Physiological and Transcriptome Analyses of Gill and Hepatopancreas of Potamocorbula ustulata Under Ammonia Exposure
Abstract
1. Introduction
2. Materials and Methods
2.1. Clam Culture and Sample Collection
2.2. Physiological Parameters Determination
2.3. Transcriptome Analysis
2.3.1. RNA Extraction and Transcriptome Sequencing
2.3.2. Transcriptome Assembly and Differential Expression Analysis
2.3.3. Functional Annotation and Enrichment of Differentially Expressed Genes
2.4. Statistical Analyses
3. Results
3.1. Enzyme Activity Determination
3.2. Characterization of Transcriptome Data
3.3. Analysis of DEGs
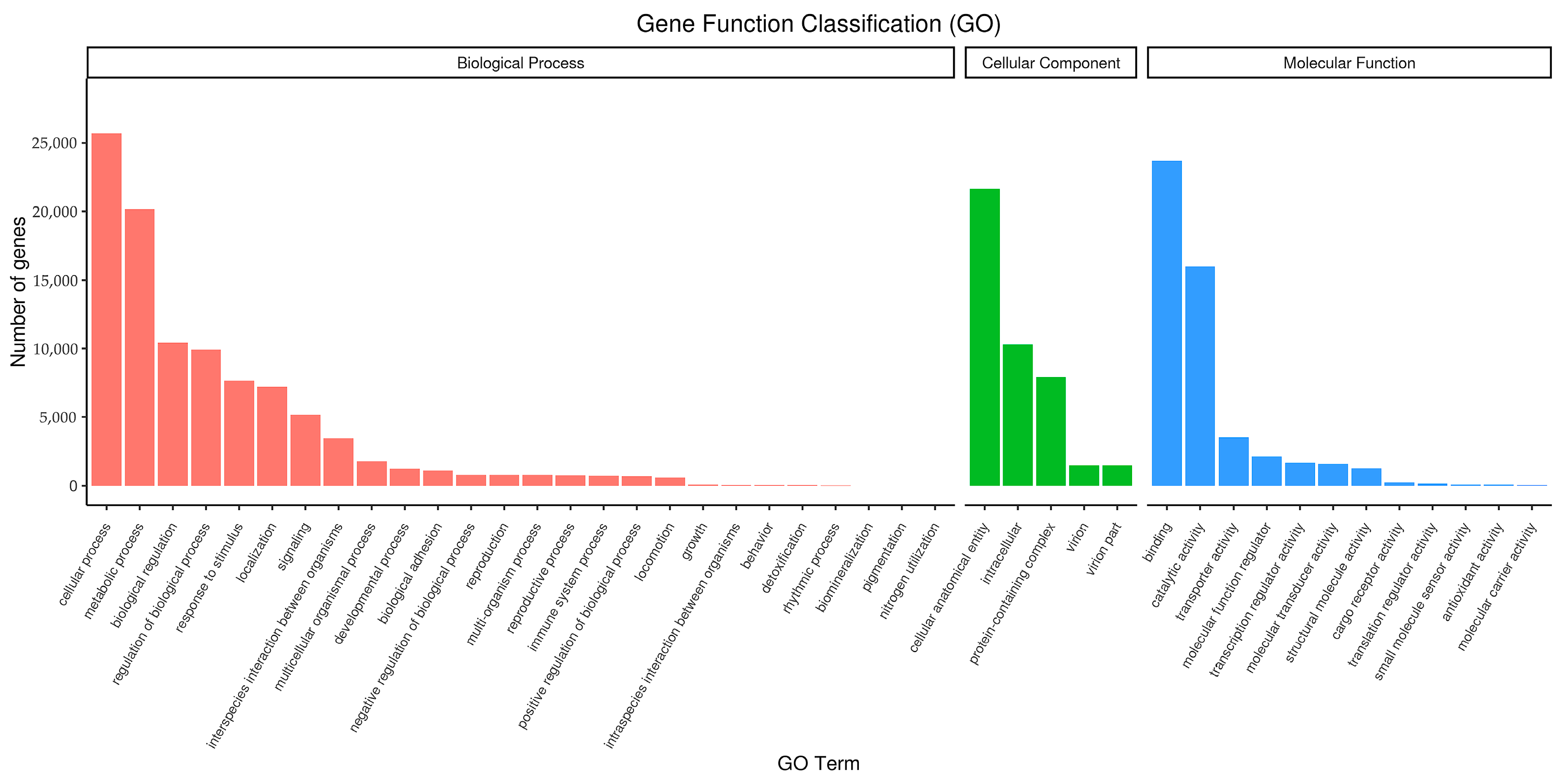
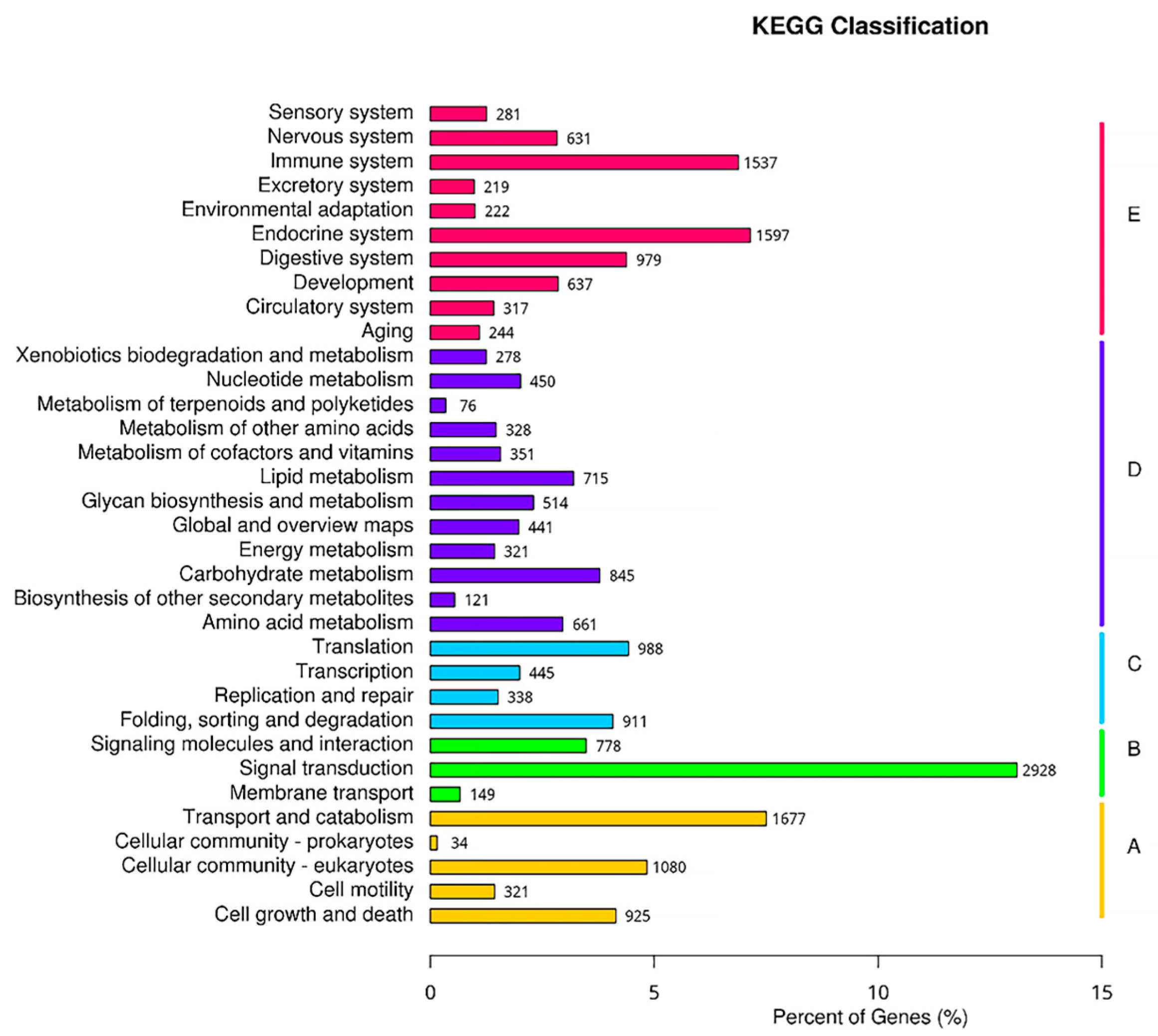
3.4. Functional Annotation of Unigenes
3.4.1. GO Functional Annotation
3.4.2. KEGG Functional Annotation
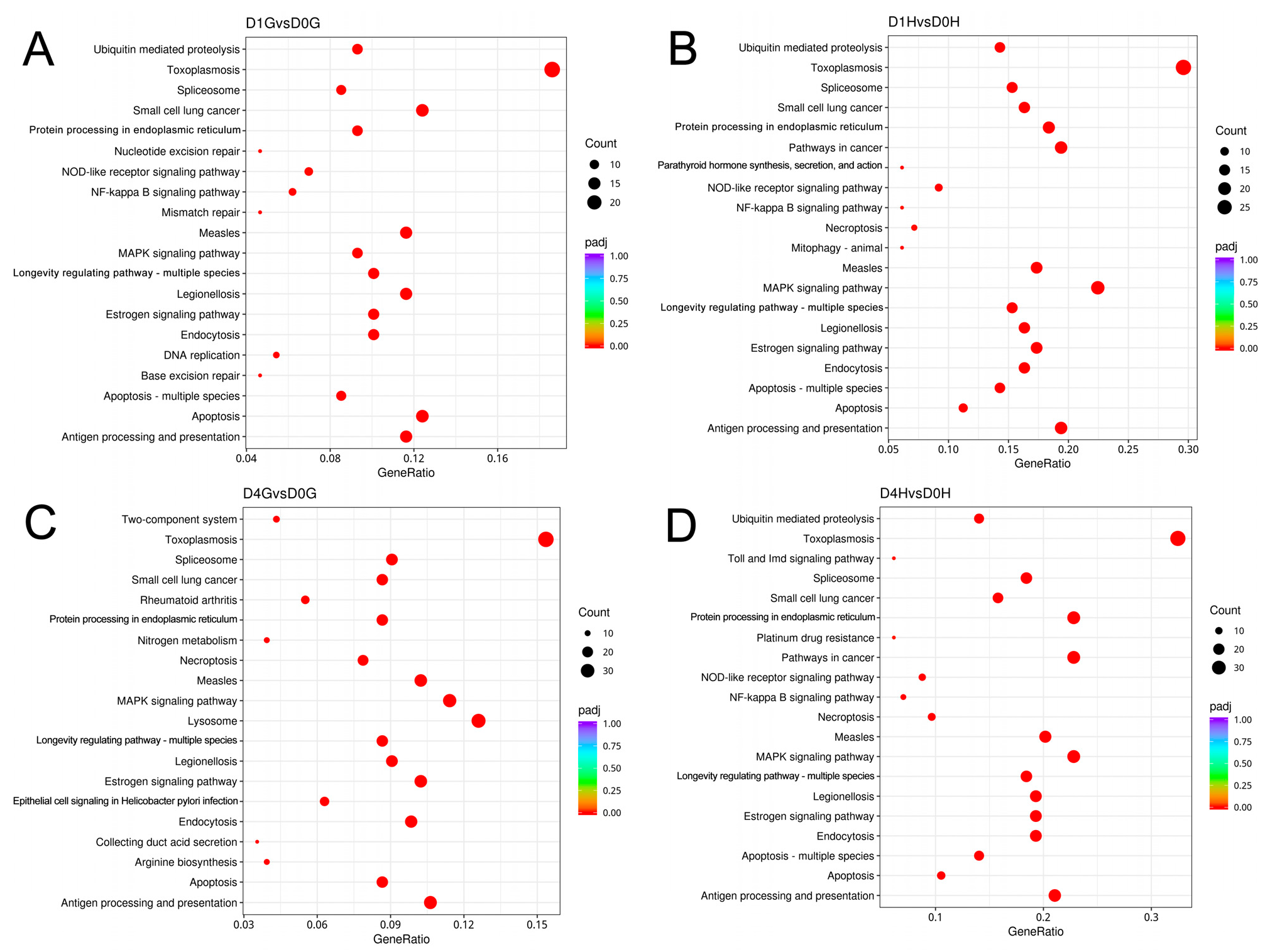
3.5. Functional Enrichment Analysis of GO and KEGG Pathways
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dauda, A.B.; Ajadi, A.; Tola-Fabunmi, A.S.; Akinwole, A.O. Waste production in aquaculture: Sources, components and managements in different culture systems. Aquacult. Fish. 2019, 4, 81–88. [Google Scholar] [CrossRef]
- Kir, M.; Topuz, M.; Sunar, M.C.; Topuz, H. Acute toxicity of ammonia in Meagre (Argyrosomus regius Asso, 1801) at different temperatures. Aquacult. Res. 2015, 47, 3593–3598. [Google Scholar] [CrossRef]
- Ip, Y.K.; Chew, S.F.; Randall, D.J. Ammonia toxicity, tolerance, and excretion. Fish Physiol. 2001, 20, 109–148. [Google Scholar] [CrossRef]
- Ren, Q.; Pan, L. Digital gene expression analysis in the gills of the swimming crab (Portunus trituberculatus) exposed to elevated ambient ammonia-N. Aquaculture 2014, 434, 108–114. [Google Scholar] [CrossRef]
- Banihashemi, E.S.; Khara, H.; Pajand, Z.; Rahnandeh, M. Histopathological study of gill, kidney and liver of Persian Sturgeon (Acipenser persicus Borodin, 1897) and Stellate (Acipenser stellatus Pallas, 1811) exposed to sublethal concentration of un-ionised ammonia UAN. J. Parasit. Dis 2016, 40, 1443–1450. [Google Scholar] [CrossRef]
- Ip, Y.K.; Chew, S.F. Ammonia Production, Excretion, Toxicity, and Defense in Fish: A Review. Front. Physiol. 2010, 1, 134. [Google Scholar] [CrossRef]
- Wright, P.A.; Wood, C.M. A new paradigm for ammonia excretion in aquatic animals: Role of Rhesus (Rh) glycoproteins. J. Exp. Biol. 2009, 212, 2303–2312. [Google Scholar] [CrossRef]
- Lv, L.; Ren, J.; Zhang, H.; Sun, C.; Dong, Y.; Lin, Z. Transcriptomic Analysis of Gill and Hepatopancreas in Razor Clam (Sinonovacula constricta) Exposed to Acute Ammonia. Front. Mar. Sci. 2022, 9, 832494. [Google Scholar] [CrossRef]
- Zhao, Q. Response and Detoxification Mechanism of Hyriopsis cumingii Exposed to Ambient Ammonia. Master’s Thesis, Southwest University, Chongqing, China, 2021. [Google Scholar]
- Liang, X. Effects of Ammonia Nitrogen Stress on Detoxification Metabolism and Functional Verification of Related Genes in Cyclina sinensis. Master’s Thesis, Jiangsu Ocean University, Lianyungang, China, 2022. [Google Scholar]
- He, L.; Xue, S.; Ye, S.; Laws, E.A.; Yuan, H.; Yang, S.; Du, X. Holocene evolution of the Liaohe Delta, a tide-dominated delta formed by multiple rivers in Northeast China. J. Asian Earth Sci. 2018, 152, 52–68. [Google Scholar] [CrossRef]
- Ryu, D.-k.; Kim, Y. Age and growth of the Asian clam Potamocorbula ustulata ustulata (Reeve, 1844) on the west coast of Korea. Korean. J. Malacol. 2007, 23, 17–23. [Google Scholar]
- Ren, P.; Fang, P.; Bao, Y.; Li, H.; Wang, H.; Gong, K. Variation in microbenthic community characteristics among different types of wetlands in Xuanmen bay. Acta Ecol. Sin. 2016, 36, 5632–5645. [Google Scholar]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Chen, J.; Huang, H.; Xu, H.; He, H.; Fan, W.; Ding, F. Ammonia effects the survival and the energy budget of clam Meretrix meretrix. Mar. Sci. 2010, 34, 40–46. [Google Scholar]
- Ge, H.; Liang, X.; Liu, J.; Cui, Z.; Guo, L.; Li, L.; Sun, Y.; Dong, Z.; Wei, M. Effects of acute ammonia exposure on antioxidant and detoxification metabolism in clam Cyclina sinensis. Ecotoxicol. Environ. Saf. 2021, 211, 111895. [Google Scholar]
- Rama, S.; Manjabhat, S.N. Protective effect of shrimp carotenoids against ammonia stress in common carp, Cyprinus carpio. Ecotoxicol. Environ. Saf. 2014, 107, 207–213. [Google Scholar] [CrossRef]
- Cheng, C.; Yang, F.; Ling, R.; Liao, S.; Miao, Y.; Ye, C.; Wang, A. Effects of ammonia exposure on apoptosis, oxidative stress and immune response in pufferfish (Takifugu obscurus). Aquat. Toxicol. 2015, 164, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yan, Z.; Zheng, X.; Wang, S.; Fan, J.; Liu, Z. Effects of acute ammonia toxicity on oxidative stress, DNA damage and apoptosis in digestive gland and gill of Asian clam (Corbicula fluminea). Fish Shellfish. Immunol. 2020, 99, 514–525. [Google Scholar] [CrossRef]
- Dong, L.; He, Y.; Wang, Y.; Dong, Z. Research progress on application of superoxide dismutase (SOD). J. Agric. Sci. Technol. 2013, 15, 53–58. [Google Scholar]
- Li, C.; Ni, D.; Song, L.; Zhao, J.; Zhang, H.; Li, L. Molecular cloning and characterization of a catalase gene from Zhikong scallop Chlamys farreri. Fish Shellfish. Immunol. 2008, 24, 26–34. [Google Scholar] [CrossRef]
- Yang, K.; Cao, F.; Xue, Y.; Tao, L.; Zhu, Y. Three classes of antioxidant defense systems and the development of Postmenopausal Osteoporosis. Front. Physiol. 2022, 13, 840293. [Google Scholar] [CrossRef]
- Chen, Y.K. Physiological, Immunological and Apoptotic Responses of Sinonovacula constricta to Ammonia Stress. Master’s Thesis, Shanghai Ocean University, Shanghai, China, 2022. [Google Scholar]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Cui, C.; Liu, Q.; Sun, J.; He, K.; Adam, A.A.; Luo, J.; Li, Z.; Wang, Y.; Yang, S. Combined exposure to hypoxia and ammonia aggravated biological effects on glucose metabolism, oxidative stress, inflammation and apoptosis in largemouth bass (Micropterus salmoides). Aquat. Toxicol. 2020, 224, 105514. [Google Scholar] [CrossRef] [PubMed]
- Cong, M.; Wu, H.; Yang, H.; Zhao, J.; Lv, J. Gill damage and neurotoxicity of ammonia nitrogen on the clam Ruditapes philippinarum. Ecotoxicology 2017, 26, 459–469. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, K.; Du, X.; Li, Y.; Niu, D. Ammonia-induced oxidative stress triggered apoptosis in the razor clam (Sinonovacula constricta). Environ. Sci. Pollut. Res. 2024, 31, 22380–22394. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wang, Z.; Luo, Z.; Yu, Q.; Jiang, Y.; Zhang, Y.; Zhou, Z.; Smith, A.J.; Cooper, P.R. LPS promote the odontoblastic differentiation of human dental pulp stem cells via MAPK signaling pathway. Cell. Physiol 2015, 230, 554–561. [Google Scholar] [CrossRef]
- He, W.; Wang, Z.; Zhou, Z.; Zhang, Y.; Zhu, Q.; Wei, K.; Lin, Y.; Cooper, P.R.; Smith, A.J.; Yu, Q. Lipopolysaccharide Enhances Wnt5a Expression through Toll-like Receptor 4, Myeloid Differentiating Factor 88, Phosphatidylinositol 3-OH Kinase/AKT and Nuclear Factor Kappa B Pathways in Human Dental Pulp Stem Cells. J. Endod. 2014, 40, 69–75. [Google Scholar] [CrossRef]
- Yu, p.; Yan, S. Effects of simulated hypoxic microenvironment on invasion, migration and Ras/MAPK /NF-kB pathway of human gastric cancer cells in Vitro. Chin. J. Clin. Res. 2019, 32, 907–910+914. [Google Scholar]
- Zou, D. Breeding of a Heat-Resistant Strain of Scapharca subcrenata and Its Response Mechanisms to Thermal Stresses. Master’s Thesis, Yantai Institute of Coastal Zone Research, Chinese Academy of Sciences, Yantai, China, 2022. [Google Scholar]
- Zhang, T.; Yan, Z.; Zheng, X.; Fan, J.; Wang, S.; Wei, Y.; Yang, L.; Wang, P.; Guo, S. Transcriptome analysis of response mechanism to ammonia stress in Asian clam (Corbicula fluminea). Aquat. Toxicol. 2019, 214, 105235. [Google Scholar] [CrossRef]
- Ma, Q.; Yan, Z.; Zheng, X.; Fan, J.; Wang, S.; Wei, Y.; Yang, L.; Wang, P.; Guo, S. Transcriptome sequencing reveals improved ammonia nitrogen tolerance in Zebra II strain of the Manila clam Ruditapes philippinarum. Comp. Biochem. Physiol. Part D Genom. Proteom. 2022, 44, 101016. [Google Scholar] [CrossRef]
- Wang, Z.G. Progress in the function of FOXO on tumor inhibition. Chin. J. Dis. Control. Prev. 2013, 17, 809–812. [Google Scholar]
- Kim, J.H.; Choi, J.S.; Lee, B.H. PI3K/Akt and MAPK pathways evoke activation of FoxO transcription factor to undergo neuronal apoptosis in brain of the silkworm Bombyx mori (Lepidoptera: Bombycidae). Cell. Mol. Biol 2012, 58, Ol1780–Ol1785. [Google Scholar]
- Jiang, P.; Ren, L.; Zhi, L.; Yu, Z.; Lv, F.; Xu, F.; Peng, W.; Bai, X.; Cheng, K.; Quan, L.; et al. Negative regulation of AMPK signaling by high glucose via E3 ubiquitin ligase MG53. Mol. Cell 2021, 81, 629–637.e625. [Google Scholar] [CrossRef]
- Garcia, D.; Shaw, R.J. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol. Cell 2017, 66, 789–800. [Google Scholar] [CrossRef]
- Nwadike, C.; Williamson, L.E.; Gallagher, L.E.; Guan, J.; Chan, E.Y.W. AMPK Inhibits ULK1-Dependent Autophagosome Formation and Lysosomal Acidification via Distinct Mechanisms. Mol. Cell. Biol. 2018, 38, MCB. 00023-18. [Google Scholar] [CrossRef]
- Song, M.; Li, M.; Li, J.; Yuan, L.; Wang, R.; Shi, G. Effects of acute ammonia exposure on gene involved in inflammation of mudskipper. J. Fish. China 2018, 42, 1704–1710. [Google Scholar]
- Weihrauch, D.; Joseph, G.; Allen, G.J.P. Ammonia excretion in aquatic invertebrates: New insights and questions. Exp. Biol. 2018, 221, jeb169219. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Walsh, P.J. High ammonia tolerance in fishes of the family Batrachoididae (Toadfish and Midshipmen). Aquat. Toxicol. 2000, 50, 205–219. [Google Scholar] [CrossRef]
- Lu, J.; Yao, T.; Shi, S.; Ye, L. Effects of acute ammonia nitrogen exposure on metabolic and immunological responses in the Hong Kong oyster Crassostrea hongkongensis. Ecotoxicol. Environ. Saf. 2022, 237, 113518. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Lv, L.; Yao, H.; Lin, Z.; Xu, N.; Dong, Y. Integrated application of multi-omics and biochemical analysis revealed the physiological response mechanism of ammonia nitrogen tolerance in the razor clam (Sinonovacula constricta). Front. Mar. Sci. 2024, 11, 1444929. [Google Scholar] [CrossRef]
- Zhang, M.; Li, M.; Wang, R.; Qian, Y. Effects of acute ammonia toxicity on oxidative stress, immune response and apoptosis of juvenile yellow catfish Pelteobagrus fulvidraco and the mitigation of exogenous taurine. Fish Shellfish. Immun. 2018, 79, 313–320. [Google Scholar] [CrossRef]
- Jahanbani, A.; Shahriari, A.; Mohammadian, T. Ureagenesis of Asian seabass (Lates calcarifer) under ammonia stress and overcrowding. Aquaculture 2023, 576, 739810. [Google Scholar] [CrossRef]
- Zhao, Q.; Feng, K.; Zhang, L.; Bai, Y.; Yao, W. Effects of acute ammonia stress on antioxidant responses, histopathology and ammonia detoxification metabolism in triangle sail mussels (Hyriopsis cumingii). Water 2021, 13, 425. [Google Scholar] [CrossRef]
- Cong, M.; Wu, H.; Cao, T.; Lv, J.; Wang, Q.; Ji, C.; Li, C.; Zhao, J. Digital gene expression analysis in the gills of Ruditapes philippinarum exposed to short- and long-term exposures of ammonia nitrogen. Aquat. Toxicol. 2018, 194, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Jayasundara, N.; Zhang, J.; Ren, X.; Gao, B.; Li, J.; Liu, P. Integrated physiological, transcriptome and metabolome analyses of the hepatopancreas of the female swimming crab Portunus trituberculatus under ammonia exposure. Ecotox. Environ. Saf. 2021, 228, 113026. [Google Scholar] [CrossRef]
- Gao, N.; Zhu, L.; Guo, Z.; Yi, M.; Zhang, L. Effects of chronic ammonia exposure on ammonia metabolism and excretion in marine medaka Oryzias melastigma. Fish Shellfish. Immunol. 2017, 65, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Clifford, A.M.; Goss, G.G.; Wilkie, M.P. Adaptations of a deep sea scavenger: High ammonia tolerance and active NH4+ excretion by the Pacific hagfish (Eptatretus stoutii). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015, 182, 64–74. [Google Scholar] [CrossRef]
- Sinha, A.K.; Zinta, G.; AbdElgawad, H.; Asard, H.; Blust, R.; de Boeck, G. High environmental ammonia elicits differential oxidative stress and antioxidant responses in five different organs of a model estuarine teleost (Dicentrarchus labrax). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2015, 174–175, 21–31. [Google Scholar] [CrossRef]
- Schram, E.; Roques, J.A.C.; Abbink, W.; Spanings, T.; de Vries, P.; Bierman, S.; van de Vis, H.; Flik, G. The impact of elevated water ammonia concentration on physiology, growth and feed intake of African catfish (Clarias gariepinus). Aquaculture 2010, 306, 108–115. [Google Scholar] [CrossRef]
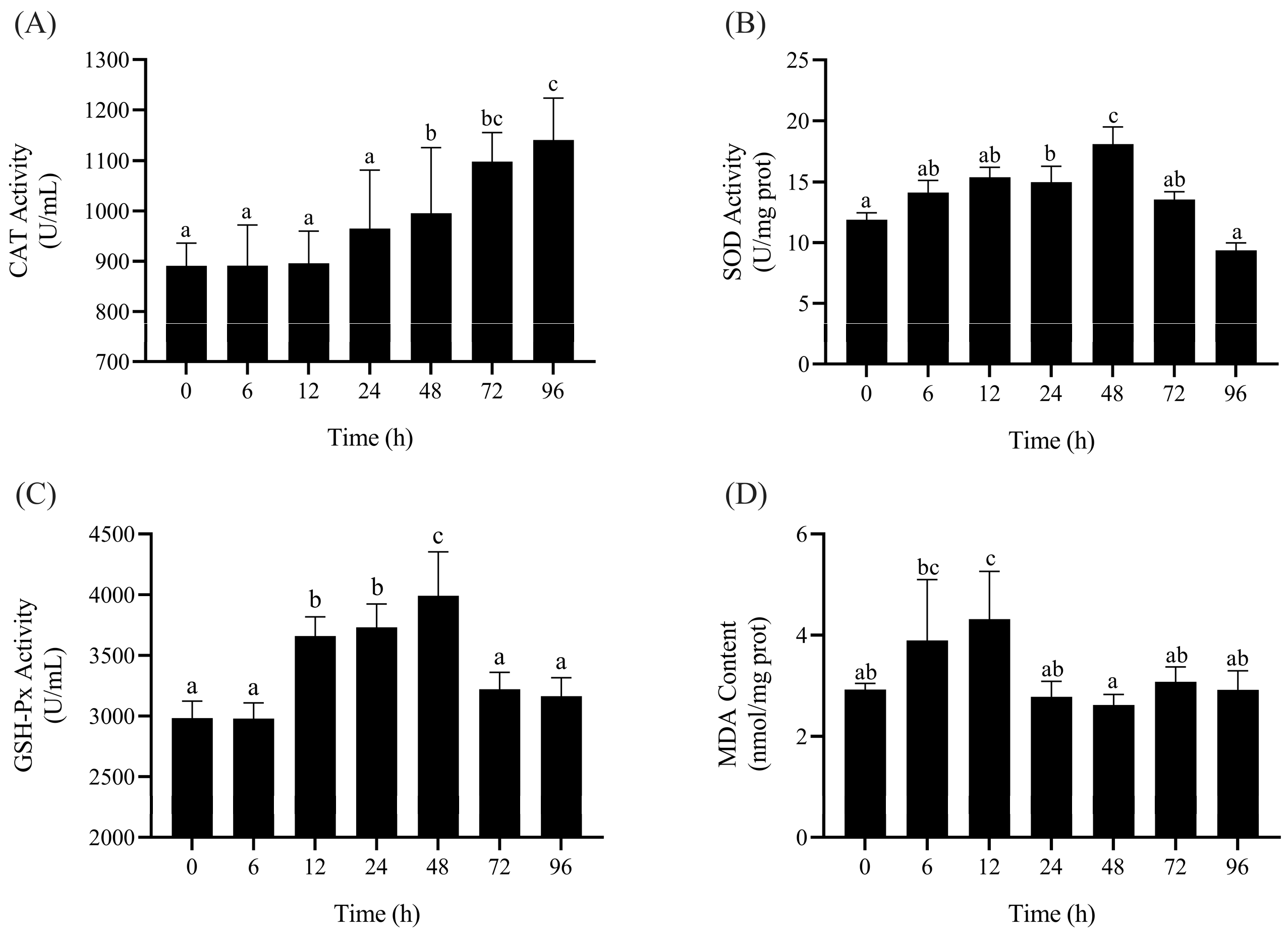
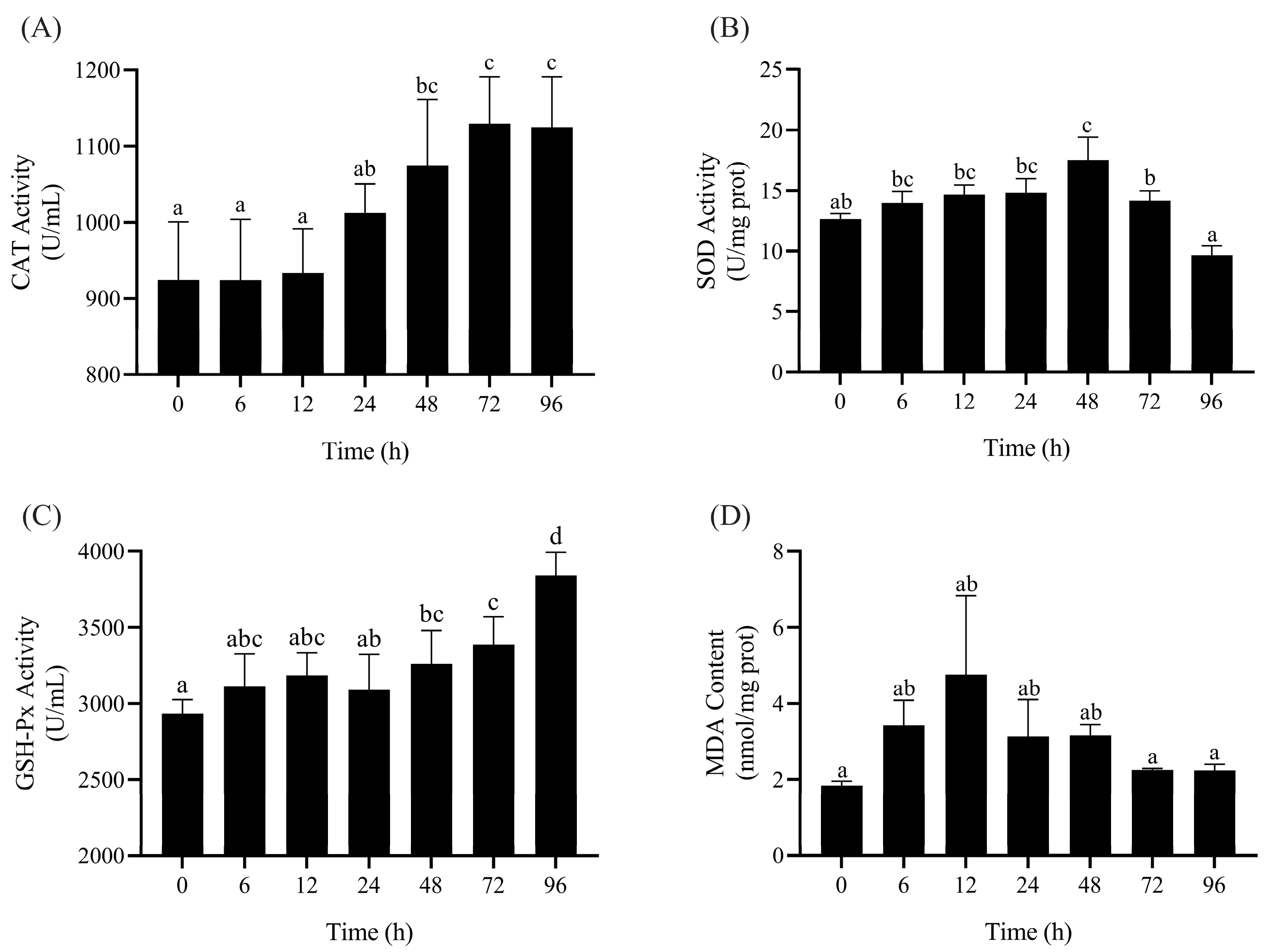


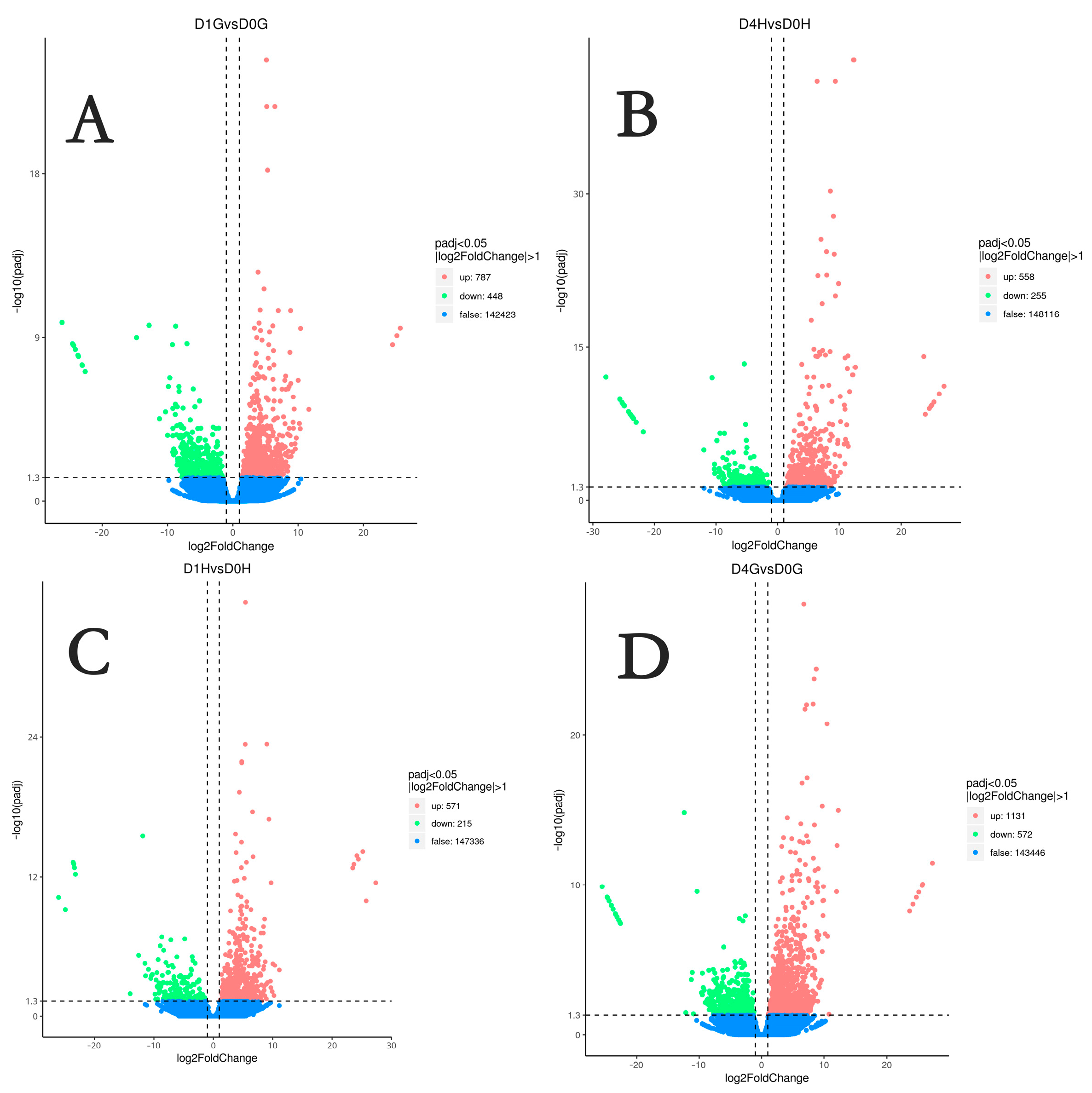
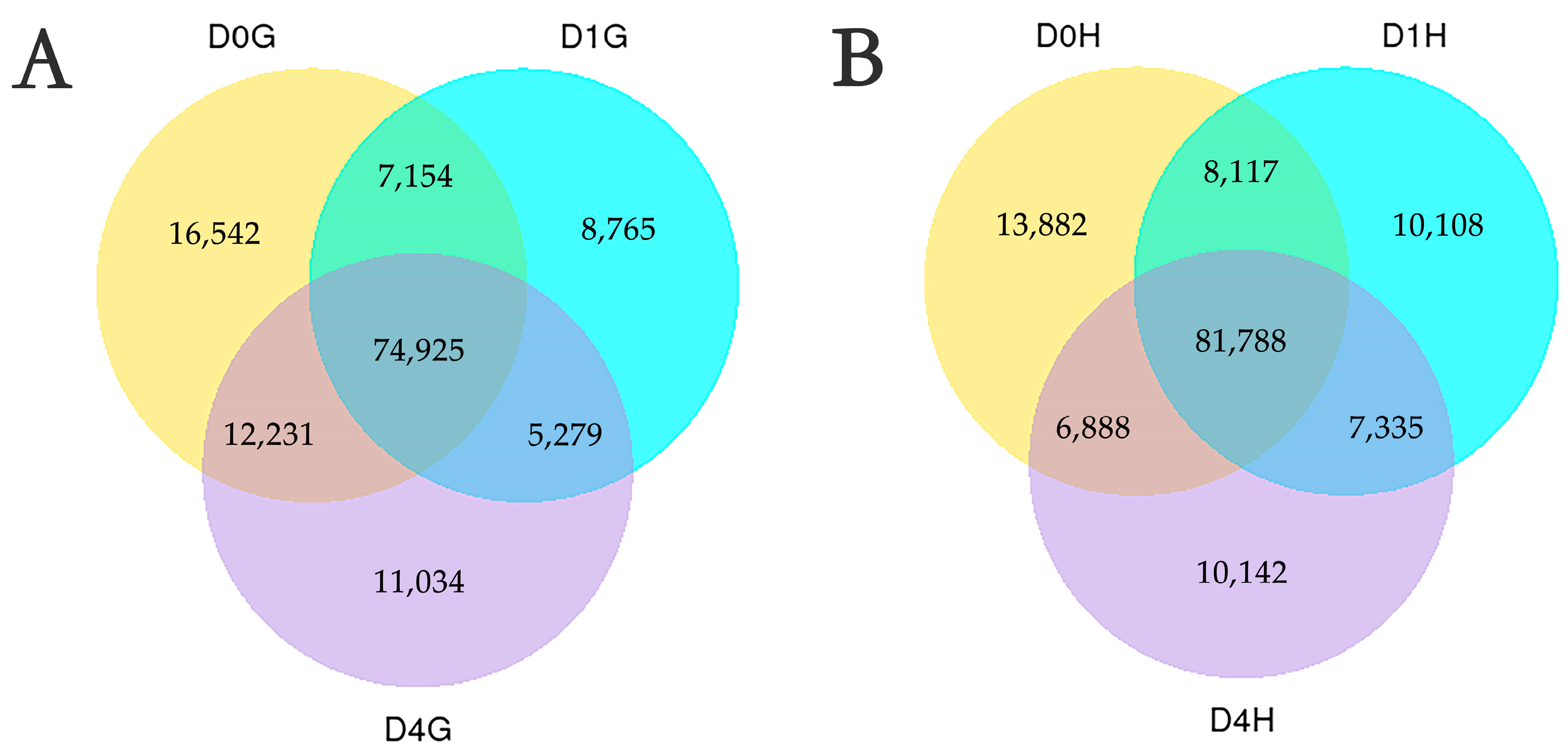
| Enzyme | Gill | Hepatopancreas |
|---|---|---|
| CAT | 0.934 | 0.888 |
| SOD | 0.557 | 0.412 |
| GSH-Px | 0.821 | 0.679 |
| MDA | 0.429 | 0.681 |
| GS | / | 0.409 |
| GDH | / | 0.663 |
| ARG | / | 0.757 |
| Urea | / | 0.909 |
| Sample | Sequence | Bases | Q30 (%) | Q20 (%) | Error Rate (%) | GC (%) |
|---|---|---|---|---|---|---|
| D0H1 | 22,842,296 | 6.9 | 92.74 | 97.30 | 0.03 | 46.29 |
| D0H2 | 23,273,599 | 7.0 | 92.32 | 97.12 | 0.03 | 42.37 |
| D0H3 | 27,418,945 | 8.2 | 92.55 | 97.21 | 0.03 | 45.26 |
| D0H4 | 23,860,226 | 7.2 | 92.48 | 97.18 | 0.03 | 45.64 |
| D1H1 | 23,994,308 | 7.2 | 91.53 | 96.79 | 0.03 | 46.80 |
| D1H2 | 22,670,194 | 6.8 | 91.83 | 96.86 | 0.03 | 46.40 |
| D1H3 | 23,711,368 | 7.1 | 92.57 | 97.20 | 0.03 | 45.68 |
| D1H4 | 23,714,877 | 7.1 | 92.33 | 97.10 | 0.03 | 45.83 |
| D4H1 | 22,935,647 | 6.9 | 92.52 | 97.20 | 0.03 | 46.42 |
| D4H2 | 23,685,353 | 7.1 | 92.52 | 97.16 | 0.03 | 46.04 |
| D4H3 | 25,613,052 | 7.7 | 92.73 | 97.28 | 0.03 | 45.89 |
| D4H4 | 23,934,056 | 7.2 | 92.66 | 97.28 | 0.03 | 45.23 |
| D0G1 | 23,564,918 | 7.1 | 92.23 | 97.14 | 0.03 | 39.38 |
| D0G2 | 23,949,276 | 7.2 | 92.54 | 97.24 | 0.03 | 42.89 |
| D0G3 | 22,637,216 | 6.8 | 92.55 | 97.22 | 0.03 | 43.49 |
| D0G4 | 23,608,151 | 7.1 | 92.44 | 97.21 | 0.03 | 40.29 |
| D1G1 | 23,043,652 | 6.9 | 92.02 | 97.04 | 0.03 | 36.88 |
| D1G2 | 22,836,559 | 6.9 | 92.62 | 97.28 | 0.03 | 39.80 |
| D1G3 | 27,259,303 | 8.2 | 92.82 | 97.36 | 0.03 | 40.51 |
| D1G4 | 25,161,300 | 7.5 | 92.74 | 97.32 | 0.03 | 40.52 |
| D4G1 | 23,078,351 | 6.9 | 92.12 | 97.06 | 0.03 | 41.31 |
| D4G2 | 23,594,571 | 7.1 | 92.55 | 97.23 | 0.03 | 42.58 |
| D4G3 | 23,397,638 | 7.0 | 91.50 | 96.76 | 0.03 | 42.71 |
| D4G4 | 23,971,951 | 7.2 | 92.45 | 97.22 | 0.03 | 43.32 |
| Group | Category | GO ID | Description | GeneRatio |
|---|---|---|---|---|
| D1G vs. D0G | BP | GO:0008219 | Cell death | 12 |
| BP | GO:0055085 | Transmembrane transport | 43 | |
| MF | GO:0022857 | Transmembrane transporter activity | 53 | |
| BP | GO:0006520 | Cellular amino acid metabolic process | 28 | |
| D4G vs. D0G | BP | GO:0055085 | Transmembrane transport | 67 |
| BP | GO:0005975 | Carbohydrate metabolic process | 36 | |
| MF | GO:0016874 | Ligase activity | 16 | |
| D1H vs. D0H | MF | GO:0003700 | DNA-binding transcription factor activity | 20 |
| CC | GO:0005654 | Nucleoplasm | 12 | |
| BP | GO:0048856 | Anatomical structure development | 13 | |
| BP | GO:0032196 | Transposition | 6 | |
| BP | GO:0071554 | Cell wall organization or biogenesis | 5 | |
| BP | GO:0006457 | Protein folding | 5 | |
| CC | GO:0005634 | Nucleus | 35 | |
| D4H vs. D0H | MF | GO:0016810 | Hydrolase activity, acting on carbon-nitrogen (but not peptide) bonds | 5 |
| BP | GO:0005975 | Carbohydrate metabolic process | 18 | |
| D4H vs. D0H | MF | GO:0003700 | DNA-binding transcription factor activity | 16 |
| MF | GO:0003677 | DNA binding | 46 | |
| BP | GO:0071554 | Cell wall organization or biogenesis | 5 | |
| BP | GO:0051301 | Cell division | 7 |
| Gene ID | Gill Log2FoldChange | Hepatopancreas Log2FoldChange | Gene Description |
|---|---|---|---|
| Cluster-22753.63857 | 2.8233 | 1.345 | V-type proton ATPase subunit B |
| Cluster-22753.75873 | 1.895 | 0.23366 | V-type H+-transporting ATPase subunit a |
| Cluster-22753.36771 | −0.63368 | −0.82744 | Na/K-ATPase alpha-subunit |
| Cluster-22753.62202 | 3.3914 | 1.9668 | Glutathione S-transferase |
| Cluster-22753.59646 | 12.094 | 10.949 | NADH dehydrogenase subunit 2 |
| Cluster-22753.15118 | 2.7716 | 1.7348 | Glutamine synthetase |
| Cluster-22753.77275 | 0.94146 | 0.57256 | Glutamate dehydrogenase |
| Cluster-22753.98962 | 2.4491 | −0.48522 | Argininosuccinate synthase |
| Cluster-16686.0 | 0.7397 | Ammonium transporter 3 | |
| Cluster-22753.117320 | 0.19705 | 0.24848 | Ammonium transporter 1 |
| Cluster-22753.129763 | 1.8164 | −0.46276 | Ammonium transporter Rh type |
| Cluster-22753.133859 | −2.8919 | 2.1372 | Carbonic anhydrase |
| Cluster-22753.2946 | −0.47581 | −2.9762 | Carbonic anhydrase 6 |
| Cluster-22753.70938 | 0.39958 | 0.39334 | Catalase |
| Cluster-21163.0 | −0.54152 | −0.41775 | Cu/Zn superoxide dismutase |
| Cluster-22753.11717 | 0.67884 | 0.14033 | Glutathione peroxidase |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Wang, X.; Wu, M.; Lin, Z.; He, L.; Zheng, X. Physiological and Transcriptome Analyses of Gill and Hepatopancreas of Potamocorbula ustulata Under Ammonia Exposure. Fishes 2025, 10, 200. https://doi.org/10.3390/fishes10050200
He J, Wang X, Wu M, Lin Z, He L, Zheng X. Physiological and Transcriptome Analyses of Gill and Hepatopancreas of Potamocorbula ustulata Under Ammonia Exposure. Fishes. 2025; 10(5):200. https://doi.org/10.3390/fishes10050200
Chicago/Turabian StyleHe, Jing, Xinhui Wang, Mingyu Wu, Zhihua Lin, Lin He, and Xiafei Zheng. 2025. "Physiological and Transcriptome Analyses of Gill and Hepatopancreas of Potamocorbula ustulata Under Ammonia Exposure" Fishes 10, no. 5: 200. https://doi.org/10.3390/fishes10050200
APA StyleHe, J., Wang, X., Wu, M., Lin, Z., He, L., & Zheng, X. (2025). Physiological and Transcriptome Analyses of Gill and Hepatopancreas of Potamocorbula ustulata Under Ammonia Exposure. Fishes, 10(5), 200. https://doi.org/10.3390/fishes10050200






