Feeding Habits of Mene maculata (Teleostei: Menidae) in the Southwestern Waters of Taiwan, Western Pacific Ocean
Abstract
1. Introduction
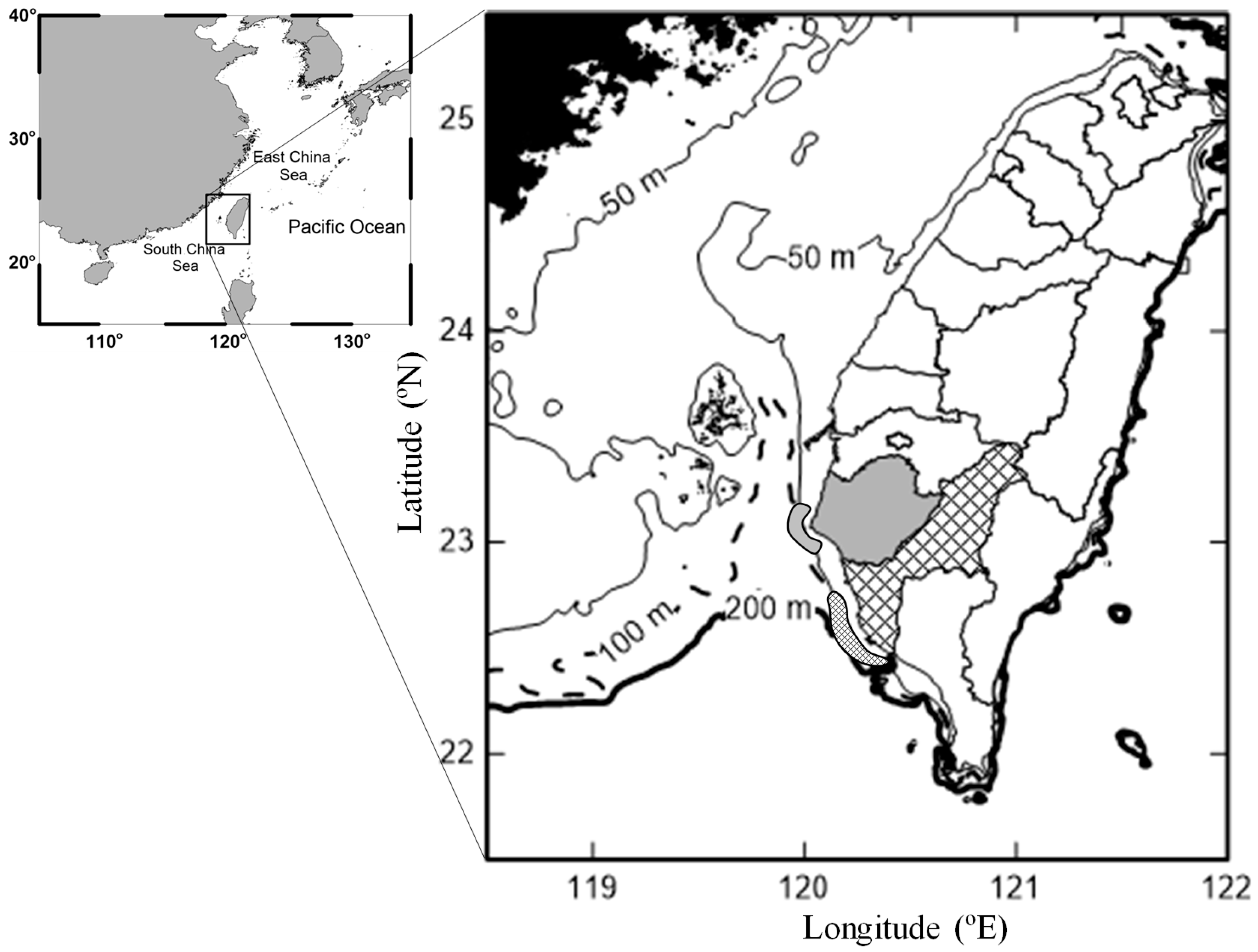
2. Materials and Methods
3. Results
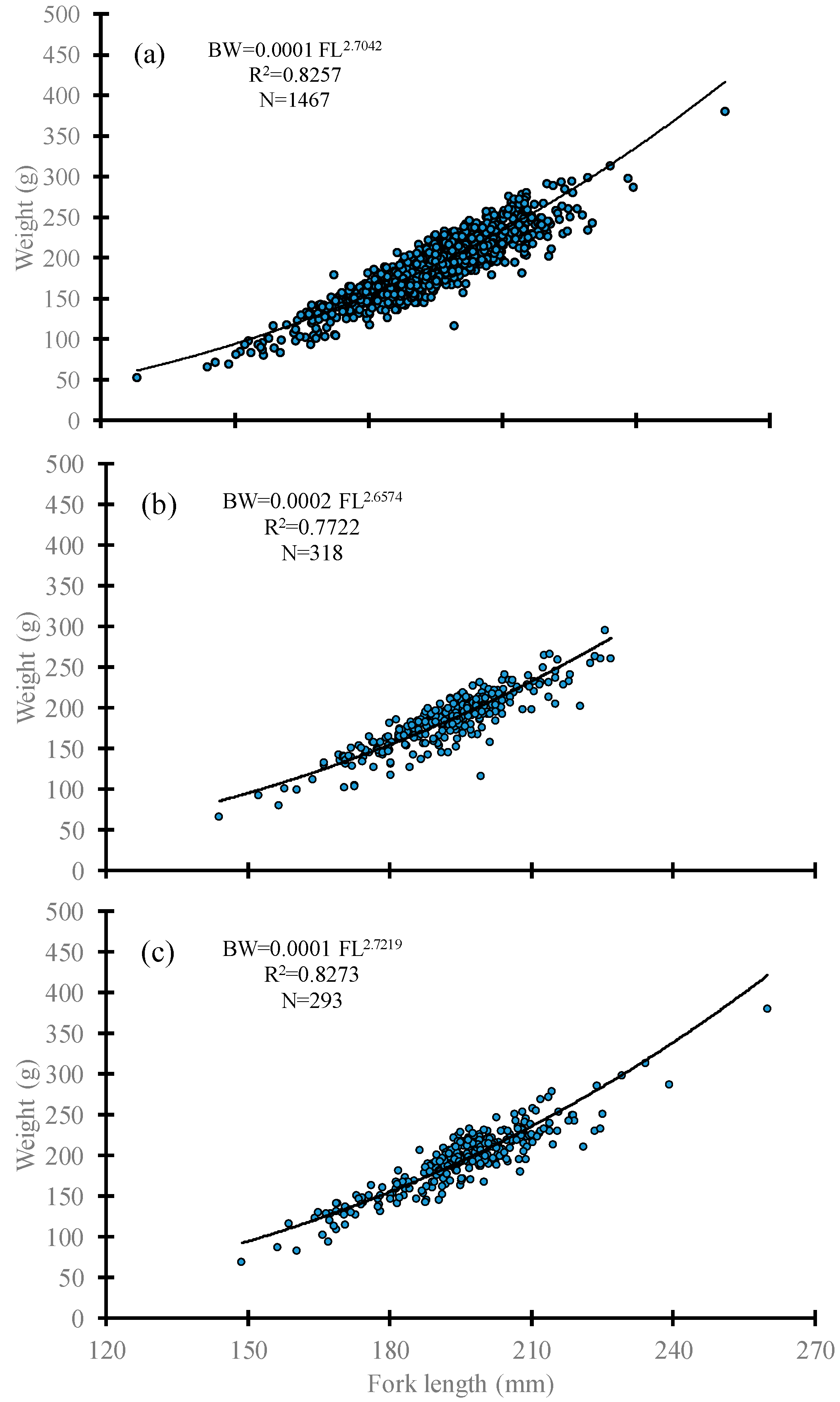
3.1. Specimen Characteristics
3.2. Stomach Fullness and Digestion State
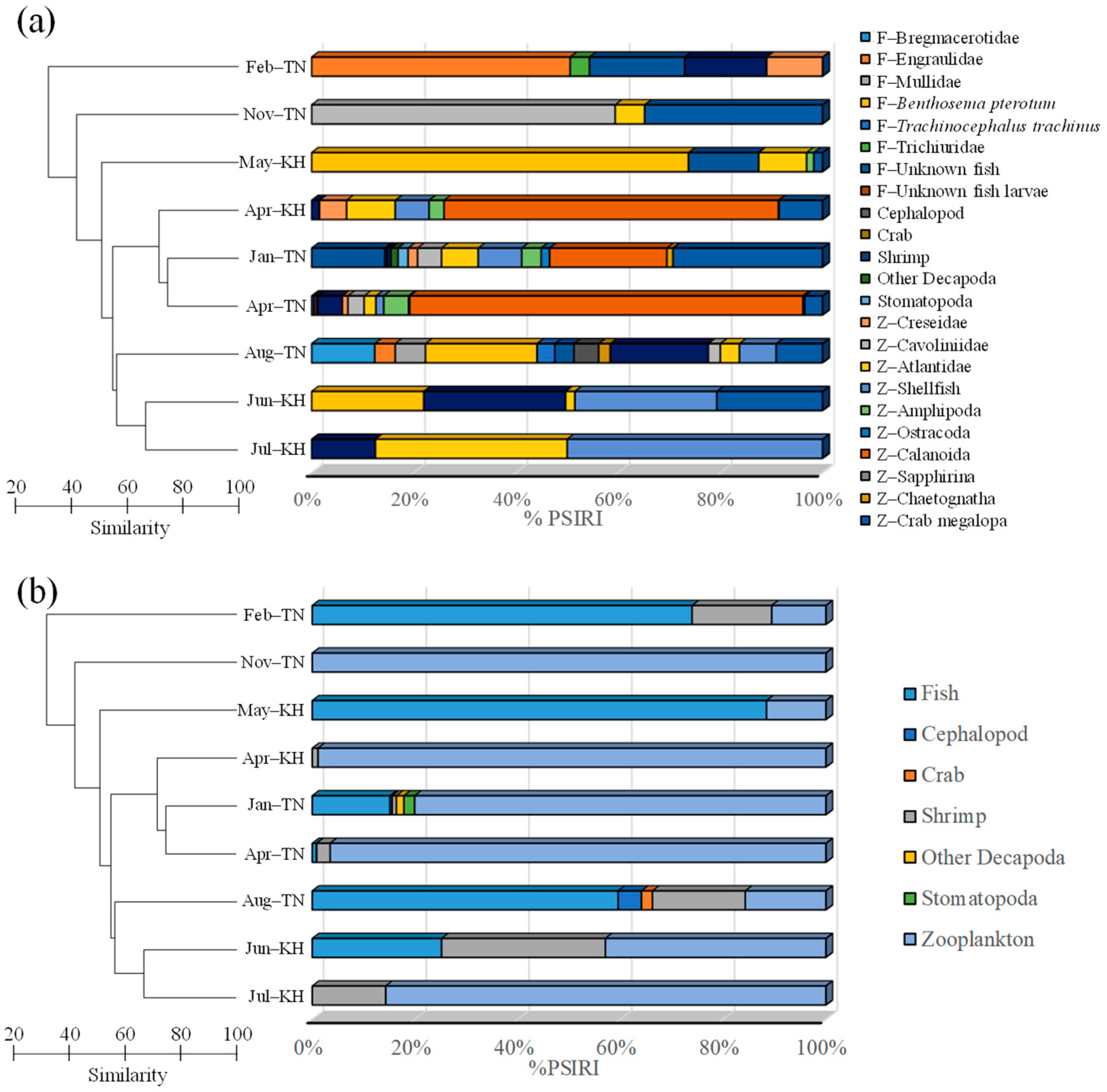
3.3. Dietary Variations Across Sampling Months
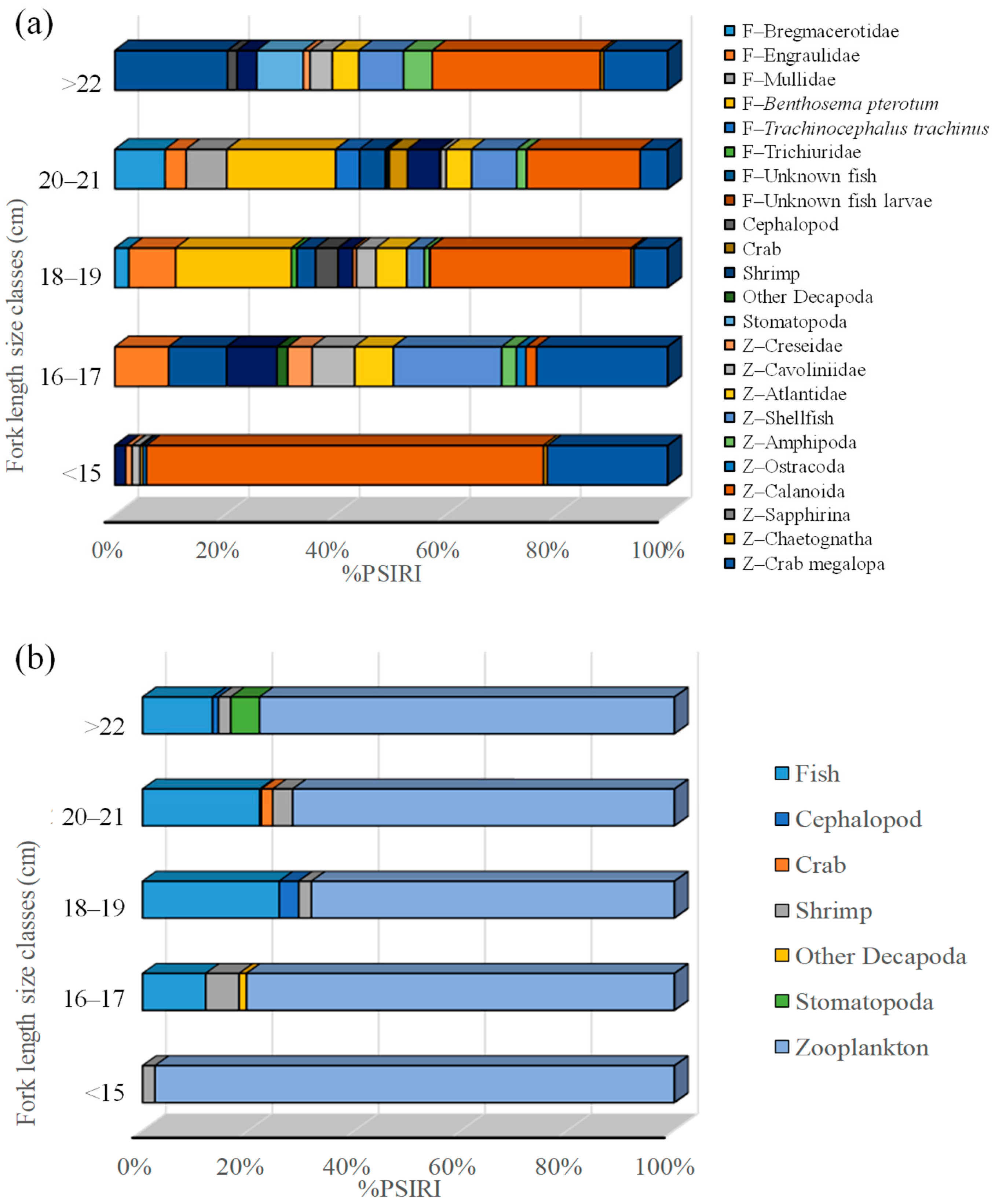
3.4. Dietary Variations Across Size Classes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Groups | G1—Summer | G2—Spring | G3—Late Spring | G4—Autumn | G5—Winter | TOTAL | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Date/City | Jul-KH | Jun-KH | Aug-TN | Apr-TN | Jan-TN | Apr-KH | May-KH | Nov-TN | Feb-TN | |||||||||||||||||||||||||||||||
| Prey Items | %N | %W | %FO | %PSIRI | %N | %W | %FO | %PSIRI | %N | %W | %FO | %PSIRI | %N | %W | %FO | %PSIRI | %N | %W | %FO | %PSIRI | %N | %W | %FO | %PSIRI | %N | %W | %FO | %PSIRI | %N | %W | %FO | %PSIRI | %N | %W | %FO | %PSIRI | %N | %W | %FO | %PSIRI |
| FISH (TOTAL) | 50 | 61.98 | 20 | 25.17 | 35.71 | 86.07 | 63.64 | 59.55 | 1.03 | 2.84 | 12.5 | 0.79 | 3.32 | 36.6 | 45.45 | 15.11 | 77.78 | 97.52 | 87.5 | 88.45 | 82.61 | 87.41 | 80 | 73.95 | 4.02 | 58.35 | 42.42 | 20.03 | ||||||||||||
| Benthosema pterotum | 50 | 61.98 | 20 | 21.93 | 10.71 | 39.03 | 27.27 | 21.82 | 72.21 | 97.39 | 75 | 73.75 | 1.19 | 34.66 | 13.13 | 19.23 | ||||||||||||||||||||||||
| Trichiuridae | 4.35 | 10.93 | 10 | 3.79 | 0.05 | 0.51 | 1.01 | 0.7 | ||||||||||||||||||||||||||||||||
| Engraulidae | 5.36 | 8.43 | 13.64 | 4 | 56.52 | 55.07 | 60 | 50.59 | 0.76 | 5.1 | 9.09 | 7.32 | ||||||||||||||||||||||||||||
| Bregmacerotidae | 8.93 | 22.55 | 22.73 | 12.34 | 0.24 | 6.84 | 5.05 | 3.57 | ||||||||||||||||||||||||||||||||
| Trachinocephalus trachinus | 3.57 | 8.9 | 9.09 | 3.49 | 0.09 | 2.7 | 2.02 | 1.01 | ||||||||||||||||||||||||||||||||
| Mullidae | 3.57 | 6.28 | 9.09 | 5.94 | 0.09 | 1.9 | 2.02 | 1.72 | ||||||||||||||||||||||||||||||||
| Unknown fish | 3.57 | 0.89 | 9.09 | 3.76 | 0.24 | 1.09 | 12.5 | 0.49 | 3.32 | 36.6 | 45.45 | 14.32 | 5.56 | 0.14 | 12.5 | 13.74 | 21.74 | 21.41 | 30 | 18.6 | 1.1 | 6.25 | 12.12 | 4.49 | ||||||||||||||||
| Unknown fish larvae | 0.79 | 1.76 | 12.5 | 0.55 | 0.48 | 0.38 | 1.01 | 0.09 | ||||||||||||||||||||||||||||||||
| CEPHALOPODS (TOTAL) | 7.14 | 1.8 | 13.64 | 4.55 | 0.24 | 0.13 | 12.5 | 0.06 | 0.55 | 0.77 | 9.09 | 0.43 | 0.43 | 0.67 | 5.05 | 0.15 | ||||||||||||||||||||||||
| Cephalopod | 7.14 | 1.8 | 13.64 | 4.83 | 0.24 | 0.13 | 12.5 | 0.1 | 0.55 | 0.77 | 9.09 | 0.41 | 0.43 | 0.67 | 5.05 | 0.25 | ||||||||||||||||||||||||
| CRABS (TOTAL) | 5.36 | 0.36 | 9.09 | 2.13 | 0.14 | 0.11 | 2.02 | 0.39 | ||||||||||||||||||||||||||||||||
| Crabs | 5.36 | 0.36 | 9.09 | 2.26 | 0.14 | 0.11 | 2.02 | 0.65 | ||||||||||||||||||||||||||||||||
| SHRIMP (TOTAL) | 28.57 | 82.99 | 12.5 | 14.29 | 8.33 | 20.66 | 20 | 31.89 | 17.86 | 10.62 | 40.91 | 18.08 | 1.43 | 6.43 | 62.5 | 2.62 | 1.39 | 0.76 | 45.45 | 0.83 | 0.59 | 0.09 | 4.55 | 1.11 | 13.04 | 12.41 | 30 | 15.5 | 2.05 | 5.9 | 25.25 | 2.42 | ||||||||
| Shrimp | 28.57 | 82.99 | 12.5 | 12.5 | 8.33 | 20.66 | 20 | 27.78 | 17.86 | 10.62 | 40.91 | 19.19 | 1.43 | 6.43 | 62.5 | 4.86 | 1.39 | 0.76 | 45.45 | 0.79 | 0.59 | 0.09 | 4.55 | 1.53 | 13.04 | 12.41 | 30 | 16.08 | 2.05 | 5.9 | 25.25 | 4 | ||||||||
| OTHER DECAPODS (TOTAL) | 0.55 | 0.66 | 9.09 | 1.46 | 0.09 | 0.08 | 1.01 | 0.18 | ||||||||||||||||||||||||||||||||
| Other decapods | 0.55 | 0.66 | 9.09 | 1.38 | 0.09 | 0.08 | 1.01 | 0.29 | ||||||||||||||||||||||||||||||||
| STOMATOPODS (TOTAL) | 1.66 | 9.05 | 9.09 | 2.1 | 0.29 | 1.17 | 1.01 | 0.25 | ||||||||||||||||||||||||||||||||
| stomatopods | 1.66 | 9.05 | 9.09 | 1.99 | 0.29 | 1.17 | 1.01 | 0.42 | ||||||||||||||||||||||||||||||||
| ZOOPLANKTON (TOTAL) | 71.43 | 17.01 | 87.5 | 85.71 | 41.67 | 17.36 | 80 | 42.94 | 33.93 | 1.15 | 40.91 | 15.69 | 97.3 | 90.6 | 100 | 96.53 | 92.53 | 52.16 | 100 | 80.07 | 99.41 | 99.91 | 100 | 98.89 | 22.22 | 2.48 | 37.5 | 11.55 | 100 | 100 | 100 | 100 | 4.35 | 0.18 | 10 | 10.55 | 92.98 | 33.72 | 82.83 | 76.58 |
| Creseidae | 1.83 | 1.47 | 25 | 1.09 | 3.05 | 0.59 | 36.36 | 1.82 | 0.3 | 0.33 | 4.55 | 5.31 | 4.35 | 0.18 | 10 | 10.94 | 1.72 | 0.42 | 8.08 | 0.46 | ||||||||||||||||||||
| Cavoliniidae | 3.57 | 0.03 | 9.09 | 2.35 | 0.87 | 3.31 | 62.5 | 3.18 | 6.65 | 5.63 | 72.73 | 4.71 | 60 | 61.29 | 80 | 59.39 | 2.2 | 1.85 | 19.19 | 1.9 | ||||||||||||||||||||
| Atlantidae | 28.57 | 9.96 | 37.5 | 37.5 | 8.33 | 0.42 | 20 | 1.81 | 14.29 | 0.21 | 13.64 | 3.73 | 0.56 | 1.78 | 37.5 | 2.31 | 9.97 | 3.33 | 81.82 | 7.17 | 3.85 | 3.82 | 36.36 | 9.52 | 11.11 | 0.12 | 25 | 9.38 | 6.67 | 6.45 | 20 | 5.77 | 3.44 | 1.18 | 30.3 | 3.79 | ||||
| Shellfish | 42.86 | 7.05 | 50 | 50 | 8.33 | 1.65 | 20 | 27.78 | 8.93 | 0.2 | 13.64 | 7.21 | 0.32 | 1.39 | 37.5 | 1.52 | 9.97 | 5.83 | 72.73 | 8.53 | 0.88 | 12.8 | 13.64 | 6.61 | 2.63 | 1.88 | 22.22 | 3.63 | ||||||||||||
| Amphipoda | 4.93 | 7.74 | 37.5 | 4.87 | 4.99 | 1.68 | 72.73 | 3.8 | 4.73 | 3.27 | 18.18 | 2.93 | 5.56 | 0.19 | 12.5 | 1.41 | 4.63 | 2.1 | 16.16 | 1.93 | ||||||||||||||||||||
| Ostracoda | 0.32 | 0.08 | 25 | 0.22 | 1.66 | 0.67 | 36.36 | 1.64 | 0.48 | 0.1 | 6.06 | 0.14 | ||||||||||||||||||||||||||||
| Calanoida | 86.8 | 70.03 | 50 | 76.95 | 31.02 | 7.84 | 54.55 | 22.96 | 84.02 | 63.18 | 59.09 | 65.47 | 71.03 | 19.53 | 23.23 | 36.79 | ||||||||||||||||||||||||
| Sapphirina | 0.3 | 0.09 | 4.55 | 0.08 | 0.05 | 0.01 | 1.01 | 0.02 | ||||||||||||||||||||||||||||||||
| Chaetognatha | 0.32 | 0.63 | 12.5 | 0.36 | 2.23 | 0.73 | 27.27 | 1.18 | 0.57 | 0.23 | 4.04 | 0.19 | ||||||||||||||||||||||||||||
| Crab megalopa | 250.1 | 15.29 | 60 | 20.7 | 7.14 | 0.7 | 9.09 | 9.08 | 1.35 | 4.16 | 62.5 | 3.5 | 22.99 | 25.86 | 90.91 | 29.3 | 5.33 | 16.42 | 40.91 | 8.55 | 5.56 | 2.16 | 12.5 | 1.72 | 33.33 | 32.26 | 80 | 34.84 | 6.25 | 6.43 | 34.34 | 7.41 | ||||||||
| Sex | Male | Female | ||||||
|---|---|---|---|---|---|---|---|---|
| Prey Items | %N | %W | %FO | %PSIRI | %N | %W | %FO | %PSIRI |
| FISH (TOTAL) | 4.34 | 56.25 | 33.93 | 18.94 | 3.79 | 59.85 | 44.19 | 21.52 |
| Benthosema pterotum | 0.84 | 18.24 | 10.71 | 12.16 | 1.42 | 46.55 | 16.28 | 22.89 |
| Trichiuridae | 0.08 | 0.87 | 2.33 | 1.36 | ||||
| Engraulidae | 1.33 | 6.47 | 10.71 | 7.84 | 0.4 | 4.11 | 6.98 | 7.72 |
| Bregmacerotidae | 0.48 | 16.27 | 7.14 | 5.98 | 0.08 | 0.01 | 2.33 | 0.32 |
| Trachinocephalus trachinus | 0.24 | 6.42 | 3.57 | 1.9 | ||||
| Mullidae | 0.24 | 4.53 | 3.57 | 3.23 | ||||
| Unknown fish | 1.2 | 4.31 | 10.71 | 2.22 | 1.03 | 7.66 | 13.95 | 7.88 |
| Unknown fish larvae | 0.79 | 0.65 | 4.65 | 0.18 | ||||
| CEPHALOPODS (TOTAL) | 0.48 | 1.3 | 5.36 | 1.8 | 0.4 | 0.22 | 4.65 | 0.06 |
| Cephalopod | 0.48 | 1.3 | 5.36 | 2.63 | 0.4 | 0.22 | 4.65 | 0.07 |
| CRABS (TOTAL) | 0.12 | 0.25 | 1.79 | 0.32 | 0.16 | 0.01 | 2.33 | 2.08 |
| Crabs | 0.12 | 0.25 | 1.79 | 0.47 | 0.16 | 0.01 | 2.33 | 2.62 |
| SHRIMP (TOTAL) | 3.86 | 8.4 | 28.57 | 4.54 | 0.87 | 4.1 | 23.26 | 1.69 |
| Shrimp | 3.86 | 8.4 | 28.57 | 6.63 | 0.87 | 4.1 | 23.26 | 2.13 |
| OTHER DECAPODS (TOTAL) | 0.16 | 0.15 | 2.33 | 0.45 | ||||
| Other decapods | 0.16 | 0.15 | 2.33 | 0.57 | ||||
| STOMATOPODS (TOTAL) | 0.47 | 2.02 | 2.33 | 0.65 | ||||
| Stomatopods | 0.47 | 2.02 | 2.33 | 0.82 | ||||
| ZOOPLANKTON (TOTAL) | 91.2 | 33.8 | 66.07 | 74.4 | 94.15 | 33.65 | 76.74 | 73.55 |
| Creseidae | 1.93 | 0.68 | 7.14 | 0.58 | 1.58 | 0.23 | 9.3 | 0.33 |
| Cavoliniidae | 2.77 | 2.03 | 16.07 | 2.05 | 1.82 | 1.71 | 27.91 | 1.71 |
| Atlantidae | 3.86 | 1.76 | 25 | 2.49 | 3.16 | 0.77 | 37.21 | 5.92 |
| Shellfish | 4.58 | 2.07 | 25 | 3.58 | 1.34 | 1.74 | 18.6 | 3.87 |
| Amphipoda | 4.7 | 2.47 | 16.07 | 2.41 | 4.58 | 1.83 | 18.6 | 1.55 |
| Ostracoda | 0.24 | 0.06 | 3.57 | 0.34 | 0.63 | 0.13 | 9.3 | 0.15 |
| Calanoida | 64.22 | 17.34 | 23.21 | 35.46 | 75.49 | 21.11 | 25.58 | 34.01 |
| Sapphirina | 0.12 | 0.01 | 1.79 | 0.05 | ||||
| Chaetognatha | 0.96 | 0.41 | 3.57 | 0.16 | 0.32 | 0.1 | 4.65 | 0.22 |
| Crab megalopa | 7.83 | 6.98 | 32.14 | 9.82 | 5.22 | 6.03 | 39.53 | 5.68 |
| Size Classes | <15 cm | 16–17 cm | 18–19 cm | 20–21 cm | >22 cm | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prey Items | %N | %W | %FO | %PSIRI | %N | %W | %FO | %PSIRI | %N | %W | %FO | %PSIRI | %N | %W | %FO | %PSIRI | %N | %W | %FO | %PSIRI |
| FISH (TOTAL) | 6.6 | 31.8 | 30.77 | 11.85 | 5.46 | 71 | 37.74 | 25.71 | 3.65 | 56.58 | 46.15 | 22.06 | 2.68 | 29.57 | 75 | 13.16 | ||||
| Benthosema pterotum | 2.84 | 54.15 | 16.98 | 20.94 | 0.58 | 25.18 | 15.38 | 19.71 | ||||||||||||
| Trichiuridae | 0.16 | 1.03 | 1.89 | 1.02 | ||||||||||||||||
| Engraulidae | 2.83 | 11.67 | 7.69 | 9.73 | 1.35 | 7.63 | 11.32 | 8.47 | 0.44 | 3.14 | 7.69 | 3.8 | ||||||||
| Bregmacerotidae | 0.28 | 6.08 | 3.77 | 2.52 | 0.44 | 11.97 | 11.54 | 9.08 | ||||||||||||
| Trachinocephalus trachinus | 0.29 | 8.38 | 7.69 | 4.33 | ||||||||||||||||
| Mullidae | 0.29 | 5.91 | 7.69 | 7.36 | ||||||||||||||||
| Unknown fish | 3.77 | 20.13 | 23.08 | 10.49 | 0.69 | 2.1 | 7.55 | 3.33 | 0.29 | 0.84 | 7.69 | 4.66 | 2.68 | 29.57 | 75 | 20.37 | ||||
| Unknown fish larvae | 0.16 | 0.01 | 1.89 | 0.01 | 1.32 | 1.16 | 3.85 | 0.26 | ||||||||||||
| CEPHALOPODS (TOTAL) | 0.28 | 0.02 | 3.77 | 3.65 | 0.73 | 1.75 | 7.69 | 0.28 | 0.45 | 0.68 | 25 | 1.11 | ||||||||
| Cephalopod | 0.28 | 0.02 | 3.77 | 4.08 | 0.73 | 1.75 | 7.69 | 0.43 | 0.45 | 0.68 | 25 | 1.72 | ||||||||
| CRABS (TOTAL) | 0.29 | 0.34 | 7.69 | 2.13 | ||||||||||||||||
| Crabs | 0.29 | 0.34 | 7.69 | 3.32 | ||||||||||||||||
| SHRIMP (TOTAL) | 1.72 | 2.9 | 66.67 | 2.35 | 3.77 | 10.58 | 30.77 | 6.32 | 1.76 | 3.86 | 18.87 | 2.4 | 1.6 | 9.23 | 30.77 | 3.75 | 2.91 | 4.71 | 50 | 2.31 |
| Shrimp | 1.72 | 2.9 | 66.67 | 1.94 | 3.77 | 10.58 | 30.77 | 9.1 | 1.76 | 3.86 | 18.87 | 2.67 | 1.6 | 9.23 | 30.77 | 5.84 | 2.91 | 4.71 | 50 | 3.58 |
| OTHER DECAPODS (TOTAL) | 1.89 | 2.85 | 7.69 | 1.37 | ||||||||||||||||
| Other decapods | 1.89 | 2.85 | 7.69 | 1.97 | ||||||||||||||||
| STOMATOPODS (TOTAL) | 1.34 | 7.95 | 25 | 5.42 | ||||||||||||||||
| Stomatopods | 1.34 | 7.95 | 25 | 8.4 | ||||||||||||||||
| ZOOPLANKTON (TOTAL) | 98.28 | 97.1 | 66.67 | 97.65 | 87.74 | 54.77 | 69.23 | 80.46 | 92.5 | 25.12 | 69.81 | 68.24 | 93.73 | 32.1 | 69.23 | 71.78 | 92.62 | 57.09 | 100 | 78 |
| Creseidae | 1.73 | 1.08 | 66.67 | 1.16 | 4.72 | 2.13 | 15.38 | 4.39 | 0.81 | 0.06 | 3.77 | 0.68 | 2.04 | 0.2 | 3.85 | 0.14 | 2.01 | 1.71 | 25 | 1.22 |
| Cavoliniidae | 0.86 | 6 | 33.33 | 1.45 | 10.38 | 9.48 | 30.77 | 7.7 | 1.89 | 1.47 | 13.21 | 3.52 | 1.46 | 1.14 | 19.23 | 1.02 | 2.24 | 2.78 | 100 | 4.05 |
| Atlantidae | 0.86 | 1.48 | 33.33 | 0.49 | 14.15 | 4.15 | 30.77 | 6.99 | 4.05 | 1 | 30.19 | 5.56 | 1.75 | 0.39 | 23.08 | 4.58 | 3.13 | 2.88 | 75 | 4.79 |
| Shellfish | 14.15 | 3.2 | 38.46 | 19.6 | 1.62 | 1.79 | 16.98 | 3.18 | 0.87 | 0.47 | 15.38 | 8.16 | 4.93 | 5.1 | 100 | 8.09 | ||||
| Amphipoda | 6.6 | 2.46 | 23.08 | 2.69 | 3.38 | 0.62 | 16.98 | 0.96 | 6.56 | 3.66 | 7.69 | 1.74 | 4.47 | 3.71 | 75 | 5.19 | ||||
| Ostracoda | 0.86 | 2.26 | 33.33 | 0.66 | 3.77 | 1.27 | 15.38 | 1.71 | 0.16 | 0.05 | 1.89 | 0.09 | 0.58 | 0.06 | 7.69 | 0.08 | ||||
| Calanoida | 91.38 | 82.94 | 66.67 | 71.85 | 3.77 | 1.71 | 15.38 | 1.92 | 72.57 | 13.23 | 30.19 | 36.34 | 77.55 | 23.23 | 11.54 | 20.53 | 69.13 | 31.48 | 25 | 30.42 |
| Sapphirina | 0.16 | 0.01 | 1.89 | 0.04 | ||||||||||||||||
| Chaetognatha | 1.73 | 1.62 | 33.33 | 0.7 | 0.81 | 0.16 | 3.77 | 0.5 | 0.89 | 0.92 | 25 | 0.61 | ||||||||
| Crab megalopa | 0.86 | 1.72 | 33.33 | 21.75 | 30.2 | 30.37 | 30.77 | 23.71 | 7.03 | 6.73 | 32.08 | 6.09 | 2.92 | 2.95 | 34.62 | 4.96 | 5.82 | 8.51 | 100 | 11.56 |
References
- Du, D.S.; Feng, B.; Hou, G.; Lu, H.S.; Yan, Y.R. Age and growth of moonfish, Mene maculata from mouth of the Beibu Gulf, South China Sea. J. Fish. China 2012, 36, 576–583. [Google Scholar] [CrossRef]
- Hwang, S.Y. Fisheries and population study of Taiwan moon fish, Mene maculate. Bull. Taiwan Fish. Res. Inst. 1981, 33, 281–295. [Google Scholar]
- Hwang, S.Y.; Chen, C.T.; Liu, K.M. Age and growth of the moon fish, Mene maculata, before and after heavy exploitation in southwestern Taiwan waters. J. Fish. Soc. Taiwan 2002, 29, 299–311. [Google Scholar] [CrossRef]
- Hwang, S.Y. Study on maturity and fecundity of moonfish, Mene maculata, in adjacent waters of Taiwan. Bull. Taiwan Fish. Res. Inst. 1984, 37, 93–100. [Google Scholar]
- Nguyen, M.L.; Jiang, X.N.; Wang, Y.C.; He, J.S.; Liao, C.H.; Lee, M.A. Preliminary study on the fishing activities of the moonfish Mene maculate fishery in the waters of southwestern Taiwan. J. Fish. Soc. Taiwan 2021, 48, 55–66. [Google Scholar] [CrossRef]
- Ray, A.; Mondal, S.; Osuka, K.E.; Sihombing, R.I.; Lee, M.A.; Wang, Y.C.; He, J.S. Impact of climate variability on moonfish (Mene maculata) catch rate in the waters off southwestern Taiwan. Fish. Oceanogr. 2024, 33, e12667. [Google Scholar] [CrossRef]
- Flemer, D.A.; Woolcott, W.S. Food habits and distribution of the fishes of Tuckahoe Creek, Virginia, with special emphasis on the bluegill, Lepomis m. macrochirus Rafinesque. Chesap. Sci. 1966, 7, 75–89. [Google Scholar] [CrossRef]
- González-Iglesias, M.D.L.C.; Paz, X.; Román-Marcote, E.; Hermida-Doval, M. Feeding habits of fish species distributed on the Grand Bank (NAFO Divisions 3NO 2002–2005). In NAFO Scientific Council Research (SCR) Documents, NAFO SCR Doc. 06/31 Serial N° N5755.; Centro Oceanográfico de Vigo: Vigo, Spain, 2006; pp. 1–22. [Google Scholar]
- Tanaka, H.; Aoki, I.; Ohshimo, S. Feeding habits and gill raker morphology of three planktivorous pelagic fish species off the coast of northern and western Kyushu in summer. J. Fish Biol. 2006, 68, 1041–1061. [Google Scholar] [CrossRef]
- Eriksen, E.; Skjoldal, H.R.; Dolgov, A.V.; Strand, E.; Keulder-Stenevik, F.; Prokopchuk, I.P.; Prokhorova, T.A.; Prozorkevich, D.; Benzik, A.N. Diet and trophic structure of fishes in the Barents Sea: Seasonal and spatial variations. Prog. Oceanogr. 2021, 197, 102663. [Google Scholar] [CrossRef]
- Hyslop, E. Stomach contents analysis—A review of methods and their application. J. Fish Biol. 1980, 17, 411–429. [Google Scholar] [CrossRef]
- Amundsen, P.A.; Sánchez-Hernández, J. Feeding studies take guts–critical review and recommendations of methods for stomach contents analysis in fish. J. Fish Biol. 2019, 95, 1364–1373. [Google Scholar] [CrossRef] [PubMed]
- da Silveira, E.L.; Semmar, N.; Cartes, J.E.; Tuset, V.M.; Lombarte, A.; Ballester, E.L.C.; Vaz-dos-Santos, A.M. Methods for trophic ecology assessment in fishes: A critical review of stomach analyses. Rev. Fish. Sci. Aquacult. 2020, 28, 71–106. [Google Scholar] [CrossRef]
- Nakabo, T. Fishes of Japan with Pictorial Keys to the Species, 3rd ed.; Tokai University Press: Tokyo, Japan, 2013; p. 877. [Google Scholar]
- Le Cren, E.D. The length-weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). J. Anim. Ecol. 1951, 20, 201–219. [Google Scholar] [CrossRef]
- Edwards, A.L. An Introduction to Linear Regression and Correlation; W. H. Freeman and Company: New York, NY, USA, 1976; p. 213. [Google Scholar]
- Brown, S.C.; Bizzarro, J.J.; Cailliet, G.M.; Ebert, D.A. Breaking with tradition: Redefining measures for diet description with a case study of the Aleutian skate Bathyraja aleutica (Gilbert 1896). Environ. Biol. Fishes 2012, 95, 3–20. [Google Scholar] [CrossRef]
- Sneha, D. Studies on Biological Aspects of the Moon fish, Mene maculata (Bloch and Schneider 1801) off Mangalore Coast. Ph.D. Thesis, Animal and Fisheries Sciences University, Bidar, Karnataka, India, 2019. [Google Scholar]
- Gani, M.J.A.; Zairion, Z.; Boer, M. Reproductive Biology Status Reproductive Biology Status of Moonfish (Mene maculata) in Palabuhanratu Bay during West Monsoon. J. Trop. Fish. Manag. 2024, 8, 9–16. [Google Scholar] [CrossRef]
- Gumanao, G.S.; Saceda-Cardoza, M.M.; Mueller, B.; Bos, A.R. Length–weight and length–length relationships of 139 Indo-Pacific fish species (Teleostei) from the Davao Gulf, Philippines. J. Appl. Ichthyol. 2016, 32, 377–385. [Google Scholar] [CrossRef]
- Viswambharan, D.; Rohit, P.; Joshi, K.K.; Thomas, S. Length-weight relationship and growth parameters of moonfish Mene maculata (Bloch and Schneider 1801) from Karnataka Coast, India. Indian J. Fish. 2018, 65, 105–109. [Google Scholar] [CrossRef]
- Pauly, D. Fish Population Dynamics in Tropical Waters: A Manual for Use with Programmable Calculators; International Center for Living Aquatic Resources Management (I.C.L.A.R.M.): Makati, Manila, Philippines, 1984; p. 325. [Google Scholar]
- Dadzie, B.S.; Abou-Seedo, F.; Al-Qattan, E. The food and feeding habits of the silver pomfret, Pampus argenteus (Euphrasen), in Kuwait waters. J. Appl. Ichthyol. 2000, 16, 61–67. [Google Scholar] [CrossRef]
- Garrido, S.; Murta, A.G.; Moreira, A.; Ferreira, M.J.; Angélico, M.M. Horse mackerel (Trachurus trachurus) stomach fullness off Portugal: Index calibration and spatio-temporal variations in feeding intensity. ICES J. Mar. Sci. 2008, 65, 1662–1669. [Google Scholar] [CrossRef]
- Valinassab, T.; Jalali, S.; Hafezieh, M.; Zarshenas, G.R. Evaluation of some feeding indices of Pomadasys kaakan in the Northern Persian Gulf. Iran. J. Fish. Sci. 2011, 10, 497–504. [Google Scholar]
- Mohammed, K.; Rohit, P.; Abdussamad, E.M.; Abdul Azeez, P.; Vase, V.K.; Bharadiya Sangita, A. Reproductive biology, diet and feeding pattern of longtail tuna Thunnus tonggol (Bleeker 1851) in the north-eastern Arabian Sea off Gujarat, India. Indian J. Fish. 2018, 65, 16–25. [Google Scholar] [CrossRef]
- Mammel, M.; Lee, M.A.; Wang, Y.C.; Ghosh, A.; Chen, Y.K.; Matilda, M.V.; Navus, S.G. Unraveling the feeding strategies of the greater amberjack: Insights into size-dependent dietary patterns and environmental influences in Taiwanese waters. Fish. Sci. 2024, 90, 931–952. [Google Scholar] [CrossRef]
- Ordiano-Flores, A.; Méndez Gudiño, K.E.; Calle-Morán, M.D.; Hernández-Téllez, A.R.; Cajas-Flores, J.M. Feeding ecology of the Patagonian toothfish, Dissostichus eleginoides, in Ecuadorian waters. Mar. Ecol. 2024, 45, e12811. [Google Scholar] [CrossRef]
- Viswambharan, D. Biology and Stock Delineation of Razor Moonfish, Mene maculata Along the Konkan Malabar Coast of India. Ph.D. Thesis, Central Instituteof Fisheries Education, Mumbai, India, 2019. [Google Scholar]
- Zahid, A.; Rahardjo, M.F.; Syafei, L.S.; Susilowati, R. Ekologi Trofik Komunitas Ikan Di Perairan Segara Menyan Subang, Jawa Barat. ILMU KELAUTAN Indones. J. Mar. Sci. 2015, 20, 170–186. [Google Scholar] [CrossRef]
- Koepper, S.; Nuryati, S.; Palm, H.W.; Wild, C.; Yulianto, I.; Kleinertz, S. Metazoan endoparasite fauna and feeding ecology of commercial fishes from Java, Indonesia. Parasitol. Res. 2022, 121, 551–562. [Google Scholar] [CrossRef]
- Wootton, R.J.; Robert, J.W. Feeding. In Ecology of Teleost Fishes; Springer: Dordrecht, The Netherlands, 1990; pp. 32–72. [Google Scholar] [CrossRef]
- St John, J. Ontogenetic changes in the diet of the coral reef grouper Plectropomus leopardus (Serranidae): Patterns in taxa, size and habitat of prey. Mar. Ecol. Prog. Ser. 1999, 180, 233–246. [Google Scholar] [CrossRef]
- Graham, B.S.; Grubbs, D.; Holland, K.; Popp, B.N. A rapid ontogenetic shift in the diet of juvenile yellowfin tuna from Hawaii. Mar. Biol. 2006, 150, 647–658. [Google Scholar] [CrossRef]
- Choi, S.H.; Suk, H.Y. The mechanisms leading to ontogenetic diet shift in a microcanivore, Pterogobius elapoides (Gobiidae). Anim. Cells Syst. 2012, 16, 343–349. [Google Scholar] [CrossRef]
- Costalago, D.; Navarro, J.; Álvarez-Calleja, I.; Palomera, I. Ontogenetic and seasonal changes in the feeding habits and trophic levels of two small pelagic fish species. Mar. Ecol. Prog. Ser. 2012, 460, 169–181. [Google Scholar] [CrossRef]
- Sánchez-Hernández, J.; Nunn, A.D.; Adams, C.E.; Amundsen, P.A. Causes and consequences of ontogenetic dietary shifts: A global synthesis using fish models. Biol. Rev. 2019, 94, 539–554. [Google Scholar] [CrossRef]
- Dolgov, A.V. The role of capelin (Mallotus villosus) in the foodweb of the Barents Sea. ICES J. Mar. Sci. 2002, 59, 1034–1045. [Google Scholar] [CrossRef]
- Troccoli, G.H.; Aguilar, E.; Martínez, P.A.; Belleggia, M. The diet of the Patagonian toothfish Dissostichus eleginoides, a deep-sea top predator off Southwest Atlantic Ocean. Polar Biol. 2020, 43, 1595–1604. [Google Scholar] [CrossRef]
- Mohamed, K.S.; Zacharia, P.U.; Muthiah, C.; Abdurahiman, K.P.; Nayak, T.H. Trophic modelling of the Arabian Sea ecosystem off Karnataka and simulation of fishery yields. Bull. Cent. Mar. Fish. Res. Inst. 2008, 51, 140. [Google Scholar]
- Ghosh, S.; Vase, V.K. Reproductive and Feeding Biology of Goldspotted Grenadier Anchovy, Coilia dussumieri Off Saurashtra Coast, Northern Arabian Sea. Thalass. Int. J. Mar. Sci. 2023, 39, 157–168. [Google Scholar] [CrossRef]
- Anulekshmi, C.; Nakhawa, A.D.; Azeez, P.A.; Akhilesh, K.V.; Bhendekar, S.; Khandagle, P.A.; Zacharia, P.U.; Shubhadeep, G.; Rohit, P. Biology of the Bombayduck Harpadon nehereus (Hamilton 1822) from the north-eastern Arabian Sea, India: Biology of Bombay duck in north eastern Arabian Sea. Indian J. Fish. 2024, 71, 70–81. [Google Scholar] [CrossRef]
- Murillo, C.; Oyarzún, C.; Fernández, I. Variación latitudinal y estacional en la dieta de Dissostichus eleginoides Smitt 1898 (Perciformes: Nototheniidae) en ambientes profundos de la costa centro-sur de Chile. Gayana 2008, 72, 94–101. [Google Scholar] [CrossRef]
- Suca, J.J.; Pringle, J.W.; Knorek, Z.R.; Hamilton, S.L.; Richardson, D.E.; Llopiz, J.K. Feeding dynamics of Northwest Atlantic small pelagic fishes. Prog. Oceanogr. 2018, 165, 52–62. [Google Scholar] [CrossRef]
- Cohen, D.L.; Beckley, L.E. Diet and prey selectivity of the mesopelagic lanternfish Myctophum asperum from the Perth Canyon, Western Australia. Jpn. J. Ichthyol. 2021, 68, 294–302. [Google Scholar] [CrossRef]
- Chiou, W.D.; Wu, C.C.; Cheng, L.Z. Spatiotemporal distribution of sergestid shrimp Acetes intermedius in the coastal waters of southwestern Taiwan. Fish. Sci. 2000, 66, 1014–1025. [Google Scholar] [CrossRef]
- Chiou, W.D.; Lee, L.K. Migration of kawakawa Euthynnus affinis in the waters near Taiwan. Fish. Sci. 2004, 70, 746–757. [Google Scholar] [CrossRef]
- Chiou, W.D.; Hsiao, Y.W. Food items of skinnycheek lantern fish (Benthosema pterotum) in the coastal waters off southwestern Taiwan. J. Fish. Soc. Taiwan 2021, 48, 211–226. [Google Scholar] [CrossRef]
- Hsieh, H.Y.; Lo, W.T.; Liao, C.C.; Meng, P.J. Shifts in the assemblage of summer mesopelagic fish larvae in the Gaoping waters of southwestern Taiwan: A comparison between El Niño events and regular years. J. Mar. Sci. Eng. 2021, 9, 1065. [Google Scholar] [CrossRef]
- Weng, J.S.; Lee, M.A.; Liu, K.M.; Hsu, M.S.; Hung, M.K.; Wu, L.J. Feeding ecology of juvenile yellowfin tuna from waters southwest of Taiwan inferred from stomach contents and stable isotope analysis. Mar. Coast. Fish. 2015, 7, 537–548. [Google Scholar] [CrossRef]
- Chang, Y.C.; Chiang, W.C.; Madigan, D.J.; Tsai, F.Y.; Chiang, C.L.; Hsu, H.H.; Lin, S.M.; Zhuang, M.Y.; Sun, C.T.; Chen, L.C.; et al. Trophic dynamics and feeding ecology of skipjack tuna (Katsuwonus pelamis) off Eastern and Western Taiwan. Molecules 2022, 27, 1073. [Google Scholar] [CrossRef]
- Chiou, W.D.; Chen, C.Y.; Wang, C.M.; Chen, C.T. Food and feeding habits of ribbonfish Trichiurus lepturus in coastal waters of south-western Taiwan. Fish. Sci. 2006, 72, 373–381. [Google Scholar] [CrossRef]

| Date (2023) | Location | N | ES% | Ns | Nf |
|---|---|---|---|---|---|
| 1/1 | TN | 159 | 0 | 66 | 63 |
| 2/17 | TN | 133 | 80 | 50 | 50 |
| 3/1 | TN | 130 | 100 | 50 | 48 |
| 4/3 | TN | 156 | 73.33 | 55 | 35 |
| 5/5 | TN | 81 | 100 | 30 | 30 |
| 8/21 | TN | 159 | 47.62 | 42 | 42 |
| 11/7 | TN | 160 | 83.33 | 30 | 30 |
| Total | 978 | 63.09 | 323 | 298 | |
| 4/27 | KH | 70 | 70.59 | 70 | 70 |
| 5/11, 5/17 | KH | 77 | 87.5 | 72 | 72 |
| 6/13 | KH | 133 | 83.33 | 30 | 30 |
| 7/13 | KH | 70 | 88.57 | 40 | 70 |
| 9/20 | KH | 77 | 100 | 43 | 77 |
| 10/11 | KH | 62 | 100 | 32 | 32 |
| Total | 489 | 88.03 | 287 | 351 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-C.; Lee, M.-A.; He, J.-S. Feeding Habits of Mene maculata (Teleostei: Menidae) in the Southwestern Waters of Taiwan, Western Pacific Ocean. Fishes 2025, 10, 182. https://doi.org/10.3390/fishes10040182
Wang Y-C, Lee M-A, He J-S. Feeding Habits of Mene maculata (Teleostei: Menidae) in the Southwestern Waters of Taiwan, Western Pacific Ocean. Fishes. 2025; 10(4):182. https://doi.org/10.3390/fishes10040182
Chicago/Turabian StyleWang, Yi-Chen, Ming-An Lee, and Jia-Sin He. 2025. "Feeding Habits of Mene maculata (Teleostei: Menidae) in the Southwestern Waters of Taiwan, Western Pacific Ocean" Fishes 10, no. 4: 182. https://doi.org/10.3390/fishes10040182
APA StyleWang, Y.-C., Lee, M.-A., & He, J.-S. (2025). Feeding Habits of Mene maculata (Teleostei: Menidae) in the Southwestern Waters of Taiwan, Western Pacific Ocean. Fishes, 10(4), 182. https://doi.org/10.3390/fishes10040182







