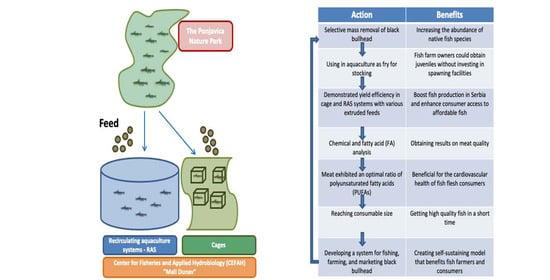
Can We Turn Harmful Invasive Non-Native Fish Species into a Valuable Food Resource?
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Experimental Rearing and Data Analysis
2.3. Methodology for Chemical Analysis of Black Bullhead Meat
2.4. Methodology for Fatty Acid (FA) Analysis of Black Bullhead Meat
2.4.1. Extraction of Total Lipids
2.4.2. Fatty Acids
2.5. Statistical Analysis
3. Results
3.1. Abiotic Parameters
3.2. Growth Performance and Survival of Black Bullhead
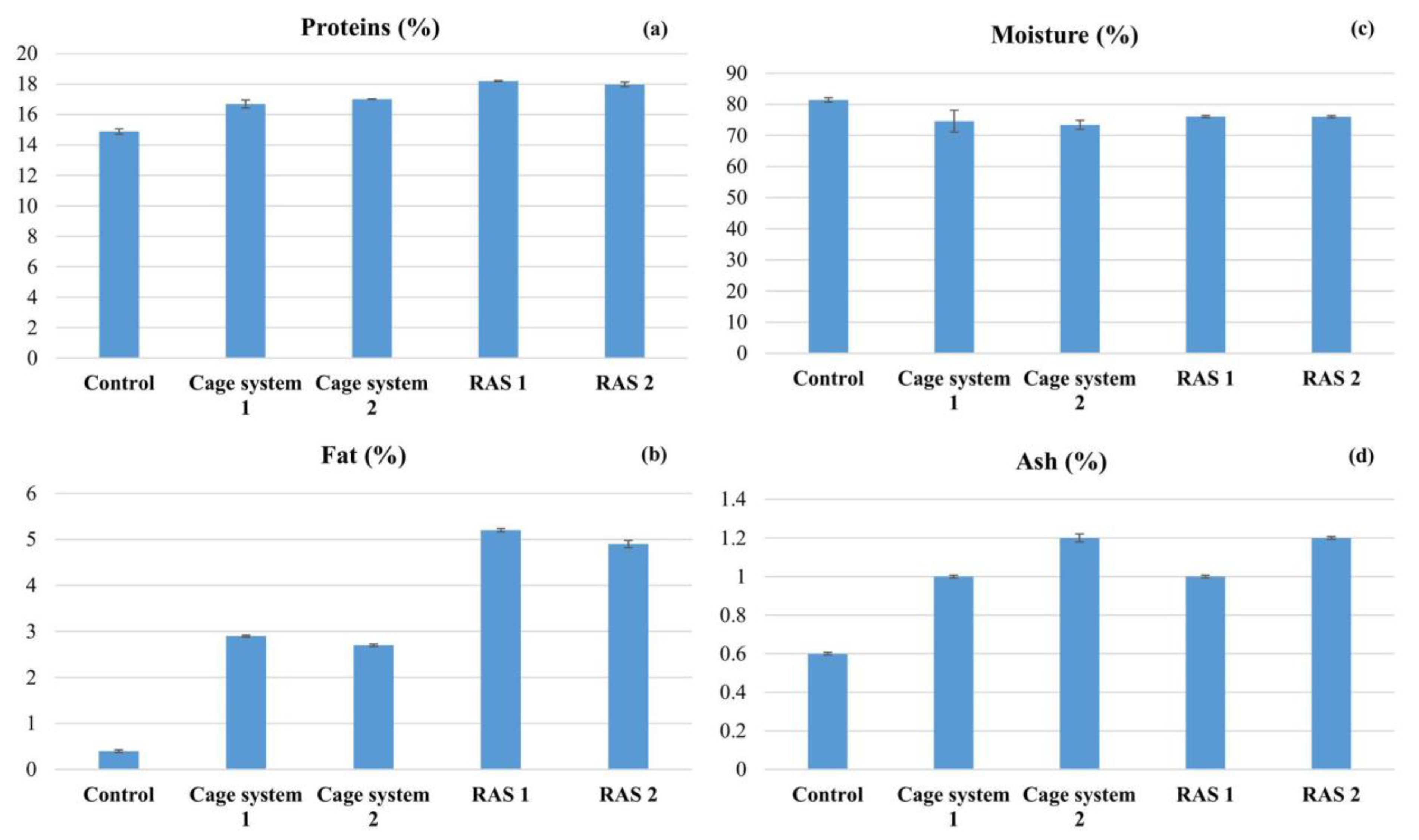
3.3. Chemical Composition of Black Bullhead Meat
4. Discussion
5. Conclusions
- 1.
- Adaptability and Growth: Both experimental setups (cage systems and RAS) demonstrated that the black bullhead adapts well to intensive fish production environments. It successfully grows in these systems, thriving on high-protein food and tolerating high population densities and stress. Notably, the cage system was generally more effective for rearing black bullheads, although the survival rate in RAS was over twice as high compared to cages. The black bullhead exhibited good growth parameters and feed conversion rates in both systems.
- 2.
- Nutritional Composition: The meat of black bullhead was found to have an optimal ratio of polyunsaturated fatty acids (PUFAs), particularly the n-6/n-3 ratio, which is beneficial for cardiovascular health. PUFAs were significantly higher in RAS and cage systems than in control.
- 3.
- Public Health: The favorable omega-6 to omega-3 fatty acid ratio in black bullhead meat suggests that increased consumption could help reduce cardiovascular diseases.
- 4.
- Efficiency and Potential: The research achieved its goals, demonstrating yield efficiency in cage and RAS systems with different types of extruded feed. It also highlighted potential challenges in breeding black bullheads and the need to improve technological conditions in future rearing systems. If production proves profitable, it could enhance fish production in Serbia and increase consumer access to affordable fish. Using juveniles from native populations could help keep market prices reasonable.
- 5.
- Ecosystem Impact: The research underscores how a non-native, invasive species can be transformed into a valuable food source, aiding in ecosystem management by removing the species from native habitats where it causes ecological disruption.
- 6.
- Economic Viability: Developing a fishing, farming, and marketing black bullhead system could be economically viable. It offers a self-sustaining model that benefits fish farmers and consumers, potentially minimizing the need for additional investments in breeding facilities. However, to avoid unintended ecological consequences, any economic valorization of invasive species such as black bullhead must be accompanied by stringent regulatory measures aimed at preventing their further spread, including restrictions on intentional breeding and mandatory traceability protocols for harvested individuals.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Upreti, G. (Ed.) Biodiversity and Ecosystem Destruction. In Ecosociocentrism—The Earth First Paradigm for Sustainable Living; Springer: Cham, Switzerland, 2023; pp. 31–64. [Google Scholar] [CrossRef]
- WWF. Living Planet Report 2024—A System in Peril; WWF: Gland, Switzerland, 2024; Available online: https://wwflpr.awsassets.panda.org/downloads/2024-living-planet-report-a-system-in-peril.pdf (accessed on 10 April 2025).
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.; Knowler, D.J.; Leveque, C.L.; Naiman, R.J.; Prieur-Richard, A.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef]
- Darwall, W.R.T.; Holland, R.A.; Smith, K.G.; Allen, D.; Brooks, E.G.E.; Katarya, V.; Pollock, C.M.; Shi, Y.; Clausnitzer, V.; Cumberlidge, N.; et al. Implications of bias in conservation research and investment for freshwater species. Conserv. Lett. 2011, 4, 474–482. [Google Scholar] [CrossRef]
- Tickner, D.; Opperman, J.J.; Abell, R.; Acreman, M.; Arthington, A.H.; Bunn, S.E.; Cooke, S.J.; Dalton, J.; Darwall, W.; Edwards, G.; et al. Bending the Curve of Global Freshwater Biodiversity Loss: An Emergency Recovery Plan. BioScience 2020, 70, 330–342. [Google Scholar] [CrossRef] [PubMed]
- van Ham, C.; Genovesi, P.; Scalera, R. Invasive Alien Species: The Urban Dimension, Case Studies on Strengthening Local Action in Europe; IUCN European Union Representative Office: Brussels, Belgium, 2013; 103p, Available online: https://iucn.org/resources/publication/invasive–alien-species-urban-dimension-case-studies-strengthening-local (accessed on 10 April 2025).
- Otero, M.; Cebrian, E.; Francour, P.; Galil, B.; Savini, D. Monitoring Marine Invasive Species in Mediterranean Marine Protected Areas (MPAs): A Strategy and Practical Guide for Managers; IUCN: Malaga, Spain, 2013; Available online: https://iucn.org/resources/publication/monitoring-marine-invasive-species-mediterranean-marine-protected-areas-mpas (accessed on 10 April 2025).
- Early, R.; Bradley, B.A.; Dukes, J.S.; Lawler, J.J.; Olden, J.D.; Blumenthal, D.M.; Gonzalez, P.; Grosholz, E.D.; Ibañez, I.; Miller, L.P. Global threats from invasive alien species in the twenty-first century and national response capacities. Nat. Commun. 2016, 7, 12485. [Google Scholar] [CrossRef] [PubMed]
- Roy, H.E.; Bacher, S.; Essl, F.; Adriaens, T.; Aldridge, D.C.; Bishop, J.D.; Blackburn, T.M.; Branquart, E.; Brodie, J.; Carboneras, C.; et al. Developing a list of invasive alien species likely to threaten biodiversity and ecosystems in the European Union. Glob. Chang. Biol. 2019, 25, 1032–1048. [Google Scholar] [CrossRef]
- Shackleton, R.T.; Foxcroft, L.C.; Pyšek, P.; Wood, L.E.; Richardson, D.M. Assessing biological invasions in protected areas after 30 years: Revisiting nature reserves targeted by the 1980s SCOPE programme. Biol. Conserv. 2020, 243, 108424. [Google Scholar] [CrossRef]
- Breithaupt, H. Aliens on the shores. Biodiversity and national economies are being threatened by the invasion of non-native species. EMBO Rep. 2003, 4, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Wittenberg, R. An Inventory of Alien Species and Their Threat to Biodiversity and Economy in Switzerland; CABI Bioscience Switzerland Centre report to the Swiss Agency for Environment, Forests and Landscape; The environment in practice no. 0629; Federal Office for the Environment: Bern, Switzerland, 2005; p. 155. Available online: https://www.researchgate.net/publication/265455571_An_Inventory_of_Alien_Species_and_Their_Threat_to_Biodiversity_and_Economy_in_Switzerland (accessed on 15 April 2025).
- Millennium Ecosystem Assessment, MEA. Ecosystems and Human Well-Being: Biodiversity Synthesis; World Resources Institute: Washington, DC, USA, 2005; Available online: https://www.millenniumassessment.org/documents/document.356.aspx.pdf (accessed on 15 April 2025).
- Clavero, M.; García-Berthou, E. Invasive species are a leading cause of animal extinctions. Trends Ecol. Evol. 2005, 20, 110. [Google Scholar] [CrossRef]
- Dueñas, M.A.; Ruffhead, H.J.; Wakefield, N.H.; Roberts, P.D.; Hemming, D.J.; Diaz-Soltero, H. The role played by invasive species in interactions with endangered and threatened species in the United States: A systematic review. Biodivers. Conserv. 2018, 27, 3171–3183. [Google Scholar] [CrossRef]
- Elliott, M. Biological pollutants and biological pollution—An increasing cause for concern. Mar. Pollut. Bull. 2003, 46, 275–280. [Google Scholar] [CrossRef]
- Cucherousset, J.; Olden, J. Ecological Impacts on Non-native Freshwater Fishes. Fish. Mag. 2011, 36, 215–230. [Google Scholar] [CrossRef]
- Gozlan, R.E.; Britton, J.R.; Cowx, I.; Copp, G.H. Current knowledge on non-native freshwater fish introductions. J. Fish Biol. 2010, 76, 751–786. [Google Scholar] [CrossRef]
- Lenhardt, M.; Marković, G.; Hegediš, A.; Maletin, S.; Ćirković, M.; Marković, Z. Non-native and translocated fish species in Serbia and their impact on the native ichthyofauna. Rev. Fish Biol. Fish. 2011, 21, 407–421. [Google Scholar] [CrossRef]
- Leunda, P.M.; Oscoz, J.; Elvira, B.; Agorreta, A.; Perea, S.; Miranda, R. Feeding habits of the exotic black bullhead Ameiurus melas (Rafinesque) in the Iberian Peninsula: First evidence of direct predation on native fish species. J. Fish Biol. 2008, 73, 96–114. [Google Scholar] [CrossRef]
- Jaćimović, M. Populaciona Dinamika i Ekotoksikologija Crnog Američkog Patuljastog Soma (Ameiurus melas Rafinesque, 1820) u Savskom jezeru Population Dynamic and Ecotoxicology of the Black Bullhead (Ameiurus melas Rafinesque, 1820) in Sava Lake. Doctoral Dissertation, Faculty of Biology, University of Belgrade, Belgrade, Serbia, 25 December 2015. Available online: http://nardus.mpn.gov.rs/handle/123456789/5170 (accessed on 5 March 2025). (In Serbian).
- Ruiz-Navarro, A.; Britton, J.R.; Jackson, M.C.; Davies, G.D.; Sheath, D. Reproductive ecology and diet of a persistent Ameiurus melas (Rafinesque, 1820) population in the UK. J. Appl. Ichthyol. 2015, 31, 201–203. [Google Scholar] [CrossRef]
- European Parliament & Council. Regulation (EU) No 1143/2014 of the European Parliament and of the Council of October 22 2014 on the Prevention and Management of the Introduction and Spread of Invasive Alien Species. Off. J. Eur. Union 2014, L 317, 35–55. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32014R1143 (accessed on 5 March 2025).
- European Parliament & Council. Directive 2000/60/EC of the European Parliament and of the Council of October 23 2000 establishing a framework for Community action in the field of water policy. Off. J. Eur. Union 2000, L 327, 1–72. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32000L0060 (accessed on 5 March 2025).
- Official Gazette of the Republic of Serbia No. 95/2018. The Law on Protection and Sustainable Use of the Fish Fund of the Republic of Serbia. 2018. Available online: https://www.paragraf.rs/propisi/zakon_o_zastiti_i_odrzivom_koriscenju_ribljeg_fonda.html (accessed on 5 March 2025). (In Serbian).
- Cucherousset, J.; Paillisson, J.M.; Carpenter, A.; Eybert, M.C.; Olden, J.D. Habitat use of an artificial wetland by the invasive catfish Ameiurus melas. Ecol. Freshw. Fish. 2006, 15, 589–596. [Google Scholar] [CrossRef]
- Cucherousset, J.; Paillisson, J.M.; Carpentier, A. Is mass removal an efficient measure to regulate the North American catfish Ameiurus melas outside of its native range? J. Freshw. Ecol. 2006, 21, 699–704. [Google Scholar] [CrossRef]
- Zogaris, S. Information on Measures and Related Costs in Relation to Species Considered for Inclusion on the Union List: Ameiurus spp. Technical Note Prepared by IUCN for the European Commission. 2017. Available online: https://circabc.europa.eu/sd/a/31fa05d1-ee5c-4919-b2bd-7f1efab8679f/TSSR-2016-003%20Ameiurus.pdf (accessed on 14 February 2025).
- Jaćimović, M.; Krpo-Ćetković, J.; Smederevac-Lalić, M.; Lenhardt, M.; Nikolić, D.; Hegediš, A. Assessment of the fyke-nets selectivity during black bullhead (Ameiurus melas) population research in Sava Lake. In Proceedings of the 8th international conference “Water & Fish”, Belgrade, Serbia, 13–15 June 2018; pp. 197–201. [Google Scholar]
- Jaćimović, M.; Lenhardt, M.; Krpo-Ćetković, J.; Jarić, I.; Gačić, Z.; Hegediš, A. Boom-bust like dynamics of invasive black bullhead (Ameiurus melas) in Lake Sava (Serbia). Fish. Manag. Ecol. 2019, 26, 153–164. [Google Scholar] [CrossRef]
- Sikora, L.W.; VanDeHey, J.A.; Sass, G.G.; Matzke, G.; Preul, M. Fish community changes associated with bullhead removals in four northern Wisconsin lakes. N. Am. J. Fish. Manag. 2021, 41, S71–S81. [Google Scholar] [CrossRef]
- Jaćimović, M.; Smederevac-Lalić, M.; Nikolić, D.; Cvijanović, G.; Spasić, S.; Višnjić-Jeftić, Ž.; Skorić, S.; Krpo-Ćetković, J. Changes to fish assemblage following the selective removal of black bullhead (Ameiurus melas). Aquat. Conserv. 2023, 33, 981–994. [Google Scholar] [CrossRef]
- FAO. Top 10 Species Groups in Global, Regional and National Aquaculture 2020. Supplementary Materials to the Factsheet on Top 10 Species Groups in Global Aquaculture 2020. World Aquaculture Performance Indicators (WAPI) Factsheet. 2022. Available online: www.fao.org/3/cc0681en/cc0681en.pdf (accessed on 14 February 2025).
- Csaba, V. Results of brown bullhead fingerling rearing in recirculating fish production systems. Acta Agrar. Debr. 2005, 16, 46–50. [Google Scholar] [CrossRef]
- Holloway, L.S. Culture of Black Bullhead (Ameiurus melas) in Cages and Open Ponds. Master Thesis, Department of Biological Sciences of the State University of New York College at Brockport, Brockport, NY, USA, 1993; p. 42. Available online: https://soar.suny.edu/bitstream/handle/20.500.12648/4568/bio_theses/88/fulltext%20.pdf?sequence=1 (accessed on 14 February 2025).
- Roncarati, A.; Mordenti, O.; Stocchi, L.; Melotti, P. Comparison of Growth Performance of ‘Common Catfish Ameiurus melas, Rafinesque1820’, Reared in Pond and in Recirculating Aquaculture System. J. Aquac. Res. Dev. 2014, 5, 218. [Google Scholar] [CrossRef]
- Bedendo, G.; Panzarin, V.; Fortin, A.; Zamperin, G.; Pretto, T.; Buratin, A.; Quartesan, R.; Sabbion, M.; Salogni, C.; Pascoli, F.; et al. Detection and characterization of a rhabdovirus causing mortality in black bullhead catfish, Ameiurus melas. J. Fish Dis. 2018, 41, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Bordignon, F.; Birolo, M.; Fanizza, C.; Trocino, A.; Zardinoni, G.; Stevanato, P.; Nicoletto, C.; Xiccato, G. Effects of water salinity in an aquaponic system with rainbow trout (Oncorhynchus mykiss), black bullhead catfish (Ameiurus melas), Swiss chard (Beta vulgaris), and cherry tomato (Solanum lycopersicum). Aquaculture 2024, 584, 740634. [Google Scholar] [CrossRef]
- Tešić, M.; Baltić, Ž.M.; Teodorović, V.; Mirilović, M.; Nedić, D.; Marković, T.; Marković, R.; Aleksić Agelidis, A. Tendency in fishing development and fish consumption in Serbia. Vet. Glas. 2013, 67, 417–427. [Google Scholar] [CrossRef]
- Baltić, B.; Aksentijević, K.; Bogunović, D.; Starčević, M.; Mitrović, R.; Mrdović, B.; Janjić, J. Investigation of the volume of fish production and catch in Serbia from 2012 to 2021. Meat Technol. 2023, 64, 329–333. [Google Scholar] [CrossRef]
- Vasić, A.; Vasiljević, Z.; Mickovski-Katalina, N.; Mandić-Rajčević, S.; Soldatović, I. Temporal Trends in Acute Coronary Syndrome Mortality in Serbia in 2005–2019: An Age-Period-Cohort Analysis Using Data from the Serbian Acute Coronary Syndrome Registry (RAACS). Int. J. Environ. Res. Public Health 2022, 19, 14457. [Google Scholar] [CrossRef]
- Bársony, P. The Pygmy Catfish and its Economic Utilization Possibilities. Fisheries Engineering Diploma Bachelor’s Thesis, University of Debrecen, Agricultural Sciences (in Hungarian) Centrum, Debrecen, Hungary, 2002. Available online: https://doksi.net/hu/get.php?lid=20652 (accessed on 25 February 2025).
- Nuez, F.; González-Moreno, P.; Gallardo, B.; García-Llorente, M. The peril of integrating invasive species into ecosystem services policies. Conserv. Lett. 2012, 5, 334–336. [Google Scholar] [CrossRef]
- Ristić, V.; Trišić, I.; Štetić, S.; Maksin, M.; Nechita, F.; Candrea, A.N.; Pavlović, M.; Hertanu, A. Institutional, Ecological, Economic, and Socio-Cultural Sustainability—Evidence from Ponjavica Nature Park. Land 2024, 13, 669. [Google Scholar] [CrossRef]
- Kottelat, M.; Freyhof, J. Handbook of European Freshwater Fishes; Publications Kottelat, Cornol and Freyhof: Berlin, Germany, 2007; p. 646. [Google Scholar]
- Simonović, P. Ribe Srbije; NNK International, Institute of Nature Protection of Republic Serbia, Biological Faculty of the Belgrade University: Belgrade, Serbia, 2001; p. 247. (In Serbian) [Google Scholar]
- Shamna, N.; Sardar, P.; Sahu, N.P.; Phulia, V.; Rajesh, M.; Fawole, F.J.; Pal, A.K.; Angel, G. Heamato-immunological and physiological responses of Labeo rohita fingerlings to dietary fermented Jatropha curcas protein concentrate. Anim. Feed. Sci. Technol. 2017, 232, 198–206. [Google Scholar] [CrossRef]
- Mohammad, E.M.; El-Etreby, S.; Sapota, M.R. Biological studies on juvenile fish of Sardinella aurita (Valenciennes 1847) of the Port-Said coast (Mediterranean Sea, Egypt). Oceanol. Stud. 2002, 31, 3–16. [Google Scholar]
- Gabriel, N.N.; Wilhem, M.R.; Habte-Tsion, H.-M.; Chimwamurombe, P.; Omoregie, E.; Iipinge, L.N.; Shimooshili, K. Effect of dietary Aloe vera polysaccharides supplementation on growth performance, feed utilization, hemato-biochemical parameters, and survival at low pH in African catfish (Clarias gariepinus) fingerlings. Int. Aquat. Res. 2019, 11, 57–72. [Google Scholar] [CrossRef]
- ISO 1443:1973; Meat and Meat Products—Determination of Total Fat Content. International Organization for Standardization: Geneva, Switzerland, 1973.
- ISO 1442:2023; Meat and Meat Products—Determination of Moisture Content (Reference Method). International Organization for Standardization: Geneva, Switzerland, 2023.
- ISO 936:1998; Meat and Meat Products—Determination of Total Ash. International Organization for Standardization: Geneva, Switzerland, 1998.
- Spirić, A.; Trbović, D.; Vranić, D.; Đinović, J.; Petronijević, R.; Matekalo-Sverak, V. Statistical evaluation of fatty acid profile and cholesterol content in fish (Common carp) lipids obtained by different sample preparation procedures. Anal. Chim. Acta 2010, 672, 66–71. [Google Scholar] [CrossRef]
- ISO 5509:2009; Animal and Vegetable Fats and Oils—Preparation of Methyl Esters of Fatty Acids. International Organization for Standardization: Geneva, Switzerland, 2009.
- Trbović, D.; Marković, Z.; Milojković-Opsenica, D.; Petronijević, R.; Spirić, D.; Đinović-Stojanović, J.; Spirić, A. Influence on diet on proximate composition and fatty acid profile in common carp (Cyprinus carpio). J. Food Compos. Anal. 2013, 31, 75–81. [Google Scholar] [CrossRef]
- Buentello, J.A.; Gatlin, D.M.; Neill, W.H. Effects of water temperature and dissolved oxygen on daily feed consumption, feed utilization and growth of channel catfish (Ictalurus punctatus). Aquaculture 2000, 182, 339–352. [Google Scholar] [CrossRef]
- Watts, M.; Munday, B.L.; Burke, C.M. Immune responses of teleost fish. Aust. Vet. J. 2001, 79, 570.e4. [Google Scholar] [CrossRef]
- Baby, S.; Philips, H.; Lochmann, R.; Chen, R. Effect of temperature on growth, feed utilization and immune status of channel catfish in a recirculating system. N. Am. J. Aquac. 2009, 71, 64–72. [Google Scholar] [CrossRef]
- Scharsack, J.P.; Franke, F. Temperature effects on teleost immunity in the light of climate change. J. Fish Biol. 2022, 101, 780–796. [Google Scholar] [CrossRef]
- Snow, R.A.; Porta, J.M.; Robinson, L.A. Seasonal diet composition of black bullhead (Ameirus melas) in Lake Carl Etling, Oklahoma. Proc. Okla. Acad. Sci. 2017, 97, 54–60. [Google Scholar]
- Melotti, P.; Roncarati, A. State of the art and future trends of European and Italian aquaculture. Vet. Res. Commun. 2009, 33, 9–13. [Google Scholar] [CrossRef]
- Robinson, E.H.; Li, M.H. Channel Catfish, Ictalurus punctatus, Size and Feed Conversion Ratio. J. World Aquac. Soc. 2010, 41, 829–833. [Google Scholar] [CrossRef]
- Copp, G.H.; Tarkan, A.S.; Masson, G.; Godard, M.J.; Koščo, J.; Kováč, V.; Novomeská, A.; Miranda, R.; Cucherousset, J.; Pedicillo, G.; et al. A review of growth and life-history traits of native and non-native European populations of black bullhead Ameiurus melas. Rev. Fish Biol. Fish. 2016, 26, 441–469. [Google Scholar] [CrossRef]
- Mráz, J.; Máchova, J.; Kozák, P.; Pickova, J. Lipid content and composition in common carp-optimization of n-3 fatty acids in different pond production systems. J. Appl. Ichthyol. 2012, 28, 238–244. [Google Scholar] [CrossRef]
- Carlson, S.J.; Fallon, E.M.; Kalish, B.T.; Gura, K.M.; Puder, M. The role of the ω-3 fatty acid DHA in the human life cycle. JPEN J. Parenter. Enteral. Nutr. 2013, 37, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Chugh, V.; Gupta, A.K. Essential fatty acids as functional components of foods-a review. J. Food Sci. Tech. 2014, 51, 2289–2303. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Lipid oxidation and improving the oxidative stability. Chem. Soc. Rev. 2010, 39, 4067. [Google Scholar] [CrossRef]
- Calder, P. Very long-chain n-3 fatty acids and human health: Fact, fiction and the future. Proc. Nutr. Soc. 2018, 77, 52–72. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Pozet, F.; Morand, M.; Moussa, A.; Torhy, C.; De Kinkelin, P. Isolation and Preliminary Characterization of a Pathogenic Icosahedral Deoxyribovirus from the Catfish Ictalurus melas. Dis. Aquat. Organ. 1992, 14, 35–42. [Google Scholar] [CrossRef]
- Alborali, L.; Bovo, G.; Lavazza, A.; Cappellaro, H.; Guadagnini, P.F. Isolation of a Herpesvirus in Breeding Catfish (Ictalurus melas). Bull. Eur. Assoc. Fish Pathol. 1996, 16, 134–137. [Google Scholar]
- Bigarré, L.; Cabon, J.; Baud, M.; Pozet, F.; Castric, J. Ranaviruses Associated with High Mortalities in Catfish in France. Bull. Eur. Assoc. Fish Pathol. 2008, 28, 163–168. [Google Scholar]
- Juhász, T.; Woynárovichné Láng, M.; Csaba, G.L.; Farkas, S.; Dán, Á. Isolation of a Ranavirus Causing Mass Mortality in Brown Bullheads (Ameiurus nebulosus) in Hungary. Magy. Állatorvosok Lapja 2013, 135, 763–768. [Google Scholar]
- Abonyi, F.; Doszpoly, A.; Czeglédi, I.; Eszterbauer, E. Molecular Detection of a Novel Poxvirus in Black Bullhead (Ameiurus melas): Emerging Pathogens in a Natural Freshwater in Hungary? J. Fish Dis. 2024, 48, e14076 . [Google Scholar] [CrossRef]
| Year | 2018 | 2021 | ||
|---|---|---|---|---|
| Abiotic Parameters | Cages Mean ± SD (Min–Max) | RAS Mean ± SD (Min–Max) | Cages Mean ± SD (Min–Max) | RAS Mean ± SD (Min–Max) |
| Temperature (°C) | 22.2 ± 4.65 a (14.3–28.2) | 19.03 ± 2.18 b (14.1–24) | 20.69 ± 4.79 a (10.3–28.4) | 18.82 ± 2.8 b (11.6–24.9) |
| Oxygen (mg/L) | 2.82 ± 0.88 b (1.51–4.36) | 5.46 ± 0.84 a (4.46–8.45) | 8.04 ± 1.94 b (3.52–11.8) | 9.09 ± 0.98 a (6.17–12.22) |
| Oxygen saturation (%) | 33.83 ± 10.78 b (15.4–55.4) | 58.98 ± 11.62 a (45.6–94.2) | 85.67 ± 23.71 b (41.5–139.3) | 97.12 ± 10.09 a (62.7–126.7) |
| pH | 8.25 ± 0.21 b (7.94–8.74) | 8.75 ± 0.15 a (8.28–8.95) | 8.72 ± 0.52 (7.69–9.6) | 8.8 ± 0.39 (7.87–9.51) |
| Electrical conductivity (µs/cm) | 2052.78 ± 247.16 a (1150–2250) | 2106.72 ± 55.07 a (1912–2270) | 2430 ± 0.17 a (2020–2990) | 2280.4 ± 97.34 b (2040–2590) |
| Year | 2018 | 2021 | ||||||
|---|---|---|---|---|---|---|---|---|
| Rearing System | Cage | RAS | Cage | RAS | ||||
| Treatment | Feed 1 | Feed 2 | Feed 1 | Feed 2 | Feed 3 | Feed 4 | Feed 3 | Feed 4 |
| Initial body mass (g) | 18.9 ± 0.0 | 19.3 ± 0.0 | 18.9 ± 0.4 | 19.3 ± 0.6 | 48.7 ± 0.5 | 49 ± 0.8 | 49 ± 0.1 | 49.1 ± 0.1 |
| Initial body length (cm) | / | / | / | / | 16.6 ± 0.2 | 16.5 ± 0.3 | 17.3 ± 0.1 | 17.3 ± 0.4 |
| Final body mass (g) | 60.3 ± 9.3 | 69.3 ± 11.7 | 41.9 ± 3.1 | 42.1 ± 2.8 | 226.2 ± 33.8 | 196.3 ± 8.7 | 200.5 ± 26.8 | 150.8 ± 11.4 |
| Final body length (cm) | / | / | / | / | 24.3 ± 0.9 | 23.7 ± 0.8 | 23.3 ± 1.2 | 21.2 ± 1.0 |
| BWG (g) | 41.4 | 50.1 | 23.0 | 22.8 | 177.5 | 147.3 | 151.5 | 100.8 |
| SGR | 1.3 | 1.4 | 0.9 | 0.8 | 1.4 | 1.2 | 1.2 | 1.0 |
| >FI (g d−1) | 1.9 | 0.9 | 0.5 | 0.5 | 4.7 | 4.1 | 3.4 | 4.6 |
| DFR (%) | 2.6 | 1.8 | 1.7 | 1.7 | 2.0 | 2.0 | 2.4 | 2.6 |
| FCR | 1.4 | 1.5 | 2.1 | 2.0 | 2.4 | 2.4 | 2.0 | 2.4 |
| MGRMBW (g kg−0.8 d−1) | 14.0 | 11.1 | 7.0 | 7.0 | 7.2 | 7.1 | 11.9 | 9.2 |
| SR | 38.1 | 73.8 | 97.6 | 100 | 20.0 | 23.3 | 63.3 | 25.00 |
| Feed 3 | Feed 4 | |||
|---|---|---|---|---|
| Indices | Cage 1 | RAS 1 | Cage 2 | RAS 2 |
| Kf | 1.4 ± 0.1 a | 1.3 ± 0.1 b | 1.8 ± 0.2 A | 1.4 ± 0.1 B |
| Kc | 1.2 ± 0.1 | 1.1 ± 0.1 | 1.5 ± 0.2 A | 1.3 ± 0.1 B |
| Ih | 5.2 ± 0.4 a | 3.9 ± 0.2 b | 4.4 ± 0.4 A | 3.9 ± 0.4 B |
| Iw | 18.5 ± 1.4 a | 14.9 ± 1.4 b | 20.3 ± 1.2 A | 16.3 ± 1.4 B |
| GSI | 0.3 ± 0.3 | 0.9 ± 0.7 | 0.7 ± 0.5 A | 0.2 ± 0.1 B |
| HSI | 3.6 ± 0.4 | 4.1 ± 0.4 | 4.0 ± 0.4 A | 3.1 ± 0.3 B |
| VSI | 9.9 ± 1.2 | 11.1 ± 1.3 | 12.0 ± 1.7 A | 9.7 ± 1.1 B |
| Fatty Acid | Control | Cage 3 | Cage 4 | RAS 3 | RAS 4 | p |
|---|---|---|---|---|---|---|
| C14:0 | 1.14 ± 0.01 | 0.53 ± 0.01 | 0.45 ± 0.01 | 0.43 ± 0.01 | 0.37 ± 0.01 | *** |
| C15:0 | 0.42 ± 0.01 | 0.24 ± 0.02 | 0.14 ± 0.02 | 0.06 ± 0.01 | 0.07 ± 0.01 | *** |
| C16:0 | 24.30 ± 0.01 | 14.39 ± 0.01 | 13.55 ± 0.02 | 15.11 ± 0.03 | 14.88 ± 0.03 | *** |
| C16:1 | 3.19 ± 0.01 | 1.99 ± 0.02 | 1.88 ± 0.01 | 1.97 ± 0.03 | 1.78 ± 0.04 | *** |
| C17:0 | 0.50 ± 0.01 | 0.26 ± 0.01 | 0.17 ± 0.01 | 0.12 ± 0.01 | 0.12 ± 0.01 | *** |
| C18:0 | 7.89 ± 0.02 | 3.54 ± 0.01 | 3.82 ± 0.01 | 3.81 ± 0.01 | 3.00 ± 0.01 | *** |
| C18:1n-9 | 26.25 ± 0.03 | 35.50 ± 0.05 | 36.92 ± 0.03 | 37.90 ± 0.05 | 38.74 ± 0.01 | *** |
| C18:2n-6 | 21.02 ± 0.18 | 30.32 ± 0.01 | 28.92 ± 0.02 | 31.96 ± 0.03 | 32.68 ± 0.02 | *** |
| C20:0 | 0.44 ± 0.01 | 0.30 ± 0.01 | 0.30 ± 0.01 | 0.28 ± 0.04 | 0.20 ± 0.04 | ** |
| C18:3n-6 | 0.37 ± 0.02 | 0.44 ± 0.03 | 0.39 ± 0.01 | 0.73 ± 0.02 | 0.78 ± 0.01 | NS |
| C18:3n-3 | 2.18 ± 0.01 | 6.49 ± 0.02 | 7.86 ± 0.01 | 3.34 ± 0.01 | 4.21 ± 0.01 | *** |
| C20:1 | 1.26 ± 0.01 | 1.34 ± 0.01 | 0.18 ± 0.01 | 1.04 ± 0.02 | 0.09 ± 0.01 | ** |
| C20:2n-6 | 0.99 ± 0.01 | 1.25 ± 0.02 | 0.98 ± 0.01 | 0.92 ± 0.03 | 0.72 ± 0.04 | NS |
| C20:3n-6 | 1.21 ± 0.01 | 0.75 ± 0.02 | 0.70 ± 0.02 | 1.04 ± 0.03 | 0.88 ± 0.03 | NS |
| C20:3n-3 | 0.26 ± 0.01 | 0.35 ± 0.02 | 0.60 ± 0.02 | 0.12 ± 0.03 | 0.15 ± 0.02 | * |
| C20:4 n-6 | 2.78 ± 0.01 | 0.66 ± 0.03 | 0.40 ± 0.04 | 0.61 ± 0.03 | 0.52 ± 0.02 | *** |
| C20:5n-3 | 1.53 ± 0.01 | 0.69 ± 0.01 | 0.85 ± 0.02 | 0.12 ± 0.04 | 0.21 ± 0.02 | *** |
| C22:5n-3 | 1.18 ± 0.01 | 0.49 ± 0.19 | 0.39 ± 0.07 | 0.20 ± 0.08 | 0.35 ± 0.52 | NS |
| C22:6n-3 | 3.07 ± 0.11 | 0.48 ± 0.03 | 0.40 ± 0.01 | 0.23 ± 0.01 | 0.25 ± 0.01 | *** |
| SFA | 34.69 ± 0.10 | 19.26 ± 0.10 | 18.43 ± 0.10 | 19.81 ± 0.14 | 18.64 ± 0.12 | *** |
| MUFA | 30.70 ± 0.13 | 38.83 ± 0.14 | 38.20 ± 0.14 | 40.91 ± 0.14 | 38.83 ± 0.14 | ** |
| PUFA | 31.83 ± 0.24 | 41.25 ± 0.29 | 41.49 ± 0.40 | 38.67 ± 0.49 | 40.75 ± 0.64 | ** |
| n-6 | 23.60 ± 0.23 | 32.76 ± 0.38 | 30.30 ± 0.24 | 34.65 ± 0.40 | 33.92 ± 0.58 | ** |
| n-3 | 8.22 ± 0.09 | 8.49 ± 0.14 | 10.10 ± 0.12 | 4.02 ± 0.14 | 5.17 ± 0.16 | ** |
| n-6/n-3 | 2.87 ± 0.25 | 3.86 ± 0.12 | 3.00 ± 0.19 | 8.62 ± 0.49 | 6.56 ± 0.33 | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaćimović, M.; Stanković, M.; Trbović, D.; Nikolić, D.; Smederevac-Lalić, M.; Marković, Z. Can We Turn Harmful Invasive Non-Native Fish Species into a Valuable Food Resource? Fishes 2025, 10, 207. https://doi.org/10.3390/fishes10050207
Jaćimović M, Stanković M, Trbović D, Nikolić D, Smederevac-Lalić M, Marković Z. Can We Turn Harmful Invasive Non-Native Fish Species into a Valuable Food Resource? Fishes. 2025; 10(5):207. https://doi.org/10.3390/fishes10050207
Chicago/Turabian StyleJaćimović, Milica, Marko Stanković, Dejana Trbović, Dušan Nikolić, Marija Smederevac-Lalić, and Zoran Marković. 2025. "Can We Turn Harmful Invasive Non-Native Fish Species into a Valuable Food Resource?" Fishes 10, no. 5: 207. https://doi.org/10.3390/fishes10050207
APA StyleJaćimović, M., Stanković, M., Trbović, D., Nikolić, D., Smederevac-Lalić, M., & Marković, Z. (2025). Can We Turn Harmful Invasive Non-Native Fish Species into a Valuable Food Resource? Fishes, 10(5), 207. https://doi.org/10.3390/fishes10050207