Abstract
The effects of different stocking densities on the growth and physiology of juvenile Piaractus brachypomus were evaluated in two experiments. Experiment I used juveniles weighing 1.5 ± 0.4 g at the following densities for 20 days: D0.68—0.68 kg/m3; D1.45—1.45 kg/m3; D4.41—4.41 kg/m3; and D7.17—7.17 kg/m3. Experiment II used juveniles weighing 6.66 ± 1.3 g at the following densities for 20 days: D1.0—1.00 kg/m3; D1.95—1.95 kg/m3; D5.63—5.63 kg/m3, and D7.90—7.90 kg/m3. Both experiments showed a reduction in dissolved oxygen levels in the water, with Experiment II showing a plateau effect from 2.34 kg/m3 (p < 0.05). Final weight, final length, weight gain, daily weight gain, and specific growth rate were inversely proportional to density (p < 0.05), while final biomass, feed intake, and feed conversion were directly related to density in both experiments (p < 0.05). At the end of Experiment II, plasma triglycerides decreased as stocking density increased (p < 0.05), and hemoglobin and mean corpuscular volume were higher at the lowest density (D1.0) (p < 0.05). High stocking densities reduced dissolved oxygen, characterizing a hypoxic state in both experiments, affecting growth and some physiological parameters. Therefore, studies testing stocking densities for P. brachypomus in normoxic situations are still needed.
Key Contribution:
The present study is the first to address different stocking densities in the production of juvenile pirapitingas Piaractus brachypomus (initial average weight of 1.5 g), in RAS, and with excellent growth responses.
1. Introduction
Closed production systems allow for greater control over production since water quality parameters can be managed compared to traditional systems [1]. Recirculating aquaculture systems (RASs) have the advantage of reduced water use, as only a small amount is replaced with clean water [2], which limits the risk of environmental contamination [3]. In addition, the versatility of the RAS allows its implementation in different climates and environments [4], enabling production in locations closer to markets. However, the assembly and maintenance of these filters and blowers, along with the uninterrupted consumption of electricity, generate costs [4], and there is a need for specialized labor to monitor and maintain the equipment.
Therefore, to make a RAS rearing system viable, it is necessary to determine the fish stocking density that allows maximum animal production without compromising their performance. Defined as the biomass or quantity of animals produced per tank [5], stocking density is an aspect of management that is of the utmost importance to produce any species [6], whatever the production system used. In addition to the efficiency of the system and the bacterial community present in the RAS [7], stocking density can directly influence fish growth and health [8,9,10,11]. Animal growth is inversely linked to stocking density due to competition for food and space [12,13,14], with influence on fish stress levels [10,15].
The health and well-being of fish can be determined through physiological analyses performed by evaluating hematological and blood biochemical indices [16]. Poorly dimensioned stocking density is considered a chronic stressor capable of affecting the physiological parameters of fish [17,18]. The so-called physiological chain is the name given to a series of physiological mechanisms that seek the adaptation of the animal to stress conditions, classified into three levels of responses [19]. In stressful situations, studies carried out with Puntius sarana show the influence of stocking density on hematological variables, increasing hematocrit and hemoglobin values [20]. Biochemical values can also be altered, since secondary responses to stress are related to glycolytic pathways, through gluconeogenesis to obtain energy for escape and/or adaptation [21], as reported by the increase in glycemia and changes in cholesterol and triglyceride levels of Ictalurus punctatus [22] and increased cortisol, glucose, cholesterol, and triglycerides of Megalobrama amblycephala [23]. Linked to energy metabolism, thyroid hormones can also be influenced by stocking density [24], as seen in I. punctatus [22].
Pirapitinga, also known as cachama blanca or “pacu de barriga vermelha” (Piaractus brachypomus), is a native fish of the Amazon and Orinoco River basins, and is easily found in northern Brazil, Colombia, Peru, Venezuela, and Ecuador [25,26]. In addition to its countries of origin, the species has been of importance in Indian [27,28] and Thai [29,30] aquaculture. In Brazil, the species is used for both consumption and for producing the hybrid tambatinga (Colossoma macropomum x P. brachypomus). It is widely produced in excavated tanks [27], and results for P. brachypomus production in intensive systems such as the RAS remain limited [31,32,33,34]. Thus, there is a need to carry out further studies to demonstrate the efficiency of producing the species in the RAS, with the aim of establishing adequate stocking densities for the different cultivation phases.
The present study, therefore, aimed to evaluate the effects of different stocking densities on the growth performance and physiological parameters of juvenile P. brachypomus.
2. Materials and Methods
The experiment was carried out at the Laboratório de Aquacultura (LAQUA) of the Universidade Federal de Minas Gerais (UFMG, Brazil), and was divided into two experiments with all procedures previously approved by the Comissão de Ética no Uso de Animais (CEUA/UFMG—n° 17/2023), in 05/08/2023.
2.1. Experiment I
Experiment I used 798 juvenile P. brachypomus, with an initial weight (IW) of 1.5 ± 0.4 g (media ± standard deviation) and measuring 4.5 ± 0.5 cm (media ± standard deviation) in total length. The animals were distributed in 16 tanks with 28 L of useful volume, maintained in a RAS with homemade mechanical filters, made of acrylic blankets changed weekly, and homemade biological filters that we use as a biofilter. The crushed stone is stored in submerged boxes that allow water to contact the surface of the stones. We used a heating system (thermostat with heater, Warner, 200 w, Ocean Tech Professional Aquarium®, Porto Alegre, Brazil) and water pumping systems (submersible pump, SB2000, 1950 L/h, 30 w, Salobetter®, São Caetano do Sul, Brazil), and supplementary aeration from a compressor (2 HP radial compressor, CR-5, Ibram Ltd.a, São Paulo, Brazil) and porous air stones connected by 4 mm silicone hoses as diffusers. The water flow was kept at 0.59 ± 0.98 L/min (media ± standard deviation) for all tanks, resulting in a total of 30.24 renewals of tank volume per day. The following four stocking densities were tested, with four replicates each: D0.68—0.68 kg/m3; D1.45—1.45 kg/m3; D4.41—4.41 kg/m3; D7.17—7.17 kg/m3.
Experiment I lasted 20 days due to rapid growth and limited space in the 28 L tank. The photoperiod was 12 h of light (Key West DNI group, digital timer).
The animals were fed a commercial extruded diet (1.3–1.5 mm in diameter) from the Wean Prime line (Total Rações®, Três Corações, Brazil) with 45% crude protein, 5% ether extract, 15% mineral matter (max.), 40% crude fiber (max.), 0.2% calcium (min.), 0.3% calcium (max.), 0.04% vitamin E (min.), and 0.1% vitamin C (min.) (manufacturer’s data). Feeding was carried out three times a day (8:00, 12:00, and 16:00), with a feeding rate of 10% of the daily biomass [35]. The amount was readjusted after the 10th day of production through partial biometrics performed to obtain the average biomass of each tank, ultimately calculating the adjustment. The remaining feed was collected, dried in a forced circulation oven at 105 °C (Nova Etica/Ethink®), and subsequently weighed to calculate consumption and apparent feed conversion.
2.2. Experiment II
Experiment II used 2850 juvenile P. brachypomus with an initial weight (IW) of 6.6 ± 1.3 g (media ± standard deviation) and measuring 8.0 ± 0.5 cm (media ± standard deviation) in total length. The animals were distributed in 12 tanks, each with a useful volume of 100 L, maintained in a RAS with homemade mechanical filters made of acrylic blanket, changed weekly, and homemade biological filters that we used as a biofilter. The crushed stone was stored in submerged boxes that allowed contact between the water and the surface of the stones. We used a heating system (thermostat with heater, Warner, 200 w, Ocean Tech Professional Aquarium®, Porto Alegre, Brazil) and water pumping systems (submersible pump, SB2000, 1950 L/h, 30 w, Salobetter®, São Caetano do Sul, Brazil), along with supplementary aeration with compressor (2 HP radial compressor, CR-5, Ibram Ltd.a, SP, Brazil) and porous air stones connected by 4 mm silicone hoses as diffusers. The water flow was kept at 1.12 ± 0.12 L/min (media ± standard deviation) for all tanks, totaling 16.12 renewals of tank volume per day. The following four different stocking densities were tested, with three replicates each: D1.0—1.00 kg/m3; D1.95—1.95 kg/m3; D5.63—5.63 kg/m3, and D7.90—7.90 kg/m3. Experiment II lasted for another 20 days due to rapid growth and limited space in the 100 L tank.
Due to the larger size of the animals, they were fed a commercial extruded diet of 2–3 mm in diameter from the Aquos Alevinos 45 line (Total Rações®, Três Corações, Brazil), containing 45% crude protein, 12% moisture, 8% ether extract, 15% mineral matter, 4% crude fiber, 0.002% calcium (min.), 0.003% calcium (max.), 0.8% phosphorus, and 0.06% vitamin C (manufacturer’s data). Feed was offered three times a day (8:00, 12:00, and 16:00), with a feeding rate of 10% of daily biomass [35]. The amount was readjusted after the 10th day of production through partial biometrics performed to obtain the average biomass of each tank and finally calculate the adjustment. The remaining feed was collected, dried in a forced circulation oven at 105 °C (Nova Etica/Ethink®), and subsequently weighed to calculate consumption and apparent feed conversion.
2.3. Water Quality Analysis
In both experiments, temperature was measured three times a week using a multiparametric probe (Hanna Instruments HI98130, Hanna®, Barueri, Brazil) together with total ammonia (LabconTest Alcon® colorimetric kit, Camburiú, Brazil). The toxic ammonia (NH3) was calculated according to the table found in the kit. Dissolved oxygen (DO) (YSI multiparametric probe, EcoSense® DO200A, Yellow Sprongs Instruments Co., Inc., Yellow Sprongs, OH, USA) and pH were measured once a week.
2.4. Growth and Survival
Biometrics were performed after 10 and 20 days in both experiments. Weight was measured using a Marte 5000 digital analytical scale (accuracy of 0.001 g), with juveniles previously anesthetized with 20 mg/L of eugenol [36], while total length was measured using an ichthyometer (accuracy of 0.1 cm). The following data were obtained:
- -
- Initial weight (g) (IW);
- -
- Initial total length (cm) (IL);
- -
- Final weight (g) (FW);
- -
- Final total length (cm) (FL);
- -
- Weight gain (g) (WG) = FW − IW;
- -
- Daily weight gain (g) (DWG) = (FW − IW)/ΔT, where ΔT is the duration of the experiment in days;
- -
- Specific growth rate (%/day) (SGR) = 100 (ln FW − ln IW)/ΔT, where ΔT is the duration of the experiment in days;
- -
- Final biomass (FB) (kg/m3), where all the weights of all the fish were added together and the value found was converted to kg/m3;
- -
- Feed consumption (FC) (kg) = (feed offered (g) − feed leftover (g))/100
- -
- Feed conversion rate (FCR) = consumption/(FB − IB), where IB is the initial biomass.
Survival was also determined after 20 days in both experiments and was calculated considering the following formula: survival (%) = (number of live juveniles × 100)/(total number of juveniles per tank).
2.5. Hematological and Biochemical Analysis
At the end of Experiment II (after 20 days of rearing), the animals were fasted for 24 h. Four fish from each tank (n = 12 per treatment) were anesthetized with eugenol (50 mg/L) [33], restrained with a damp cloth, and subjected to blood collection by caudal venipuncture with previously heparinized 1 mL syringes, collecting a total of 300 µL of whole blood per fish.
Blood samples (50 µL) were dispensed into microtubes containing sodium heparin anticoagulant (10%) for determination of hemoglobin (tHb) using a commercial colorimetric kit (Quibasa-Bioclin, Belo Horizonte, MG, Brazil) and hematocrit (Ht) by the microhematocrit method [37] using capillary tubes.
Total plasma protein (TPP) was determined with an analog refractometer (0 to 90% Brix)—RHB0-90 after rupture of the microhematocrit tube. The number of erythrocytes (RBCs) was determined by diluting 10 µL of whole blood in 2 mL of citrate formalin and then counting them in a Neubauer chamber.
The remaining whole blood (250 µL) was centrifuged at 4000 RPM for 10 min (microcentrifuge SL-5AM SPINLAB CO®, Shenzhen, China) to separate the plasma (125 µL) and determine the following biochemical parameters: glucose (GLU), triglycerides (TG), cholesterol (TC), alanine aminotransferase (ALT), and aspartate aminotransferase (AST). All analyses were performed by colorimetric method using commercial kits (Quibasa-Bioclin, Belo Horizonte, MG, Brazil), with readings on a Biochrom Libra S22 UV-VIS spectrophotometer (Biochrom Instruments, Cambridge, UK).
The hematimetric indices (mean corpuscular volume—MCV, mean corpuscular hemoglobin—MCH, and mean corpuscular hemoglobin concentration—MCHC) were calculated according to the formulations established by [38]. These analyses were not performed at the end of Experiment I due to the small size of the juveniles for blood collection.
2.6. Viscerosomatic and Hepatosomatic Indexes
After blood collection, the same four animals from each tank (n = 12 per treatment) were euthanized with 285 mg/L eugenol solution [39], and their viscera (stomach, intestine, pyloric cecum, liver, gallbladder, and perivisceral fat) and liver were collected to determine the following indices:
- -
- Viscerosomatic Index (VSI) (%) = 100 × ((weight of total viscera (g)/body weight (g)).
- -
- Hepatosomatic Index (HSI) (%) = 100 × (weight of liver/body weight).
2.7. Statistical Analysis
The data from both experiments were tested for homoscedasticity and normality using Shapiro–Wilk and Levene’s tests, respectively. The data were then subjected to ANOVA followed by regression tests. Variables that were not significant in regression tests were subjected to mean comparison using Tukey’s test at 5% probability.
3. Results
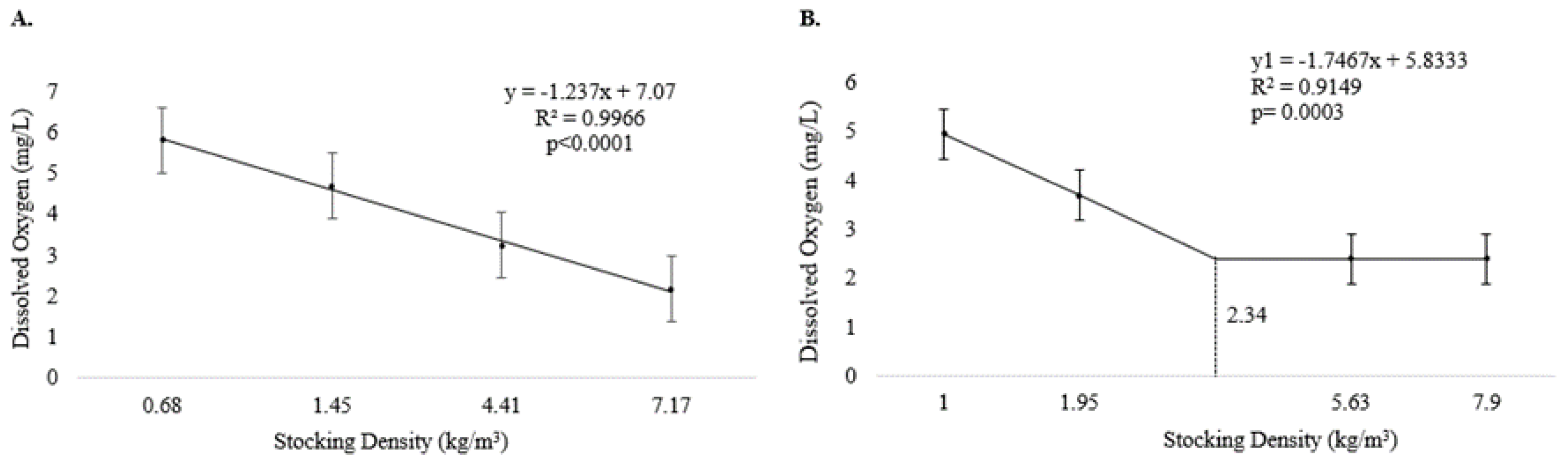
During Experiment I, dissolved oxygen levels decreased linearly with increasing stocking density (p < 0.05) (Figure 1A). Dissolved oxygen levels also decreased with increasing density during Experiment II, but only up to D1.95, with constant values from 2.34 kg/m3 onwards, reflecting a plateau effect (p < 0.05) (Figure 1B). The other water quality parameters were not influenced by stocking density (p > 0.05) (Table 1).
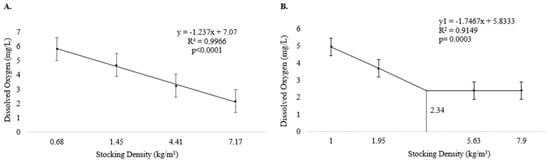
Figure 1.
Dissolved oxygen level (means and standard error) in culture tanks during the production of pirapitinga (Piaractus brachypomus) at different stocking densities. (A) Phase 1. (B) Phase 2.

Table 1.
Water quality parameters (means ± standard deviations) in culture tanks during the production of pirapitinga (Piaractus brachypomus) after 20 days of production in Experiment I and Experiment II at different stocking densities in RAS.
Survival was not affected in either experiment, being 94.05 ± 12.13% for Experiment I and 99.28 ± 1.19% for Experiment II.
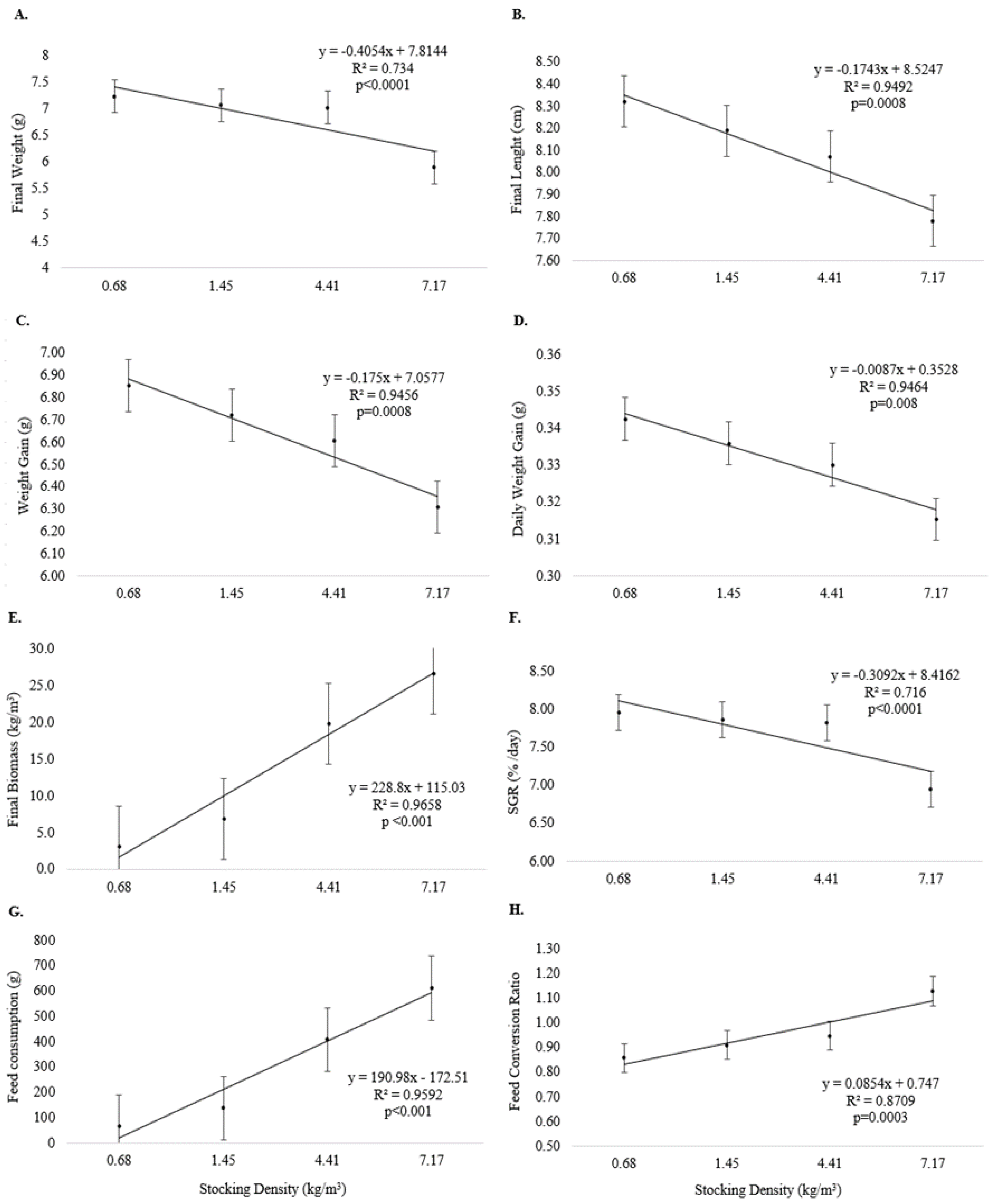
Final weight (Figure 2A), final length (Figure 2B), weight gain (Figure 2C), daily weight gain (Figure 2D), and SGR (Figure 2F) were inversely related to increasing stocking density (p < 0.05) at the end of Experiment I. Final biomass (Figure 2E), feed consumption (Figure 2G), and feed conversion (Figure 2H) were directly related to increasing stocking density (p < 0.05).
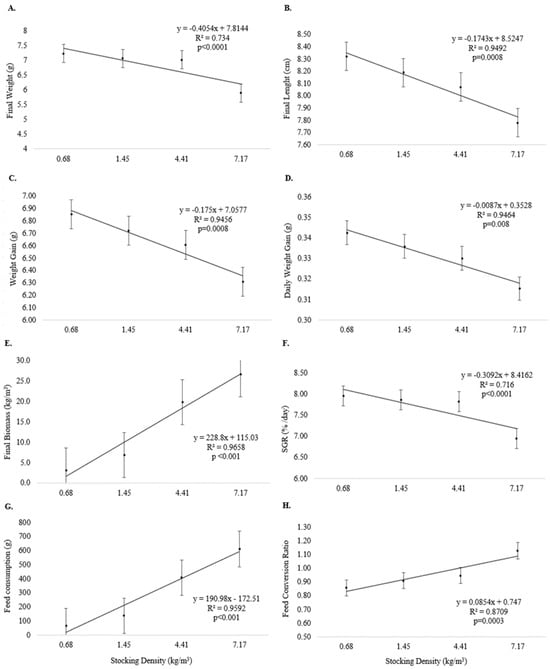
Figure 2.
Growth performance (means and standard error) of juvenile pirapitinga (Piaractus brachypomus) after 20 days of culture in Phase 1 at different stocking densities in RAS. (A) Final weight, (B) final length, (C) weight gain, (D) daily weight gain, (E) final biomass, (F) specific growth rate, (G) feed consumption, and (H) feed conversion.
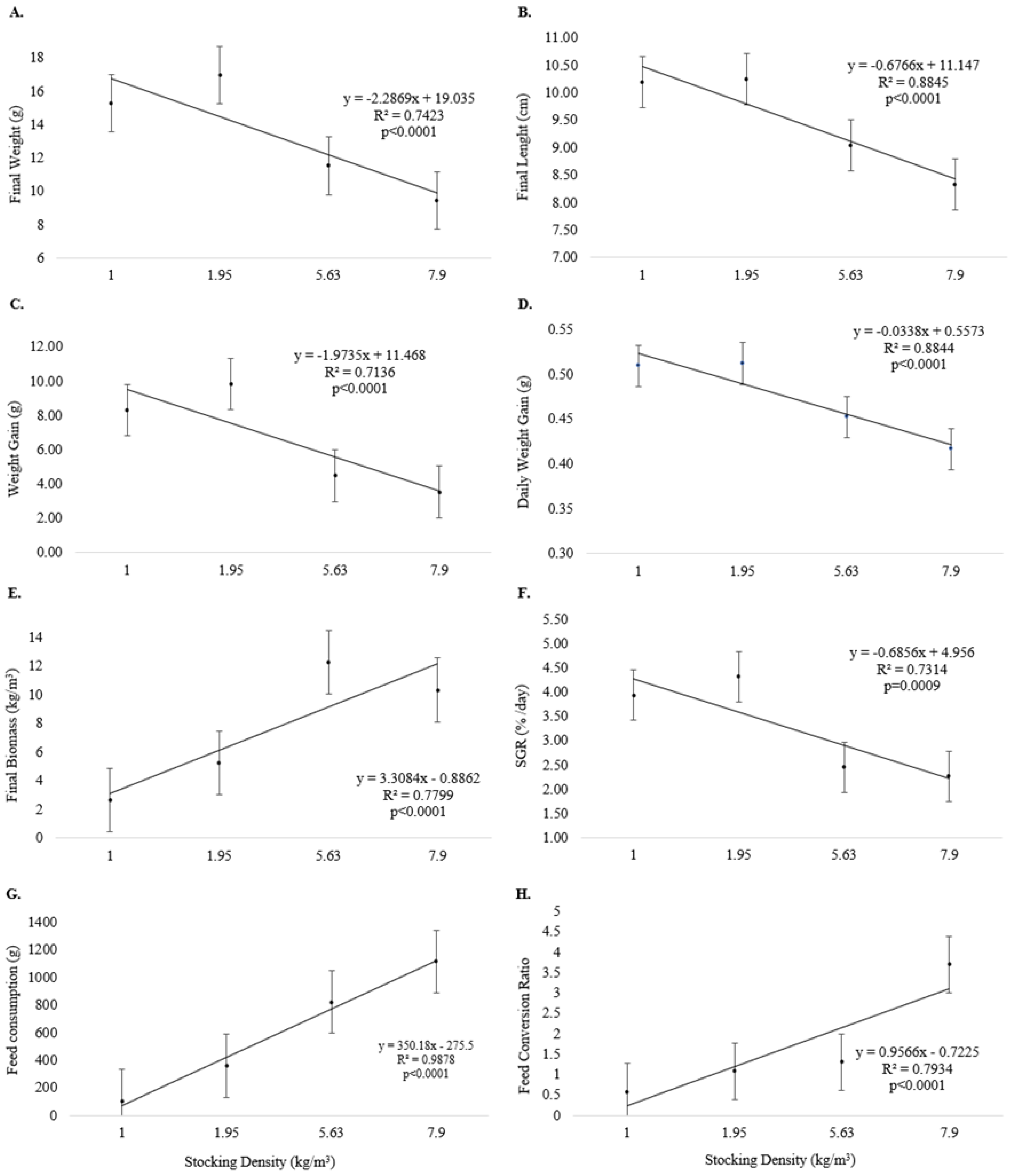
Final weight (Figure 3A), final length (Figure 3B), weight gain (Figure 3C), daily weight gain (Figure 3D), and SGR (Figure 3F) were inversely related to increasing stocking density (p < 0.05) at the end of Experiment II. Final biomass (Figure 3E), feed consumption (Figure 3G), and feed conversion (Figure 3H) were directly related to increasing stocking density (p < 0.05).
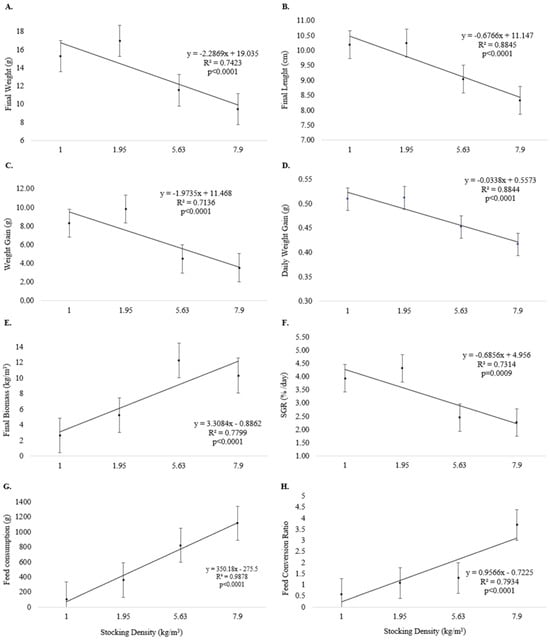
Figure 3.
Growth performance (means and standard error) of pirapitinga (Piaractus brachypomus) after 20 days of culture in Phase 2 at different stocking densities in RAS. (A) Final weight, (B) final length, (C) weight gain, (D) daily weight gain, (E) final biomass, (F) specific growth rate, (G) feed consumption, and (H) feed conversion.
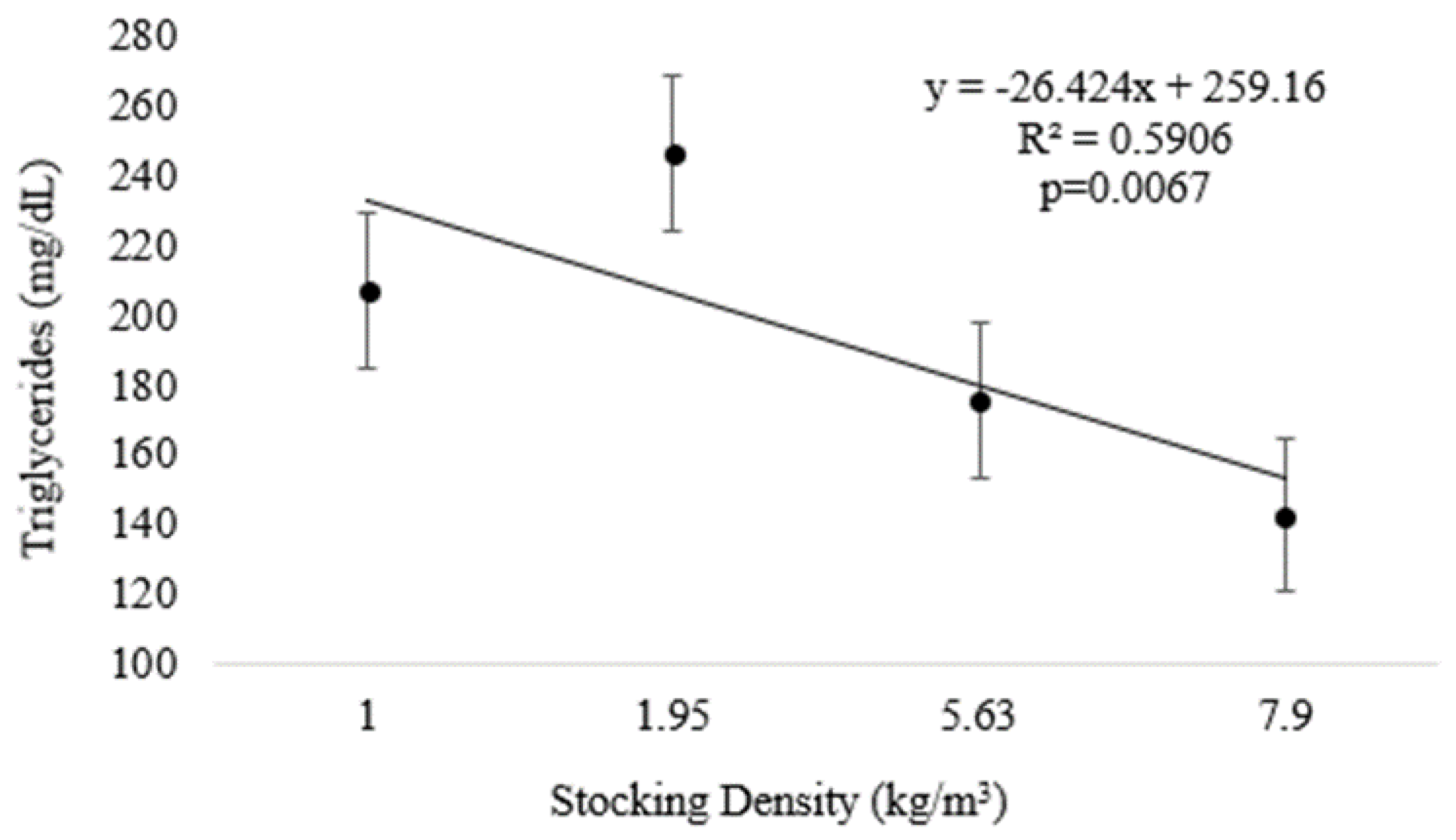
At the end of Experiment II, plasma triglycerides were inversely related to increasing density (Figure 4 and Table 2) (p < 0.05). There were no differences in the regression analyses for tHb and MCV; however, higher values were observed for the lowest density (D1.0) (p < 0.05) (Table 1). The other variables analyzed, as well as VSI and HSI, did not differ significantly among treatments (Table 2).
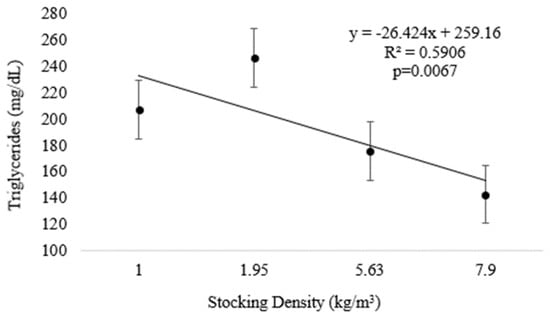
Figure 4.
Plasma triglycerides (means and standard error) of pirapitinga (Piaractus brachypomus) after 20 days of culture in Phase 2 at different stocking densities in RAS.

Table 2.
Hematological and biochemical parameters and viscerosomatic and hepatosomatic indices (means ± standard error) for juvenile pirapitinga (Piaractus brachypomus) after 20 days of production in Experiment II at different stocking densities in RAS.
4. Discussion
Dissolved oxygen was reduced at the higher stocking densities in both experiments, which may have affected the growth of juveniles kept at D4.41 and D7.17 during Experiment I, and D5.63 and D7.9 during Experiment II. The high survival and physiological changes observed in tHb, MCV, and TG highlight the oxiconforming characteristics of P. brachypomus. However, the adaptability of the species and the possibility of its production in the RAS are reinforced by survival rates exceeding 90% after the two experiments, along with unchanged somatic indices, as observed in previous studies [31,32,33,34].
Among the physico–chemical parameters of the water, only dissolved oxygen was influenced by stocking density in both experiments, which influenced the growth of juveniles. The ideal concentration of dissolved oxygen for rearing P. brachypomus has not yet been determined. However, rivers in the Amazon region have a large amount of organic matter and high water temperatures, variables that result in low levels of available dissolved oxygen [40,41,42]. These conditions have led Amazonian species to develop morphological and physiological adaptations to resist low oxygen concentrations [43,44]. The reduction in oxygen with increasing density in both phases of the experiment was a result like that found in studies with tambaqui (C. macropomum) in the RAS, also an Amazonian species [12,14]. For example, C. macropomum can withstand hypoxic conditions [45,46], including excellent recovery after periods of exposure to air [47]. The pacu (Piaractus mesopotamicus), although endemic to other river basins, belongs to the same genus as P. brachypomus and can also be reared in lower concentrations of dissolved oxygen, around 3 mg/L [48]. The high survival and growth of the juveniles after the experimental period of this study confirms the adaptation and production capacity of P. brachypomus in conditions of low oxygen availability, demonstrating its oxiconforming capacity even though its growth is reduced. However, there is still a need for more studies examining the tolerance of P. brachypomus in hypoxic situations, how their adaptation mechanisms are activated, as well as research on stocking density in normoxic conditions.
However, the maintenance of the other parameters means that the RAS used was efficient in maintaining water quality. As all the tanks were managed in the same way, future studies should aim to improve the aeration system of the RAS at high densities so that dissolved oxygen levels remain higher than those recorded here. Little is known about NH3 tolerance levels for P. brachypomus culture; however, P. mesopotamicus, used as a study object for NH3 toxicity, could withstand concentrations of up to 1.49 mg/L of total ammonia without symptoms of discomfort [49]. Similar studies were carried out with the tambacu hybrid (C. macropomum x P. mesopotamicus), which indicated an LC of 1.63 mg/L of NH3 [50]. Even with the low temperature for Amazonian fish in Experiment II, the daily weight gain, although affected by oxygen concentrations and stocking densities, together with the high survival rate, demonstrates the possibility of rearing P. brachypomus in regions with mild temperatures. Results like production in C. macropomum have been observed, allowing Amazonian species to be produced in colder regions [51,52].
The two experiments showed similar patterns in relation to growth parameters, except for biomass, feed consumption, and feed conversion ratio, all of which were inversely proportional to the increase in stocking density. These results suggest that space and low dissolved oxygen concentrations are limiting factors for the growth of P. brachypomus. Factors such as the species’ biology, age, growth stage, and the type of system used determine the stocking density variable [53]. In addition to space limitations, there is an increase in energy demand due to competition for food at high stocking densities [13,54]. The low oxygen concentrations at the highest densities tested in the two experiments should also be considered when reducing the growth of juveniles. In situations of chronic stress, such as hypoxia resulting from the experiments and stocking densities, growth is reduced due to energy reorganization for homeostasis [55]. A study carried out with C. macropomum (0.54 g) at different densities (0.3, 0.6, and 0.9 kg/m3) also showed similar results, with a reduction in dissolved oxygen levels at higher densities and consequently lower growth at the same densities [14]. However, even under normoxic conditions, juvenile C. macropomum with different size classifications suffered the influence of the stocking densities tested (34.88 g—0.5; 1.0; 1.6 kg/m3); (150.61 g—1.5; 3.0; 4.5 kg/m3); (300–400; 400–500 g—3.9 kg/m3) on their growth [13]. High stocking densities also reduced the development of individuals of Prochilodus cearaensis (0.09; 0.15; 0.3 g/L) [56], Micropterus salmoides (50; 75; 100 peixes/m3) [9], and Clarias gariepinus (100; 200; 400 kg/m3) [10], which is a common response for different species.
One of the indices used to assess the productivity of the system is the final biomass produced [57]. The final biomass, feed consumption, and feed conversion ratio increased with the increase in stocking density in both experiments. The increase in biomass is explained by the greater number of animals kept at the higher densities. However, the feed conversion ratio values remained between the averages of 0.86 and 1.13, results that have already been observed for the species [32,58]. Stocking density influences feeding competition behavior, oxygen consumption, and the response to stress in fish, consequently affecting the feeding rate [59], an influence that varies according to the species [60]. Feed consumption is linked to nutrient retention in fish; when it increases, retention is reduced, interfering with growth [60] and increasing feed conversion. However, in situations of hypoxia, feed consumption and feed conversion ratio are worsened, as reported for C. macropomum [14,61], channel catfish hybrids (Ictalurus punctatus x Ictalurus furcatus) [62], and Atlantic salmon (Salmo salar) [63].
Hematological and biochemical parameters and somatic indices can be used to assess an animal’s stress condition. Somatic indices are linked to an animal’s nutritional status [64], while biochemical and hematological parameters show the influence of stress factors on its health and metabolism [65]. The limited influence of stocking density in Experiment II of the study on unchanged biochemical and hematological parameters, combined with survival and growth performance data, shows the species’ adaptation to the RAS. Changes in triglyceride levels are related to energy metabolism linked to glycolytic pathways [66]. Decreases in value may be linked to energy consumption as a way of adapting to stressful situations. Triglycerides are considered important indicators in conditions of high stocking density [67]. The reduction in triglyceride levels observed with the increase in stocking density and the hypoxic conditions resulting from overcrowding may indicate a form of energy consumption by the organism to adapt to its environment. The reduction in the lipedogram of fish under stressful conditions is related to glycolytic pathways, mainly gluconeogenesis, to produce glucose [21]. Nutritional studies carried out on juvenile P. brachypomus, testing cycles of food restriction [32] and low water temperature and feeding time [34] as stressors for the species, resulted in a decrease in plasma triglyceride levels. Different supplementations at two different stocking densities for P. mesopotamicus under normoxic conditions for the species promoted a reduction in triglyceride levels at the high density tested, regardless of the type of supplementation used [68]. However, studies with different densities of juvenile C. macropomum have also reported variations in lipid concentrations, although with an increase in triglyceride levels as stocking density increased [13,14]. The results suggest species-specific responses to this production management.
Hematimetric indices and variables such as tHb, Ht, and RBC are related to oxygen transport capacity in stressful situations [65]. The low MCV and tHb values observed at the highest densities (D1.95, D5.63, and D7.90) may be related not only to the increase in stocking density but also to the low oxygen concentrations available at the respective densities tested. In stressful situations, an increase in the indices related to the fish’s blood count is to be expected, given the increased demand for oxygen in order to achieve homeostasis [65]. However, the results observed can be explained by the reduction in oxygen pressure in the water, which facilitates its uptake by increasing the affinity of hemoglobin for oxygen [69]. The results observed in this study may indicate an oxyconforming mechanism of the species. However, more studies are needed to prove this hypothesis and to report on the low oxygen conformation characteristics of P. brachypomus. Even with the change in hemoglobin and MCV values, they are still within the limits observed in other studies with the species [32,33,34], indicating that even with the influence of low oxygen and stocking densities, the well-being of P. brachypomus has not suffered major damage. Juveniles of C. macropomum kept at densities of 4 and 6 kg/m3 in a normoxic situation also showed lower hemoglobin levels than those kept at a lower density (2 kg/m3) [14]. Specimens of Paralichthys olivaceus kept at five different stocking densities and two dissolved oxygen concentrations showed changes in hemoglobin levels, but their quantity increased with the increase in available oxygen [70].
The other hematological and plasma biochemical indices were not influenced by stocking densities and hypoxia resulting from overcrowding. The responses to stress depend not only on the species and age of the animals, but also on the intensity of the stressor [65], so the conditions of this study did not cause serious problems for the physiology of P. brachypomus. C. macropomum juveniles kept at three stocking densities also showed no changes in TC, ALS, and AST levels [14].
5. Conclusions
Dissolved oxygen levels were reduced at the higher stocking densities, resulting in a hypoxic situation, directly influencing the growth of P. brachypomus juveniles in both experiments and the levels of triglycerides, hemoglobin rate, and MCV in Experiment II. The increase in feed consumption and feed conversion ratio made it impossible to produce P. brachypomus juveniles weighing 1.5 g at stocking densities above 4.41 kg/m3 and juveniles weighing 6.6 g at stocking densities above 5.63 kg/m3. More studies are still needed on the production of P. brachypomus juveniles at high stocking densities under normoxic conditions.
Author Contributions
Conceptualization, methodology, validation, formal analysis, resources, investigation, writing—original draft, writing—reviewing and editing, visualization: I.d.M.C.A.; conceptualization, methodology, validation, formal analysis, investigation: S.d.S.S., A.d.S.S., F.A.C.d.S., T.B.M., and W.J.d.F.M.; conceptualization, methodology, validation, formal analysis, investigation, writing—reviewing and editing, visualization, supervision, project administration, funding acquisition: G.C.F. and R.K.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-Brasil—402952/2021-9, 310170/2023-0, 316901/2021-0 and 402840/2023-2); Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG-Brasil); Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES-Brasil—finance code 001).
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee on Animal Use of Universidade Federal de Minas Gerais. (Protocol number: n° 17/2023; Approval date: 5 August 2023).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data needed to evaluate the conclusions in the paper are present in the main text. Detailed numerical data will be made available to individuals upon request.
Acknowledgments
The authors thank Conselho Nacional de Desenvolvimento Científico e Tecnológico, Fundação de Amparo à Pesquisa do Estado de Minas Gerais and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior for financial support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ray, A. Biofloc technology for super-intensive shrimp culture. In Biofloc Technology—A Practical Guide Book, 2nd ed.; The World Aquaculture Society: Baton Rouge, LO, USA, 2012; Volume 167, p. 188. [Google Scholar]
- Chun, S.-J.; Cui, Y.; Ahn, C.-Y.; Oh, H.-M. Improving water quality using settleable microalga Ettlia sp. and the bacterial community in freshwater recirculating aquaculture system of Danio rerio. Water Res. 2018, 135, 112–121. [Google Scholar] [PubMed]
- Verdegem, M.C.J.; Bosma, R.H.; Verreth, J.A.J. Reducing water use for animal production through aquaculture. Int. J. Water Resour. Dev. 2006, 22, 101–113. [Google Scholar]
- Martins, C.I.M.; Eding, E.H.; Verdegem, M.C.J.; Heinsbroek, L.T.N.; Schneider, O.; Blancheton, J.P.; d’Orbcastel, E.R.; Verreth, J.A.J. New developments in recirculating aquaculture systems in Europe: A perspective on environmental sustainability. Aquac. Eng. 2010, 43, 83–93. [Google Scholar] [CrossRef]
- Sousa, R.M.; Silva, R.R.D.S.; Santos, A.S.D.; Silva, C.V.D.; Magalhães, J.A.; Fogaça, F.H.D.S.; Lopes, J.M. Tambatinga juveniles performance in a recirculation aquaculture system with different stocking densities. RSD 2020, 9, e178953317. [Google Scholar]
- Karnatak, G.; Das, B.K.; Mishal, P.; Tayung, T.; Kumari, S.; Sarkar, U.K.; Das, A.K.; Ali, Y. Impact of stocking density on growth, feed utilization and survival of cage reared minor carp, Labeo bata (Hamilton, 1822) in Maithon reservoir, India. Aquaculture 2021, 532, 736078. [Google Scholar]
- Clols-Fuentes, J.; Nguinkal, J.A.; Unger, P.; Kreikemeyer, B.; Palm, H.W. Bacterial community in African catfish (Clarias gariepinus) recirculating aquaculture systems under different stocking densities. Front. Mar. Sci. 2023, 10, 1073250. [Google Scholar]
- Yarahmadi, P.; Miandare, H.K.; Fayaz, S.; Caipang, C.M.A. Increased stocking density causes changes in expression of selected stress- and immune-related genes, humoral innate immune parameters and stress responses of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2016, 48, 43–53. [Google Scholar]
- Wang, L.; Jia, S.; Guo, X.-R.; Lu, K.; Zhang, L.; Gong, J.; Guo, X.-P.; Hu, Y.; Cheng, T.; Shang, Q.; et al. Effect of stocking density on growth of largemouth bass (Micropterus salmoides) cultured in containers in a land-based recirculating aquaculture system (C-RAS). Aquac. Res. 2021, 53, 1518–1526. [Google Scholar]
- Bassmann, B.; Hahn, L.; Rebl, A.; Wenzel, L.C.; Hildebrand, M.-C.; Verleih, M.; Palm, H.W. Effects of stocking density, size, and external stress on growth and welfare of African catfish (Clarias gariepinus Burchell, 1822) in a commercial RAS. Fishes 2023, 8, 74. [Google Scholar] [CrossRef]
- Rebl, A.; Zebunke, M.; Borchel, A.; Bochert, R.; Verleih, M.; Goldammer, T. Microarray-predicted marker genes and molecular pathways indicating crowding stress in rainbow trout (Oncorhynchus mykiss). Aquaculture 2017, 473, 355–365. [Google Scholar]
- Costa, O.T.F.D.; Dias, L.C.; Malmann, C.S.Y.; Lima Ferreira, C.A.D.; Carmo, I.B.D.; Wischneski, A.G.; Sousa, R.L.D.; Cavero, B.A.S.; Lameiras, J.L.V.; Santos, M.C. The effects of stocking density on the hematology, plasma protein profile and immunoglobulin production of juvenile tambaqui (Colossoma macropomum) farmed in Brazil. Aquaculture 2019, 499, 260–268. [Google Scholar]
- Santos, F.A.C.; Boaventura, T.P.; Julio, G.S.C.; Cortezzi, P.P.; Figueiredo, L.G.; Favero, G.C.; Palheta, G.D.A.; Melo, N.F.A.C.; Luz, R.K. Growth performance and physiological parameters of Colossoma macropomum in a recirculating aquaculture system (RAS): Importance of stocking density and classification. Aquaculture 2021, 534, 736274. [Google Scholar]
- Ananias, I.D.M.C.; Silva, S.D.S.; Santos, F.A.C.D.; Souza, A.D.S.; Magalhães, T.B.; Reis, P.A.R.; Favero, G.C.; Luz, R.K. Tambaqui production at different stocking densities in RAS: Growth and physiology. Fishes 2024, 9, 19. [Google Scholar]
- Sánchez, P.; Ambrosio, P.P.; Flos, R. Stocking density affects Senegalese sole (Solea senegalensis, Kaup) growth independently of size dispersion, evaluated using an individual photo-identification technique. Aquac. Res. 2013, 44, 231–241. [Google Scholar]
- Ahmed, I.; Reshi, Q.M.; Fazio, F. The influence of the endogenous and exogenous factors on hematological parameters in different fish species: A review. Aquacult Int 2020, 28, 869–899. [Google Scholar]
- Ruane, N.M.; Komen, H. Measuring cortisol in the water as an indicator of stress caused by increased loading density in common carp (Cyprinus carpio). Aquaculture 2003, 218, 685–693. [Google Scholar]
- Khansari, A.R.; Balasch, J.C.; Vallejos-Vidal, E.; Teles, M.; Fierro-Castro, C.; Tort, L.; Reyes-López, F.E. Comparative study of stress and immune-related transcript outcomes triggered by Vibrio anguillarum bacterin and air exposure stress in liver and spleen of gilthead seabream (Sparus aurata), zebrafish (Danio rerio) and rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2019, 86, 436–448. [Google Scholar]
- Barton, B.A. Stress in fishes: A diversity of responses with particular reference to changes in circulating corticosteroids. Integr. Comp. Biol. 2002, 42, 517–525. [Google Scholar]
- Upadhyay, A.; Swain, H.S.; Das, B.K.; Ramtke, M.H.; Kumar, V.; Krishna, G.; Mohanty, B.P.; Chadha, N.K.; Das, A.K. Stocking density matters in open water cage culture: Influence on growth, digestive enzymes, haemato-immuno and stress responses of Puntius sarana (Ham, 1822). Aquaculture 2022, 30, 73745. [Google Scholar]
- Nakano, T.; Kameda, M.; Shoji, Y.; Hayashi, S.; Yamaguchi, T.; Sato, M. Effect of severe environmental thermal stress on redox state in salmon. Redox. Biol. 2014, 5, 772–776. [Google Scholar]
- Refaey, M.M.; Li, D.; Tian, X.; Zhang, Z.; Zhang, X.; Li, L.; Tang, R. High stocking density alters growth performance, blood biochemistry, intestinal histology, and muscle quality of Channel catfish Ictalurus punctatus. Aquaculture 2018, 492, 73–81. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, J.; Ge, X.; Sun, S.; Su, Y.; Li, B.; Hou, Y.; Ren, M. Effects of stocking density on the growth performance, digestive enzyme activities, antioxidant resistance, and intestinal microflora of Blunt Snout Bream (Megalobrama Amblycephala) juveniles. Aquac. Res. 2019, 50, 236–246. [Google Scholar] [CrossRef]
- Fatima, S.; Izhar, S.; Usman, Z.; Rashid, F.; Kanwal, Z.; Jabeen, G.; Latif, A.A. Effects of high stocking density on condition factor and profile of free thyroxine and cortisol in Catla catla (Hamilton, 1822) and Labeo rohita (Hamilton, 1822). Turk. J. Fish. Aquat. Sci. 2018, 18, 217–221. [Google Scholar]
- Escobar, L.M.D.; Ota, R.P.; Machado-Allison, A.; Andrade-López, J.; Farias, I.P.; Hrbek, T. A new species of Piaractus (Characiformes: Serrasalmidae) from the Orinoco Basin with a redescription of Piaractus brachypomus. J. Fish Biol. 2019, 95, 411–427. [Google Scholar] [CrossRef]
- Kolmann, M.A.; Kalacska, M.; Lucanus, O.; Sousa, L.; Wainwright, D.; Arroyo-Mora, J.P.; Andrade, M.C. Hyperspectral data as a biodiversity screening tool can differentiate among diverse. Sci. Rep. 2021, 11, 16157. [Google Scholar] [CrossRef]
- Kumar, A.; Pradhan, P.K.; Das, P.C.; Srivastava, S.M.; Lal, K.K.; Jena, J.K. Growth performance and compatibility of pacu, Piaractus brachypomus with Indian major carps in polyculture system. Aquaculture 2018, 490, 236–239. [Google Scholar] [CrossRef]
- Bharane, P.R.; Bethi, C.M.S.; Kudre, T.G. Effect of Catla catla roe protein isolate on textural and sensorial properties of surimi gel from Piaractus brachypomus. Food Meas. 2020, 14, 1391–1401. [Google Scholar] [CrossRef]
- Theerawoot, L. Diversity and distribution of external parasites from potentially cultured freshwater fishes in Nakhonsithammarat, Southern Thailand. Dis. Asian Aquac. 2008, 6, 235–244. [Google Scholar]
- Xiong, W.; Sui, X.; Liang, S.-H.; Chen, Y. Non-native freshwater fish species in China. Rev. Fish Biol. Fish. 2015, 25, 651–687. [Google Scholar] [CrossRef]
- Garcia, L.D.O.; Gutiérrez-Espinosa, M.; Wásquez-Torres, W.; Baldisserotto, B. Dietary protein levels in Piaractus brachypomus submitted to extremely acidic or alkaline pH. Cienc. Rural 2014, 44, 301–306. [Google Scholar] [CrossRef]
- Favero, G.C.; Santos, F.A.C.; Júlio, G.S.C.; Pedras, P.P.C.; Ferreira, A.L.; Silva, W.S.; Ferreira, N.S.; Neves, L.C.; Luz, R.K. Effects of short feed restriction cycles in Piaractus brachypomus juveniles. Aquaculture 2021, 536, 736465. [Google Scholar] [CrossRef]
- Ferreira, A.L.; Bonifácio, C.T.; Silva, W.S.; Takata, R.; Favero, G.C.; Luz, R.K. Anesthesia with eugenol and menthol for Piaractus brachypomus (Cuvier, 1818): Induction and recovery times, ventilation frequency and hematological and biochemical responses. Aquaculture 2021, 544, 737076. [Google Scholar] [CrossRef]
- Favero, G.C.; Santos, F.A.C.; Júlio, G.S.C.; Batista, F.S.; Bonifácio, C.T.; Torres, I.F.A.; Paranhos, C.O.; Luz, R.K. Effects of water temperature and feeding time on growth performance and physiological parameters of Piaractus brachypomus juveniles. Aquaculture 2022, 548, 737716. [Google Scholar] [CrossRef]
- Corrêa, R.O. Manejo alimentar. In Criação de Tambaqui; Correa, R.O., Sousa, A.R.B., Martins-Junior, H., Eds.; Embrapa: Brasília, Brazil, 2018; pp. 13–19. [Google Scholar]
- Santos, F.A.C.; Costa Julio, G.S.; Luz, R.K. Stocking density in Colossoma macropomum larviculture, a freshwater fish, in recirculating aquaculture system. Aquac. Res. 2021, 52, 1185–1191. [Google Scholar] [CrossRef]
- Goldenfarb, P.B.; Bowyer, F.P.; Hall, E.; Brosious, E. Reproducibility in the hematology laboratory: The microhematocrit determination. Am. J. Clin. Pathol. 1971, 56, 35–39. [Google Scholar] [CrossRef]
- Wintrobe, M.M. Variations in the size and hemoglobin content of erythrocytes in the blood of various vertebrates. Folia Haematol. 1934, 51, 32–49. [Google Scholar]
- Mattioli, C.C.; Takata, R.; Leme, F.O.P.; Costa, D.C.; Melillo Filho, R.; Silva, W.S.; Luz, R.K. The effects of acute and chronic exposure to water salinity on juveniles of the carnivorous freshwater catfish Lophiosilurus alexandri. Aquaculture 2017, 481, 255–266. [Google Scholar] [CrossRef]
- Silva, E.R.M.D.; Costa, L.G.S.; Silva, A.D.S.; Souza, E.C.D.; Barbosa, I.C.D.C. Physical-chemical, chemistry and chemometric characterization of underground waters from pirabas and Barreiras Aquifers in municipalities of the state of Pará. Rev. Bras. Geog. Fis. 2018, 11, 1026–1041. [Google Scholar]
- Gomes, D.F.; Sanches, N.A.D.O.; Andrade, D.D.P.; Bastos, W.R. Occurrence of aquatic macroinvertebrates in an extrativist reserve of brazilian Amazon. Rev. Biol. Neotrop. 2019, 16, 50–60. [Google Scholar] [CrossRef]
- Silva, E.C.D.; Gutjahr, A.L.N.; Braga, C.E.D.S. Caracterização físico-química da água de um rio urbano amazônico, Capanema, Pará, Brasil. RSD 2021, 10, e51101622866. [Google Scholar] [CrossRef]
- Burggren, W.W.; Arriaga-Bernal, J.C.; Méndez-Arzate, P.M.; Méndez-Sánchez, J.F. Metabolic physiology of the Mayan cichlid fish (Mayaheros Uropthalmus): Re-examination of classification as an oxyconformer. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2019, 237, 110538. [Google Scholar] [CrossRef]
- Braz-Mota, S.; Almeida-Val, V.M.F. Ecological adaptations of Amazonian fishes acquired during evolution under environmental variations in dissolved oxygen: A review of responses to hypoxia in fishes, featuring the hypoxia-tolerant Astronotus spp. J. Exp. Zool. A 2021, 335, 771–786. [Google Scholar] [CrossRef]
- Saint-Paul, U. Diurnal routine O2 consumption at different O2 concentrations by Colossoma macropomum and Colossoma brachypomus (Teleostei: Serrasalmidae). CBPA 1988, 89, 675–682. [Google Scholar]
- Neves, L.C.; Favero, G.C.; Beier, S.L.; Ferreira, N.S.; Palheta, G.D.A.; Melo, N.F.A.C.; Luz, R.K. Physiological and metabolic responses in juvenile Colossoma macropomum exposed to hypoxia. Fish Physiol. Biochem. 2020, 46, 2157–2167. [Google Scholar] [CrossRef]
- Neves, L.C.; Silva, W.S.; Ferreira, A.L.; Favero, G.C.; Beier, S.L.; Palheta, G.D.A.; Melo, N.F.A.C.; Luz, R.K. Physiological responses of juvenile Colossoma macropomum after different periods of air exposure. Aquaculture 2022, 548, 737583. [Google Scholar] [CrossRef]
- Lima, J.A.F.; Ferrari, V.A.; Colares de Melo, J.S.; Gaspar, L.A.; Chabalin, E.; Santos, E.P. Comportamento do pacu, Colossoma mitrei, em um cultivo experimental, no centro-oeste do Brasil. Bol. Técnico CEPTA Cent. Pesqui. Treinamento Aquicultura 1988, 1, 15–29. [Google Scholar]
- Pinheiro, M.S.L.; Selini Dorce, L.; Momo Ziemniczak, H.; Honorato Da Silva, C.A.; Hertes Neu, D. Toxicidade aguda da amônia em pacu (Piaractus mesopotamicus). Rev. Acad. Ciênc. Anim. 2021, 19, 1. [Google Scholar] [CrossRef]
- Quaresma, F.D.S.; Santos, F.L.B.D.; Ribeiro, P.F.; Leite, L.A.; Sampaio, A.H. Acute toxicity of non-ionized ammonia on tambacu (Colossoma macropomum x Piaractus mesopotamicus). Rev. Ciên. Agron. 2020, 51, e20186277. [Google Scholar] [CrossRef]
- Dairiki, J.K.; Da Silva, T.B.A. Revisão de Literatura: Exigências Nutricionais do Tambaqui—Compilação de Trabalhos, Formulação de Ração a e Desafios Futuros; Embrapa Amazônica Ocidental: Manaus, Brazil, 2011; 44p. [Google Scholar]
- Izel, A.C.U.; Crescencio, R.; O’Sullivan, F.L.d.A.; Chagas, E.C.; Boijink, C.d.L. Cultivo do Tambaqui no Amazonas; ABC da Agricultura Familiar; Embrapa Amazônica Ocidental: Manaus, Brazil, 2014; 51p. [Google Scholar]
- Maeda, H.; Silva, P.C.; Aguiar, M.; Padua, D.M.C.; Oliveira, R.P.C.; Machado, N.P.; Rodrigues, V.; Silva, R.H. Efeitos da densidade de estocagem na segunda alevinagem de tilápia nilótica (Oreochromis niloticus), em sistema raceway. R. Bras. Zootec. 2006, 7, 265–272. [Google Scholar]
- Li, H.W.; Brocksen, R.W. Approaches to the analysis of energetic costs of intraspecific competition for space by rainbow trout (Salmo gairdneri). J. Fish Biol. 1977, 11, 329–341. [Google Scholar] [CrossRef]
- Melo, D.C.; Oliveira, D.A.A.; Melo, M.M.; Júnior, D.V.; Teixeira, E.A.; Guimarães, S.R. Perfil proteico de tilápia nilótica chitralada (Oreochromis niloticus), submetida ao estresse crônico por hipóxia. Arq. Bras. Med. Vet. Zootec. 2009, 61, 1183–1190. [Google Scholar]
- Carvalho, M.A.M.D.; Costa, R.B.D.; Silva, L.D.A.; Oliveira, C.G.D.; Miranda, L.A.M.; Martins, L.P.; Sales, R.D.O.; Farias, J.O. Crescimento do curimatã comum, Prochilodus cearaensis (Steindachner,1911) em sistema de recirculação de água (SRA) em três diferentes densidades de estocagem. Rev. Bras. Hig. San. Anim. 2020, 14, 1–8. [Google Scholar]
- Ali, M.; Iqbal, F.; Salam, A.; Sial, F.; Athar, M. Comparative study of body composition of four fish species in relation to pond depth. Int. J. Environ. Sci. Technol. 2006, 2, 359–364. [Google Scholar]
- Angeles-Escobar, B.E.; Silva, S.M.B.C.; Severi, W. Growth, red blood cells, and gill alterations of red pacu (Piaractus brachypomus) fingerlings by chronic exposure to different total suspended solids in biofloc. J. World Aquac. Soc. 2022, 53, 652–668. [Google Scholar]
- Houlihan, D.; Boujard, T.; Jobling, M. (Eds.) Food Intake in Fish; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Liu, Z.-S.; Zhang, L.; Chen, W.-L.; He, C.-F.; Qian, X.-Y.; Liu, W.-B.; Li, X.-F. Insights into the interaction between stocking density and feeding rate in fish Megalobrama ambylcephala based on growth performance, innate immunity, antioxidant activity, and the GH-IGF1 axis. Aquaculture 2024, 580, 740355. [Google Scholar]
- Izel-Silva, J.; Ono, E.A.; Queiroz, M.N.; Dos-Santos, R.B.; Affonso, E.G. Aeration strategy in the intensive culture of tambaqui, Colossoma macropomum, in the tropics. Aquaculture 2020, 529, 735644. [Google Scholar]
- Torrans, L.; Ott, B.; Bosworth, B. Impact of minimum daily dissolved oxygen concentration on production performance of hybrid female channel catfish × male blue catfish. N. Am. J. Aquac. 2015, 77, 485–490. [Google Scholar]
- Gamperl, A.K.; Ajiboye, O.O.; Zanuzzo, F.S.; Sandrelli, R.M.; Peroni, E.D.F.C.; Beemelmanns, A. The impacts of increasing temperature and moderate hypoxia on the production characteristics, cardiac morphology and haematology of Atlantic salmon (Salmo salar). Aquaculture 2020, 519, 734874. [Google Scholar]
- Luckenbach, J.A.; Murashige, R.; Daniels, H.V.; Godwin, J.; Borski, R.J. Temperature affects insulin-like growth factor I and growth of juvenile southern flounder, Paralichthys lethostigma. Comp. Biochem. Physiol. A 2007, 146, 95–104. [Google Scholar]
- Urbinati, E.C.; Zanuzzo, F.S.; Biller-Takahashi, J.D. Estresse e sistema imune em peixes. In Biologia e Fisiologia de Peixes Neotropicais de Água Doce; Baldisseroto, B., Cyrino, J.E.P., Urbinati, E.C., Eds.; FUNEP: Jaboticabal, Brazil, 2014; pp. 87–105. [Google Scholar]
- Barton, B.A.; Iwama, G.K. Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Annu. Rev. Fish Dis. 1991, 1, 3–26. [Google Scholar]
- Qi, C.; Xie, C.; Tang, R.; Qin, X.; Wang, D.; Li, D. Effect of stocking density on growth, physiological responses, and body composition of juvenile blunt snout bream, Megalobrama amblycephala. J World Aquac. Soc. 2016, 47, 358–368. [Google Scholar] [CrossRef]
- Bacchetta, C.; Rossi, A.S.; Ale, A.; Cazenave, J. Physiological effects of stocking density on the fish Piaractus mesopotamicus fed with red seaweed (Pyropia columbina) and β-carotene-supplemented diets. Aquac. Res. 2020, 51, 1992–2003. [Google Scholar] [CrossRef]
- Baldisserotto, B. Fisiologia de Peixes Aplicada à Piscicultura; UFSM: Santa Maria, Brazil, 2002; 49p. [Google Scholar]
- Guo, H.-Y.; Dong, X.-Y.; Zhang, X.-M.; Zhang, P.-D.; Li, W.-T. Survival, growth and physiological responses of juvenile Japanese flounder (Paralichthys olivaceus, Temminck & Schlegel, 1846) exposed to different dissolved oxygen concentrations and stocking densities. J. Appl. Ichthyol. 2017, 33, 731–739. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).