1. Introduction
Microplastic (MP) pollution has emerged as a significant environmental concern due to its persistence, bioavailability, and potential ecological risks [
1]. These microscopic plastic particles (<5 mm) originate from various sources, including industrial processes, urban runoff, and the degradation (attrition) of larger plastic debris [
2]. Aquatic ecosystems, particularly freshwater bodies, serve as major sinks for MPs, where they accumulate and pose threats to aquatic organisms across the entire food web, including fish [
3].
Freshwater fish are particularly vulnerable to MP contamination due to direct exposure through the ingestion of water, sediments, and prey [
4]. Studies worldwide have documented the ingestion of MPs by fish, raising concerns about their potential impact on fish health and food safety [
5]. However, data on MP contamination in freshwater fish from South Asia, including Pakistan, remain limited. Given the increasing amount of plastic production, the diverse routes of primary and secondary MP formation, and inadequate waste management infrastructure in this region, there is an urgent need to assess MP pollution in freshwater fish to understand its ecological and human health implications [
6].
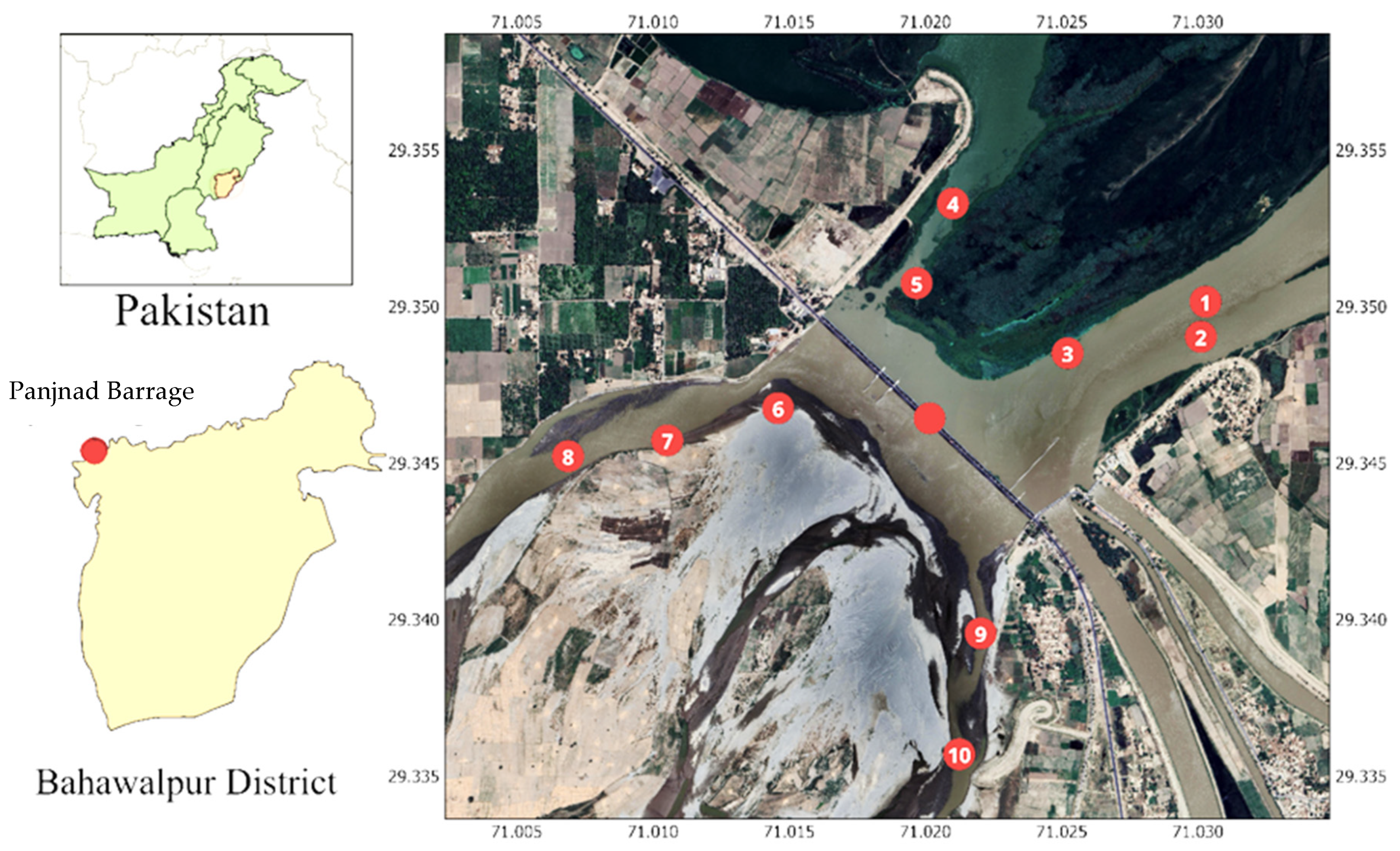
In Pakistan, studies on MP pollution are still in their infancy, with only a few reports documenting their presence in freshwater environments. The Panjnad Barrage, a critical confluence of five major rivers [
7], serves as an important site for fisheries and local livelihoods, making it a priority location for investigating persistent MP contamination. Despite its ecological and economic significance, there is limited research on the MP prevalence in fish species inhabiting this region.
This study aimed to address this knowledge gap by systematically assessing MP contamination in freshwater fish from the Panjnad Barrage. Specifically, we sought to determine the prevalence and characteristics of MPs in fish, thereby contributing to a better understanding of MP pollution in Pakistan’s freshwater ecosystems. By filling these critical gaps, this research will provide valuable insights for policymakers, environmental agencies, and conservationists striving to mitigate plastic pollution and its impacts on aquatic biodiversity and food security.
3. Results
Table 1 presents statistical comparisons of MP contamination across different body parts (GIT, gills, muscles) and MP characteristics (no. of MPs and density of MPs per gram) in three fish species from the Panjnad Barrage. The data reveal that MPs tended to accumulate more in the GIT and gills than in muscle tissues across all species. Specifically, in
Labeo rohita, the GIT had the highest number of MP particles (7.80 ± 0.34) and the lowest MP particle density (0.59 ± 0.04 per gram). In contrast, the gills of
Labeo rohita had a significantly higher MP particle density (3.17 ± 0.31 per gram), followed by the muscles with the lowest density (0.02 ± 0.00). The
Wallago attu species showed a similar trend, with the GIT having the highest number of MP particles (11.87 ± 0.60), but the gills had the highest MP particle density (3.79 ± 0.39 per gram), indicating a higher concentration of MPs in the gills compared to other body parts. For
Cirrhinus mrigala, the GIT (8.10 ± 0.35) once again contained the highest number of MP particles, with the gills exhibiting the highest density (3.01 ± 0.26 per gram), while the muscles showed the lowest MP count and density.
Statistically, there were significant differences in MP accumulation across body parts. For example, in Labeo rohita, the GIT and gills differed significantly in terms of the MP particle number and density, with the gills showing a much higher concentration. Similarly, for W. attu and C. mrigala, the gills significantly differed from both the GIT and muscles in terms of the MP density. These results suggest that while the number of MP particles may have varied across body parts, the gills generally exhibited the highest concentration of MP particles per gram, followed by the GIT, with the muscles showing the least contamination. This highlights the differential bioaccumulation and potential impacts of MPs in various tissues of fish species.
Table 2 also supports this, showing a correlation matrix for different body parts, the MP particle number, and the MP density of
Labeo rohita from the Panjnad Barrage.
The correlation matrix for the weight, MPN, and MPW of different body parts of
W. attu from the Panjnad Barrage (
Table 3) indicated that MP accumulation varied not only by the species but also by the body part, with the GIT often showing the highest number of MP particles, while the gills exhibited a higher concentration per gram. The overall weight of the body part also influenced the number of MP particles it accumulated, with larger body parts such as muscles generally accumulating more in total but less per gram.
The correlation matrix for the comparison of the weight, MP particle number, and MP density in different body parts of
C. mrigala from the Panjnad Barrage (
Table 4) suggested that in
C. mrigala, the GIT accumulated the highest number of MP particles, while the gills accumulated the highest concentration of MPs per gram, a pattern like that observed in
Labeo rohita. The muscles accumulated significantly fewer MP particles in terms of both the total number and concentration, likely due to their composition and function as a less absorptive tissue for MPs.
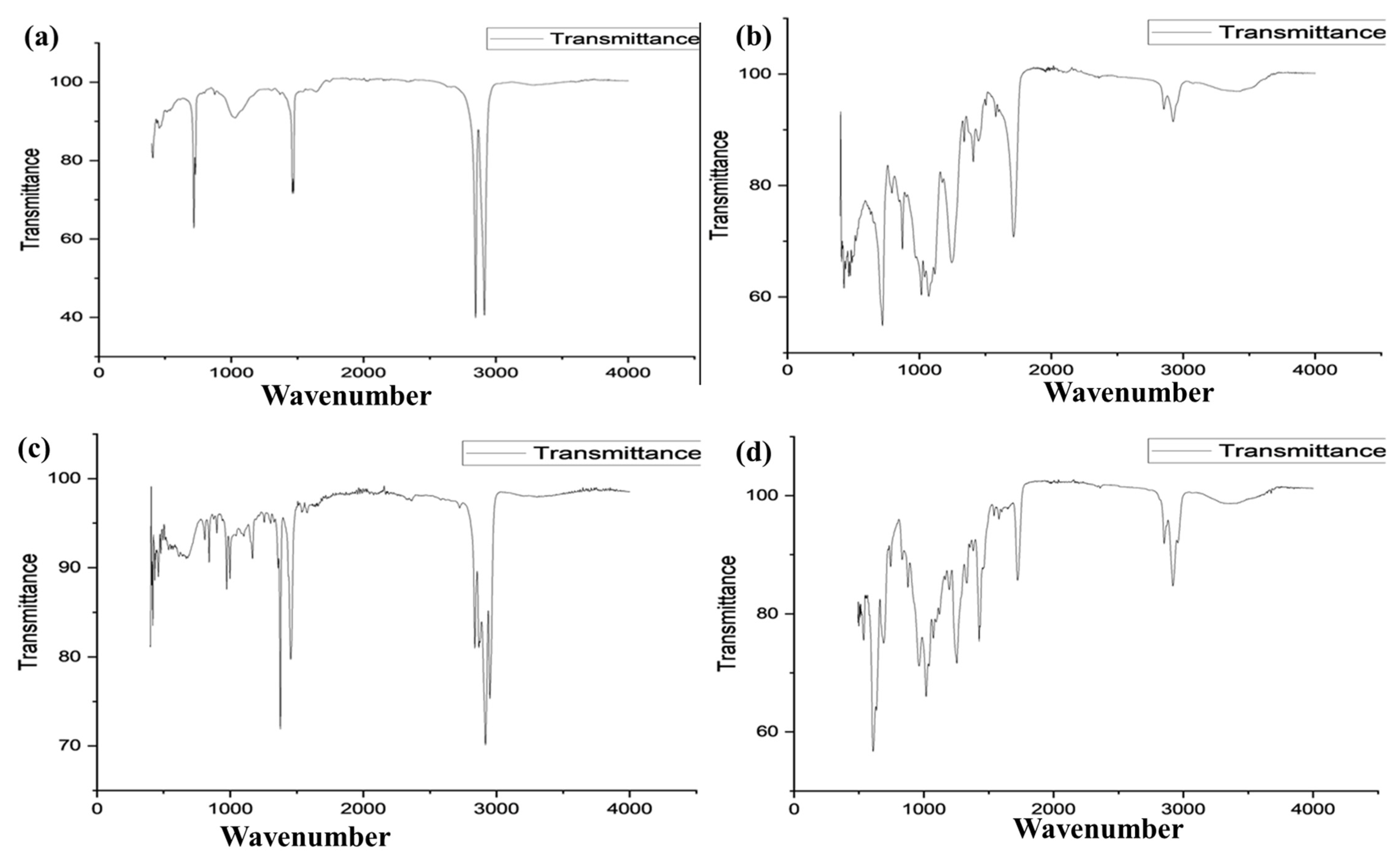
3.1. Fourier Transform Infrared Spectroscopy (FTIR)
FTIR analysis was performed to determine the types of polymers in the particles identified as plastic. FTIR spectra were established as standards for the comparison of functional groups in the sample spectra. (
Figure 2 and
Table 5)
3.2. Energy-Dispersive X-Ray Spectroscopy (EDS) Results for Plastic Particles
The EDX data allowed for a comparative analysis of the elemental composition of four different polymers: PE, PET, PP, and PVC. Each polymer exhibited a distinct elemental profile consistent with its chemical structure, while also revealing oxidation and possible contamination.
PE was primarily composed of carbon (83.3%) with a notable presence of oxygen (16.6%), suggesting surface oxidation or contamination, likely due to environmental exposure. The trace amount of chlorine (0.1%) was negligible but could indicate minor impurities. PP exhibited a similar composition, with a slightly higher carbon content (84.1%) and 15.8% oxygen, reinforcing its close structural resemblance to PE. PET showed a significantly different composition due to the ester functional groups in its structure. Its carbon content (63.0%) was lower than that of PE and PP, while the amount of oxygen (36.8%) was considerably higher, reflecting the presence of ester linkages. A small amount of chlorine (0.2%) was detected, likely from environmental contamination or processing residues. PVC demonstrated the most distinct composition, with the content of carbon (39.7%) and chlorine (9.4%) confirming the presence of a chloride-containing polymer backbone. However, the unexpectedly high oxygen content (50.9%) suggested oxidation.
The EDX results confirmed the expected elemental composition of these polymers while highlighting oxidation and minor contamination. The relatively high oxygen levels across all samples suggest potential environmental interactions, which may influence the polymers’ degradation properties and suitability for various applications. These findings are crucial for assessing polymer stability, aging behavior, and potential modifications for enhanced performance. (
Table 6)
3.3. Scanning Electron Microscopy
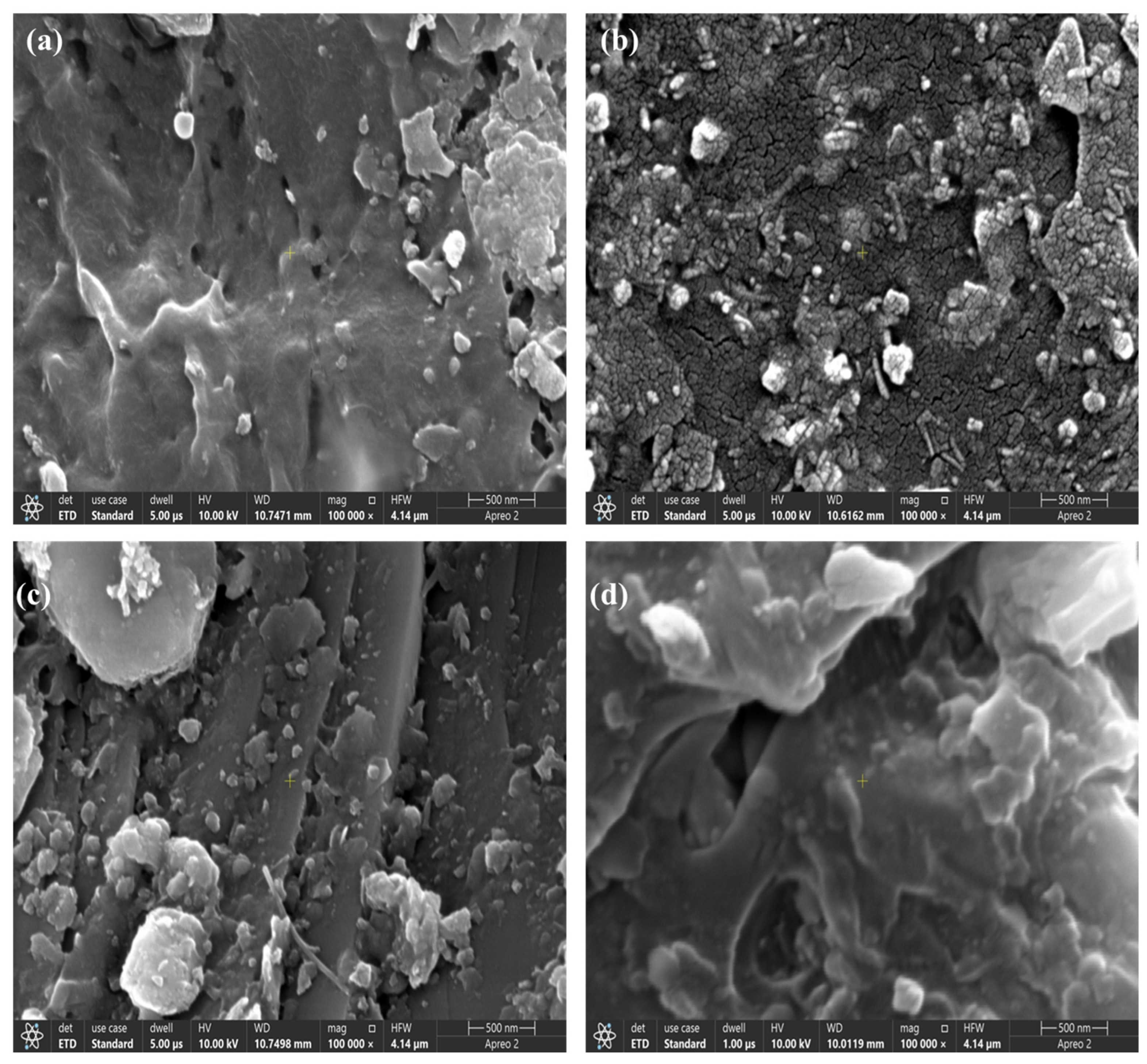
The SEM images taken at a 100,000× magnification provided a detailed insight into the surface morphology of four polymers: PE, PET, PP, and PVC. Each polymer exhibited distinct textural features influenced by its chemical composition, processing, and potential degradation mechanisms (
Figure 3).
Figure 3a shows a relatively smooth surface with minor cracks and scattered particulate debris. The presence of small rough patches suggests some degree of surface oxidation or contamination, likely due to environmental exposure. The continuous polymer matrix indicates a high degree of flexibility, but the appearance of cracks could signal early signs of degradation or mechanical stress, which may impact the polymer’s long-term mechanical properties.
Figure 3b exhibits a much rougher and more fragmented surface morphology. The highly granular and porous structure suggests the occurrence of crystallization effects or degradation due to environmental conditions, such as hydrolysis or oxidation.
Figure 3c shows a more heterogeneous texture with embedded particulate matter, possibly from additives or fillers incorporated during processing.
Figure 3d displays the most irregular and deformed surface morphology, with deep cracks and large voids. The SEM analysis highlighted the varying degradation and surface characteristics of these polymers. PE and PP exhibited smoother surfaces, suggesting better flexibility and environmental stability, whereas PET and PVC showed more pronounced roughness and porosity, indicative of structural weakening due to environmental factors. The highly granular texture of PET and the extensive cracking in PVC suggest substantial material breakdown, which could significantly influence their applications and longevity.
3.4. Gas Chromatography/Mass Spectrometry
The GC-MS analysis of multiple samples revealed the presence of various organic compounds, with a recurring pattern of sulfoxides, nitriles, fatty acid derivatives, and pharmaceutical-related compounds across different chromatograms. In the first sample, ten distinct compounds were detected, with significant peaks at 32.0, 32.9, and 41.8 min, corresponding to (2,3-diphenylcyclopropyl) methyl phenyl sulfoxide, trans- (C22H20OS, 332 g/mol), 9,12,15-octadecatrienoic acid, 2,3-dihydroxypropyl ester (C21H36O4, 352 g/mol), and octadecanenitrile, 6-aza-2,8-bis (3,4-dimethoxyphenyl)-6-methyl-2-propyl- (C27H38N2O4, 454 g/mol), respectively. The presence of sulfoxide and nitrile compounds indicates potential oxidative activity and synthetic applications, while the identification of a glycerolipid derivative suggests a biological origin, possibly from plant-based sources.
In the second sample, the retention times ranged from 7.0 to 47.2 min, with major peaks at 8.8, 31.7, and 47.2 min. The detected compounds included Hexanedinitrile (C6H8N2, 108 g/moL), (2,3-diphenylcyclopropyl) methyl phenyl sulfoxide, trans- (C22H20OS, 332 g/moL), and benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, octadecyl ester (C35H62O3, 530 g/mol). The presence of Hexanedinitrile suggests a role in polymer synthesis, while the detection of the sulfoxide compound further reinforces its recurring presence in the analyzed materials. The identification of benzenepropanoic acid ester, a known lipophilic antioxidant, indicates potential applications in stabilizing polymers, oils, or food products against oxidative degradation.
The third sample exhibited retention times from 32.0 to 37.4 min, with significant peaks at 32.0, 33.7, and 36.1 min. The major identified compounds were (2,3-diphenylcyclopropyl) methyl phenyl sulfoxide, trans- (C22H20OS, 332 g/mol), thiocarbamic acid, N,N-dimethyl, S-1,3-diphenyl-2-butenyl ester (C19H21NOS, 311 g/moL), and 2-Phenylbutyrophenone. The presence of thiocarbamate derivatives suggests potential industrial or pharmaceutical relevance, while phenylketones such as 2-Phenylbutyrophenone are widely utilized in organic synthesis. Notably, discrepancies were observed in the molecular formula attribution for this compound, necessitating further validation. The fourth sample demonstrated retention times from 32.0 to 38.2 min, with three primary peaks at 32.0, 32.9, and 36.1 min, corresponding to (2,3-diphenylcyclopropyl) methyl phenyl sulfoxide, trans- (C22H20OS, 332 g/mol), 9,12,15-octadecatrienoic acid, 2,3-dihydroxypropyl ester (C21H36O4, 352 g/mol), and 5-benzamido-4-oxo-6-phenylhexanoic acid (C19H19NO4, 325 g/mol). The repeated presence of the sulfoxide compound across multiple samples underscores its chemical significance in the analyzed material. The identification of a triglyceride derivative suggests a biological or plant-derived component, whereas 5-benzamido-4-oxo-6-phenylhexanoic acid, with its benzamido and oxo functionalities, hints at potential bioactive or pharmaceutical applications.
Collectively, the GC-MS analysis highlighted a consistent chemical profile across the samples, with a strong presence of sulfoxides, nitriles, fatty acid derivatives, and pharmaceutical-related compounds. The recurring detection of (2,3-diphenylcyclopropyl) methyl phenyl sulfoxide across multiple samples suggests its stability and prominence in the analyzed material. The identification of antioxidants and lipid derivatives indicates potential biological or synthetic applications, while nitrile and thiocarbamate compounds suggest relevance to polymer and pharmaceutical industries. The findings provide valuable insights into the chemical diversity and potential functional applications of the analyzed samples, warranting further investigation into their specific roles and industrial significance (
Figure 4).
The GIT of
Labeo rohita predominantly contained fibers (54.31%), followed by fragments (21.44%) and pellets/beads (10.11%). The primary color was yellow (36.11%), accompanied by notable amounts of red (16.19%) and orange (20.57%). The predominant chemical composition was PP at 40.63%, followed by PE at 26.61%. Fragments (26.44%) and fibers (53.30%) were the most prevalent in the gills. Yellow MPs constituted 48.20% of the total MPs, making this the predominant color, while PP accounted for 39.39% and PE for 25.74% of the MPs, representing the primary materials. In the muscles, pellets/beads constituted 41.29% of the MPs, followed by fragments at 24.96% and fibers at 17.92%. Yellow MPs constituted 47.68% of the total MPs, making this the most prevalent color, while PP accounted for 39.51% of the primary chemical composition (
Table 7).
In Wallago attu, the predominant types in the GIT were fibers (49.64%) and fragments (20.27%). Yellow MPs constituted 35.18% of the occurrences, while PP accounted for 40.54% as the predominant material. In the gills, fibers constituted 51.76% of the MPs, while fragments accounted for 24.10%, with yellow representing the most prevalent color at 47.28%. PP constituted 38.45% of the MPs, while PE accounted for 28.05% of the chemical compositions. The primary types found in the muscles were pellets/beads (42.54%) and fragments (25.79%). Yellow MPs constituted 46.88% of the occurrences, while PP accounted for 41.28% and PE for 24.24% of the chemical compositions.
In Cirrhinus mrigala, the GIT predominantly contained fibers (52.47%) and fragments (20.58%), with yellow (41.93%) being the most prevalent color. The main materials consisted of PP at 42.12% and PE at 22.60%. In the gills, fibers constituted 51.64% of the MPs and fragments accounted for 25.89%, with yellow representing the most common color at 44.45%. The primary chemical compositions were PP at 39.29% and PE at 27.49%. Pellets/beads constituted 42.13% of the MPs and fragments accounted for 23.78% in the muscles. Yellow constituted the most prevalent color at 46.32%, while PP and PE represented the primary chemical compositions at 38.17% and 24.79%, respectively.
It was observed that fibers and fragments represented the predominant categories of MPs observed across various species and organ types. Yellow was the dominant color across all three species, with PP and PE identified as the most prevalent chemical compositions. The GIT of all species demonstrated a greater occurrence of fibers, whereas the muscles showed a higher ratio of pellets and beads.
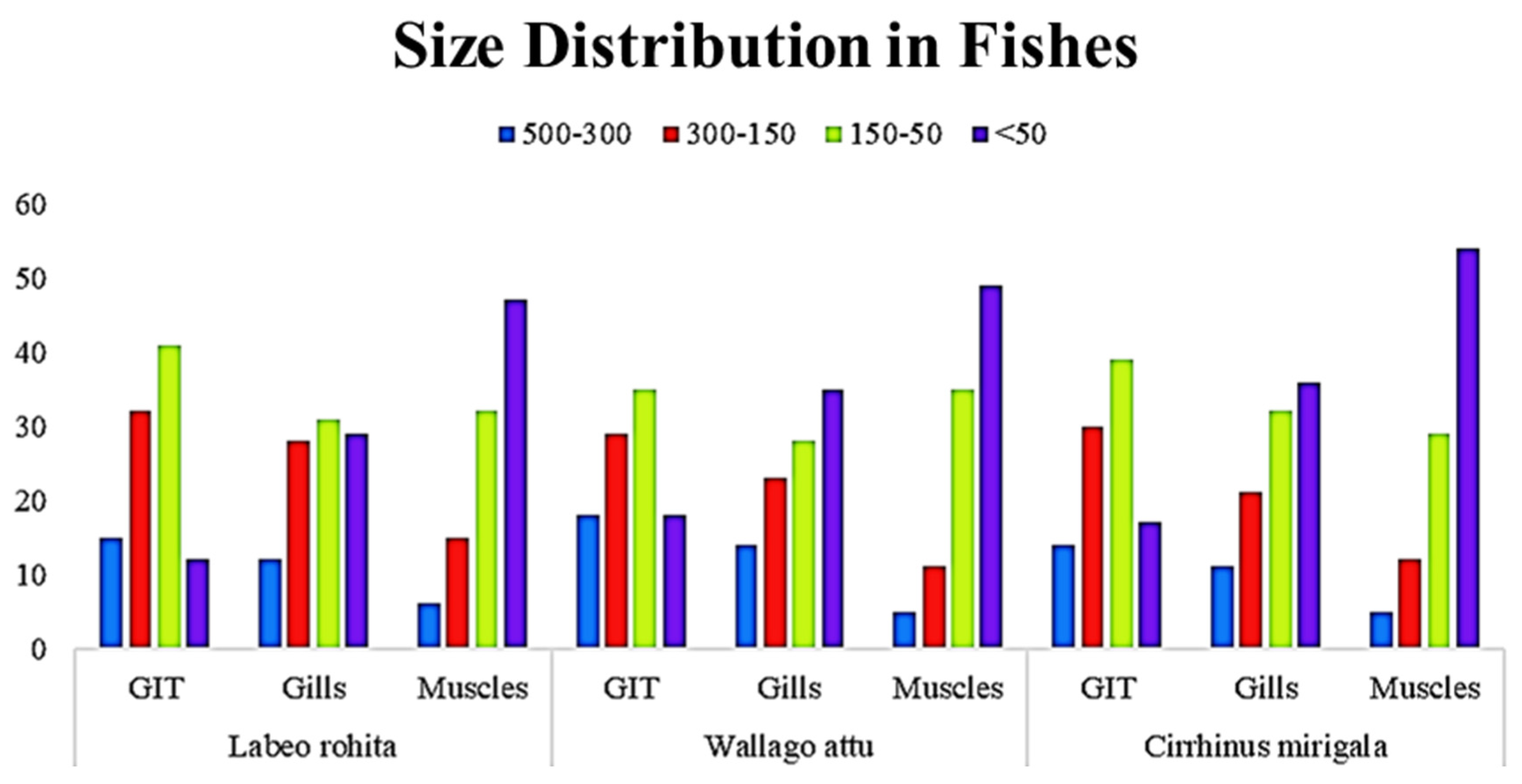
3.5. Organ-Wise Distribution of Microplastics by Size in Fish from Head Pajnad
The data presented show the size distribution of MPs found in different body parts (GIT, gills, and muscles) of three fish species: Labeo rohita, Wallago attu, and Cirrhinus mrigala. The MPs were categorized into four size ranges: 500–300 µm, 300–150 µm, 150–50 µm, and <50 µm.
In Labeo rohita, the 150–50 µm range represented the highest percentage of MPs in most body parts. In the GIT, 41% of the MPs were in the 150–50 µm size range. In the muscles, this category also represented the largest proportion at 32%. Similarly, the 150–50 µm size range was dominant in Wallago attu, with a proportion of 35% in the GIT and 49% in the muscles, indicating the tendency toward smaller MPs in muscle tissues. In Cirrhinus mrigala, this trend continued, where the 150–50 µm size category made up 39% of the MPs in the GIT and 29% in the muscles. Particles belonging to the <50 µm size category were more abundant in the muscles of Labeo rohita, where 47% of the MPs fell into this category. This was significantly higher than in the GIT (12%) and gills (29%). The smallest particles also had a higher presence in the muscles of Wallago attu, where 49% of the MPs were <50 µm. This contrasted with the findings for the GIT (18%) and gills (35%). Particles of <50 µm made up 54% of the MPs in the muscles of Cirrhinus mrigala, which was the highest percentage across all species and body parts. The GIT and gills had lower percentages of smaller MPs, 17% and 36%, respectively. Larger MPs in the 500–300 µm and 300–150 µm categories were present in smaller proportions, 15% and 32% in the GIT, respectively, in Labeo rohita. These particles were less prominent in the muscles (a proportion of 6% in the 500–300 µm range and 15% in the 300–150 µm range). Larger MPs followed a similar trend, with 18% in the 500–300 µm range in the GIT and 5% in the muscles. The 300–150 µm range represented 29% of the MPs in the GIT and 11% in the muscles of Wallago attu. Cirrhinus mrigala also showed a similar distribution, with 14% of the MPs in the 500–300 µm range in the GIT and 5% in the muscles. The 300–150 µm range made up 30% of the MPs in the GIT and 12% in the muscles.
Across all species, muscle tissues consistently showed the highest percentage of MPs in the <50 µm size category, suggesting that smaller particles may more easily penetrate and accumulate in muscle tissues. This was particularly pronounced in
Cirrhinus mrigala, where 54% of the MPs in the muscles were in the <50 µm range. The 150–50 µm size range was the most abundant in the GIT and gills across all species, with MP particles of this size category making up 30–41% of the total MPs. This suggests that mid-sized particles were more readily ingested or filtered by the fish. While the general trend of higher MP accumulation in muscle tissues was observed across all species, the
Cirrhinus mrigala species showed a significantly higher proportion of smaller particles (<50 µm) in the muscles compared to
Labeo rohita and
Wallago attu. MPs, particularly in the 150–50 µm and <50 µm size categories, were more abundant in the muscle tissues of all three fish species. Smaller MPs appeared to accumulate more in muscle tissue, suggesting that these particles may be more easily absorbed by muscle cells. (
Figure 5)
4. Discussion
The pervasive presence of MPs in freshwater ecosystems represents a critical threat to aquatic biodiversity and human food security. This study elucidated the bioaccumulation dynamics of MPs in three commercially vital fish species (
L. rohita,
W. attu, and
C. mrigala) from the Panjnad Barrage, Pakistan, while contextualizing the findings within mechanistic, ecological, and socio-political frameworks. The observed tissue-specific accumulation patterns (GIT > gills > muscles) are underpinned by a confluence of behavioral, physiological, and environmental factors. Detritivorous feeding behavior in
L. rohita and
C. mrigala likely exacerbates MP ingestion, as these species forage in benthic sediments, known reservoirs for dense polymer fragments (PP/PE) derived from degraded packaging materials [
11]. The significantly higher MP load in
W. attu (11.87 ± 0.60 MP particles/GIT), a carnivorous species, suggests secondary ingestion via MP-contaminated prey, a trophic transfer mechanism increasingly documented in piscivorous fish [
12]. This aligns with global studies demonstrating the presence of MPs at higher trophic levels, including in marine mammals [
13], underscoring the ubiquity of plastic pollution across ecosystems. These results agree with those of Raza et al. [
11], who showed that the mean occurrence of MPs in organs was in the order of the GIT > gills > muscles for
Labeo rohita collected from the River Ravi (a 725 km long river in northwestern India and eastern Pakistan) at two sites (site I, Dhand Nano Dogar, and site II, Jhamra). Other fish species, such as
Scomber japonicas, Sardinops pilcardus, and
Sardinella aurita, (253.3 ± 43.4 n·kg
−1) [
14]
Trichiurus lepturus (46.00 n/g
−1) [
15],
Heteropneustes fossilis (0.33–1.57 g
−1, 7–15 μm) [
16], and
Sardinops sagax (5.50 ± 1.6 items/g, 500–1000 μm) [
17], have also been reported in the River Ravi, which flows near to industrial cities.
Gill tissues exhibited the highest MP concentration per gram (3.79 ± 0.39 MP/g) in
W. attu, a phenomenon mechanistically tied to the respiratory biology of fish. In contrast to our findings, the average abundance of MPs was 0.49 ± 0.54 particles/gill in fishes from the Zhoushan fishing ground, China [
18]. As water passes over gill filaments, buoyant MPs (e.g., PE, with a density of <1 g/cm
3) are trapped via size exclusion mechanisms and mucociliary entrapment [
19]. The preferential accumulation of fibrous MPs in gills observed here and in
Mugil cephalus [
20] may impair respiratory efficiency by inducing epithelial hyperplasia, as demonstrated in laboratory exposures with
Oncorhynchus mykiss [
21]. The smaller MPs (<50 µm) detected in muscle tissues were likely a result of systemic translocation via the bloodstream, facilitated by trans-epithelial uptake in the GIT or gills. Recent work using fluorescent PS nanoparticles in
Danio rerio revealed that particles of <100 nm can cross intestinal barriers via clathrin-mediated endocytosis, accumulating muscle and hepatic tissues within 72 h [
22]. This raises alarming implications for human consumers, as muscle tissue is a primary edible component, potentially serving as a conduit for the introduction of NPs and adsorbed contaminants (e.g., heavy metals, PCBs) into the human diet.
The dominance of PP and PE polymers, constituting > 70% of the identified MPs, reflects their global production volumes and environmental persistence [
23]. PP’s resistance to chemical degradation and widespread use in fishing gear (e.g., monofilament lines) and agricultural mulch films [
24] explains its prevalence in the aquatic system fo Panjnad, a region intensively farmed for cotton and wheat. PE’s buoyancy and hydrophobic surface promote biofilm formation, enhancing its resemblance to organic prey and facilitating ingestion [
25]. For instance,
Oreochromis mossambicus (Mozambique tilapia) was found to exhibit a high concentration of PP and PE in the Guangdong Coastal Area of south China [
26].
Oreochromis niloticus (Nile tilapia) contains PET, PE, and PS in large amounts [
27]. In
Mugil cephalus (flathead gray mullet), PP and PE particles are found in high concentrations [
28]. In
Danio rerio (Zebrafish), PE is dominant [
29].
Perca fluviatilis (European perch) contains PE in large concentrations [
30]. In
Chanos chanos (milkfish), fibers and granules are found in abundance [
31]. Black (42.97%) MPs accounted for a large proportion of the MPs ingested by shellfish caught in Jiaozhou Bay, China. The additives identified in this study, including plasticizers (thiocarbamic acid derivatives) and thermal stabilizers (octadecanenitrile), exacerbate toxicity risks. For instance, phthalate esters, common plasticizers in PVC, are known to disrupt steroidogenesis in fish by inhibiting cytochrome P450 enzymes, as shown in
Clarias gariepinus [
32].
Ecologically, the prevalence of mid-sized MPs (150–50 µm) in GITs and gills signals the ongoing fragmentation of MPs within the Barrage, likely accelerated by seasonal monsoon flows and UV exposure [
33]. Conversely, the accumulation of NPs (<50 µm) in muscles mirrors trends in urbanized Asian water bodies, such as Dhaka’s Hatir Jheel Lake, where 78% of the MPs in
Anabas testudineus were <100 µm [
34]. Panjnad’s MP contamination levels, while moderate compared to those of industrial hubs [
35], highlight the role of anthropogenic pressures. The dominance of yellow/red MPs, contrasting with findings for white-dominated European estuaries [
36], points to localized pollution sources, including synthetic textile dyes from upstream garment industries and agricultural runoff containing colored mulch fragments.
From a policy perspective, these findings necessitate multi-tiered interventions. First, source reduction strategies must target Pakistan’s unregulated plastic waste sector, which generates 250 million tons of plastic annually, with <10% recycled [
37]. Adopting the EU’s Single-Use Plastics Directive (2019/904), which bans specific PE/PP products, could mitigate the MP influx. Second, wastewater treatment upgrades implementing membrane bioreactors (MBRs) and rapid sand filtration could capture up to 98% of fibrous MPs, as demonstrated in Cartagena, Spain [
38]. Third, community-led initiatives, such as the Indus River Plastic Cleanup Consortium, could enhance local stewardship through citizen science programs.
Future research should prioritize longitudinal studies to track the seasonal MP flux in the Barrage and experimental assays quantifying additive leaching kinetics under varying pH and salinity conditions. Additionally, harmonizing the MP quantification methodologies (e.g., spectroscopic vs. thermogravimetric analysis) used across South Asian studies would enable robust regional risk assessments. By bridging mechanistic insights, ecological monitoring, and policy innovation, this study contributes to a growing paradigm for combating plastic pollution in Global South freshwater ecosystems.