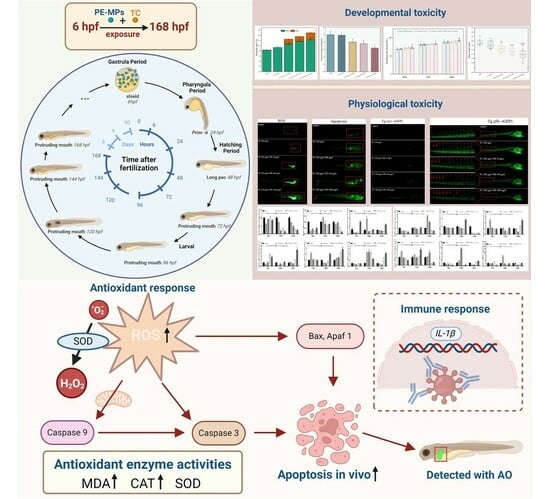
Microplastics Enhance the Toxic Effects of Tetracycline on the Early Development of Zebrafish in a Dose-Dependent Manner
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Zebrafish Maintenance
2.2. Exposure Experiment and Sample Collection
2.3. PE-MP Fluorescence Intensity Measurement
2.4. Developmental Toxicity Endpoint Detection
2.5. Reactive Oxygen Species (ROS) Level Measurement
2.6. Determination of the Oxidative Stress Index
2.7. Acridine Orange (AO) Staining
2.8. Zebrafish Expressing Fluorescent Markers to Detect Relevant Gene Expression
2.9. RT-qPCR
2.10. Statistical Analysis
3. Results and Discussion
3.1. Polyethylene MP Accumulation in Zebrafish Larvae
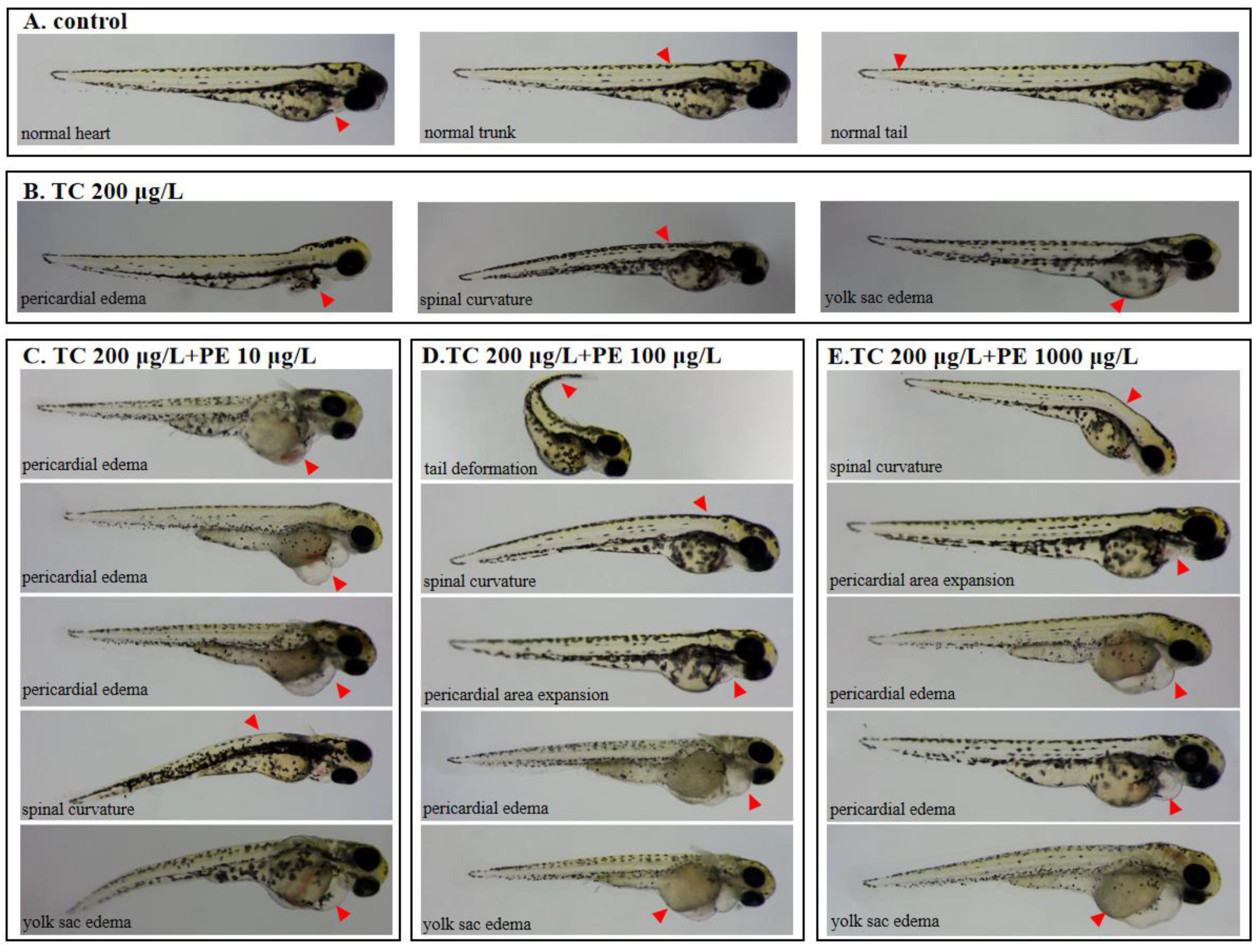
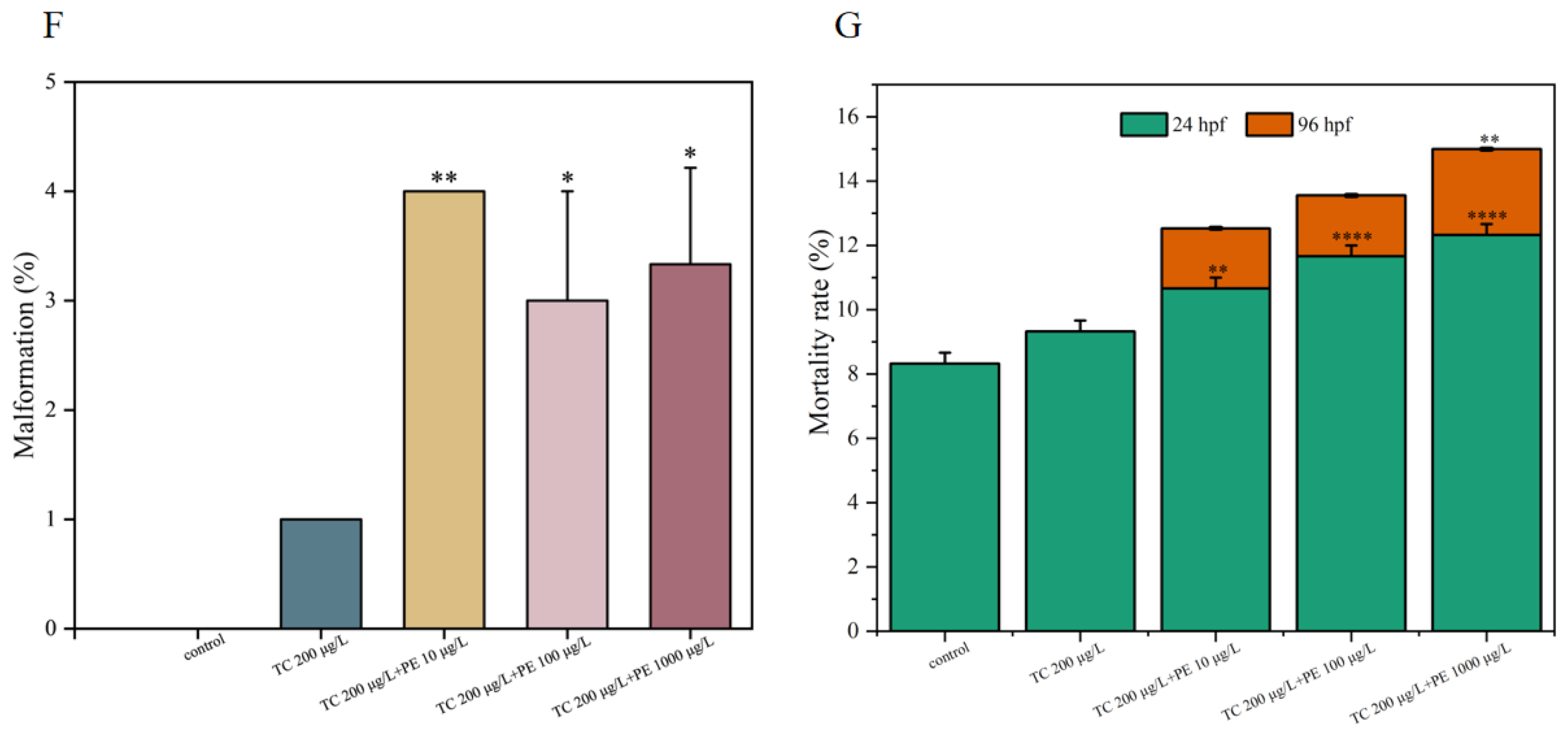
3.2. Exposure to PE-TC Induced Mortality and Malformation in Zebrafish
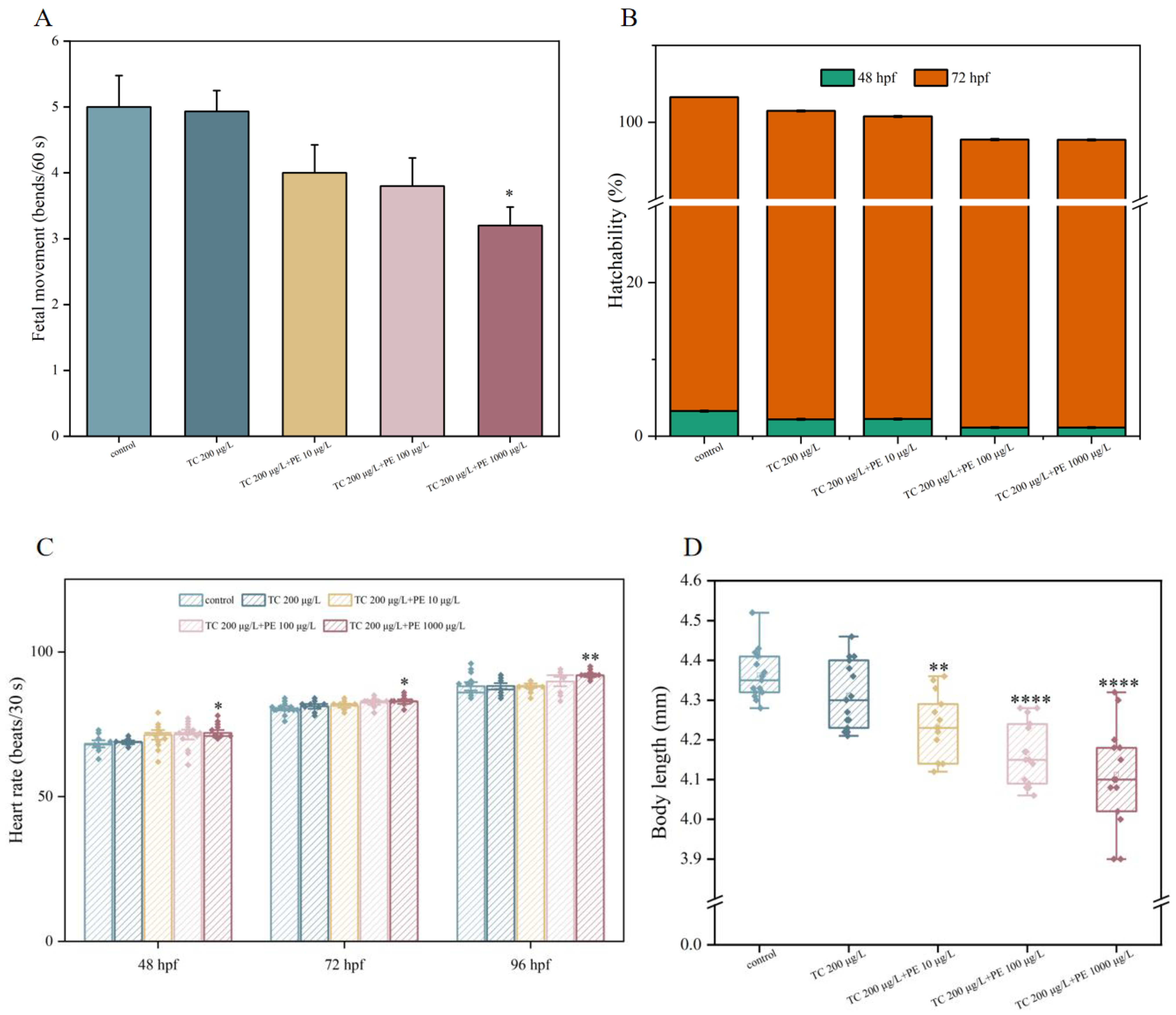
3.3. Developmental Toxicity of TC and PE Treatments in Zebrafish
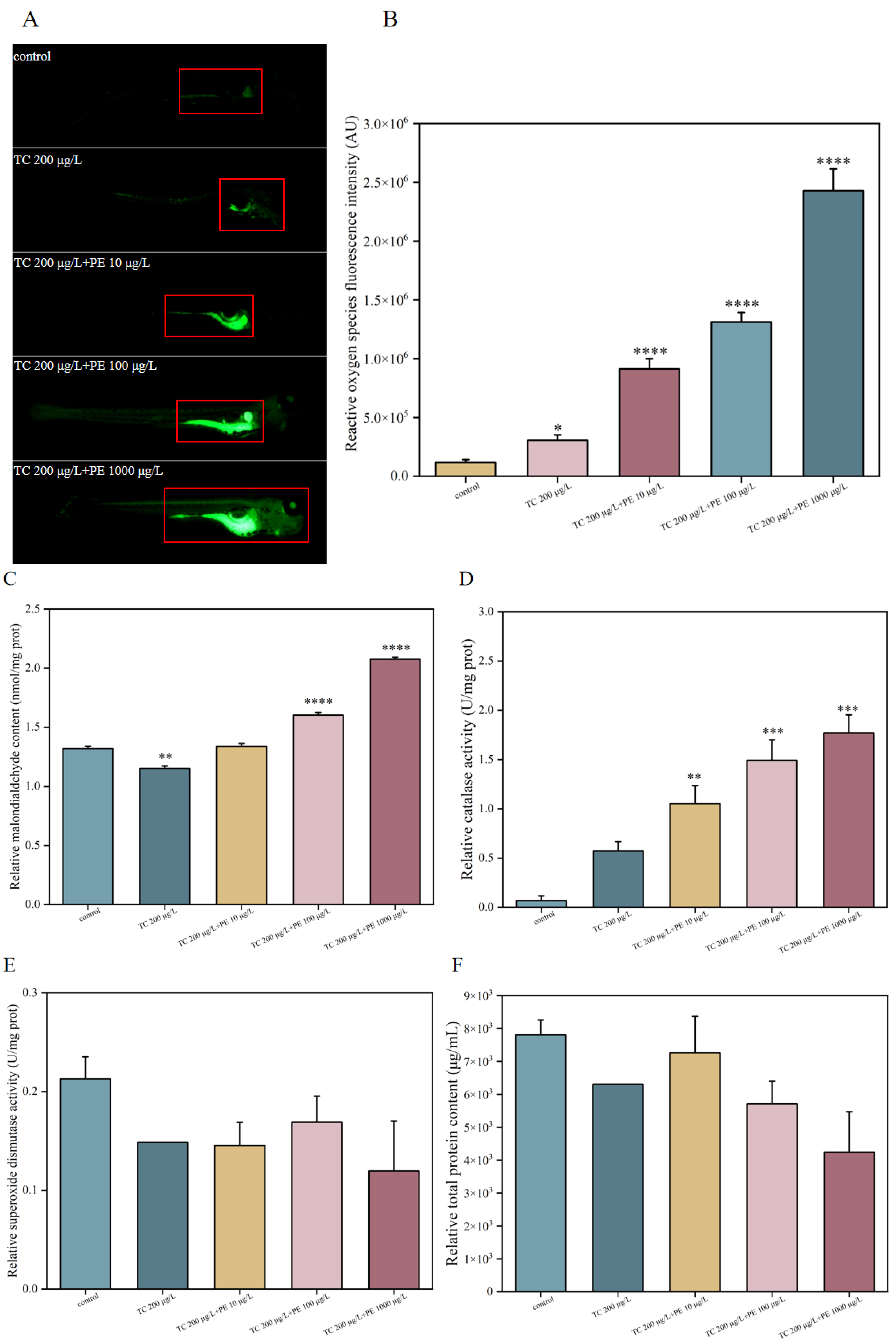
3.4. Oxidative Stress-Related Biochemical Indicators
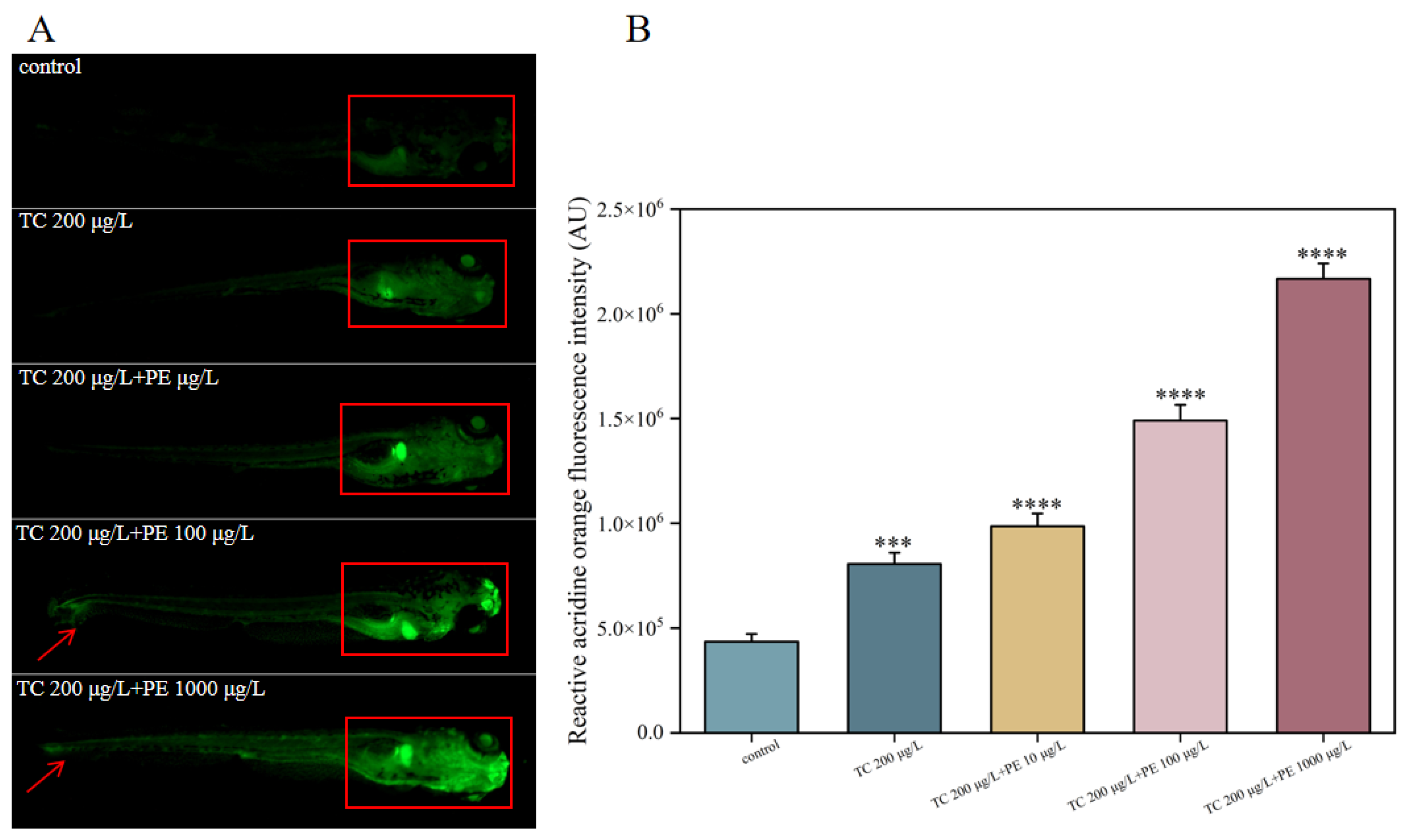
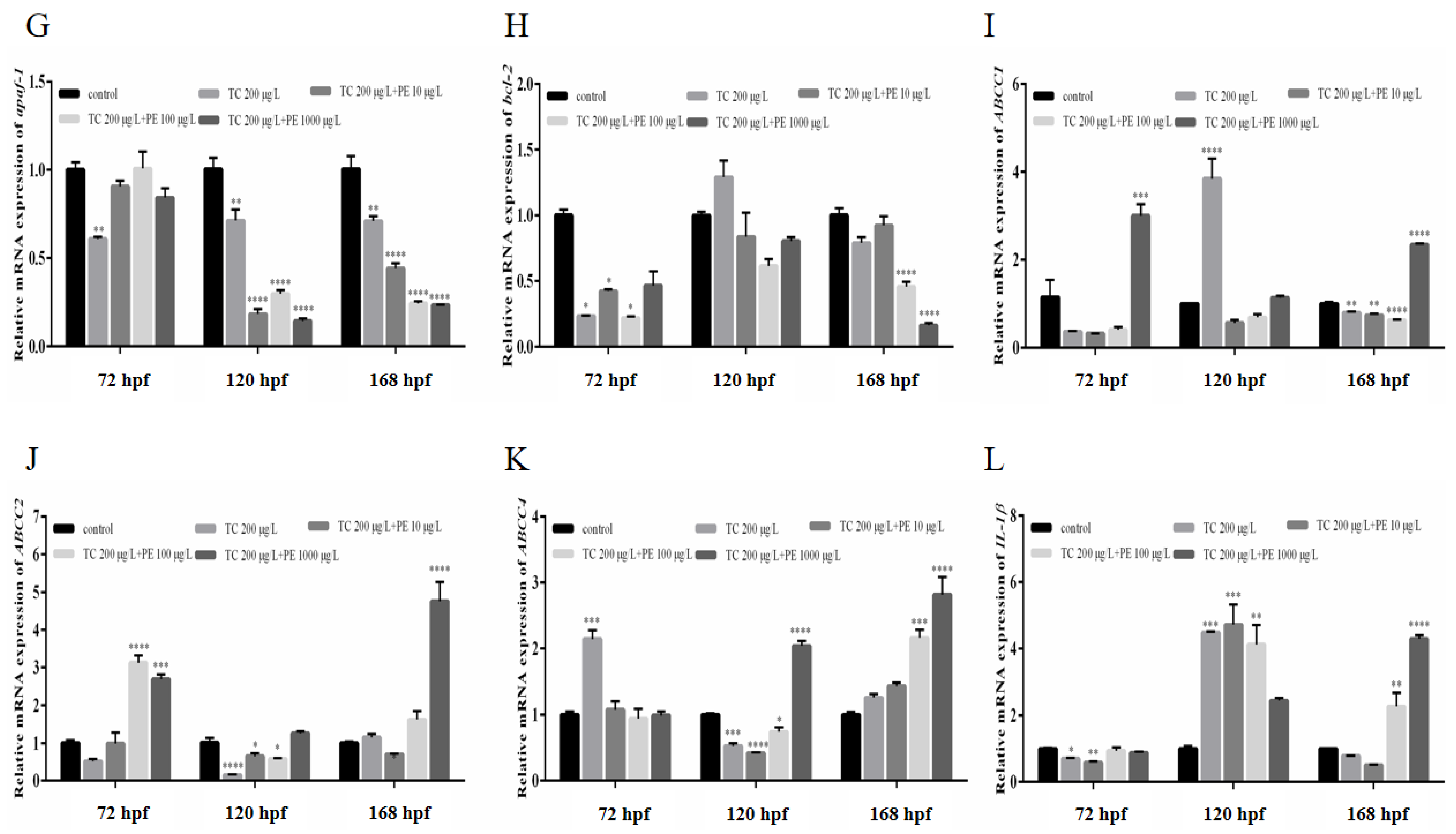
3.5. Effects of Combined TC-PE Exposure on Apoptosis in Zebrafish Larvae
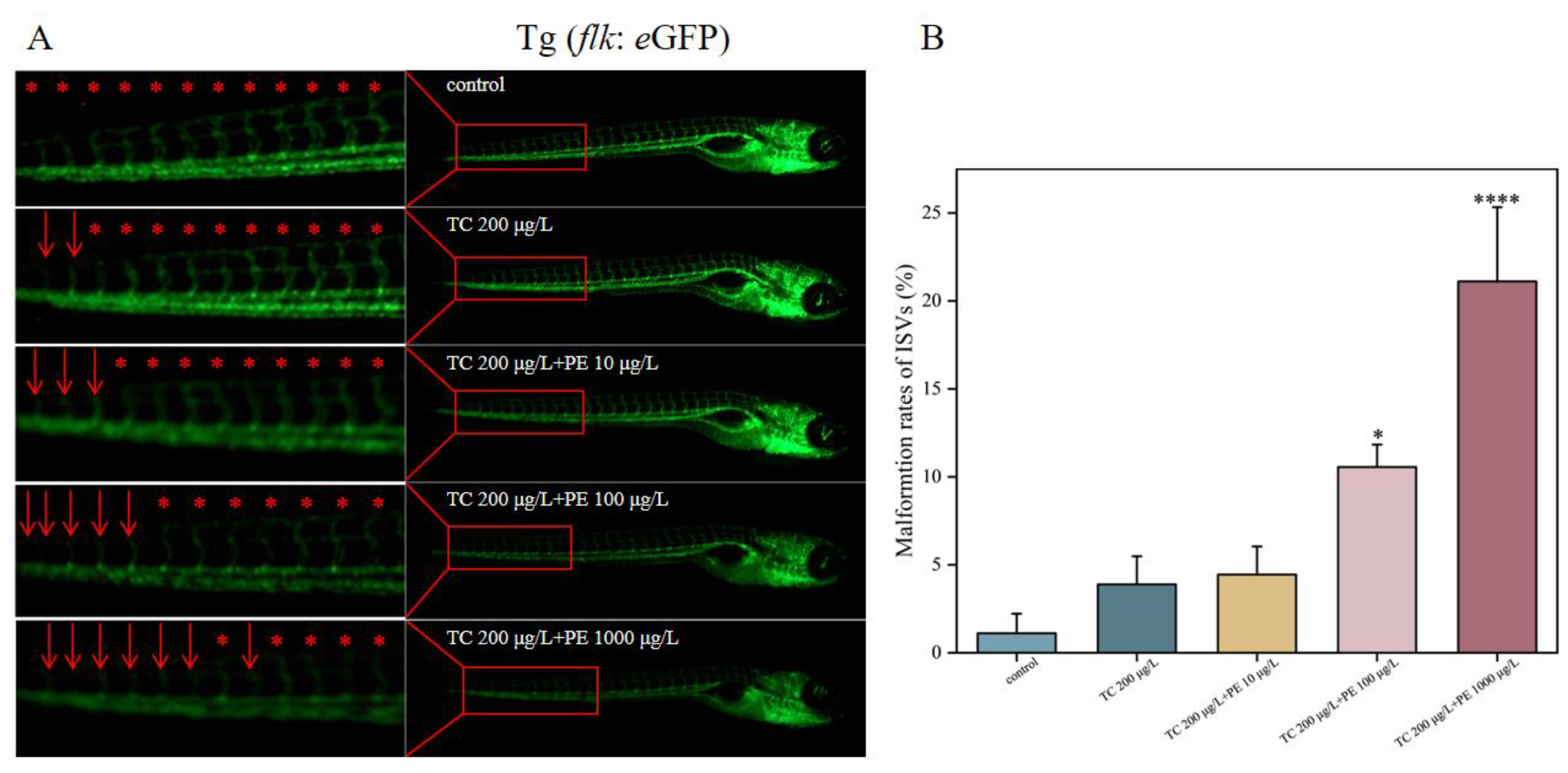
3.6. TC and PE Exposure Induces Ectopic Sprouting of Intersegmental Blood Vessels (ISVs) in Zebrafish Larvae
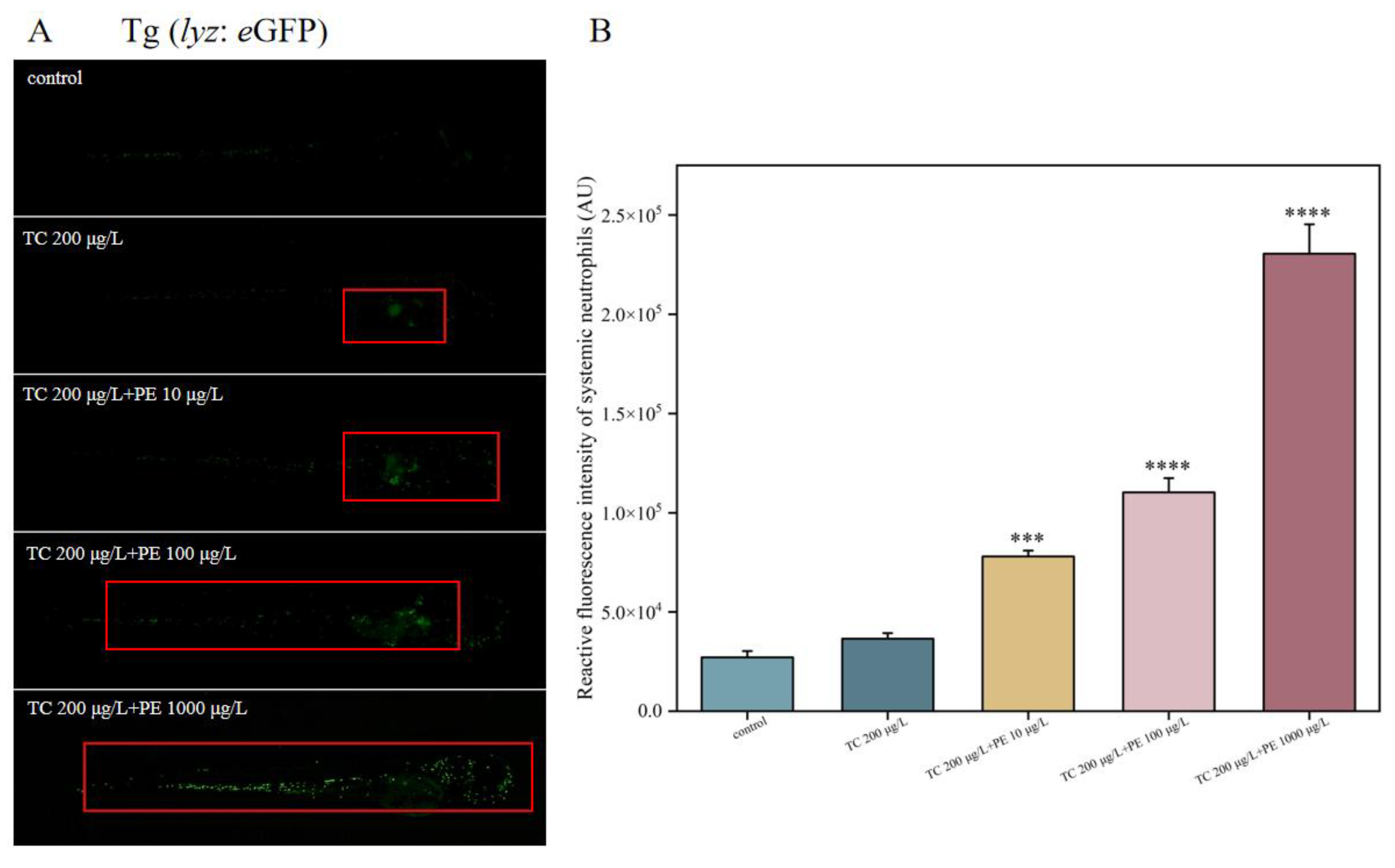
3.7. Different Forms of Exposure Cause Severe Inflammation in Zebrafish Larvae
3.8. Impacts on Apoptotic and Inflammatory Gene Expression
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hale, R.C.; Seeley, M.E.; Guardia, M.J.L.; Mai, L.; Zeng, E.Y. A Global Perspective on Microplastics. J. Geophys. Res. Ocean. 2020, 125, e2018JC014719. [Google Scholar] [CrossRef]
- Ivleva, N.P.; Wiesheu, A.C.; Niessner, R. Microplastic in Aquatic Ecosystems. Angew. Chem. Int. Ed. Engl. 2017, 56, 1720–1739. [Google Scholar] [CrossRef] [PubMed]
- Hammer, J.; Kraak, M.H.; Parsons, J.R. Plastics in the marine environment: The dark side of a modern gift. Rev. Environ. Contam. Toxicol. 2012, 220, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zuo, L.Z.; Peng, J.P.; Cai, L.Q.; Fok, L.; Yan, Y.; Li, H.X.; Xu, X.R. Occurrence and distribution of microplastics in an urban river: A case study in the Pearl River along Guangzhou City, China. Sci. Total Environ. 2018, 644, 375–381. [Google Scholar] [CrossRef]
- Lebreton, L.; Slat, B.; Ferrari, F.; Sainte-Rose, B.; Aitken, J.; Marthouse, R.; Hajbane, S.; Cunsolo, S.; Schwarz, A.; Levivier, A.; et al. Evidence that the Great Pacific Garbage Patch is rapidly accumulating plastic. Sci. Rep. 2018, 8, 4666. [Google Scholar] [CrossRef]
- Gigault, J.; Halle, A.T.; Baudrimont, M.; Pascal, P.Y.; Gauffre, F.; Phi, T.L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current opinion: What is a nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef]
- Karim, M.E.; Sanjee, S.A.; Mahmud, S.; Shaha, M.; Moniruzzaman, M.; Das, K.C. Microplastics pollution in Bangladesh: Current scenario and future research perspective. Chem. Ecol. 2019, 36, 83–99. [Google Scholar] [CrossRef]
- Koehler, A.; Anderson, A.; Andrady, A.; Arthur, C.; Wyles, K. Sources, Fate and Effects of Microplastics in the Marine Environment: A Global Assessment; UNESCO: Paris, France, 2015. [Google Scholar]
- Tirkey, A.; Upadhyay, L.S.B. Microplastics: An overview on separation, identification and characterization of microplastics. Mar. Pollut. Bull. 2021, 170, 112604. [Google Scholar] [CrossRef]
- Andrady, A.L. The plastic in microplastics: A review. Mar. Pollut. Bull. 2017, 119, 12–22. [Google Scholar] [CrossRef]
- Abdurahman, A.; Li, S.; Li, Y.; Song, X.; Gao, R. Ecotoxicological effects of antibiotic adsorption behavior of microplastics and its management measures. Environ. Sci. Pollut. Res. Int. 2023, 30, 125370–125387. [Google Scholar] [CrossRef]
- da Costa, J.P.; Santos, P.S.M.; Duarte, A.C.; Rocha-Santos, T. (Nano)plastics in the environment-Sources, fates and effects. Sci. Total Environ. 2016, 566–567, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Conkle, J.L.; Báez Del Valle, C.D.; Turner, J.W. Are We Underestimating Microplastic Contamination in Aquatic Environments? Environ. Manag. 2018, 61, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kovochich, M.; Liong, M.; Parker, J.A.; Oh, S.C.; Lee, J.P.; Xi, L.; Kreider, M.L.; Unice, K.M. Chemical mapping of tire and road wear particles for single particle analysis. Sci. Total Environ. 2021, 757, 144085. [Google Scholar] [CrossRef]
- Sahu, S.; Kaur, A.; Khatri, M.; Singh, G.; Arya, S.K. A review on cutinases enzyme in degradation of microplastics. J. Environ. Manag. 2023, 347, 119193. [Google Scholar] [CrossRef]
- Gallagher, A.; Rees, A.; Rowe, R.; Stevens, J.; Wright, P. Microplastics in the Solent estuarine complex, UK: An initial assessment. Mar. Pollut. Bull. 2016, 102, 243–249. [Google Scholar] [CrossRef]
- Zurub, R.E.; Cariaco, Y.; Wade, M.G.; Bainbridge, S.A. Microplastics exposure: Implications for human fertility, pregnancy and child health. Front. Endocrinol. 2023, 14, 1330396. [Google Scholar] [CrossRef]
- Sadri, S.S.; Thompson, R.C. On the quantity and composition of floating plastic debris entering and leaving the Tamar Estuary, Southwest England. Mar. Pollut. Bull. 2014, 81, 55–60. [Google Scholar] [CrossRef]
- Li, J.; Zhang, K.; Zhang, H. Adsorption of antibiotics on microplastics. Environ. Pollut. 2018, 237, 460–467. [Google Scholar] [CrossRef]
- Chen, G.; Li, Y.; Wang, J. Occurrence and ecological impact of microplastics in aquaculture ecosystems. Chemosphere 2021, 274, 129989. [Google Scholar] [CrossRef]
- Dong, H.; Chen, Y.; Wang, J.; Zhang, Y.; Zhang, P.; Li, X.; Zou, J.; Zhou, A. Interactions of microplastics and antibiotic resistance genes and their effects on the aquaculture environments. J. Hazard. Mater. 2021, 403, 123961. [Google Scholar] [CrossRef]
- Rubin, A.E.; Sarkar, A.K.; Zucker, I. Questioning the suitability of available microplastics models for risk assessment-A critical review. Sci. Total Environ. 2021, 788, 147670. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Manna, C.; Padha, S.; Verma, A.; Sharma, P.; Dhar, A.; Ghosh, A.; Bhattacharya, P. Micro(nano)plastics pollution and human health: How plastics can induce carcinogenesis to humans? Chemosphere 2022, 298, 134267. [Google Scholar] [CrossRef] [PubMed]
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Distribution and importance of microplastics in the marine environment: A review of the sources, fate, effects, and potential solutions. Environ. Int. 2017, 102, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Ragu Prasath, A.; Sudhakar, C.; Selvam, K. Microplastics in the environment: Types, sources, and impact on human and aquatic systems. Bioresour. Technol. Rep. 2025, 29, 102055. [Google Scholar] [CrossRef]
- Cherian, T.; Ragavendran, C.; Vijayan, S.; Kurien, S.; Peijnenburg, W.J.G.M. A review on the fate, human health and environmental impacts, as well as regulation of antibiotics used in aquaculture. Environ. Adv. 2023, 13, 100411. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, Y.; Yuan, Y.; Xie, Y. A systematic review on antibiotics misuse in livestock and aquaculture and regulation implications in China. Sci. Total Environ. 2021, 798, 149205. [Google Scholar] [CrossRef]
- Chen, X.; Yang, Y.; Ke, Y.; Chen, C.; Xie, S. A comprehensive review on biodegradation of tetracyclines: Current research progress and prospect. Sci. Total Environ. 2022, 814, 152852. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, H.; Xiong, P.; Zhu, Q.; Liao, C.; Jiang, G. Occurrence, fate, and risk assessment of typical tetracycline antibiotics in the aquatic environment: A review. Sci. Total Environ. 2021, 753, 141975. [Google Scholar] [CrossRef]
- Lundström, S.V.; Östman, M.; Bengtsson-Palme, J.; Rutgersson, C.; Thoudal, M.; Sircar, T.; Blanck, H.; Eriksson, K.M.; Tysklind, M.; Flach, C.F.; et al. Minimal selective concentrations of tetracycline in complex aquatic bacterial biofilms. Sci. Total Environ. 2016, 553, 587–595. [Google Scholar] [CrossRef]
- Lulijwa, R.; Rupia, E.J.; Alfaro, A.C. Antibiotic use in aquaculture, policies and regulation, health and environmental risks: A review of the top 15 major producers. Rev. Aquac. 2020, 12, 640–663. [Google Scholar] [CrossRef]
- Farid, M.U.; Choi, P.J.; Kharraz, J.A.; Lao, J.-Y.; St-Hilaire, S.; Ruan, Y.; Lam, P.K.S.; An, A.K. Hybrid nanobubble-forward osmosis system for aquaculture wastewater treatment and reuse. Chem. Eng. J. 2022, 435, 135164. [Google Scholar] [CrossRef]
- Chen, J.; Sun, R.; Pan, C.; Sun, Y.; Mai, B.; Li, Q.X. Antibiotics and Food Safety in Aquaculture. J. Agric. Food Chem. 2020, 68, 11908–11919. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Chen, C.; Wang, J. Sorption of sulfamethoxazole onto six types of microplastics. Chemosphere 2019, 228, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, J.; Zhou, B.; Zhou, Y.; Dai, Z.; Zhou, Q.; Chriestie, P.; Luo, Y. Enhanced adsorption of oxytetracycline to weathered microplastic polystyrene: Kinetics, isotherms and influencing factors. Environ. Pollut. 2018, 243, 1550–1557. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Wang, F.; Yang, H.; Liu, L. Adsorption of tetracyclines onto polyethylene microplastics: A combined study of experiment and molecular dynamics simulation. Chemosphere 2021, 265, 129133. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, L.; Huang, Q.; Dong, S.; Wang, X.; Yan, C. Combined effects of micro-/nano-plastics and oxytetracycline on the intestinal histopathology and microbiome in zebrafish (Danio rerio). Sci. Total Environ. 2022, 843, 156917. [Google Scholar] [CrossRef]
- Liao, X.; Zhao, P.; Hou, L.; Adyari, B.; Xu, E.G.; Huang, Q.; Hu, A. Network analysis reveals significant joint effects of microplastics and tetracycline on the gut than the gill microbiome of marine medaka. J. Hazard. Mater. 2023, 442, 129996. [Google Scholar] [CrossRef]
- Han, Y.; Zhou, W.; Tang, Y.; Shi, W.; Shao, Y.; Ren, P.; Zhang, J.; Xiao, G.; Sun, H.; Liu, G. Microplastics aggravate the bioaccumulation of three veterinary antibiotics in the thick shell mussel Mytilus coruscus and induce synergistic immunotoxic effects. Sci. Total Environ. 2021, 770, 145273. [Google Scholar] [CrossRef]
- Xu, K.; Tang, Z.; Liu, S.; Liao, Z.; Xia, H.; Liu, L.; Wang, Z.; Qi, P. Effects of low concentrations copper on antioxidant responses, DNA damage and genotoxicity in thick shell mussel Mytilus coruscus. Fish. Shellfish. Immunol. 2018, 82, 77–83. [Google Scholar] [CrossRef]
- Dai, L.; Luo, J.; Feng, M.; Wang, M.; Zhang, J.; Cao, X.; Yang, X.; Li, J. Nanoplastics exposure induces vascular malformation by interfering with the VEGFA/VEGFR pathway in zebrafish (Danio rerio). Chemosphere 2023, 312, 137360. [Google Scholar] [CrossRef]
- Santos, A.L.; Rodrigues, L.C.; Rodrigues, C.C.; Cirqueira, F.; Malafaia, G.; Rocha, T.L. Polystyrene nanoplastics induce developmental impairments and vasotoxicity in zebrafish (Danio rerio). J. Hazard. Mater. 2024, 464, 132880. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Ding, R.; Ma, Y.; Sun, Q.; Ren, X.; Sun, Z.; Duan, J. Cardiovascular toxicity assessment of polyethylene nanoplastics on developing zebrafish embryos. Chemosphere 2021, 282, 131124. [Google Scholar] [CrossRef]
- You, X.; Cao, X.; Zhang, X.; Guo, J.; Sun, W. Unraveling individual and combined toxicity of nano/microplastics and ciprofloxacin to Synechocystis sp. at the cellular and molecular levels. Environ. Int. 2021, 157, 106842. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Cai, Y.; Ma, C.; Han, L.; Yang, Z. Combined toxicity of micro/nano scale polystyrene plastics and ciprofloxacin to Corbicula fluminea in freshwater sediments. Sci. Total Environ. 2021, 789, 147887. [Google Scholar] [CrossRef]
- Fred-Ahmadu, O.H.; Bhagwat, G.; Oluyoye, I.; Benson, N.U.; Ayejuyo, O.O.; Palanisami, T. Interaction of chemical contaminants with microplastics: Principles and perspectives. Sci. Total Environ. 2020, 706, 135978. [Google Scholar] [CrossRef]
- Dmytriw, A.A. The microplastics menace: An emerging link to environment and health. Sci. Total Environ. 2020, 707, 135558. [Google Scholar] [CrossRef]
- Suzuki, S.; Nakanishi, S.; Tamminen, M.; Yokokawa, T.; Sato-Takabe, Y.; Ohta, K.; Chou, H.Y.; Muziasari, W.I.; Virta, M. Occurrence of sul and tet(M) genes in bacterial community in Japanese marine aquaculture environment throughout the year: Profile comparison with Taiwanese and Finnish aquaculture waters. Sci. Total Environ. 2019, 669, 649–656. [Google Scholar] [CrossRef]
- Sarmah, A.K.; Meyer, M.T.; Boxall, A.B. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 2006, 65, 725–759. [Google Scholar] [CrossRef]
- Zhao, Y.; Qin, Z.; Huang, Z.; Bao, Z.; Luo, T.; Jin, Y. Effects of polyethylene microplastics on the microbiome and metabolism in larval zebrafish. Environ. Pollut. 2021, 282, 117039. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, J.; Xin, Q. Effects of tetracycline on developmental toxicity and molecular responses in zebrafish (Danio rerio) embryos. Ecotoxicology 2015, 24, 707–719. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Z.; Zhang, Y.; Pan, Y.; Wang, T.; Wang, Z.; Li, Z.; Zeng, Q.; Qian, Y.; Qiu, J.; et al. Developmental effects and lipid disturbances of zebrafish embryos exposed to three newly recognized bisphenol A analogues. Environ. Int. 2024, 189, 108795. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Q.; Liu, Y.; Bi, L.; Jin, L.; Xu, K.; Peng, R. High glucose-induced ROS-accumulation in embryo-larval stages of zebrafish leads to mitochondria-mediated apoptosis. Apoptosis 2022, 27, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Peng, T.; Xiang, Y.; Liao, G.; Zou, F.; Meng, X. Neurotoxicity and gene expression alterations in zebrafish larvae in response to manganese exposure. Sci. Total Environ. 2022, 825, 153778. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Silic, M.R.; Schaber, A.; Wasel, O.; Freeman, J.L.; Sepúlveda, M.S. Exposure route affects the distribution and toxicity of polystyrene nanoplastics in zebrafish. Sci. Total Environ. 2020, 724, 138065. [Google Scholar] [CrossRef]
- LeMoine, C.M.R.; Kelleher, B.M.; Lagarde, R.; Northam, C.; Elebute, O.O.; Cassone, B.J. Transcriptional effects of polyethylene microplastics ingestion in developing zebrafish (Danio rerio). Environ. Pollut. 2018, 243, 591–600. [Google Scholar] [CrossRef]
- Tsay, H.J.; Wang, Y.H.; Chen, W.L.; Huang, M.Y.; Chen, Y.H. Treatment with sodium benzoate leads to malformation of zebrafish larvae. Neurotoxicol. Teratol. 2007, 29, 562–569. [Google Scholar] [CrossRef]
- Wiegand, J.; Avila-Barnard, S.; Nemarugommula, C.; Lyons, D.; Zhang, S.; Stapleton, H.M.; Volz, D.C. Triphenyl phosphate-induced pericardial edema in zebrafish embryos is dependent on the ionic strength of exposure media. Environment International 2023, 172, 107757. [Google Scholar] [CrossRef]
- Lett, Z.; Hall, A.; Skidmore, S.; Alves, N.J. Environmental microplastic and nanoplastic: Exposure routes and effects on coagulation and the cardiovascular system. Environ. Pollut. 2021, 291, 118190. [Google Scholar] [CrossRef]
- Asharani, P.V.; Lian Wu, Y.; Gong, Z.; Valiyaveettil, S. Toxicity of silver nanoparticles in zebrafish models. Nanotechnology 2008, 19, 255102. [Google Scholar] [CrossRef]
- Lu, J.; Wu, J.; Gong, L.; Cheng, Y.; Yuan, Q.; He, Y. Combined toxicity of polystyrene microplastics and sulfamethoxazole on zebrafish embryos. Environ. Sci. Pollut. Res. Int. 2022, 29, 19273–19282. [Google Scholar] [CrossRef]
- Tshering, G.; Plengsuriyakarn, T.; Na-Bangchang, K.; Pimtong, W. Embryotoxicity evaluation of atractylodin and β-eudesmol using the zebrafish model. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021, 239, 108869. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Yang, Q.; Jiang, W.; Lu, J.; Xiang, Z.; Guo, R.; Chen, J. Integrated toxic evaluation of sulfamethazine on zebrafish: Including two lifespan stages (embryo-larval and adult) and three exposure periods (exposure, post-exposure and re-exposure). Chemosphere 2018, 195, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Shao, Y.; Hu, Z.; Gao, H. Effects of soluble sulfide on zebrafish (Danio rerio) embryonic development. Environ. Toxicol. Pharmacol. 2016, 42, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Felker, A.; Prummel, K.D.; Merks, A.M.; Mickoleit, M.; Brombacher, E.C.; Huisken, J.; Panáková, D.; Mosimann, C. Continuous addition of progenitors forms the cardiac ventricle in zebrafish. Nat. Commun. 2018, 9, 2001. [Google Scholar] [CrossRef]
- Xu, L.; Yang, X.; He, Y.; Hu, Q.; Fu, Z. Combined exposure to titanium dioxide and tetracycline induces neurotoxicity in zebrafish. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2023, 267, 109562. [Google Scholar] [CrossRef]
- Yang, X.; Sun, Z.; Wang, W.; Zhou, Q.; Shi, G.; Wei, F.; Jiang, G. Developmental toxicity of synthetic phenolic antioxidants to the early life stage of zebrafish. Sci. Total Environ. 2018, 643, 559–568. [Google Scholar] [CrossRef]
- Wang, M.; Chen, X.; Zhang, R.; Zhao, J.; Yang, C.; Wu, L. Developmental toxicity and transcriptome analysis of 4-epianhydrotetracycline to zebrafish (Danio rerio) embryos. Sci. Total Environ. 2020, 734, 139227. [Google Scholar] [CrossRef]
- Lin, Y.; Yu, J.; Wang, M.; Wu, L. Toxicity of single and combined 4-epianhydrotetracycline and cadmium at environmentally relevant concentrations on the zebrafish embryos (Danio rerio). Environ. Pollut. 2023, 316, 120543. [Google Scholar] [CrossRef]
- Cormier, B.; Le Bihanic, F.; Cabar, M.; Crebassa, J.C.; Blanc, M.; Larsson, M.; Dubocq, F.; Yeung, L.; Clérandeau, C.; Keiter, S.H.; et al. Chronic feeding exposure to virgin and spiked microplastics disrupts essential biological functions in teleost fish. J. Hazard. Mater. 2021, 415, 125626. [Google Scholar] [CrossRef]
- Tarasco, M.; Gavaia, P.J.; Bensimon-Brito, A.; Cordelières, F.P.; Santos, T.; Martins, G.; de Castro, D.T.; Silva, N.; Cabrita, E.; Bebianno, M.J.; et al. Effects of pristine or contaminated polyethylene microplastics on zebrafish development. Chemosphere 2022, 303, 135198. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Li, J.; Pan, Y.; Zhuang, Z.; Zhang, X.; Chen, C.; Liu, Y.; Zhang, L.; Luo, Y.; et al. The combined toxic effects of polystyrene microplastics and different forms of arsenic on the zebrafish embryos (Danio rerio). Sci. Total Environ. 2023, 887, 164017. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pang, S.; Chen, Z.; Wang, J.; Liu, L.; Zhang, L.; Wang, F.; Song, M. Surface Modification Determines the Distribution and Toxicity of Quantum Dots during the Development of Early Staged Zebrafish. Environ. Sci. Technol. 2023, 57, 10574–10581. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Weck, J.; Sundaram, R.; Goldstone, A.E.; Louis, G.B.; Kannan, K. Urinary concentrations of phthalates in couples planning pregnancy and its association with 8-hydroxy-2′-deoxyguanosine, a biomarker of oxidative stress: Longitudinal investigation of fertility and the environment study. Environ. Sci. Technol. 2014, 48, 9804–9811. [Google Scholar] [CrossRef]
- Tugasworo, D.; Prasetyo, A.; Kurnianto, A.; Retnaningsih, R.; Andhitara, Y.; Ardhini, R.; Budiman, J. Malondialdehyde (MDA) and 8-hydroxy-2′-deoxyguanosine (8-OHdG) in ischemic stroke: A systematic review. Egypt. J. Neurol. Psychiatry Neurosurg. 2023, 59, 87. [Google Scholar] [CrossRef]
- Cheng, H.; Duan, Z.; Wu, Y.; Wang, Y.; Zhang, H.; Shi, Y.; Zhang, H.; Wei, Y.; Sun, H. Immunotoxicity responses to polystyrene nanoplastics and their related mechanisms in the liver of zebrafish (Danio rerio) larvae. Environ. Int. 2022, 161, 107128. [Google Scholar] [CrossRef]
- Huang, W.; Mo, J.; Li, J.; Wu, K. Exploring developmental toxicity of microplastics and nanoplastics (MNPS): Insights from investigations using zebrafish embryos. Sci. Total Environ. 2024, 933, 173012. [Google Scholar] [CrossRef]
- Wan, Z.; Wang, C.; Zhou, J.; Shen, M.; Wang, X.; Fu, Z.; Jin, Y. Effects of polystyrene microplastics on the composition of the microbiome and metabolism in larval zebrafish. Chemosphere 2019, 217, 646–658. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Deng, Y.; Jiang, W.; Zhao, Y.; Geng, J.; Ding, L.; Ren, H. Uptake and Accumulation of Polystyrene Microplastics in Zebrafish (Danio rerio) and Toxic Effects in Liver. Environ. Sci. Technol. 2016, 50, 4054–4060. [Google Scholar] [CrossRef]
- Qiao, R.; Sheng, C.; Lu, Y.; Zhang, Y.; Ren, H.; Lemos, B. Microplastics induce intestinal inflammation, oxidative stress, and disorders of metabolome and microbiome in zebrafish. Sci. Total Environ. 2019, 662, 246–253. [Google Scholar] [CrossRef]
- Qiang, L.; Cheng, J. Exposure to microplastics decreases swimming competence in larval zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2019, 176, 226–233. [Google Scholar] [CrossRef]
- Lee, J.H.; Kang, J.C.; Kim, J.H. Toxic effects of microplastic (Polyethylene) on fish: Accumulation, hematological parameters and antioxidant responses in Korean Bullhead, Pseudobagrus fulvidraco. Sci. Total Environ. 2023, 877, 162874. [Google Scholar] [CrossRef] [PubMed]
- Blum, Y.; Belting, H.G.; Ellertsdottir, E.; Herwig, L.; Lüders, F.; Affolter, M. Complex cell rearrangements during intersegmental vessel sprouting and vessel fusion in the zebrafish embryo. Dev. Biol. 2008, 316, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kim, K. Microplastics induced developmental toxicity with microcirculation dysfunction in zebrafish embryos. Chemosphere 2022, 286, 131868. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Duan, X.; Zhao, S.; Wang, X.; Wang, J.; Liu, Y.; Peng, Y.; Gong, Z.; Wang, L. Barrier function of zebrafish embryonic chorions against microplastics and nanoplastics and its impact on embryo development. J. Hazard. Mater. 2020, 395, 122621. [Google Scholar] [CrossRef]
- Lu, K.; Qiao, R.; An, H.; Zhang, Y. Influence of microplastics on the accumulation and chronic toxic effects of cadmium in zebrafish (Danio rerio). Chemosphere 2018, 202, 514–520. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Iftikhar, N.; Konig, I.; English, C.; Ivantsova, E.; Souders, C.L., 2nd; Hashmi, I.; Martyniuk, C.J. Sulfamethoxazole (SMX) Alters Immune and Apoptotic Endpoints in Developing Zebrafish (Danio rerio). Toxics 2023, 11, 178. [Google Scholar] [CrossRef]
- Xiong, Q.; Xie, P.; Li, H.; Hao, L.; Li, G.; Qiu, T.; Liu, Y. Involvement of Fas/FasL system in apoptotic signaling in testicular germ cells of male Wistar rats injected i.v. with microcystins. Toxicon 2009, 54, 1–7. [Google Scholar] [CrossRef]
- Liu, C.; Yu, K.; Shi, X.; Wang, J.; Lam, P.K.; Wu, R.S.; Zhou, B. Induction of oxidative stress and apoptosis by PFOS and PFOA in primary cultured hepatocytes of freshwater tilapia (Oreochromis niloticus). Aquat. Toxicol. 2007, 82, 135–143. [Google Scholar] [CrossRef]
- Gao, D.; Xu, Z.; Zhang, X.; Zhu, C.; Wang, Y.; Min, W. Cadmium triggers kidney cell apoptosis of purse red common carp (Cyprinus carpio) without caspase-8 activation. Dev. Comp. Immunol. 2013, 41, 728–737. [Google Scholar] [CrossRef]
- Morales-Cano, D.; Calviño, E.; Rubio, V.; Herráez, A.; Sancho, P.; Tejedor, M.C.; Diez, J.C. Apoptosis induced by paclitaxel via Bcl-2, Bax and caspases 3 and 9 activation in NB4 human leukaemia cells is not modulated by ERK inhibition. Exp. Toxicol. Pathol. 2013, 65, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Kingtong, S.; Chitramvong, Y.; Janvilisri, T. ATP-binding cassette multidrug transporters in Indian-rock oyster Saccostrea forskali and their role in the export of an environmental organic pollutant tributyltin. Aquat. Toxicol. 2007, 85, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Yu, S.; Chen, Y.; Chen, W. Integrated biomarker responses in zebrafish exposed to sulfonamides. Environ. Toxicol. Pharmacol. 2014, 38, 444–452. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Zhu, Z.; Zhong, R.; Fang, X.; Wang, X.; Huang, Y.; Gong, H.; Yan, M. Microplastics Enhance the Toxic Effects of Tetracycline on the Early Development of Zebrafish in a Dose-Dependent Manner. Fishes 2025, 10, 150. https://doi.org/10.3390/fishes10040150
Wu Y, Zhu Z, Zhong R, Fang X, Wang X, Huang Y, Gong H, Yan M. Microplastics Enhance the Toxic Effects of Tetracycline on the Early Development of Zebrafish in a Dose-Dependent Manner. Fishes. 2025; 10(4):150. https://doi.org/10.3390/fishes10040150
Chicago/Turabian StyleWu, Yanqing, Ziying Zhu, Riying Zhong, Xilin Fang, Xiaocui Wang, Yuanyin Huang, Han Gong, and Muting Yan. 2025. "Microplastics Enhance the Toxic Effects of Tetracycline on the Early Development of Zebrafish in a Dose-Dependent Manner" Fishes 10, no. 4: 150. https://doi.org/10.3390/fishes10040150
APA StyleWu, Y., Zhu, Z., Zhong, R., Fang, X., Wang, X., Huang, Y., Gong, H., & Yan, M. (2025). Microplastics Enhance the Toxic Effects of Tetracycline on the Early Development of Zebrafish in a Dose-Dependent Manner. Fishes, 10(4), 150. https://doi.org/10.3390/fishes10040150