://doi.org/10.3390/fishes10050235
APA StyleAldridge, C. A., & Colvin, M. E. (2025). A Review of Standardization in Mississippi’s Multidecadal Inland Fisheries Monitoring Program. Fishes, 10(5), 235. https://doi.org/10.3390/fishes10050235






