Abstract
The study aimed to evaluate the effectiveness of the cryopreservation protocols for Pseudoplatystoma magdaleniatum semen using dimethyl sulfoxide (DMSO) or methanol (MET) as permeable cryoprotectants at two concentrations (5% and 10%) combined with 12% egg yolk (Y12%) or 5% skimmed milk powder (SMP5%) and glucose (6%), resulting in eight treatments. A semen pool (n = 8) was diluted in a 1:4 ratio, packed in 2.5 mL straws, and frozen in nitrogen vapors. It was thawed at 35 °C for 90 s. Sperm kinetics and motility duration of fresh, prefrozen, and thawed semen were analyzed using a CASA system. The osmolarity of seminal plasma and cryosolutions was estimated. Fertilization (F) and embryo viability (E) rates of thawed semen were evaluated. The osmolarity of seminal plasma was 251.1 ± 3.3 mOsmol/kg and, in the cryosolutions, ranged between 1248.3 ± 19.9 mOsmol/kg (DMSO5% + Y12%) and 3488.2 ± 1.5 mOsmol/kg (MET10% + Y12%). After thawing, total motility ranged from 38.2% to 60.5%, representing a significant reduction compared to fresh semen (95.4 ± 2.1%) (p < 0.05). The best fertilization and embryo viability rates of thawed semen were obtained with DMSO5% + SMP 5% (F = 20.7%, E = 11.7%) and MET10% + SMP5% (F = 20.1%, E = 11.5%) (p < 0.05). A cryopreservation protocol for P. magdaleniatum semen with 5%DMSO or 10%MET combined with SMP5% is possible, but further study is necessary to optimize its fertilizing capacity.
Key Contribution:
Although there are previous studies on cryopreservation of P. magdaleniatum semen with different permeable and non-permeable cryoprotectants, this study is the first report of fertilization and embryo viability rates of semen thawed with DMSO and methanol. Cryopreservation of P. magdaleniatum semen is possible with 5% dimethyl sulfoxide or 10% methanol combined with 5% skimmed milk powder and 6% glucose. The fertilizing capacity of semen thawed using cryomedium with skimmed milk powder was better than that of semen thawed using cryomedium with egg yolk as a non-permeable cryoprotectant.
1. Introduction
The Neotropical catfish genus Pseudoplatystoma (Siluriformes: Pimelodidae) comprises eight large-sized species widely distributed across South American river basins [1]. This genus is significant for South American aquaculture due to its high commercial value and exceptional meat quality, serving as a vital resource for both commercial fisheries and the sportfishing industry [2,3,4].
The catfish Pseudoplatystoma magdaleniatum is an endemic species in Colombia [5,6], distributed in the Magdalena, Cauca, Sogamoso, and San Jorge River basins [7]. In the Magdalena–Cauca basin fishery, it is the largest and most commercially valuable species [7,8]. However, its catches have decreased by 34.9%, from 435.6 t in 2014 to 283.4 t in 2022 [8]. The causes of the decline are mainly attributed to environmental degradation caused by deforestation, mining, agriculture, livestock, organic pollution from wastewater, and the loss of longitudinal connectivity (dams) and lateral connectivity (sedimentation of channels) [9,10]. As a result of these pressures, P. magdaleniatum is classified as critically endangered [11], highlighting the need to develop management and conservation strategies.
Semen cryopreservation is a tool for biodiversity conservation and managing species at risk of extinction [12,13,14]. This tool allows the storage of genetic material for long periods without compromising its viability, which facilitates the creation of germplasm banks [13,14]. In addition, semen cryopreservation represents a strategy that makes semen available for assisted reproduction and the production of fry, particularly in species with reproductive asynchrony in captivity [13,15].
However, semen cryopreservation involves multiple technical challenges, and its success depends on optimizing several critical factors [12], including semen quality, cryomedium composition, cryoprotectant concentration, and freezing/thawing protocols [16,17]. The choice of cryoprotectants is particularly relevant, as they directly influence sperm cell integrity, motility, and fertilizing ability. In Neotropical catfish species, both permeable and non-permeable cryoprotectants have been compared and tested with variable results, highlighting the need to develop species-specific cryopreservation protocols [16,18,19].
Information on semen cryopreservation in P. magdaleniatum is scarce, and existing studies have not evaluated its fertilizing capacity after thawing [16,18,19]. There are studies about catfish semen cryopreservation with various permeable (dimethyl sulfoxide, dimethylacetamide, and ethylene glycol) and non-permeable cryoprotectants (skimmed milk powder) [16,18,19], but the fertilizing capacity of the thawed semen has not been reported. In this context, the present study aimed to assess the cryopreservation of P. magdaleniatum semen using two permeable cryoprotectants (methanol and dimethyl sulfoxide) at two concentrations (5% and 10%) combined with non-permeable cryoprotectants (5% skimmed milk powder and 12% egg yolk).
2. Materials and Methods
2.1. Location and Biological Material
Pseudoplatystoma magdaleniatum broodstock (eight males and two females) were captured from the wild in a sexually mature state and maintained in earthen ponds at 0.5 kg/m2 at the Santacruz fish farm (Caucasia, Antioquia, Colombia; 7°56′28.1″ N, 75°08′30.2″ W). The male biomass was 17.6 kg (2.2 ± 0.3 kg, 71.0 cm) and the female biomass was 22.0 kg (11.0 kg, 140 cm). Hormonal induction was performed by administering salmon GnRH analog (sGnRH, Syndel, Canada) at a single dose of 18 µg/kg body weight. Gamete collection was performed 12 h after induction. The males released between 5 and 20 mL of semen, and females spawned between 1.1 and 1.8 kg of eggs per female
2.2. Cryopreservation of Semen, Freezing and Thawing
Glucose (6%, 333 mM) was used as an extender. Dimethyl sulfoxide (DMSO) (AppliChem GmbH, Darmstadt, Germany) or methanol (MET) (Merck KGaA, Darmstadt, Germany) was used as permeable cryoprotectant at two inclusion levels (5 or 10%), and skimmed milk powder (SMP 5%) or hen’s egg yolk (Y 12%) were used as non-permeable cryoprotectant (six straws per treatment). For cryopreservation, semen samples from eight males (n = 8) with a total motility greater than 90% were selected. The diluted semen was packed in 2.5 mL cryogenic tubes (Minitüb, Tiefenbach, Germany) in a ratio of 1:4 (semen: cryosolution, 27 ± 1 °C). Samples were kept for 10 min at room temperature before being frozen in a 4 L dry shipper (MVE, St Paul, MN, USA) with nitrogen vapors for 30 min. The cooling curve was: 28 to −20 °C at 27.3 °C/min, −20 to −100 °C at 29.9 °C/min, and −100 to −196 °C at 5.5 °C/min [15]. The straws with cryopreserved semen were transferred in less than 10 s to a 34 L cryogenic thermos (MVE, St Paul, MN, USA) and immersed directly in liquid nitrogen. The straws were thawed in a serological bath (Memmert, WNB 7-45, Schwabach, Germany) at 35 °C for 90 s.
The osmolarity of each male’s seminal plasma and the different cryoprotectant solutions (DMSO 5, 10%) or (MET 5, 10%) combined with egg yolk (Y 12%) or (SMP 5%) was measured using a freezing point osmometer (Gonotec, Osmomat 3000, Berlin, Germany).
2.3. Semen Quality
In fresh, pre-frozen and thawed semen, a 0.25 µL aliquot of semen was placed in a Makler chamber (Sefi Medical Instruments, Haifa, Israel), activated with 75 µL of distilled water and analyzed using a computer-assisted semen analysis (CASA) system (Microptic SL, SCA® VET 6.5, Barcelona, Spain) and a phase contrast optical microscope (Nikon, E50i, Tokyo, Japan). Parameters such as total motility (Mt), types of motility (rapid, medium, and slow), total progressivity, curvilinear (VCL), and linear (VSL) velocities were evaluated. Motility types were classified based on curvilinear velocity (VCL) values. Rapid motility corresponds to the percentage of sperm with a velocity greater than 100 µm/s, medium to sperm with a velocity between 45 and 100 µm/s, and slow to sperm with a velocity between 10 and 45 µm/s [20].
The motility duration (Md) was estimated from the moment of activation until approximately 90% of the sperm stopped moving, using a stopwatch and a phase contrast optical microscope (Nikon, E50i, Tokyo, Japan) [20,21]. Sperm concentration (Sc) was estimated only in fresh semen, for which 1 µL of semen was diluted in 699 µL of 6% glucose (1:699 dilution). This dilution was homogenized at 1200 rpm for five seconds in a vortex mixer (Velp Scientifica, Zxclasic, Shanghai, China). A 10 µL aliquot was placed in the Makler chamber (Sefi Medical Instruments, Israel), and the concentration was determined using the CASA system (Microptic SL, SCA® VET 6.5, Barcelona, Spain) [20,21].
2.4. Fertilizing Capacity of Thawed Semen
A sample of 1.0 g of oocytes (~1500 oocytes) was taken and fertilized with 400 μL of thawed semen from the different treatments to estimate fertility and embryo viability rates. As a control, an oocyte sample was inseminated with fresh semen [20]. For the fertilizing capacity trial, the sperm-to-oocyte ratio was adjusted based on semen quality: a proportion of 160,000 spermatozoa per oocyte was used for fresh semen, and 320,000 spermatozoa per oocyte for cryopreserved semen to compensate for the expected reduction in motility and viability after thawing. The fertilization rate (F) was estimated at five hours post-fertilization (hpf) (gastrulation phase), and the embryo viability rate (E) was measured at 10 hpf (pharyngulation phase). Viable embryos were identified as translucent, while nonviable embryos were observed as opaque, with detachments of cellular material. Fertilization and embryo viability rates were calculated by dividing the number of viable embryos by the total number of embryos analyzed [20].
Water quality parameters such as temperature, dissolved oxygen (YSI, 550A, Yellow Springs, OH, USA), and pH (YSI, 100, Yellow Springs, OH, USA) were measured during the experiments. In addition, hardness, total alkalinity, non-ionized ammonium, and nitrite were measured with a photometer (YSI, 9500, Yellow Springs, OH, USA). Temperature (26.5–27.5 °C), dissolved oxygen (7.2–9.5 mg/L), pH (7.5–8.2), total alkalinity (40.0–65.0 mg CaCO3/L), total hardness (45.0–55.0 mg CaCO3/L), unionized ammonia (0.09–0.10 mg/L), and nitrite (0.03–0.04 mg/L) were within normal values for incubation of Neotropical fish species [22,23].
2.5. Statistical Analysis
A completely randomized design with eight treatments was applied; fresh semen was also evaluated as a control group. All variables were tested for normality (Kolmogorov–Smirnov test) and homogeneity of variance (Levene test). Variables that met these assumptions were analyzed using ANOVA, and when significant differences were detected, Tukey’s post hoc test was applied to compare means. In all cases, p < 0.05 was considered statistically significant. Data are expressed as mean ± standard error (SE). Statistical analysis was performed using Statgraphics Centurion version 19.3.03.
3. Results
3.1. Semen Osmolarity and Cryomedia
The osmolarity of P. magdaleniatum seminal plasma ranged between 247 and 260 mOsmol/Kg, averaging 251.1 ± 3.3 mOsmol/Kg. The osmolarity of the different cryoprotectant solutions ranged between 1248.3 ± 19.9 (DMSO 5% + SMP 5%) and 3488.2 ± 1.5 mOsmol/kg (MET 10% + Y 12%). The lowest osmolarities (<1600 mOsmol/Kg) were observed in the combinations containing 5% DMSO with either 5% SMP or 12% Y (p < 0.05). In contrast, osmolarities above 2000 mOsmol/Kg were recorded in all combinations that included MET (5% or 10%) or 10% DMSO, regardless of the non-permeable cryoprotectant used (p < 0.05) (Table 1).

Table 1.
Osmolarity of Pseudoplatystoma magdaleniatum seminal plasma and glucose-based cryomedia (6%).
3.2. Pre-Frozen
Compared to fresh semen (94.4 ± 0.6% total motility), all treatments showed a reduction in total motility ranging from 30.8% to 57.2%, depending on the cryoprotective solution used. The highest pre-frozen semen motility values were recorded with 5% DMSO combined with 5% SMP (total motility: 65.3 ± 5.8%, progressivity: 16.5 ± 1.1%, medium motility: 20.2 ± 1.7%, VCL: 38.5 ± 1.8 µm/s, VSL: 22.2 ± 1.8 µm/s) (p < 0.05). In contrast, the lowest values for these parameters were obtained when 10% MET was combined with either 12% Y or 5% SMP, or when 10% DMSO was combined with 5% SMP (p < 0.05). Rapid motility across all cryoprotective solutions ranged between 0.6 ± 0.9% and 2.3 ± 1.5%, showing no significant differences among treatments (p > 0.05) (Table 2).

Table 2.
Quality of pre-frozen semen from Pseudoplatystoma magdaleniatum diluted (1:4) in different cryoprotective solutions.
3.3. Thawed Semen
In all treatments, a reduction in total motility of approximately 38.2% to 60.5% was observed compared to fresh semen (95.4 ± 2.1%). The highest values of total motility in thawed semen were recorded in treatments with 5% or 10% DMSO combined with 12% Y (p < 0.05). Notably, VSL was significantly higher (16.7 ± 2.0 µm/s) when 10% DMSO was associated with 12% Y (p < 0.05). In contrast, the lowest values of motility parameters (total, rapid, medium) and sperm velocities (VCL and VSL) were observed in treatments with 10% DMSO combined with 5% SMP, as well as with MET (5% or 10%) combined with either of the non-permeable cryoprotectants (p < 0.05), while slow motility did not show a statistically defined trend among treatments (Table 3).

Table 3.
Quality of thawed semen from Pseudoplatystoma magdaleniatum cryopreserved in different cryoprotectant solutions.
3.4. Fertility and Embryo Viability Rates
Fertilization and embryo viability rates were significantly reduced when cryopreserved semen was used, with reductions of up to 67% and 81%, respectively, compared to fresh semen (73.0 ± 1.3% and 59.7 ± 1.6%, p < 0.05). The highest values in thawed semen for both parameters were obtained with DMSO 5% + SMP 5% (F = 20.7 ± 1.3%, E = 11.8 ± 2.0%) or MET 10% + SMP 5% (F = 20.1 ± 2.3%, E = 11.5 ± 2.3%), with no significant differences between these treatments (p > 0.05). In the treatments DMSO 5% + Y 12%, MET 5% + Y 12%, SMP 5%, and MET 10% + Y 12%, the thawed semen had no fertilizing capacity (Figure 1).
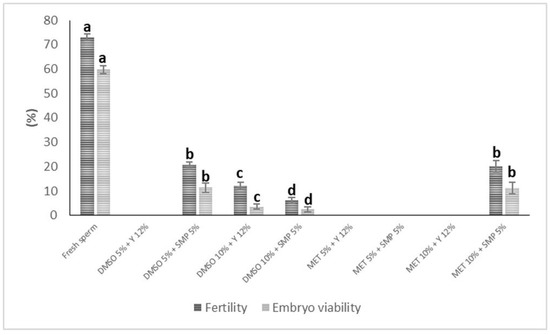
Figure 1.
Fertility and embryo viability rates for thawed semen of Pseudoplatystoma magdaleniatum cryopreserved with different solutions. DMSO: dimethyl sulfoxide, MET: methanol, SMP: skimmed milk powder, Y: egg yolk. Different letters indicate significant differences (p < 0.05).
4. Discussion
Cryopreservation significantly impacts seminal quality at multiple stages—pre-freezing, freezing, and thawing—due to cryoprotectant toxicity and osmotic and thermal stress. These factors contribute to structural damage such as flagellar detachment, plasma membrane rupture, weakening of the midpiece, and alterations in mitochondria and DNA [14,20,24]. In particular, mitochondrial disruption reduces ATP production, which compromises energy availability for sperm motility [25,26]. Since sperm needs ATP to support motility and biochemical processes essential for fertilization, any disruption directly affects reproductive potential. Although this phenomenon is well documented in other species, little information exists for P. magdaleniatum, reinforcing the relevance of the present study.
The osmolarity of seminal plasma in P. magdaleniatum (251.1 ± 3.3 mOsmol/Kg) is like that reported by other authors in migratory pimelodid catfish such as Pseudoplatystoma metaense (259.3 ± 3.5 mOsmol/Kg) [16] and Sorubim cuspicaudus (273.3 ± 7.2 mOsmol/kg) [20].
The results of the present study show that all cryoprotective solutions tested were hyperosmotic (1248.3 ± 19.9 to 3488.2 ± 1.5 mOsmol/Kg). In pre-frozen semen, the best sperm kinetic values were observed in solutions containing 5% DMSO or MET combined with 5% SMP or 12% Y, which recorded osmolarities lower than ~2200 mOsmol/kg. In thawed semen, the best sperm kinetic values were obtained with 5% or 10% DMSO combined with 12% Y. However, the best fertilization and embryo viability rates in thawed semen were recorded when 5% DMSO or 10% MET was combined with 5% SMP, suggesting that SMP may play a key role in preserving the fertilizing capacity of cryopreserved sperm, likely through mechanisms related to membrane stabilization under osmotic and thermal stress. A recent review highlights the use of powdered milk as a non-permeable cryoprotectant and its effects against free radicals and thermal shocks in the cryopreservation of fish semen [27].
Furthermore, when DMSO was included at 10%, combined with 5% SMP or 12% Y, or MET was included at 10%, combined with 12% Y, no fertilizing capacity of the semen was recorded. These solutions recorded osmolarity above 2900 mOsmol/kg. These findings indicate that the evaluation of sperm kinetics (total motility and types of motility) does not constitute a reliable criterion to predict the fertilizing capacity of cryopreserved semen [28]. The fertilizing capacity of cryopreserved semen is influenced by the type of cryoprotectant, its concentration, and the combinations used. Furthermore, it does not depend solely on motility but mainly on the integrity of DNA and the plasma membrane [12,29].
Herrera-Cruz et al. [19], using DMSO (5 or 10%) with 3% SMP in cryopreservation of striped catfish semen, obtained lower values of total motility (17.4–17.6%) and rapid (0.4–0.1%) and medium (0.5–1.0%) spermatozoa than those obtained in the present study with DMSO (5 or 10%) combined with 5% SMP, which suggests a better performance of sperm kinetics in thawed semen when SMP is included at 5%. Ramirez-Merlano et al. [18], in the cryopreservation of Pseudoplatystoma metaense semen, obtained lower total motility values using 12% MET and 12% Y (23.3 ± 5.4%) or 10% DMSO and 5% SMP (22.5 ± 4.4%) than those obtained in the present study with similar cryoprotectants: MET 10% + Y 12% (34.9 ± 1.9%) and DMSO 10% + SMP 5% (35.3 ± 1.7%). Comparable results have been reported in other siluriform catfishes. For example, Handayani et al. [30] observed acceptable post-thaw sperm motility in Clarias batrachus (Clariidae), while Pereira et al. [31] reported 33.1 ± 1.54% motility in Steindachneridion scriptum (Pimelodidae). Both studies demonstrated good sperm viability using 10% DMSO, which is consistent with our observations in Pseudoplatystoma magdaleniatum.
In Sorubim cuspicaudus, cryo-damages to DNA and mitochondria were recorded using cryoprotective solutions with osmolarities above 1500 mOsmol/Kg [20]. It has been suggested that cryo-damages to spermatozoa are severe when hypertonic solutions above 1500 mOsmol/Kg are used [32]. The results of the present study show that all cryoprotective solutions used in the cryopreservation of P. magdaleniatum semen were hyperosmotic. Even though sperm viability was recorded after exposure to these media, their fertilizing capacity was affected.
In all treatments, thawed semen recorded a reduction in total motility (38.2% to 60.5%) compared to fresh semen. Similar trends were observed in motility types (rapid and medium) and progressivity, with the lowest values recorded in treatments combining 10% DMSO or MET (5% or 10%) with either of the two non-permeable cryoprotectants analyzed in the study. These reductions reflect the cryoinjury associated with specific cryoprotectant combinations.
Another cause affecting sperm motility is damage to the sperm membrane, which causes the loss of some internal components of the cell and reduces its enzymatic activity [33]. Martínez-Páramo et al. [34] consider that the freezing and thawing processes induce alterations in the lipid bilayer of the sperm membrane, causing its destabilization and the loss of cellular components. These alterations not only affect sperm motility and viability but also increase lipid peroxidation and the percentage of static sperm. Some studies report that proteins, which are generally reduced in sperm during cryopreservation, are associated with energy availability for movement, such as those with catalytic activity and ATP [33,35].
Sperm quality is the ability and capacity of a sperm to successfully fertilize an egg and trigger the development of a viable embryo [36]. In addition, quality parameters suggest the efficiency of a cryopreservation protocol [14], but fertilizing capacity is the key test in evaluating seminal quality [37]. The results of the present study suggest that the cryoprotective solutions that showed the best fertilizing capacity of cryopreserved semen, in terms of fertility and embryo viability rates, were those using 5% DMSO or 10% methanol combined with 5% SMP. Semen cryopreserved with these cryoprotective solutions registered a fertilizing capacity equivalent to one-fourth or one-fifth of that obtained with fresh semen (F = 73.0 ± 1.3%, E = 59.7 ± 1.6%). In a recent review on permeable and non-permeable cryoprotectants in the cryopreservation of fish semen, it was suggested that 10% methanol combined with 15% milk powder recorded the best motility of thawed semen using motility as a criterion [27]. In the present study, fertilizing capacity was used in addition to motility, and the results suggest 10% methanol or 5% DMSO combined with 5% milk powder.
Fertilizing capacity requires more than high sperm motility; it also requires the overall well-being of the sperm because cryopreservation processes cause damage to DNA, mitochondria, and flagella, among others [24,38,39]. Therefore, it is suggested that relatively high percentages of total motility are not necessarily reflected in high fertility rates [40]. Müller et al. [41], using methanol in Clarias gariepinus, recorded embryo viability rates (12.5 ± 9.3%) similar to those obtained in the present study when MET 10%+SMP5% was used (E = 11.5%).
According to the results, the percentages of inclusion of the cryoprotectant significantly affected the fertilizing capacity. Each cryoprotectant in the different inclusion percentages performed differently on the sperm cells of P. magdaleniatum, highlighting the use of DMSO at 5% and MET at 10%. Cellular stress caused by exposure to cryoprotectant solutions (pre-freezing) during freezing and thawing is well known and widely reported. These phases induce physical and biochemical damage that compromises sperm structure and function [41,42,43,44,45,46].
In the present study, sperm kinetics and fertilization capacity of thawed semen were evaluated. The results of this study demonstrate that a combination of 10% methanol or 5% DMSO with 5% skimmed milk powder (SMP) is a suitable protocol for cryopreservation of P. magdaleniatum semen. However, the concentration of 5% SMP was selected based on previous studies in related fish species. Further studies evaluating higher SMP inclusions (e.g., 10–15%) are suggested to determine whether they could improve sperm quality and fertilization capacity of cryopreserved P. magdaleniatum semen.
The cryopreservation of Pseudoplatystoma corruscans semen was successfully achieved using methanol combined with egg yolk or powdered milk, resulting in fertilization rates between 11% and 38% [47], similar to those in the present study (~20%). However, this study is the first to report successful embryo viability from cryopreserved semen in the genus Pseudoplatystoma. Despite the modest embryo viability rates (~11%), this result represents a significant advance in the reproductive biotechnology of this Neotropical catfish.
5. Conclusions
Cryosolutions composed of 5% dimethyl sulfoxide or 10% methanol, combined with 5% skimmed milk powder and diluted in 6% glucose, are suitable for the cryopreservation of Pseudoplatystoma magdaleniatum semen. Despite the need for further refinement to enhance fertilizing and embryo viability outcomes, this study reports, for the first time, successful embryo viability rates using cryopreserved semen in the genus Pseudoplatystoma. This finding represents a significant advancement in reproductive biotechnology for Neotropical catfish.
Author Contributions
Conceptualization, V.A.-G., J.E.-A. and C.M.-P.; methodology, V.A.-G., B.R.-P., D.M.-M., C.T.-P., J.E.-A. and C.M.-P.; validation, V.A.-G., J.E.-A. and C.M.-P.; formal analysis, C.M.-P. and J.E.-A.; investigation, V.A.-G., B.R.-P., C.T.-P., J.E.-A., D.M.-M. and C.M.-P.; resources, V.A.-G., J.E.-A. and C.M.-P.; data curation, V.A.-G., C.T.-P., J.E.-A. and C.M.-P.; writing—original draft preparation, V.A.-G., J.E.-A. and C.M.-P.; writing—review and editing, V.A.-G., J.E.-A. and C.M.-P.; visualization, C.M.-P. and J.E.-A.; supervision, J.E.-A.; project administration, V.A.-G. and D.M.-M.; funding acquisition, V.A.-G. All authors have read and agreed to the published version of the manuscript.
Funding
Empresas Públicas de Medellín (EPM) funded this study. It is part of the project’s ex-situ conservation strategies for the main migratory fish species of the Middle and Lower Cauca River Basin (CW 246358, EPM-U. of Córdoba).
Institutional Review Board Statement
The study was carried out following the Standards of Conduct for the Use of Animals in Teaching and Research of the Central Bioethics Committee (Technical Room of Knowledge of Veterinary Medicine and Animal Husbandry) of the University of Córdoba (Colombia) (11 August 2023, CW246358).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data provided in this manuscript were appropriately cited in the tables, figures, and references section.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- García-Castro, K.; Rangel-Medrano, J.; Landínez-García, R.; Márquez, E. Population genetics of the endangered catfish Pseudoplatystoma magdaleniatum (Siluriformes: Pimelodidae) based on species-specific microsatellite loci. Neotrop. Ichthyol. 2021, 19, e200120. [Google Scholar] [CrossRef]
- Barzotto, E.; Oliveira, M.; Mateus, L. Reproductive biology of Pseudoplatystoma corruscans (Spix and Agassiz, 1829) and Pseudoplatystoma reticulatum (Eigenmann and Eigenmann, 1889), two species of fisheries importance in the Cuiabá River basin, Brazil. J. Appl. Ichthyol. 2017, 33, 29–36. [Google Scholar] [CrossRef]
- Balboni, L.; Vargas, F.; Colautti, D. Age and growth of Pseudoplatystoma corruscans (Siluriformes: Pimelodidae) at the confluence of the Paraná and Paraguay rivers. Neotrop. Ichthyol. 2021, 19, e200101. [Google Scholar] [CrossRef]
- Velarde, J.; Bastos, N.; Carneiro-Leite, L.; Borges, L.; Vieira, E.; Veríssimo-Silveira, R.; Ninhaus-Silveira, A. Dimethyl acetamide and dimethyl sulfoxide associated at glucose and egg yolk for cryopreservation of Pseudoplatystoma corruscans semen. Neotrop. Ichthyol. 2023, 21, e220071. [Google Scholar] [CrossRef]
- Buitrago-Suárez, A.; Burr, B. Taxonomy of the catfish genus Pseudoplatystoma Bleeker (Siluriformes: Pimelodidae) with recognition of eight species. Zootaxa 2007, 1512, 1–38. [Google Scholar] [CrossRef]
- Maldonado-Ocampo, J.; Vari, R.; Usma, J. Checklist of the freshwater fishes of Colombia. Biota Colomb. 2008, 9, 143–237. [Google Scholar]
- García-Alzate, C.; DoNascimiento, C.; Villa-Navarro, F.; García-Melo, J.; Herrera, R.G. Diversidad de peces de la cuenca del río Magdalena, Colombia. In Peces de la Cuenca del río Magdalena, Colombia: Diversidad, Conservación y Uso Sostenible; Jiménez Segura, L., Lasso, C.A., Eds.; Instituto de Investigación de Recursos Biológicos Alexander von Humboldt: Bogotá, Colombia, 2020; pp. 85–113. Available online: http://hdl.handle.net/20.500.11761/35752 (accessed on 3 March 2025).
- Duarte, L.; García, E.; Tejeda, K.; Cuello, F.; Gil-Manrique, B.; De León, G.; Curiel, J.; Cuervo, C.; Vargas, O.; Isaza, E.; et al. Estadísticas de Desembarco y Esfuerzo de las Pesquerías Artesanales de Colombia–Enero a Octubre de 2022; Autoridad Nacional de Acuicultura y Pesca (AUNAP); Universidad del Magdalena: Santa Marta, Colombia, 2022; p. 136. Available online: http://sepec.aunap.gov.co/Estadisticas (accessed on 3 March 2025).
- Marrugo-Negrete, J.; Pinedo-Hernández, J.; Díez, S. Assessment of heavy metal pollution, spatial distribution and origin in agricultural soils along the Sinú River Basin, Colombia. Environ. Res. 2017, 154, 380–388. [Google Scholar] [CrossRef]
- Gutiérrez-Moreno, F.; De la Parra-Guerra, A. Contaminación en la cuenca del río Magdalena (Colombia) y su relación con los peces. In Peces de la Cuenca del río Magdalena, Colombia: Diversidad, Conservación y uso Sostenible; Jiménez Segura, L., Lasso, C.A., Eds.; Instituto de Investigación de Recursos Biológicos Alexander von Humboldt: Bogotá, Colombia, 2020; pp. 239–263. Available online: http://hdl.handle.net/20.500.11761/35752 (accessed on 3 March 2025).
- Mojica, J.; Castellanos, C.; Usma, J.; Álvarez, R.; Lasso, C. Libro rojo de Peces Dulceacuícolas de Colombia; Serie Libros Rojos de Especies Amenazadas de Colombia; Universidad Nacional de Colombia; WWF: Manizales, Colombia, 2012; 319p. [Google Scholar]
- Cabrita, E.; Martínez-Páramo, S.; Gavaia, P.; Riesco, M.; Valcarce, D.; Sarasquete, C.; Herráez, M.; Robles, V. Factors enhancing fish sperm quality and emerging tools for sperm analysis. Aquaculture 2014, 432, 389–401. [Google Scholar] [CrossRef]
- Medina-Robles, V.; Duarte-Trujillo, A.; Cruz-Casallas, P. Crioconservación seminal en peces de agua dulce: Aspectos biotecnológicos, celulares y bioquímicos. Orinoquia 2020, 24, 51–78. [Google Scholar] [CrossRef]
- Bernáth, G.; Csorbai, B.; Nagy, B.; Csókás, E.; Molnár, J.; Bartucz, T.; Láng, Z.; Gyurcsák, M.; Hegyi, Á.; Kobolák, J.; et al. The investigation of post-thaw chilled storage and the applicability of large-scale cryopreservation in chub (Squalius cephalus) sperm. Cryobiology 2023, 113, 1–9. [Google Scholar] [CrossRef]
- Cruz-Casallas, P.; Medina-Robles, V.; Velasco-Santamaría, Y. Protocolo para la crioconservación de semen de yamú (Brycon amazonicus Spix & Agassiz 1829). Rev. Colomb. Cienc. Pecu. 2006, 19, 146–151. [Google Scholar] [CrossRef]
- Ramirez-Merlano, J.; Medina-Robles, V.; Cruz-Casallas, P. Variación estacional de las características seminales del bagre rayado Pseudoplatystoma metaense (Telostei, Pimelodidae). Rev. MVZ Córdoba 2011, 16, 2336–2348. [Google Scholar] [CrossRef][Green Version]
- França, T.; Motta, N.; Egger, R.; Oliveira, A.; Murgas, L. Impact of activation solutions on fresh and frozen-thawed sperm motility and fertilization success for two species of migratory freshwater fishes. Theriogenology 2020, 149, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Merlano, J.; Medina-Robles, V.; Cruz-Casallas, P. Crioconservación seminal de bagre rayado Pseudoplatystoma metaense (Teleostei, Pimelodidae), bajo diferentes protocolos de congelación. Arch. Med. Vet. 2011, 43, 135–144. [Google Scholar] [CrossRef]
- Herrera-Cruz, E.; Aristizabal-Regino, J.; Yepes-Blandón, J.; Estrada-Posada, A.; Espinosa-Araujo, J.; Atencio-García, V. Criopreservación de semen de bagre rayado Pseudoplatystoma magdaleniatum con tres diferentes crioprotectoras. Rev. Colomb. Biotecnol. 2019, 21, 55–62. [Google Scholar] [CrossRef]
- Atencio-García, V.; Padilla-Izquierdo, D.; Robles-González, J.; Prieto-Guevara, M.; Pardo-Carrasco, S.; Espinosa-Araujo, J. Damage to Sorubim cuspicaudus sperm cryopreserved with ethylene glycol. Animals 2023, 13, 235. [Google Scholar] [CrossRef]
- Montes-Petro, C.; Yepes-Escobar, J.; Tapia Pacheco, C.; Madariaga-Mendoza, D.; Espinosa-Araujo, J.; Atencio-García, V. Madurez testicular y calidad seminal de Ichthyoelephas longirostris (Prochilodontidae) del Medio río Cauca. Acta Biol. Colomb. 2024, 29, 179–188. [Google Scholar] [CrossRef]
- Vinatea-Arana, L. Principios Químicos de Calidad del Agua en Acuicultura: Una Revisión Para Peces y Camarones; UAM: Unidad Xochimilco, México, 2002; 154p, Available online: https://www.academia.edu/50857947/ (accessed on 3 March 2025).
- Trejo-Albarrán, R.; Flores-Ibarra, K.; Trujillo-Jiménez, P.; Granados-Ramírez, J.; Gómez-Márquez, J.; Delgado Sánchez, L. Water quality in fish farming ponds using some physical and chemical parameters. Braz. J. Anim. Environ. Res. 2021, 4, 5490–5509. [Google Scholar] [CrossRef]
- Martínez, J.; Atencio-García, V.; Pardo-Carrasco, S. DNA fragmentation and membrane damage of bocachico Prochilodus magdalenae (Ostariophysi, Prochilodontidae) sperm following cryopreservation with dimethylsulfoxide and glucose. Neotrop. Ichthyol. 2012, 10, 577–586. [Google Scholar] [CrossRef]
- Figueroa, E.; Merino, O.; Risopatrón, J.; Isachenko, V.; Sánchez, R.; Effer, B.; Isachenko, E.; Farias, J.; Valdevenito, I. Effect of seminal plasma on Atlantic salmon (Salmo salar) sperm vitrification. Theriogenology 2015, 83, 238–245. [Google Scholar] [CrossRef]
- Figueroa, E.; Valdebenito, I.; Zepeda, A.; Figueroa, C.; Dumorné, K.; Castillo, R.; Farias, J. Effects of cryopreservation on mitochondria of fish spermatozoa. Rev. Aquac. 2017, 9, 76–87. [Google Scholar] [CrossRef]
- Neyrão, I.; Santos, F.; Rodrigues, R.; Streit, D.; Godoy, L. Use of powdered milk in semen cryopreservation protocols for fish: A systematic review. Biopreserv. Biobank 2024, 22, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Riesco, M.F.; Oliveira, C.; Soares, F.; Gavaia, P.J.; Dinis, M.T.; Cabrita, E. Solea senegalensis sperm cryopreservation: New insights on sperm quality. PLoS ONE 2017, 12, e0186542. [Google Scholar] [CrossRef] [PubMed]
- Xin, M.; Niksirat, H.; Shaliutina-Kolešová, A.; Siddique, M.A.M.; Sterba, J.; Boryshpolets, S.; Linhart, O. Molecular and subcellular cryoinjury of fish spermatozoa and approaches to improve cryopreservation. Rev. Aquac. 2019, 12, 909–924. [Google Scholar] [CrossRef]
- Handayani, L.; Maulida, S.; Rahayu, S.; Razi, N.M.; Kocabas, M.; Kocabas, F.K.; Wilkes, M.; Siti-Azizah, M.N.; Eriani, K.; Fadli, N.; et al. Effect of cryoprotectant and concentration on the sperm quality of walking catfish, Clarias batrachus, post-cryopreservation. CryoLetters 2024, 45, 320–328. [Google Scholar] [CrossRef]
- Pereira, J.R.; Pereira, F.A.; Perry, C.T.; Pires, D.M.; Muelbert, J.R.E.; Garcia, J.R.E.; Corcini, C.D.; Varela-Junior, A.S. Dimethylsulfoxide, methanol and methylglycol in the seminal cryopreservation of Suruvi, Steindachneridion scriptum. Anim. Reprod. Sci. 2019, 200, 7–13. [Google Scholar] [CrossRef]
- Pardo-Carrasco, S.; Salas-Villalva, J.; Reza-Gaviria, L.; Espinosa-Araújo, J.; Atencio-García, V. Criopreservación de semen de bagre blanco (Sorubim cuspicaudus) con dimetilacetamida como crioprotector. Rev. CES Med. Zootec. 2015, 10, 122–131. [Google Scholar]
- Dietrich, M.; Arnold, J.; Fröhlich, T.; Otte, K.; Dietrich, G.; Ciereszko, A. Proteomic analysis of extracellular medium of cryopreserved carp (Cyprinus carpio L.) semen. Comp. Biochem. Physiol. (CBPD) 2015, 15, 49–57. [Google Scholar] [CrossRef]
- Martínez-Páramo, S.; Horváth, Á.; Labbé, C.; Zhang, T.; Robles, V.; Herráez, P.; Suquet, M.; Adams, S.; Viveiros, A.; Tiersch, T.; et al. Cryobanking of aquatic species. Aquaculture 2017, 472, 156–177. [Google Scholar] [CrossRef]
- Nynca, J.; Arnold, G.; Fröhlich, T.; Ciereszko, A. Cryopreservation induced alterations in protein composition of rainbow trout semen. Proteomics 2015, 15, 2643–2654. [Google Scholar] [CrossRef]
- Dumorné, K.; Figueroa, E.; Cosson, J.; Lee-Estevez, M.; Ulloa-Rodríguez, P.; Valdebenito, I.; Farías, J. Protein phosphorylation and ions effects on salmonid sperm motility activation. Aquaculture 2017, 10, 727–737. [Google Scholar] [CrossRef]
- Galo, M.; Streit-Junior, D.; Oliveira, C.; Povh, J.; Fornari, C.; Digmayer, M.; Ribero, P. Quality of fresh and cryopreserved semen and their influence on the rates of fertilization, hatching and quality of the larvae of Piaractus mesopotamicus. Braz. J. Biol. 2019, 79, 438–445. [Google Scholar] [CrossRef]
- El Kamouh, M.; Brionne, A.; Sayyari, A.; Laurent, A.; Labbé, C. Cryopreservation effect on DNA methylation profile in rainbow trout spermatozoa. Sci. Rep. 2023, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, B.; Lassen, P.; Streit, P. Cryopreservation-induced morphological changes in freshwater fish sperm: A systematic review. Biopreserv. Biobank. 2024, 22, 416–427. [Google Scholar] [CrossRef]
- Martínez, J.; Pardo, S. Crioconservación de semen en peces: Efectos sobre la movilidad espermática y la fertilidad. Acta Biol. Colomb. 2010, 15, 3–24. [Google Scholar]
- Müller, T.; Szabó, T.; Kollár, T.; Csorbai, B.; Marinović, Z.; Horváth, L.; Kucska, B.; Bodnár, Á.; Urbányi, B.; Horváth, Á. Artificial insemination of African catfish (Clarias gariepinus) using cryopreserved sperm. Theriogenology 2019, 123, 145–150. [Google Scholar] [CrossRef]
- Mazur, P.; Leibo, S.; Chu, E. A two-factor hypothesis of freezing injury: Evidence from Chinese hamster tissue-culture cells. Exp. Cell Res. 1972, 71, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Yeste, M. Sperm cryopreservation update: Cryodamage, markers and factors affecting the sperm freezability in pigs. Theriogenology 2016, 85, 47–64. [Google Scholar] [CrossRef]
- Sieme, H.; Oldenhof, H.; Wolkers, W. Sperm membrane behaviour during cooling and cryopreservation. Reprod. Domest Anim. 2015, 50 (Suppl. 3), 20–26. [Google Scholar] [CrossRef]
- Roca, J.; Parrilla, I.; Gil, M.; Cuello, C.; Martinez, E.; Rodriguez-Martinez, H. Non-viable sperm in the ejaculate: Lethal escorts for contemporary viable sperm. Anim. Reprod. Sci. 2016, 169, 24–31. [Google Scholar] [CrossRef]
- Kumar, A.; Prasad, J.; Srivastava, N.; Ghosh, S.K. Strategies to minimize various stress-related freeze-thaw damages during conventional cryopreservation of mammalian spermatozoa. Biopreserv. Biobank. 2019, 17, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Carolsfeld, J.; Godinho, H.; Zaniboni-Filho, E.; Harvey, B. Cryopreservation of sperm in Brazilian migratory fish conservation. J. Fish Biol. 2003, 63, 472–489. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).