Assessing the Effects of Pesticides on Aquacultured Fish and Ecosystems: A Comprehensive Environmental Health Review
Abstract
1. Introduction
2. Pesticides
2.1. Classification of Pesticides
2.2. Organochlorines
2.3. Organophosphates
2.4. Carbamates
2.5. Pyrethroids
3. Pesticides and Fish Health
3.1. Insecticides and Its Effect on Fish Species
| Fish Species | Insecticide | Toxic Dose | Molecular Effect on Fish | Reference |
|---|---|---|---|---|
| Oncorhynchus mykiss | endosulfan | 19.78 μg | ↑lamellar edema in fish gills, ↑lamellar–epithelial separation, ↑lamellar fusion, ↑swelling and necrosis, ↑melanomacrophage centers | [60] |
| Clarias batrachus | phorate | 0.27 ppm (1/3 LC50) | ↑serum glucose, ↑ALP, and ↑ bilirubin levels | [61] |
| carbaryl | 15.3 ppm (1/3 LC30) | ↑serum glucose, ↑ALP, and ↑bilirubin levels | [61] | |
| Channa punctatus | chlorpyrifos | 0.365 ppm | ↑ALP, ↑acid phosphatase, and ↑cholesterol, and ↓glycogen in the liver | [62] |
| Rhamdia quelen | trichlorfon | 11 mg trichlorfon/L water | ↑cerebral ROS and ↑lipid peroxidation | [63] |
| Oreochromis niloticus | chlorpyrifos | 42.0 µg/L (20% EC) | ↓hepatic glycogen, ↓ALP, ↓AChE, ↓catalase activity in the liver, and ↑plasma glucose | [64] |
| Folidol 600® | 17.82 mg/L | ↓ ChE, ↓AChE, ↓BChE, ↓oxygen consumption, and ↑ammonium excretion. | [35] | |
| Brycon cephalus | 2 ppm (1/3 of 96 h-LC50) | ↓plasma and brain AChE activity, ↓liver ALT, ↑plasma ALT, and ↓hepatic glycogen and glucose | [65] | |
| Mystus cavasius | 5.9 ppm | ↓oxygen consumption | [66] | |
| Anguilla anguilla | dichlorvos | 1.5 mg/L (the 96 h LC85) | ↑GSH, ↑GSH/GSSG ratio, ↑hepatic glutathione reductase, ↑GST, ↑glutamate: cysteine ligase, ↑γ-glutamyl transferase activities, ↓GSH loss and oxidation, and ↑AChE and caspase-3 inactivation | [67] |
| Cyprinus carpio | dichlorvos | 1 ppm | ↓AChE and ↑oxidative stress | [68] |
| Ictalurus nebulosus | dichlorvos | 1 ppm | ↓AChE and ↑oxidative stress | [68] |
| chlorpyrifos | I50 28–33 nM | ↓AChE | [69] | |
| parathion | I50 446–578 nM | ↓AChE | [69] | |
| Anguilla anguilla | diazinon | 0.042 mg/L (0.50 of the 96-h LC50) | ↓ChE activity in the brain, plasma, and eye | [70] |
| fenitrothion | Sublethal 0.02 | ↓brain AChE activity, ↓Na+, K+-ATPase activity in gill tissue, ↑blood glucose, and ↑blood lactate values | [71] | |
| Tilapia guineensis | chlorpyrifos | 0.002 mg/L | ↓leucocyte and erythrocyte number | [72] |
| Oreochromis niloticus | diazinon | 7.830 ppm | ↓intracellular calcium flux, ↓ERK1/2, ↓pERK1/2, ↓apoptosis, ↓senescence, and | [73] |
| ↓mitochondrial membrane potential in spleen mononuclear cells ↑ROS production, | [74] | |||
| ↓phagocytic active cells, ↓splenocyte proliferation, ↓phagocytic indices | [75] | |||
| ↓AChE activity, ↑ACh concentration | [75] | |||
| ↑AChE, ↓nicotinic AChR, ↓muscarinic AChR, and ↓AChE activity immune cells | [76] | |||
| Cyprinus carpio | atrazine | 428 μg/L | ↑IL-1β, ↑IL-1RI expression in spleen and head kidney | [77] |
| chlorpyrifos | 116 μg/L | ↑IL-1β and ↑IL-1R1 expression significantly after exposure in the spleen and head kidney | [2] | |
| artazine | 428 μg/L | ↓GSTs | [78] | |
| chlorpyrifos | 116 μg/L | ↓GSTs | [78] | |
| Channa striata | diazinon | 500 g/ha | Long-term brain ChE inhibition | [79] |
| diazinon | 0.35 mg/L | Long-term inhibition of brain ChE activity and growth inhibition | [79] | |
| Oncorhynchus mykiss | AzMe | 0.007 (0.004–0.009) (LC50) | ↓brain and muscular ChE | [80] |
| carbamate carbaryl | 5.40 (4.27–6.18) (LC50) | ↓brain and muscular ChE | [80] | |
| B. arenarum Larvae | AzMe | 10.44 (LC50) | ↓ChE | [80] |
| carbamate carbaryl | 24.64 (17.68–34.77) (LC50) | ↓ChE | [80] | |
| Oncorhynchus mykiss | AzMe | 0.007 mg/L | ↓brain ChE | [81] |
| azinphos carbaryl | ↓brain ChE | [81] | ||
| methiocarb | 5.43 ± 0.19 mg/L | ↑lamellar edema, ↑lamellar–epithelial separation, ↑lamellar fusion, and ↑necrosis | [82] | |
| Labeo rohita | profenofos | 0.06 mg/L | ↓the AChE in the brain, gills, muscle, blood, liver, and kidney, and ↓BuChE in the liver and blood | [83] |
| carbofuran | 0.28 mg/L | ↓the AChE in the brain, gills, muscle, blood, liver, and kidney, and ↓BuChE in the liver and blood | [83] | |
| Channa punctatus | sevin | Sublethal concentration 1.05 mg/L | ↓glycogen in the liver and muscles, ↑lactic acid in the liver and muscles, ↑ LDH activity in the liver, muscles, brain, and gills, ↓LDH in the kidney and intestine, ↓PDH activity in the liver, muscles, brain, gills, kidney, and intestines, ↓succinate dehydrogenase in muscle, and ↑succinate dehydrogenase in kidney and intestine | [84] |
| Colossoma macropomum | dichlorvos | 0.04 µmol/L | ↓brain AChE | [85] |
| chlorpyrifos | 7.6 µmol/L | ↓brain AChE | [85] | |
| Colossoma macropomum | TEPP | 3.7 µmol/L | ↓brain AChE | [85] |
| carbaryl | 33.8 µmol/L | ↓brain AChE | [85] | |
| Carassius carassius L. | endosulfan | 0.070 ppm | ↑lipid peroxidation and ↓ GSH | [86] |
| Colossoma macropomum | carbofuran | 0.92 µmol/L | ↓brain AChE | [85] |
| Puntius conchonius | carbaryl | 2.142 ppm | ↑hyperglycemia and ↑glycogenesis in the liver, brain, and heart, and ↑hypercholesterolemia, Long-term exposure: ↑hypoglycemia, ↓liver glycogen, ↑glycogenesis in the heart, ↓glycogen levels in the brain, and ↓blood and liver cholesterol | [87] |
| Puntius conchonius | dimethoate | 4.784 ppm | ↑hyperglycemia and ↑glycogenesis in the liver, brain, and heart, and ↑hypercholesterolemia. Long-term exposure: ↑hypoglycemia, ↓liver glycogen, ↑glycogenesis in the heart, ↓glycogen levels in the brain, and ↓blood and liver cholesterol Reduction in blood and liver cholesterol | [87] |
| Clarias batrachus | carbofuran | 23 mg/L | ↓total ATPase in the tissue of kidney, muscle, liver, and gills | [88] |
| Oncorhynchus kisutch | carbofuran | 10.4 μg/L (EC 50) | ↓brain and olfactory rosette AChE activity | [89] |
| Clarias batrachus | endosulfan | Sublethal concentration 0.06 mg/L | ↓activity of citrate synthase, ↓G6PDH in the brain, liver, and skeletal muscle, ↓activity of lactase dehydrogenase in the brain, and ↓RNA and ↓protein content of the brain, liver, and skeletal muscle tissue | [90] |
| Clarias gariepinus | carbaryl | 0.003 μM (IC 50) | ↓brain AChE | [91] |
| chlorfenvinphos | 0.03 μM (IC 50) | ↓the AChE in plasma and eye homogenate | [91] | |
| diazinon | 0.15 μM (IC 50) | ↓brain AChE | [91] | |
| dimethoate | 190 μM (IC 50) | ↓brain AChE | [91] | |
| fenitrothion | 0.02 μM (IC 50) | ↓brain AChE | [91] | |
| pirimiphosmethyl | 0.003 μM (IC 50) | ↓brain AChE | [91] | |
| profenofos | 0.003 μM (IC 50) | ↓brain AChE | [91] | |
| Salmo salar L. | carbofuran | 1.0 µg/L (Not LC50) | ↓priming pheromonal system in mature males, and ↓the ability of the olfactory system to detect PGF2α | [92] |
| Cirrhinus mrigala | Cartap hydrochloride | 0.339–0.436 mg/L | ↓glycogen, ↓total protein, and ↓ nucleic acids in gill, liver, brain, kidney and muscle tissues | [93] |
| Chironomus riparius | carbofuran | 27.2 μg/L | ↓AChE activity | [94] |
| pirimiphos-methyl | 63.8 μg/L | ↓AChE activity | [94] | |
| permethrin | 16.6 μg/L | ↓AChE activity | [94] | |
| Rana perezi | ZZ-aphox® | 0.02% and 0.14% | ↑histological damage to gills, liver, gallbladder, heart and notochord | [55] |
| Monopterus albus | endosulfan | 0.42 μg/L | Abnormal behavioral response, ↓erythrocyte and leukocyte count, ↑size of erythrocyte cell, ↓HB, and ↓hematocrit | [95] |
| Cichlasoma dimerus | endosulfan | 2.6 μg/L | ↓erythrocyte volume, ↓corpuscular hemoglobin, ↑hyperplasia if there is ↑ interlamellar epithelium, ↑blood congestion in secondary lamellae, ↑mucous cells hyperplasia, and ↑hypertrophy in gills, ↑pyknotic nuclei, ↑hydropic degeneration in the liver, and ↑testicular damage | [96] |
| Channa punctatus | endosulfan | 5 ppb | ↑glutathione peroxidase, ↑glutathione S-transferase activity and ↑GSH levels in all organs, ↓catalase activity, and ↑lipid peroxidation | [97] |
| Oncorhynchus mykiss | endosulfan | 0.6 and 1.3 micro g/L | ↑histological lesions in the gill, liver, spleen and trunk, kidney | [54] |
| Prochilodus lineatus | endosulfan | 2.4 μg/L | ↓HB, ↓mean cell hemoglobin, ↓total plasma protein, ↑white blood cell count, ↑plasma glucose, ↑lipid peroxidation in the intestine, liver, and brain | [98] |
| Lepomis macrochirus | endosulfan | 1.2 μg/L (exposure dose) | ↑damage to connective tissue and ↑seminiferous tubules in the testis | [99] |
| endosulfan | 1.2 μg/L | ↓AChE activity | [100] | |
| Chanos chanos | endosulfan | 21.5 g/L | At a moderate dose: ↑the activity of catalase, ↑SOD, and ↑GST in the brain, gill, and liver; brain AChE, ↑LDH, and ↑MDH activity in the brain, liver, and gill; ↑activity of ↑ALT, ↑AST, and ↑G6PDH in the liver and gill; curling of secondary lamellae, ↑thickening of primary epithelium, ↑shorting of secondary lamellae, ↑epithelial hyperplasia, ↑a fusion of secondary lamellae, ↑aneurysm, ↑collapsed secondary lamellae in gills, ↑cloudy swelling, ↑necrosis with pyknotic nuclei in the liver, At a high dose: ↑necrosis of hepatic cells in the liver | [101] |
| Jordanella floridae | endosulfan | 10.8 μg/L (LOEC) | ↑hyperactivity, ↑convulsions, ↑axis malformations, ↑adverse effects on growth, reproduction, and survivability | [102] |
| Oncorhynchus mykiss | maneb | 1.19 mg/L | ↑lamellar edema, ↑lamellar–epithelial separation, ↑lamellar fusion, ↑swelling, and ↑necrosis of epithelial cells, ↑focal lamellar hyperplasia in gills, ↑inflammation, ↑focal necrosis in the liver, trunk kidney, and spleen | [53] |
| carbaryl | 2.52 mg/L | ↑lamellar edema, ↑lamellar–epithelial separation, ↑lamellar fusion, ↑swelling, and ↑necrosis of epithelial cells, ↑focal lamellar hyperplasia in gills, ↑inflammation, ↑focal necrosis in the liver, trunk kidney, and spleen | [53] |
3.1.1. Effect of Insecticide Exposure on Histopathological Abnormalities
3.1.2. Effect of Insecticide Exposure on Neurobehavioral Abnormalities
3.1.3. Effect of Insecticide Exposure on Hepatic Dysfunctions, Metabolism Disorders, and Oxidative Stress Responses
3.1.4. Effect of Insecticide Exposure on Hematological Abnormalities
3.1.5. Effect of Insecticide Exposure on the Immune Response
3.1.6. Effect of Insecticide Exposure on Reproduction and Growth
3.2. Herbicides and Their Effect on Fish Health
| Fish Species | Herbicide | Toxic Dose | Effect on Fish | Reference |
|---|---|---|---|---|
| Cnesterodon decemmaculatus | DIC and 2,4-dichlorophenoxyacetic acid (2,4-D) | 410 mg/L | ↑Oxidative damage, ↑catalase, ↑GST, and ↑AChE activity. | [109] |
| Anguilla anguilla | molinate | 11.15 mg/L (one-third of the 96 h LC(50) | ↓ChE activity, ↓blood proteins, ↓hematocrit, ↓HG, ↓erythrocytes, and ↓leukocytes | [110] |
| Leporinus obtusidens | clomazone | 0.5 mg/L | ↓AChE brain activity | [111] |
| quinclorac | 0.375 mg/L | ↓AChE brain activity and ↑AChE tissue activity | [111] | |
| propanil | 3.6 mg/L | ↑AChE tissue activity | [111] | |
| metsulfuron methyl | 0.002 mg/L | ↑AChE tissue activity | [111] | |
| Astyanax sp. | diuron | 30 mg/L | ↑GST activity and ↓catalase activity | [112] |
| glyphosate | 0.006 mL/L | ↑membrane damage | [112] | |
| Pimephales promelas | bromacil | 185 mg/L | ↓growth | [113] |
| diuron | 23.3 mg/L | abnormal or dead fry and ↓survival | [113] | |
| Cnesterodon decemmaculatus | Panzer | 15.68–16.70 mg/L | ↑micronuclei frequency (genotoxic effect) | [114] |
| Credit | 91.73–98.50 mg/L | ↑micronuclei frequency, | [114] | |
| Leporinus obtusidens | clomazone | 376 μg/L | ↓AChE activity in the brain and muscles, ↓TBARS in brain, muscle, and liver tissues, and ↓catalase in the liver | [115] |
| propanil | 1644 μg/L | ↓AChE activity in the brain and muscles, ↓ TBARS in brain, muscle, and liver tissues, and ↓catalase in the liver | [115] | |
| Oreochromis niloticus | acetochlor | 2.625 mg/L | Alteration in erythrocytes, ↓RBC count, ↑cholesterol, ↑total plasma protein, ↑urea, ↑creatinine, ↑albumin, ↑globulin, ↑albumin/globulin ratio, ↑ALP activity, ↑ALT activity, ↑and AST activity. | [116] |
| bispyribac-sodium | 0.800 mg/L | Alteration in erythrocytes, ↓RBC count, ↑cholesterol, ↑total plasma protein, ↑urea, ↑creatinine, ↑albumin, ↑globulin, ↑albumin/globulin ratio, ↑ALP activity, ↑ALT activity, ↑and AST activity. | [116] | |
| bentazon | 36.00 mg/L | Alteration in erythrocytes, ↓RBC count, ↑cholesterol, ↑total plasma protein, ↑urea, ↑creatinine, ↑albumin, ↑globulin, ↑albumin/globulin ratio, ↑ALP activity, ↑ALT activity, ↑and AST activity. | [116] | |
| bensulfuron-methyl | 2.50 mg/L | Alteration in erythrocytes, ↓RBC count, ↑cholesterol, ↑total plasma protein, ↑urea, ↑creatinine, ↑albumin, ↑globulin, ↑albumin/globulin ratio, ↑ALP activity, ↑ALT activity, ↑and AST activity. | [116] | |
| halosulfuron-methyl | 1.275 mg/L | Alteration in erythrocytes, ↓RBC count, ↑cholesterol, ↑total plasma protein, ↑urea, ↑creatinine, ↑albumin, ↑globulin, ↑albumin/globulin ratio, ↑ALP activity, ↑ALT activity, and ↑AST activity. | [116] | |
| quinclorac | 11.250 mg/L | Alteration in erythrocytes, ↓RBC count, ↑cholesterol, ↑total plasma protein, ↑urea, ↑creatinine, ↑albumin, ↑globulin, ↑albumin/globulin ratio, ↑ALP activity, ↑ALT activity, ↑and AST activity. | [116] | |
| Leporinus obtusidens | glyphosate | 3 mg/L | ↓ AChE brain activity, ↑hepatic glycogen and glucose, ↓glycogen and glucose in the liver, and ↑ammonia in liver and muscle tissue. | [117] |
3.2.1. Impact of Herbicide Exposure on Hepatic Dysfunctions, Metabolism Disorders, and Oxidative Stress Responses
3.2.2. Effect of Herbicide Exposure on Neuronal Abnormalities
3.3. Fungicides and Their Effect on Fish Health
| Fish Species | Fungicide | Toxic Dose | Effect on Fish | Reference |
|---|---|---|---|---|
| Danio rerio | kresoxim-methyl | 195 μg/L | ↑catalase, ↑peroxidase, ↑carboxylesterase activities, ↑malondialdehyde content in larvae, and ↑oxidative stress in adults | [118] |
| pyraclostrobin | 81.3 μg/L | ↑catalase, ↑peroxidase, ↑carboxylesterase activities, ↑malondialdehyde content in larvae, and ↑oxidative stress in adults | [118] | |
| Oryzias latipes | triadimefon | 2.0 and 3.5 μM | ↑CYP3A, ↑CYP1A activity, ↑ CYP3A38 and ↑CYP3A40, ↑CYP26B, ↑pregnant x receptor, ↑retinoid acid receptor γ1, and ↑p53 gene expression | [119] |
| myclobutanil | 2.0 and 3.5 μM | ↑CYP3A activity, ↑CYP3A38, ↑CYP3A40, ↑CYP1A, ↑pregnant x receptor, ↑p53 and ↑catalase expression | [119] | |
| Pimephales promelas | prochloraz | 0.1 mg/L | ↓CYP19 aromatase activity in brain and ovarian homogenates, ↓productiveness of fish, and ↓steroidogenesis | [120] |
| fenarimol | 1.0 mg/L | ↓CYP19 aromatase activity in brain and ovarian homogenates, ↓productiveness of fish, and ↓steroidogenesis | [120] | |
| Carassius auratus | Tattoo (mancozeb) | 10 mg/L (Tattoo) Corresponding to 3 mg/L (mancozeb) | ↑SOD, ↑catalase, ↑glutathione peroxidase, ↑protein carbonyls in the liver and kidney, ↓GAPDH activity in the kidney, ↑lipid peroxide level in the brain, ↓glutathione reductase activity in the brain | [121] |
| Oncorhynchus kisutch | mancozeb | 2.05 mg/L (EC 50) | ↑AChE brain activity | [89] |
| Carassius auratus | Tattoo (mancozeb) | 10 mg/L | Moderate lymphopenia, ↑protein carbonyl groups in the blood, ↓thiols levels, ↑lipid peroxide, ↑SOD, ↑catalase, and ↑GAPDH activity | [122] |
| Clarius batrachus | mancozeb | 22.87 mg/L | ↓protein, ↓amino acids, ↓glycogen, ↓nucleic acids, and ↓enzyme succinic dehydrogenase in the liver and muscles, ↑lactic dehydrogenase, ↑protease, ↑GOT, and ↑DPT in the liver and muscles | [123] |
3.3.1. Impact of Fungicide Exposure on Hepatic Dysfunctions, Metabolism Disorders, and Oxidative Stress Responses
3.3.2. Effect of Fungicide Exposure on Reproductive Abnormalities and Neuronal Abnormalities
4. Pesticides and the Nutritional Value of Fish
4.1. Protein
4.2. Fat
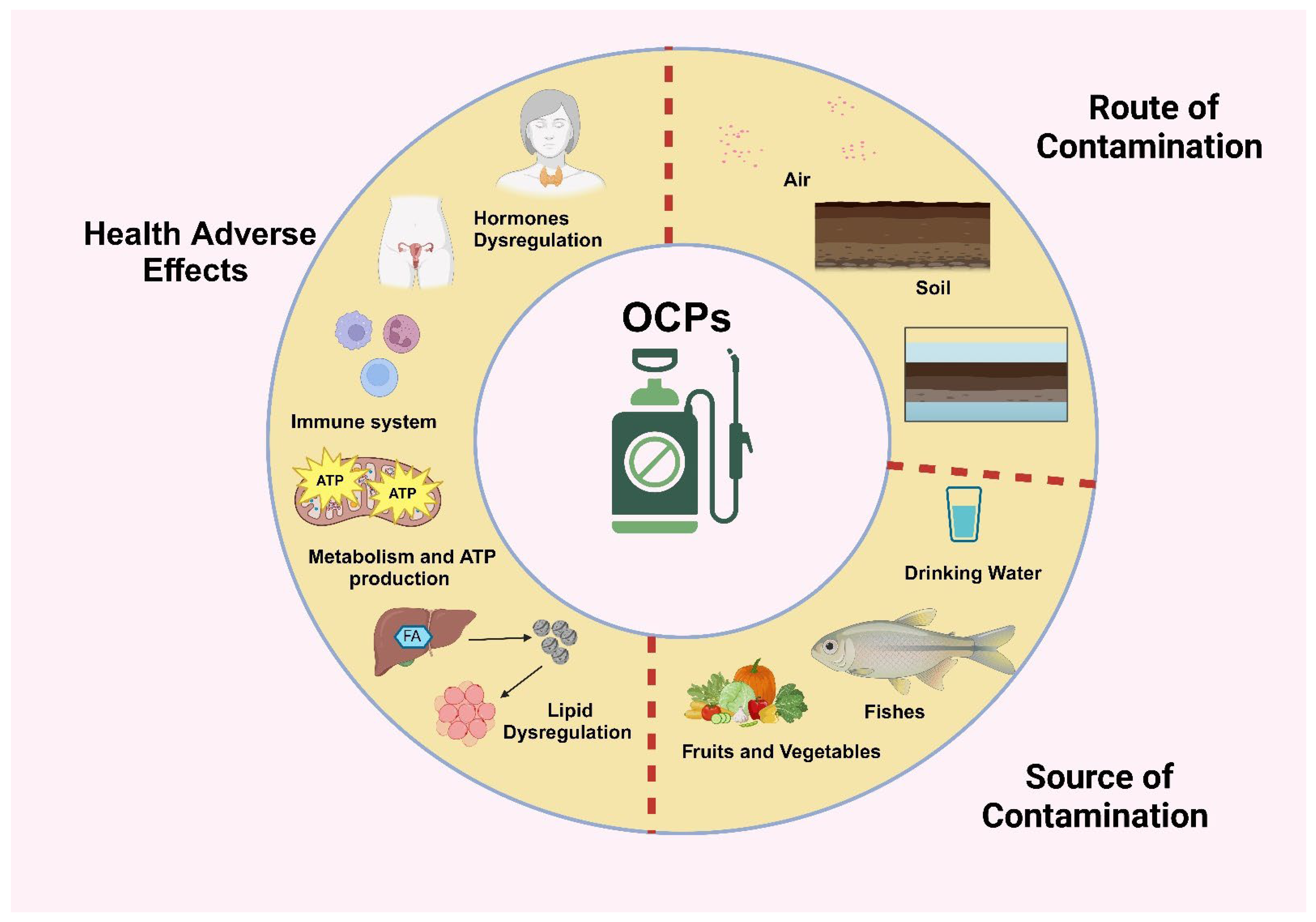
5. The Impact of Pesticides on Fish and Human Health
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AChR | acetylcholine receptor |
| ALT | alanine transaminase |
| ALP | alkaline phosphatase |
| ARA | arachidonic acid |
| EPA | eicosapentaenoic acid |
| DHA | docosahexaenoic acid |
| AST | aspartate aminotransferase |
| AzMe | azinphos methyl |
| BChE | butyrylcholinesterase |
| CFP | complement factor properdin |
| ChE | cholinesterase |
| DDE | dichlorodiphenyldichloroethylene |
| DDT | dichlorodiphenyltrichloroethane |
| DPT | dihydropyrimidine dehydrogenase |
| ERK1/2 | extracellular signal-regulated kinase 1/2 |
| G6PDH | glucose-6-phosphate dehydrogenase |
| GOT | glutamic-oxaloacetic transaminase |
| GSH | glutathione |
| GSSG | glutathione disulfide |
| GST | glutathione S-transferase isoenzymes |
| IL-1RI | interleukin receptor I |
| IL-1Î2 | interleukin-1Î2 |
| LDH | lactate dehydrogenase |
| MDH | malate dehydrogenase |
| OPs | organophosphates |
| PCBs | polychlorinated biphenyl |
| OCPs | organochlorines |
| PDH | pyruvate dehydrogenase |
| pERK1/2 | phospho extracellular signal-regulated kinase 1/2 |
| PGF2α | prostaglandin f2alpha |
| POPs | persistent organic pollutants |
| PUFAs | polyunsaturated fatty acids |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
| TBARS | thiobarbituric acid-reactive substances |
| TEPP | tetraethyl pyrophosphate |
References
- Kautsky, N.; Folke, C.; Rönnbäck, R.; Troell, M.; Beveridge, M.; Primavera, J. Aquaculture. In Encyclopedia of Biodiversity; Elsevier: Amsterdam, The Netherlands, 2001; pp. 185–198. Available online: https://books.google.com.eg/books?id=N9IIVBBmbrsC (accessed on 6 July 2024).
- Wang, S.-Y.; Fodjo, E.K.; Kong, C.; Yu, H.-J. Multi-Residue Screening of Pesticides in Aquaculture Waters through Ultra-High-Performance Liquid Chromatography-Q/Orbitrap Mass Spectrometry. Water 2020, 12, 1238. [Google Scholar] [CrossRef]
- Laffoley, D.; Baxter, J.M.; Day, J.C.; Wenzel, L.; Bueno, P.; Zischka, K. Marine Protected Areas. In World Seas: An Environmental Evaluation, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 549–569. [Google Scholar]
- Cole, D.W.; Cole, R.; Gaydos, S.J.; Gray, J.; Hyland, G.; Jacques, M.L.; Powell-Dunford, N.; Sawhney, C.; Au, W.W. Aquaculture: Environmental, toxicological, and health issues. Int. J. Hyg. Environ. Health 2009, 212, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Tidwell, J.H.; Allan, G.L. Fish as food: Aquaculture’s contribution. Ecological and economic impacts and contributions of fish farming and capture fisheries. EMBO Rep. 2001, 2, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Wei, S.; Ali, A.; Khan, I.; Sun, Q.; Xia, Q.; Wang, Z.; Han, Z.; Liu, Y.; Liu, S. Research Progress on Nutritional Value, Preservation and Processing of Fish-A Review. Foods 2022, 11, 3669. [Google Scholar] [CrossRef]
- Hosomi, R.; Yoshida, M.; Fukunaga, K. Seafood consumption and components for health. Glob. J. Health Sci. 2012, 4, 72–86. [Google Scholar] [CrossRef]
- Béné, C.; Arthur, R.; Norbury, H.; Allison, E.H.; Beveridge, M.; Bush, S.; Campling, L.; Leschen, W.; Little, D.; Squires, D.; et al. Contribution of Fisheries and Aquaculture to Food Security and Poverty Reduction: Assessing the Current Evidence. World Dev. 2016, 79, 177–196. [Google Scholar] [CrossRef]
- Gormaz, J.G.; Fry, J.P.; Erazo, M.; Love, D.C. Public Health Perspectives on Aquaculture. Curr. Environ. Health Rep. 2014, 1, 227–238. [Google Scholar] [CrossRef]
- Shefali; Kumar, R.; Sankhla, M.S.; Kumar, R.; Sonone, S.S. Impact of Pesticide Toxicity in Aquatic Environment. Biointerface Res. Appl. Chem. 2020, 11, 10131–10140. [Google Scholar] [CrossRef]
- Khan, M.Z. Adverse Effects of Pesticides and Related Chemicals on Enzyme and Hormone Systems of Fish, Amphibians and Reptiles: A Review. Environ. Sci. Biol. 2005, 42, 315–323. [Google Scholar]
- Gonçalves, A.M.M.; Rocha, C.P.; Marques, J.C.; Gonçalves, F.J.M. Fatty acids as suitable biomarkers to assess pesticide impacts in freshwater biological scales–A review. Ecol. Indic. 2021, 122, 107299. [Google Scholar] [CrossRef]
- Hassaan, M.A.; El Nemr, A. Pesticides pollution: Classifications, human health impact, extraction and treatment techniques. Egypt. J. Aquat. Res. 2020, 46, 207–220. [Google Scholar] [CrossRef]
- Jayaraj, R.; Megha, P.; Sreedev, P. Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip. Toxicol. 2016, 9, 90–100. [Google Scholar] [CrossRef]
- Alavanja, M.C. Introduction: Pesticides use and exposure extensive worldwide. Rev. Environ. Health 2009, 24, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Akashe, M.M.; Pawade, U.V.; Nikam, A.V. Classification of Pesticides: A Review. Int. J. Res. Ayurveda Pharm. 2018, 9, 144–150. [Google Scholar] [CrossRef]
- Naqvi, G.-e.-Z.; Shoaib, N.; Ali, A.M. Pesticides impact on protein in fish (Oreochromis mossambicus) tissues. Indian J. Geo-Mar. Sci. 2017, 46, 1864–1868. [Google Scholar]
- Kaur, R.; Mavi, G.K.; Raghav, S.; Khan, I. Pesticides Classification and its Impact on Environment. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1889–1897. [Google Scholar] [CrossRef]
- Mrema, E.J.; Rubino, F.M.; Brambilla, G.; Moretto, A.; Tsatsakis, A.M.; Colosio, C. Persistent organochlorinated pesticides and mechanisms of their toxicity. Toxicology 2013, 307, 74–88. [Google Scholar] [CrossRef]
- Chang, G.-R. Persistent organochlorine pesticides in aquatic environments and fishes in Taiwan and their risk assessment. Environ. Sci. Pollut. Res. 2018, 25, 7699–7708. [Google Scholar] [CrossRef]
- Philbert, A.; Lyantagaye, S.L.; Nkwengulila, G. Farmers’ pesticide usage practices in the malaria endemic region of North-Western Tanzania: Implications to the control of malaria vectors. BMC Public. Health 2019, 19, 1456. [Google Scholar] [CrossRef]
- Matowo, N.S.; Tanner, M.; Munhenga, G.; Mapua, S.A.; Finda, M.; Utzinger, J.; Ngowi, V.; Okumu, F.O. Patterns of pesticide usage in agriculture in rural Tanzania call for integrating agricultural and public health practices in managing insecticide-resistance in malaria vectors. Malar. J. 2020, 19, 257. [Google Scholar] [CrossRef]
- Bai, Y.; Ruan, X.; van der Hoek, J.P. Residues of organochlorine pesticides (OCPs) in aquatic environment and risk assessment along Shaying River, China. Environ. Geochem. Health 2018, 40, 2525–2538. [Google Scholar] [CrossRef] [PubMed]
- Girones, L.; Arias, A.H.; Oliva, A.L.; Recabarren-Villalon, T.; Marcovecchio, J.E. Occurrence and spatial distribution of organochlorine pesticides in the southwest Buenos Aires using the freshwater snail Chilina parchappii as environmental biomonitor. Reg. Stud. Mar. Sci. 2020, 33, 100898. [Google Scholar] [CrossRef]
- Bajwa, A.; Ali, U.; Mahmood, A.; Chaudhry, M.J.I.; Syed, J.H.; Li, J.; Zhang, G.; Jones, K.C.; Malik, R.N. Organochlorine pesticides (OCPs) in the Indus River catchment area, Pakistan: Status, soil–air exchange and black carbon mediated distribution. Chemosphere 2016, 152, 292–300. [Google Scholar] [CrossRef]
- Freire, C.; Koifman, R.J.; Koifman, S. Serum levels of organochlorine pesticides in blood donors: A biomonitoring survey in the North of Brazil, 2010–2011. Sci. Total Environ. 2017, 598, 722–732. [Google Scholar] [CrossRef]
- Koureas, M.; Karagkouni, F.; Rakitskii, V.; Hadjichristodoulou, C.; Tsatsakis, A.; Tsakalof, A. Serum levels of organochlorine pesticides in the general population of Thessaly, Greece, determined by HS-SPME GC-MS method. Environ. Res. 2016, 148, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.H.B.; Polder, A.; Brynildsrud, O.B.; Karimi, M.; Lie, E.; Manyilizu, W.B.; Mdegela, R.H.; Mokiti, F.; Murtadha, M.; Nonga, H.E.; et al. Organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) in human breast milk and associated health risks to nursing infants in Northern Tanzania. Environ. Res. 2017, 154, 425–434. [Google Scholar] [CrossRef]
- Martyniuk, C.J.; Mehinto, A.C.; Denslow, N.D. Organochlorine pesticides: Agrochemicals with potent endocrine-disrupting properties in fish. Mol. Cell Endocrinol. 2020, 507, 110764. [Google Scholar] [CrossRef]
- Awe, Y.T.; Sangodoyin, A.Y.; Ogundiran, M.B. Assessment of organophosphate pesticide residues in environmental media of Araromi farm settlement, Osun State, Nigeria. Environ. Anal. Health Toxicol. 2022, 37, e2022030–e2022035. [Google Scholar] [CrossRef]
- Syawal, M.; Ibrahim, A.; Yustiawati; Nasution, S.; Taufik, I.; Saraswati, M.; Ardiwinata, A. Organophosphate pesticide residues in surface water and bilih fish (Mystacoleucus padangensis Blkr.) in Lake Singkarak, West Sumatra. IOP Conf. Ser. Earth Environ. Sci. 2023, 1221, 012080. [Google Scholar] [CrossRef]
- Yao, R.; Yao, S.; Ai, T.; Huang, J.; Liu, Y.; Sun, J. Organophosphate Pesticides and Pyrethroids in Farmland of the Pearl River Delta, China: Regional Residue, Distributions and Risks. Int. J. Environ. Res. Public. Health 2023, 20, 1017. [Google Scholar] [CrossRef]
- Wongta, A.; Sawang, N.; Tongjai, P.; Jatiket, M.; Hongsibsong, S. The Assessment of Organophosphate Pesticide Exposure among School Children in Four Regions of Thailand: Analysis of Dialkyl Phosphate Metabolites in Students’ Urine and Organophosphate Pesticide Residues in Vegetables for School Lunch. Toxics 2022, 10, 434. [Google Scholar] [CrossRef] [PubMed]
- Bajgar, J. Organophosphates/nerve agent poisoning: Mechanism of action, diagnosis, prophylaxis, and treatment. Adv. Clin. Chem. 2004, 38, 151–216. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, E.; Ferreira, L.A.A. Effects of the organophosphate pesticide Folidol 600® on the freshwater fish, Nile Tilapia (Oreochromis niloticus). Pestic. Biochem. Physiol. 2011, 99, 209–214. [Google Scholar] [CrossRef]
- Shalaby, S. Levels of pesticide residues in water, sediment, and fish samples collected from Nile River in Cairo, Egypt. Environ. Forensics 2019, 19, 228–238. [Google Scholar] [CrossRef]
- Duc, N.; Quynh, N.; Phuong, T.; Thuy, T.T. Development and Validation of Analytical Method for Carbamate Pesticide Residues in Vietnam Agricultural Products. JST Eng. Technol. Sustain. Dev. 2023, 33, 048–057. [Google Scholar] [CrossRef]
- Siriwat, S.; Ong-Artborirak, P.; Ponrachom, C.; Siriwong, W.; Nganchamung, T. Non-carcinogenic health risk from carbamate pesticide exposure of toddlers living in agricultural areas of Thailand. Int. J. Environ. Health Res. 2022, 33, 1738–1748. [Google Scholar] [CrossRef]
- Sogorb, M.A.; Vilanova, E. Enzymes involved in the detoxification of organophosphorus, carbamate and pyrethroid insecticides through hydrolysis. Toxicol. Lett. 2002, 128, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Mojiri, A.; Zhou, J.; Robinson, B.; Ohashi, A.; Ozaki, N.; Kindaichi, T.; Farraji, H.; Vakili, M. Pesticides in aquatic environments and their removal by adsorption methods. Chemosphere 2020, 253, 126646. [Google Scholar] [CrossRef]
- Brander, S.; Gabler, M.; Fowler, N.; Connon, R.; Schlenk, D. Pyrethroid Pesticides as Endocrine Disruptors: Molecular Mechanisms in Vertebrates with a Focus on Fishes. Environ. Sci. Technol. 2016, 50, 8977–8992. [Google Scholar] [CrossRef]
- Tang, T.; Wu, R.; Zhang, L.; Wang, Y.; Ling, J.; Du, W.; Shen, G.; Chen, Y.; Zhao, M.-R. Distribution and partitioning of pyrethroid insecticides in agricultural lands: Critical influencing factors. Environ. Int. 2021, 156, 106736. [Google Scholar] [CrossRef]
- Song, X.; Zhang, X.; You, M.; Zhao, X.; Wang, X. Characteristics of Pyrethroid Pesticide Residues in Soil of Shenyang, China. IOP Conf. Ser. Earth Environ. Sci. 2021, 821, 012029. [Google Scholar] [CrossRef]
- Wang, J.-Z.; Bai, Y.-S.; Wu, Y.; Zhang, S.; Chen, T.-H.; Peng, S.-C.; Xie, Y.; Zhang, X.-W. Occurrence, compositional distribution, and toxicity assessment of pyrethroid insecticides in sediments from the fluvial systems of Chaohu Lake, Eastern China. Environ. Sci. Pollut. Res. 2016, 23, 10406–10414. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.; Reif, R.; Luo, Y.-C.; Gan, J. Distribution of pesticides in dust particles in urban environments. Environ. Pollut. 2016, 214, 290–298. [Google Scholar] [CrossRef]
- Agnandji, P.; Ayi-Fanou, L.; Gbaguidi, M.; Cachon, B.; Hounha, M.; Tchibozo, M.; Gbewonyo, W.; Cazier, F.; Sanni, A. Assessment of Organophosphorus and Pyrethroid Pesticide Residues in Lactuca sativa L. and Solanum macrocarpum L. cultivated in Benin. Glob. J. Sci. Front. Res. C Biol. Sci. 2018, 18, 4–12. [Google Scholar] [CrossRef]
- Huong, D.; Nga, T.; Ha, D. Residue Pesticides (Pyrethroid Group) in Vegetable and Their Health Risk Assessment via Digestion on Consumers in Ha Nam Province, Vietnam. IOP Conf. Ser. Earth Environ. Sci. 2020, 505, 012052. [Google Scholar] [CrossRef]
- Fosu-Mensah, B.; Okoffo, E.; Mensah, M. Synthetic Pyrethroids Pesticide Residues in Soils and Drinking Water Sources from Cocoa Farms in Ghana. Environ. Pollut. 2016, 5, 60–72. [Google Scholar] [CrossRef]
- Quijano, L.; Yusa, V.; Font, G.; Pardo, O. Chronic cumulative risk assessment of the exposure to organophosphorus, carbamate and pyrethroid and pyrethrin pesticides through fruit and vegetables consumption in the region of Valencia (Spain). Food Chem. Toxicol. 2016, 89, 39–46. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; El-Shamaa, I.S.; Abdel-Razik, N.I.; Elkomy, A.H.; Gewaily, M.S.; Abdo, S.E.; Soliman, A.A.; Paray, B.A.; Abdelkhalek, N. The effect of mannanoligosaccharide on the growth performance, histopathology, and the expression of immune and antioxidative related genes in Nile tilapia reared under chlorpyrifos ambient toxicity. Fish. Shellfish. Immunol. 2020, 103, 421–429. [Google Scholar] [CrossRef]
- Zahran, E.; Risha, E.; Awadin, W.; Palic, D. Acute exposure to chlorpyrifos induces reversible changes in health parameters of Nile tilapia (Oreochromis niloticus). Aquat. Toxicol. 2018, 197, 47–59. [Google Scholar] [CrossRef]
- Hussein, K. Article Review: Heavy Metals and Pesticides in Aquaculture. Eur. J. Acad. Essays 2015, 2, 15–22. [Google Scholar]
- Boran, H.; Altinok, I.; Capkin, E. Histopathological changes induced by maneb and carbaryl on some tissues of rainbow trout, Oncorhynchus mykiss. Tissue Cell 2010, 42, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Altinok, I.; Capkin, E. Histopathology of rainbow trout exposed to sublethal concentrations of methiocarb or endosulfan. Toxicol. Pathol. 2007, 35, 405–410. [Google Scholar] [CrossRef]
- Pilar Honrubia, M.; Paz Herraez, M.; Alvarez, R. The carbamate insecticide ZZ-Aphox induced structural changes of gills, liver, gall-bladder, heart, and notochord of Rana perezi tadpoles. Arch. Environ. Contam. Toxicol. 1993, 25, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Ledda, C.; Cannizzaro, E.; Cina, D.; Filetti, V.; Vitale, E.; Paravizzini, G.; Di Naso, C.; Iavicoli, I.; Rapisarda, V. Oxidative stress and DNA damage in agricultural workers after exposure to pesticides. J. Occup. Med. Toxicol. 2021, 16, 1. [Google Scholar] [CrossRef]
- Sule, R.O.; Condon, L.; Gomes, A.V. A Common Feature of Pesticides: Oxidative Stress-The Role of Oxidative Stress in Pesticide-Induced Toxicity. Oxid. Med. Cell Longev. 2022, 2022, 5563759. [Google Scholar] [CrossRef]
- Hilgert Jacobsen-Pereira, C.; Dos Santos, C.R.; Troina Maraslis, F.; Pimentel, L.; Feijo, A.J.L.; Iomara Silva, C.; de Medeiros, G.D.S.; Costa Zeferino, R.; Curi Pedrosa, R.; Weidner Maluf, S. Markers of genotoxicity and oxidative stress in farmers exposed to pesticides. Ecotoxicol. Environ. Saf. 2018, 148, 177–183. [Google Scholar] [CrossRef]
- Ansari, M.S.; Moraiet, M.A.; Ahmad, S. Insecticides: Impact on the Environment and Human Health. In Environmental Deterioration and Human Health; Springer: Berlin/Heidelberg, Germany, 2014; pp. 99–123. [Google Scholar]
- Capkin, E.; Altinok, I.; Karahan, S. Water quality and fish size affect toxicity of endosulfan, an organochlorine pesticide, to rainbow trout. Chemosphere 2006, 64, 1793–1800. [Google Scholar] [CrossRef] [PubMed]
- Jyothi, B.; Narayan, G. Certain pesticide-induced carbohydrate metabolic disorders in the serum of freshwater fish Clarias batrachus (Linn.). Food Chem. Toxicol. 1999, 37, 417–421. [Google Scholar] [CrossRef]
- Jaroli, D.P.; Sharma, B. Effect of Organophosphate Insecticide on the Organic Constituents in Liver of Channa punctatus. Asian J. Exp. Sci. 2005, 19, 121–129. [Google Scholar]
- Baldissera, M.D.; Souza, C.F.; Parmeggiani, B.; Vendrusculo, R.G.; Ribeiro, L.C.; Muenchen, D.K.; Zeppenfeld, C.C.; Meinhart, A.D.; Wagner, R.; Zanella, R.; et al. Protective effects of diet containing rutin against trichlorfon-induced muscle bioenergetics disruption and impairment on fatty acid profile of silver catfish Rhamdia quelen. Ecotoxicol. Environ. Saf. 2020, 205, 111127. [Google Scholar] [CrossRef]
- Majumder, R.; Kaviraj, A. Acute and sublethal effects of organophosphate insecticide chlorpyrifos on freshwater fish Oreochromis niloticus. Drug Chem. Toxicol. 2019, 42, 487–495. [Google Scholar] [CrossRef] [PubMed]
- de Aguiar, L.H.; Moraes, G.; Avilez, I.M.; Altran, A.E.; Correa, C.F. Metabolical effects of Folidol 600 on the neotropical freshwater fish matrinxa, Brycon cephalus. Environ. Res. 2004, 95, 224–230. [Google Scholar] [CrossRef]
- Murty, A.S.; Ramani, A.V.; Christopher, K.; Rajabhushanam, B.R. Toxicity of methyl parathion and fensulfothion to the fish Mystus cavasius. Environ. Pollut. Ser. A Ecol. Biol. 1984, 34, 37–46. [Google Scholar] [CrossRef]
- Pena-Llopis, S.; Ferrando, M.D.; Pena, J.B. Fish tolerance to organophosphate-induced oxidative stress is dependent on the glutathione metabolism and enhanced by N-acetylcysteine. Aquat. Toxicol. 2003, 65, 337–360. [Google Scholar] [CrossRef] [PubMed]
- Hai, D.Q.; Varga, S.I.; Matkovics, B. Organophosphate effects on antioxidant system of carp (Cyprinus carpio) and catfish (Ictalurus nebulosus). Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1997, 117, 83–88. [Google Scholar] [CrossRef]
- Straus, D.L.; Chambers, J.E. Inhibition of acetylcholinesterase and aliesterases of fingerling channel catfish by chlorpyrifos, parathion, and S,S,S-tributyl phosphorotrithioate (DEF). Aquat. Toxicol. 1995, 33, 311–324. [Google Scholar] [CrossRef]
- Ceron, J.J.; Ferrando, M.D.; Sancho, E.; Gutierrez-Panizo, C.; Andreu-Moliner, E. Effects of diazinon exposure on cholinesterase activity in different tissues of European eel (Anguilla anguilla). Ecotoxicol. Environ. Saf. 1996, 35, 222–225. [Google Scholar] [CrossRef]
- Sancho, E.; Ferrando, M.D.; Andreu, E. Sublethal effects of an organophosphate insecticide on the European eel, Anguilla anguilla. Ecotoxicol. Environ. Saf. 1997, 36, 57–65. [Google Scholar] [CrossRef]
- Chindah, A.C.; Sikoki, F.D.; Ijeoma, V.-A. Toxicity of an Organophosphate Pesticide (chloropyrifos) on a common Niger Delta Wetland Fish -Tilapia guineensis. J. Appl. Sci. Environ. Mgt. 2004, 8, 11–17. [Google Scholar]
- Diaz-Resendiz, K.J.G.; Ortiz-Lazareno, P.C.; Covantes-Rosales, C.E.; Trujillo-Lepe, A.M.; Toledo-Ibarra, G.A.; Ventura-Ramon, G.H.; Giron-Perez, M.I. Effect of diazinon, an organophosphate pesticide, on signal transduction and death induction in mononuclear cells of Nile tilapia fish (Oreochromis niloticus). Fish. Shellfish. Immunol. 2019, 89, 12–17. [Google Scholar] [CrossRef]
- Covantes-Rosales, C.E.; Trujillo-Lepe, A.M.; Diaz-Resendiz, K.J.G.; Toledo-Ibarra, G.A.; Ventura-Ramon, G.H.; Ortiz-Lazareno, P.C.; Giron-Perez, M.I. Phagocytosis and ROS production as biomarkers in Nile tilapia (Oreochromis niloticus) leukocytes by exposure to organophosphorus pesticides. Fish. Shellfish. Immunol. 2019, 84, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Giron-Perez, M.I.; Santerre, A.; Gonzalez-Jaime, F.; Casas-Solis, J.; Hernandez-Coronado, M.; Peregrina-Sandoval, J.; Takemura, A.; Zaitseva, G. Immunotoxicity and hepatic function evaluation in Nile tilapia (Oreochromis niloticus) exposed to diazinon. Fish. Shellfish. Immunol. 2007, 23, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Giron-Perez, M.I.; Zaitseva, G.; Casas-Solis, J.; Santerre, A. Effects of diazinon and diazoxon on the lymphoproliferation rate of splenocytes from Nile tilapia (Oreochromis niloticus): The immunosuppresive effect could involve an increase in acetylcholine levels. Fish. Shellfish. Immunol. 2008, 25, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Ibarra, G.A.; Diaz-Resendiz, K.J.; Pavon-Romero, L.; Rojas-Garcia, A.E.; Medina-Diaz, I.M.; Giron-Perez, M.I. Effects of diazinon on the lymphocytic cholinergic system of Nile tilapia fish (Oreochromis niloticus). Vet. Immunol. Immunopathol. 2016, 176, 58–63. [Google Scholar] [CrossRef]
- Wang, X.; Xing, H.; Li, X.; Xu, S.; Wang, X. Effects of atrazine and chlorpyrifos on the mRNA levels of IL-1 and IFN-gamma2b in immune organs of common carp. Fish. Shellfish. Immunol. 2011, 31, 126–133. [Google Scholar] [CrossRef]
- Xing, H.; Wang, X.; Sun, G.; Gao, X.; Xu, S.; Wang, X. Effects of atrazine and chlorpyrifos on activity and transcription of glutathione S-transferase in common carp (Cyprinus carpio L.). Environ. Toxicol. Pharmacol. 2012, 33, 233–244. [Google Scholar] [CrossRef]
- Cong, N.V.; Phuong, N.T.; Bayley, M. Effects of repeated exposure of diazinon on cholinesterase activity and growth in snakehead fish (Channa striata). Ecotoxicol. Environ. Saf. 2009, 72, 699–703. [Google Scholar] [CrossRef]
- Ferrari, A.; Venturino, A.; Pechén de D’Angelo, A.M. Muscular and brain cholinesterase sensitivities to azinphos methyl and carbaryl in the juvenile rainbow trout Oncorhynchus mykiss. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2007, 146, 308–313. [Google Scholar] [CrossRef]
- Ferrari, A.; Anguiano, O.L.; Soleno, J.; Venturino, A.; Pechen de D’Angelo, A.M. Different susceptibility of two aquatic vertebrates (Oncorhynchus mykiss and Bufo arenarum) to azinphos methyl and carbaryl. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2004, 139, 239–243. [Google Scholar] [CrossRef]
- Altinok, I.; Capkin, E.; Karahan, S.; Boran, M. Effects of water quality and fish size on toxicity of methiocarb, a carbamate pesticide, to rainbow trout. Environ. Toxicol. Pharmacol. 2006, 22, 20–26. [Google Scholar] [CrossRef]
- Ghazala; Mahboob, S.; Ahmad, L.; Sultana, S.; Alghanim, K.; Al-Misned, F.; Ahmad, Z. Fish cholinesterases as biomarkers of sublethal effects of organophosphorus and carbamates in tissues of Labeo rohita. J. Biochem. Mol. Toxicol. 2014, 28, 137–142. [Google Scholar] [CrossRef]
- Sastry, K.V.; Siddiqui, A.A. Chronic toxic effects of the carbamate pesticide sevin on carbohydrate metabolism in a freshwater snakehead fish, Channa punctatus. Toxicol. Lett. 1982, 14, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Assis, C.R.; Castro, P.F.; Amaral, I.P.; Carvalho, E.V.; Carvalho, L.B., Jr.; Bezerra, R.S. Characterization of acetylcholinesterase from the brain of the Amazonian tambaqui (Colossoma macropomum) and in vitro effect of organophosphorus and carbamate pesticides. Environ. Toxicol. Chem. 2010, 29, 2243–2248. [Google Scholar] [CrossRef] [PubMed]
- Dar, S.A.; Yousuf, A.R.; Balkhi, M.U.; Ganai, F.A.; Bhat, F.A. Assessment of endosulfan induced genotoxicity and mutagenicity manifested by oxidative stress pathways in freshwater cyprinid fish crucian carp (Carassius carassius L.). Chemosphere 2015, 120, 273–283. [Google Scholar] [CrossRef]
- Pant, J.C.; Singh, T. Inducement of metabolic dysfunction by carbamate and organophosphorus compounds in a fish, Puntius conchonius. Pestic. Biochem. Physiol. 1983, 20, 294–298. [Google Scholar] [CrossRef]
- Begum, G. Organ-specific ATPase and phosphorylase enzyme activities in a food fish exposed to a carbamate insecticide and recovery response. Fish. Physiol. Biochem. 2011, 37, 61–69. [Google Scholar] [CrossRef]
- Jarrard, H.E.; Delaney, K.R.; Kennedy, C.J. Impacts of carbamate pesticides on olfactory neurophysiology and cholinesterase activity in coho salmon (Oncorhynchus kisutch). Aquat. Toxicol. 2004, 69, 133–148. [Google Scholar] [CrossRef]
- Tripathi, G.; Verma, P. Endosulfan-mediated biochemical changes in the freshwater fish Clarias batrachus. Biomed. Environ. Sci. 2004, 17, 47–56. [Google Scholar]
- Mdegela, R.H.; Mosha, R.D.; Sandvik, M.; Skaare, J.U. Assessment of acetylcholinesterase activity in Clarias gariepinus as a biomarker of organophosphate and carbamate exposure. Ecotoxicology 2010, 19, 855–863. [Google Scholar] [CrossRef]
- Waring, C.P.; Moore, A. Sublethal effects of a carbamate pesticide on pheromonal mediated endocrine function in mature male Atlantic salmon (Salmo salar L.) parr. Fish Physiol. Biochem. 1997, 11, 203–211. [Google Scholar] [CrossRef]
- Vani, G.; Veeraiah, K.; Kumar, M.V.; Parveen, S.; Rao, G.D.V.P. Biochemical Changes Induced by Cartap Hydrochloride (50% SP), Carbamate Insecticide in Freshwater Fish Cirrhinus mrigala (Hamilton, 1822). Nat. Environ. Pollut. Technol. 2020, 19, 1801–1829. [Google Scholar] [CrossRef]
- Ibrahim, H.; Kheir, R.; Helmi, S.; Lewis, J.; Crane, M. Effects of organophosphorus, carbamate, pyrethroid and organochlorine pesticides, and a heavy metal on survival and cholinesterase activity of Chironomus riparius Meigen. Bull. Environ. Contam. Toxicol. 1998, 60, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Hii, Y.S.; Lee, M.Y.; Chuah, T.S. Acute toxicity of organochlorine insecticide endosulfan and its effect on behaviour and some hematological parameters of Asian swamp eel (Monopterus albus, Zuiew). Pestic. Biochem. Physiol. 2007, 89, 46–53. [Google Scholar] [CrossRef]
- Da Cuna, R.H.; Rey Vazquez, G.; Piol, M.N.; Guerrero, N.V.; Maggese, M.C.; Lo Nostro, F.L. Assessment of the acute toxicity of the organochlorine pesticide endosulfan in Cichlasoma dimerus (Teleostei, Perciformes). Ecotoxicol. Environ. Saf. 2011, 74, 1065–1073. [Google Scholar] [CrossRef]
- Pandey, S.; Ahmad, I.; Parvez, S.; Bin-Hafeez, B.; Haque, R.; Raisuddin, S. Effect of Endosulfan on Antioxidants of Freshwater Fish Channa punctatus Bloch: 1. Protection Against Lipid Peroxidation in Liver by Copper Preexposure. Arch. Environ. Contam. Toxicol. 2001, 41, 345–352. [Google Scholar] [CrossRef]
- Bacchetta, C.; Cazenave, J.; Parma, M.J. Responses of Biochemical Markers in the Fish Prochilodus lineatus Exposed to a Commercial Formulation of Endosulfan. Water Air Soil. Pollut. 2011, 216, 39–49. [Google Scholar] [CrossRef]
- Dutta, H.M.; Misquitta, D.; Khan, S. The effects of endosulfan on the testes of bluegill fish, Lepomis macrochirus: A histopathological study. Arch. Environ. Contam. Toxicol. 2006, 51, 149–156. [Google Scholar] [CrossRef]
- Dutta, H.M.; Arends, D.A. Effects of endosulfan on brain acetylcholinesterase activity in juvenile bluegill sunfish. Environ. Res. 2003, 91, 157–162. [Google Scholar] [CrossRef]
- Kumar, N.; Ambasankar, K.; Krishnani, K.K.; Gupta, S.K.; Bhushan, S.; Minhas, P.S. Acute toxicity, biochemical and histopathological responses of endosulfan in Chanos chanos. Ecotoxicol. Environ. Saf. 2016, 131, 79–88. [Google Scholar] [CrossRef]
- Beyger, L.; Orrego, R.; Guchardi, J.; Holdway, D. The acute and chronic effects of endosulfan pulse-exposure on Jordanella floridae (Florida flagfish) over one complete life-cycle. Ecotoxicol. Environ. Saf. 2012, 76, 71–78. [Google Scholar] [CrossRef]
- MagarR, S.; Shaikh, A. Biochemical Changes in Proteins and Amino Acids in Channa Punctatus in Responses to Sublethal Treatment with the Insecticide Malathion. Trends Life Sci. 2012, 1, 19–23. [Google Scholar]
- Bharti, S.; Rasool, F. Analysis of the biochemical and histopathological impact of a mild dose of commercial malathion on Channa punctatus (Bloch) fish. Toxicol. Rep. 2021, 8, 443–455. [Google Scholar] [CrossRef]
- Baldissera, M.D.; Souza, C.F.; Zanella, R.; Prestes, O.D.; Meinhart, A.D.; Da Silva, A.S.; Baldisserotto, B. Behavioral impairment and neurotoxic responses of silver catfish Rhamdia quelen exposed to organophosphate pesticide trichlorfon: Protective effects of diet containing rutin. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021, 239, 108871. [Google Scholar] [CrossRef]
- Morrison, H.I.; Wilkins, K.; Semenciw, R.; Mao, Y.; Wigle, D. Herbicides and cancer. J. Natl. Cancer Inst. 1992, 84, 1866–1874. [Google Scholar] [CrossRef]
- Solomon, K.R.; Dalhoff, K.; Volz, D.; Van Der Kraak, G. 7-Effects of Herbicides on Fish. In Fish Physiology; Tierney, K.B., Farrell, A.P., Brauner, C.J., Eds.; Academic Press: Cambridge, MA, USA, 2013; Volume 33, pp. 369–409. [Google Scholar]
- Es Ruiz de Arcaute, C.; Ossana, N.A.; Perez-Iglesias, J.M.; Soloneski, S.; Larramendy, M.L. Auxinic herbicides induce oxidative stress on Cnesterodon decemmaculatus (Pisces: Poeciliidae). Environ. Sci. Pollut. Res. Int. 2019, 26, 20485–20498. [Google Scholar] [CrossRef] [PubMed]
- Sancho, E.; Ceron, J.J.; Ferrando, M.D. Cholinesterase activity and hematological parameters as biomarkers of sublethal molinate exposure in Anguilla anguilla. Ecotoxicol. Environ. Saf. 2000, 46, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Moraes, B.S.; Loro, V.L.; Glusczak, L.; Pretto, A.; Menezes, C.; Marchezan, E.; de Oliveira Machado, S. Effects of four rice herbicides on some metabolic and toxicology parameters of teleost fish (Leporinus obtusidens). Chemosphere 2007, 68, 1597–1601. [Google Scholar] [CrossRef]
- Rossi, S.C.; Dreyer da Silva, M.; Piancini, L.D.; Oliveira Ribeiro, C.A.; Cestari, M.M.; Silva de Assis, H.C. Sublethal effects of waterborne herbicides in tropical freshwater fish. Bull. Environ. Contam. Toxicol. 2011, 87, 603–607. [Google Scholar] [CrossRef]
- Call, D.J.; Brooke, L.T.; Kent, R.J.; Knuth, M.L.; Poirier, S.H.; Huot, J.M.; Lima, A.R. Bromacil and diuron herbicides: Toxicity, uptake, and elimination in freshwater fish. Arch. Environ. Contam. Toxicol. 1987, 16, 607–613. [Google Scholar] [CrossRef]
- Vera-Candioti, J.; Soloneski, S.; Larramendy, M.L. Evaluation of the genotoxic and cytotoxic effects of glyphosate-based herbicides in the ten spotted live-bearer fish Cnesterodon decemmaculatus (Jenyns, 1842). Ecotoxicol Env. Saf. 2013, 89, 166–173. [Google Scholar] [CrossRef]
- Moraes, B.S.; Loro, V.L.; Pretto, A.; da Fonseca, M.B.; Menezes, C.; Marchesan, E.; Reimche, G.B.; de Avila, L.A. Toxicological and metabolic parameters of the teleost fish (Leporinus obtusidens) in response to commercial herbicides containing clomazone and propanil. Pestic. Biochem. Physiol. 2009, 95, 57–62. [Google Scholar] [CrossRef]
- Fathy, M.; Mohamed, I.A.; Farghal, A.I.A.; Temerak, S.A.H.; Sayed, A.E.-D.H. Hemotoxic effects of some herbicides on juvenile of Nile tilapia Oreochromis niloticus. Environ. Sci. Pollut. Res. 2019, 26, 30857–30865. [Google Scholar] [CrossRef] [PubMed]
- Glusczak, L.; dos Santos Miron, D.; Crestani, M.; Braga da Fonseca, M.; de Araujo Pedron, F.; Duarte, M.F.; Vieira, V.L. Effect of glyphosate herbicide on acetylcholinesterase activity and metabolic and hematological parameters in piava (Leporinus obtusidens). Ecotoxicol. Environ. Saf. 2006, 65, 237–241. [Google Scholar] [CrossRef]
- Mao, L.; Jia, W.; Zhang, L.; Zhang, Y.; Zhu, L.; Sial, M.U.; Jiang, H. Embryonic development and oxidative stress effects in the larvae and adult fish livers of zebrafish (Danio rerio) exposed to the strobilurin fungicides, kresoxim-methyl and pyraclostrobin. Sci. Total Environ. 2020, 729, 139031. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Chou, P.H.; Chen, P.J. Two azole fungicides (carcinogenic triadimefon and non-carcinogenic myclobutanil) exhibit different hepatic cytochrome P450 activities in medaka fish. J. Hazard. Mater. 2014, 277, 150–158. [Google Scholar] [CrossRef]
- Ankley, G.T.; Jensen, K.M.; Durhan, E.J.; Makynen, E.A.; Butterworth, B.C.; Kahl, M.D.; Villeneuve, D.L.; Linnum, A.; Gray, L.E.; Cardon, M.; et al. Effects of two fungicides with multiple modes of action on reproductive endocrine function in the fathead minnow (Pimephales promelas). Toxicol. Sci. 2005, 86, 300–308. [Google Scholar] [CrossRef]
- Atamaniuk, T.M.; Kubrak, O.I.; Husak, V.V.; Storey, K.B.; Lushchak, V.I. The Mancozeb-containing carbamate fungicide tattoo induces mild oxidative stress in goldfish brain, liver, and kidney. Environ. Toxicol. 2014, 29, 1227–1235. [Google Scholar] [CrossRef]
- Kubrak, O.I.; Atamaniuk, T.M.; Husak, V.V.; Drohomyretska, I.Z.; Storey, J.M.; Storey, K.B.; Lushchak, V.I. Oxidative stress responses in blood and gills of Carassius auratus exposed to the mancozeb-containing carbamate fungicide Tattoo. Ecotoxicol. Environ. Saf. 2012, 85, 37–43. [Google Scholar] [CrossRef]
- Pallavi Srivastava, A.S. In vivo study of effects of dithiocarbamates fungicide. (Mancozeb) and its metabolite ethylenethiourea (ETU) on fresh water fish Clarius batra. J. Biol. Earth Sci. 2013, 3, B228–B235. [Google Scholar]
- Remia, K.M.; Logaswamy, S.; Logankumar, K.; Rajmohan, D. Effect of an insecticide (Monocrotophos) on some biochemical constituents of the fish Tilapia mossambica. Poll. Res. 2008, 27, 523–526. [Google Scholar]
- Murty, A.S.; Devi, A.P. The effect of endosulfan and its isomers on tissue protein, glycogen, and lipids in the fish Channa punctata. Pestic. Biochem. Physiol. 1982, 17, 280–286. [Google Scholar] [CrossRef]
- Olsvik, P.A.; Hammer, S.K.; Sanden, M.; Softeland, L. Chlorpyrifos-induced dysfunction of lipid metabolism is not restored by supplementation of polyunsaturated fatty acids EPA and ARA in Atlantic salmon liver cells. Toxicol. Vitr. 2019, 61, 104655. [Google Scholar] [CrossRef] [PubMed]
- Lal, B.; Singh, T.P. The effect of malathion and γ-BHC on the lipid metabolism in relation to reproduction in the tropical teleost, Clarias batrachus. Environ. Pollut. 1987, 48, 37–47. [Google Scholar] [CrossRef]
- Karami-Mohajeri, S.; Abdollahi, M. Toxic influence of organophosphate, carbamate, and organochlorine pesticides on cellular metabolism of lipids, proteins, and carbohydrates: A systematic review. Hum. Exp. Toxicol. 2011, 30, 1119–1140. [Google Scholar] [CrossRef] [PubMed]
- Parrish, C.C. Essential fatty acids in aquatic food webs. In Lipids in Aquatic Ecosystems; Kainz, M., Brett, M.T., Arts, M.T., Eds.; Springer: New York, NY, USA, 2009; pp. 309–326. [Google Scholar]
- Olsvik, P.A.; Larsen, A.K.; Berntssen, M.H.G.; Goksoyr, A.; Karlsen, O.A.; Yadetie, F.; Sanden, M.; Kristensen, T. Effects of Agricultural Pesticides in Aquafeeds on Wild Fish Feeding on Leftover Pellets Near Fish Farms. Front. Genet. 2019, 10, 794. [Google Scholar] [CrossRef]
- Panseri, S.; Chiesa, L.; Ghisleni, G.; Marano, G.; Boracchi, P.; Ranghieri, V.; Malandra, R.M.; Roccabianca, P.; Tecilla, M. Persistent organic pollutants in fish: Biomonitoring and cocktail effect with implications for food safety. Food Addit. Contam. Part. A Chem. Anal. Control Expo. Risk Assess. 2019, 36, 601–611. [Google Scholar] [CrossRef]
- Islam, R.; Kumar, S.; Karmoker, J.; Kamruzzaman, M.; Rahman, M.A.; Biswas, N.; Tran, T.K.A.; Rahman, M.M. Bioaccumulation and adverse effects of persistent organic pollutants (POPs) on ecosystems and human exposure: A review study on Bangladesh perspectives. Environ. Technol. Innov. 2018, 12, 115–131. [Google Scholar] [CrossRef]
- Baibergenova, A.; Kudyakov, R.; Zdeb, M.; Carpenter, D.O. Low birth weight and residential proximity to PCB-contaminated waste sites. Environ. Health Perspect. 2003, 111, 1352–1357. [Google Scholar] [CrossRef]
- Cox, S.; Niskar, A.S.; Narayan, K.M.; Marcus, M. Prevalence of self-reported diabetes and exposure to organochlorine pesticides among Mexican Americans: Hispanic health and nutrition examination survey, 1982–1984. Environ. Health Perspect. 2007, 115, 1747–1752. [Google Scholar] [CrossRef]
- Dickman, M.D.; Leung, K.M. Mercury and organochlorine exposure from fish consumption in Hong Kong. Chemosphere 1998, 37, 991–1015. [Google Scholar] [CrossRef]
- Marti, M.; Ortiz, X.; Gasser, M.; Marti, R.; Montana, M.J.; Diaz-Ferrero, J. Persistent organic pollutants (PCDD/Fs, dioxin-like PCBs, marker PCBs, and PBDEs) in health supplements on the Spanish market. Chemosphere 2010, 78, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Porta, M. Persistent organic pollutants and the burden of diabetes. Lancet 2006, 368, 558–559. [Google Scholar] [CrossRef] [PubMed]
- Ram, M.A. Route of Exposure and Impact of Pesticides Pollution on Human Health and Aquatic Ecosystem. Int. J. Zool. Investig. 2022, 8, 85–99. [Google Scholar] [CrossRef]
- Elbialy, Z.; Ismail, T.; Abdelhady, D.; Elasely, A. Assessment of Genotoxic Effects of Pesticide Residues and Related Haemato-Biochemical Parameters on Farmed Nile Tilapia (Oreochromis niloticus L.) in Kafrelsheikh Governorate, Egypt. Alex. J. Vet. Sci. 2015, 44, 136–146. [Google Scholar] [CrossRef]
- Ergenler, A.; Turan, F. DNA Damage in Fish Due to Pesticide Pollution. Nat. Eng. Sci. 2023, 8, 195–201. [Google Scholar] [CrossRef]
- Kapeleka, J.; Sauli, E.; Ndakidemi, P. Pesticide exposure and genotoxic effects as measured by DNA damage and human monitoring biomarkers. Int. J. Environ. Health Res. 2019, 31, 805–822. [Google Scholar] [CrossRef]


| Fish Species | Pesticide | Toxic Dose | Effect on Fish | Reference |
|---|---|---|---|---|
| Oreochromis mossambicus | cypermethrin | 10% EC | ↓total protein | [17] |
| lambda-cyhalothrin | 2.5% EC | ↓total protein | [17] | |
| malathion | 57% EC | ↓total protein | [17] | |
| Channa punctatus | malathion | 0.l mg/L | ↑ free amino acid in muscle, kidney, heart, and stomach, ↓protein content in muscle, gill, and liver | [104] |
| chlorpyrifos | 0.365 ppm | ↑cholesterol and ↓protein in the liver | [62] | |
| Anguilla anguilla | fenitrothion | Sublethal 0.02 ppm | ↓protein | [71] |
| Cnesterodon decemmaculatus | DIC and 2,4-dichlorophenoxyacetic acid (2,4-D) | 410 mg/L | ↓total protein | [109] |
| Oreochromis niloticus | acetochlor | 2.625 mg/L | ↑total plasma protein | [116] |
| Tilapia mossambica | monocrotophos | 52.36 mg/L | ↓protein, ↑carbohydrate levels in the gill, muscle, and kidney, and ↓cholesterol in the gill, muscle, and kidney | [124] |
| Clarius batrachus | mancozeb | 22.87 mg/L | ↓protein, ↓amino acids, ↓glycogen, ↓and nucleic acids in the liver and muscles | [123] |
| Cirrhinus mrigala | Cartap hydrochloride | 0.339–0.436 mg/L | ↓glycogen, ↓total protein, ↓nucleic acids in gill, liver, brain, kidney and muscle tissues | [93] |
| Channa punctata | endosulfan | Endosulfan A (0.05 and 0.45 ppb) and Endosulfan B (3 and 11 ppb) | ↓protein, ↓glycogen, ↓lipid in the liver, ↓glycogen in muscle, ↑protein and ↑glycogen in the kidney, and ↑protein in the brain | [125] |
| malathion | 0.4 mg/L; 1/20th of 96 h LC50 value | ↓serum glucose and ↑protein content | [105] | |
| Gadus morhua | chlorpyrifos | 0.5 mg/kg | ↑omega-6 and ↓omega-3 | [126] |
| Clarias batrachus | γ-BHC | 2 and 8 ppm | Altered lipid metabolism | [127] |
| malathion | 1 and 4 ppm | ↑liver lipids and Altered lipid metabolism | ||
| Rhamdia quelen | trichlorfon | 11 mg/L | ↓total PUFAs | [63] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burch, E.; Hussein, M.A.; Zaki, M.; Kamal, L.T.; Zaki, G.; Shoeib, T.; Dawood, M.; Sewilam, H.; Abdelnaser, A. Assessing the Effects of Pesticides on Aquacultured Fish and Ecosystems: A Comprehensive Environmental Health Review. Fishes 2025, 10, 223. https://doi.org/10.3390/fishes10050223
Burch E, Hussein MA, Zaki M, Kamal LT, Zaki G, Shoeib T, Dawood M, Sewilam H, Abdelnaser A. Assessing the Effects of Pesticides on Aquacultured Fish and Ecosystems: A Comprehensive Environmental Health Review. Fishes. 2025; 10(5):223. https://doi.org/10.3390/fishes10050223
Chicago/Turabian StyleBurch, Emily, Mohamed Ali Hussein, Manar Zaki, Lereen T. Kamal, Ghada Zaki, Tamer Shoeib, Mahmoud Dawood, Hani Sewilam, and Anwar Abdelnaser. 2025. "Assessing the Effects of Pesticides on Aquacultured Fish and Ecosystems: A Comprehensive Environmental Health Review" Fishes 10, no. 5: 223. https://doi.org/10.3390/fishes10050223
APA StyleBurch, E., Hussein, M. A., Zaki, M., Kamal, L. T., Zaki, G., Shoeib, T., Dawood, M., Sewilam, H., & Abdelnaser, A. (2025). Assessing the Effects of Pesticides on Aquacultured Fish and Ecosystems: A Comprehensive Environmental Health Review. Fishes, 10(5), 223. https://doi.org/10.3390/fishes10050223






