A Catch Community Diversity Analysis of Purse Seine in the Tropical Western and Central Pacific Ocean
Abstract
1. Introduction
2. Materials and Methods
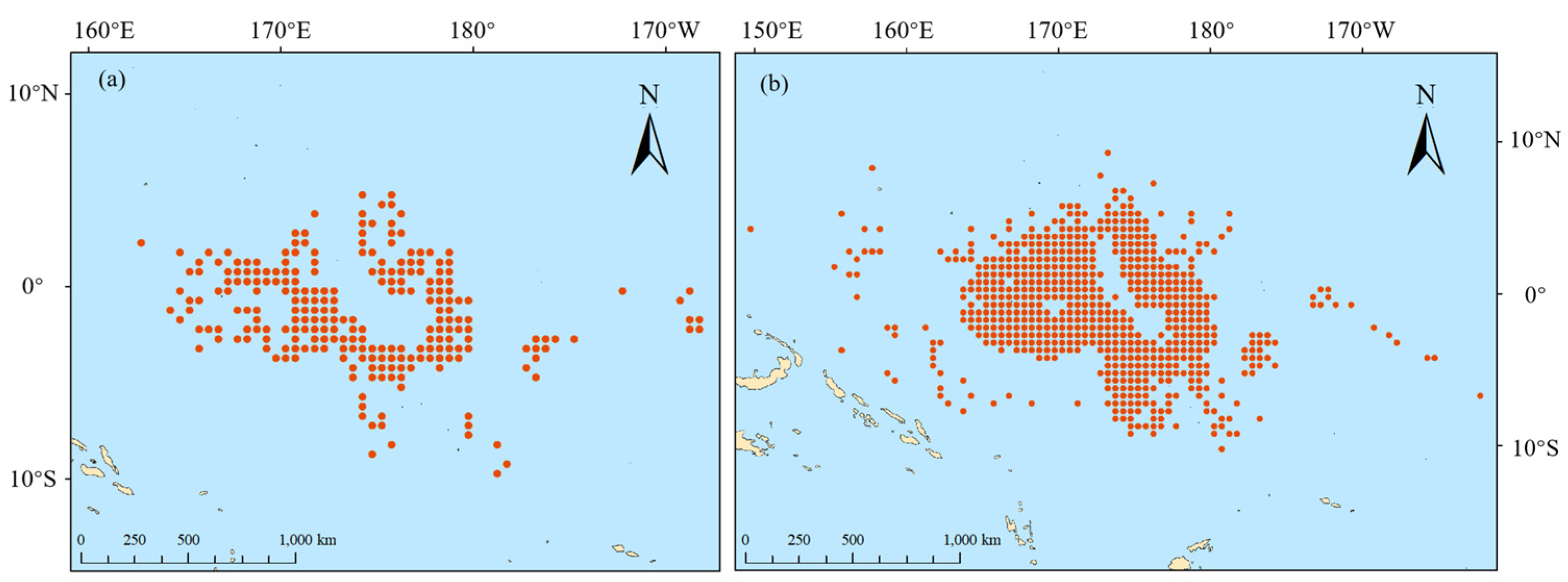
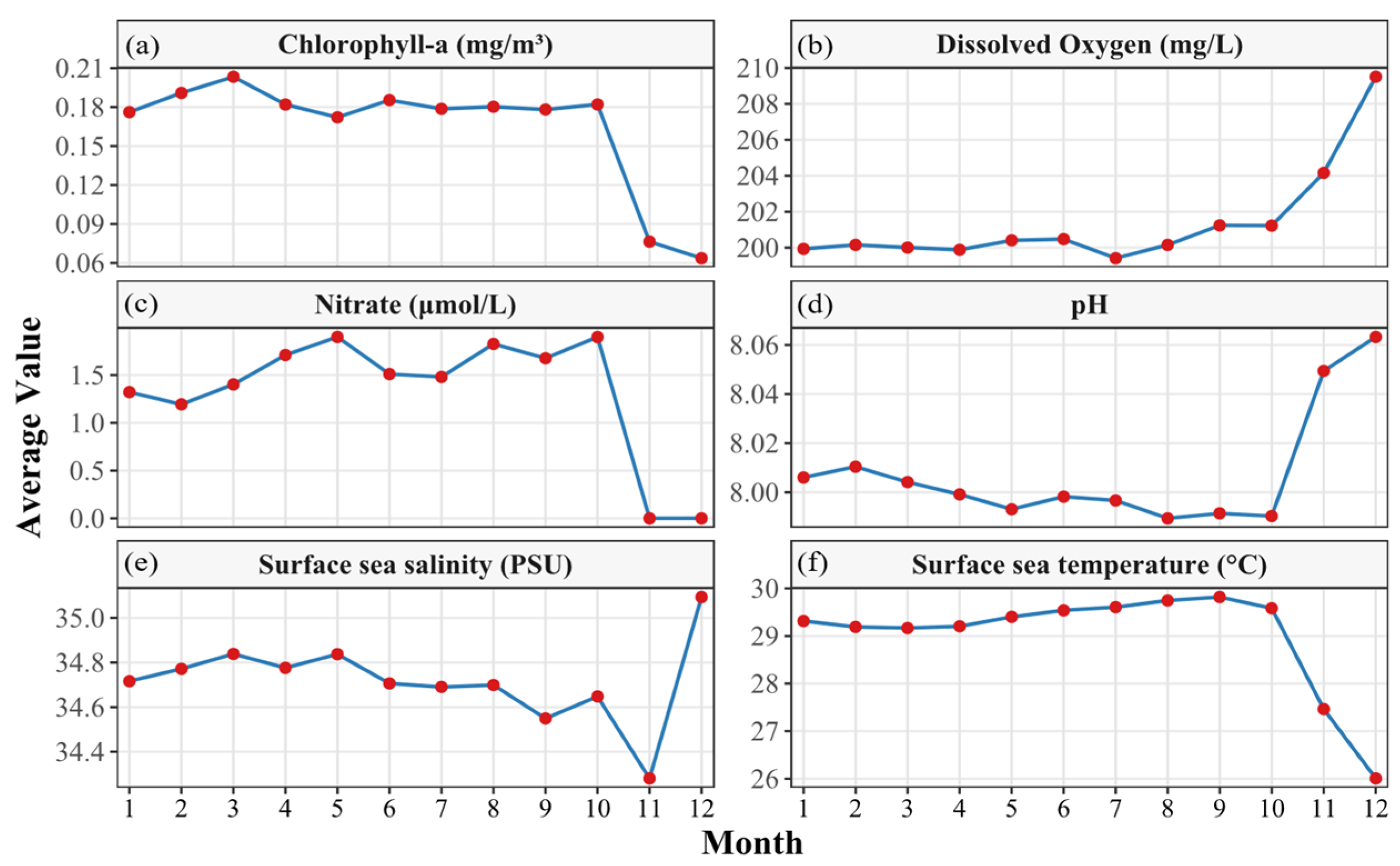
2.1. Data Sources
2.2. Data Processing
2.2.1. Community Composition and Dominant Species
2.2.2. Analysis of Community Diversity
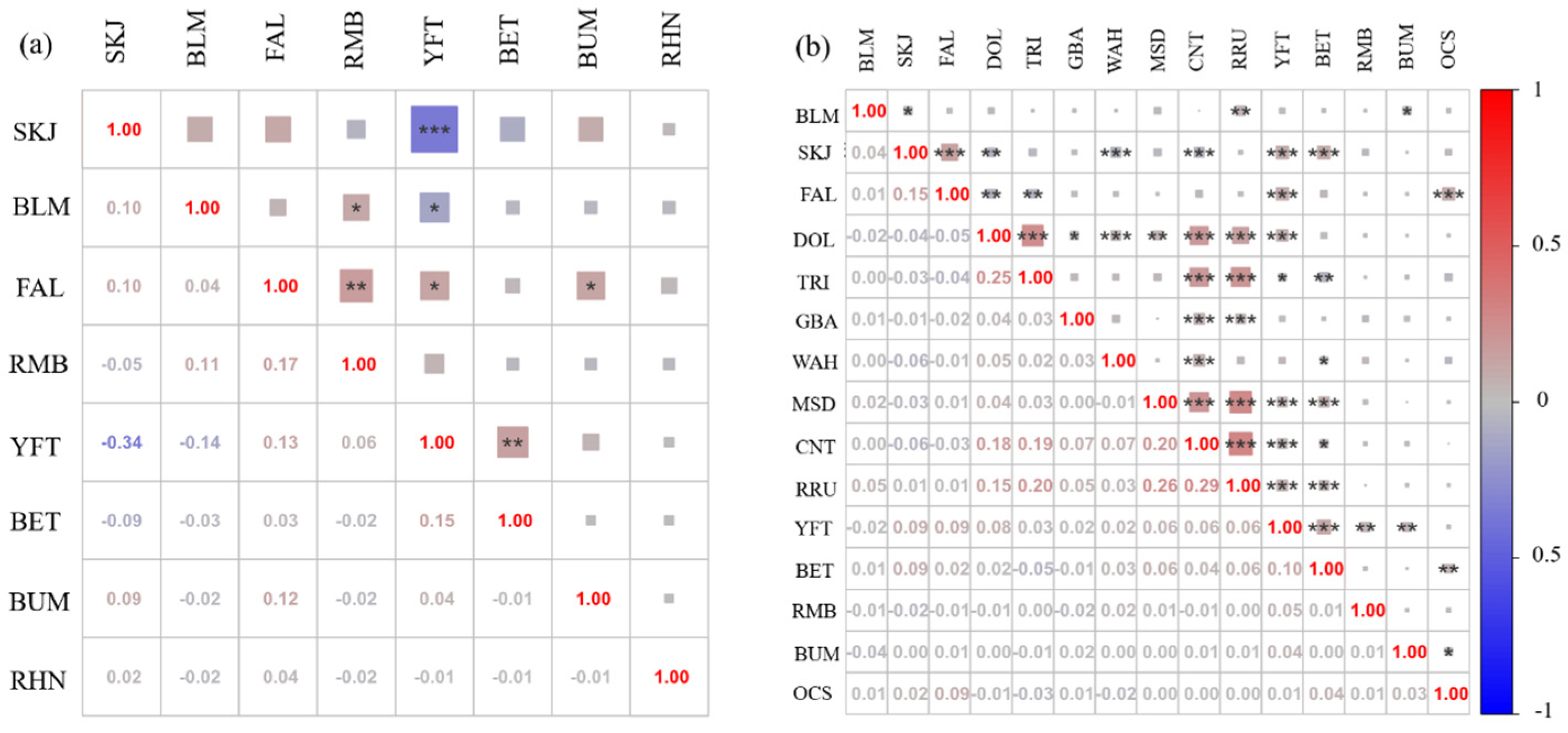
2.2.3. Correlation Analysis of Fish Species
3. Results
3.1. Differences in the Composition of Catch Species
3.2. Community Diversity Analysis
3.3. Correlations Between Community Species
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, C.; Long, H.; Yang, S.; Zhang, Y.; Tang, F.; Jin, W.; Wang, G.; Chang, W.; Pi, Y.; Gao, L.; et al. eDNA assessment of pelagic fish diversity, distribution, and abundance in the central Pacific Ocean. Reg. Stud. Mar. Sci. 2022, 56, 102661. [Google Scholar] [CrossRef]
- Ñacari, L.A.; Escribano, R.; Harrod, C.; Oliva, M.E. Combined use of carbon, nitrogen and sulfur stable isotopes reveal trophic structure and connections in deep-sea mesopelagic and demersal fish communities from the Southeastern Pacific Ocean. Deep-Sea Res. Part I 2023, 197, 104069. [Google Scholar] [CrossRef]
- Escalle, L.; Mourot, J.; Hamer, P.; Hare, S.R.; Phillip, N.B.; Pilling, G.M. Towards non-entangling and biodegradable drifting fish aggregating devices—Baselines and transition in the world’s largest tuna purse seine fishery. Mar. Policy 2023, 149, 105500. [Google Scholar] [CrossRef]
- Basurko, O.C.; Gabiña, G.; Lopez, J.; Granado, I.; Murua, H.; Fernandes, J.A.; Krug, I.; Ruiz, J.; Uriondo, Z. Fuel consumption of free-swimming school versus FAD strategies in tropical tuna purse seine fishing. Fish. Res. 2022, 245, 106139. [Google Scholar] [CrossRef]
- de Mesquita, G.C.; Menezes, R.; da Cunha-Neto, M.A.; Dantas-Neto, A.B.; da Silva, G.B. Feeding strategy of pelagic fishes caught in aggregated schools and vulnerability to ingesting anthropogenic items in the western equatorial Atlantic Ocean. Environ. Pollut. 2021, 282, 117021. [Google Scholar] [CrossRef]
- Tolotti, M.T.; Forget, F.; Capello, M.; Filmalter, J.D.; Hutchinson, M.; Itano, D.; Holland, K.; Dagorn, L. Association dynamics of tuna and purse seine bycatch species with drifting fish aggregating devices (FADs) in the tropical eastern Atlantic Ocean. Fish. Res. 2020, 226, 105521. [Google Scholar] [CrossRef]
- Wan, R.; Zhang, T.; Zhou, C.; Zhao, F.; Wang, W. Experimental and numerical investigations of hydrodynamic response of biodegradable drifting Fish Aggregating Devices (FADs) in waves. Ocean Eng. 2022, 244, 110436. [Google Scholar] [CrossRef]
- Sinopoli, M.; Castriota, L.; Vivona, P.; Gristina, M.; Andaloro, F. Assessing the fish assemblage associated with FADs (Fish Aggregating Devices) in the southern Tyrrhenian Sea using two different professional fishing gears. Fish. Res. 2012, 123–124, 56–61. [Google Scholar] [CrossRef]
- Crespo-Neto, O.; Díaz-Delgado, E.; Acosta-Pachón, T.A.; Martínez-Rincón, R.O. Spatial segregation by size of billfishes bycaught by the tuna purse-seine fishery in the Eastern Pacific Ocean. Fish. Res. 2021, 241, 106001. [Google Scholar] [CrossRef]
- Tang, H.; Xu, L.; Zhou, C.; Wang, X.; Zhu, G.; Hu, F. The effect of environmental variables, gear design and operational parameters on sinking performance of tuna purse seine setting on free-swimming schools. Fish. Res. 2017, 196, 151–159. [Google Scholar] [CrossRef]
- Blowes, S.A.; Daskalova, G.N.; Dornelas, M.; Engel, T.; Gotelli, N.J.; Magurran, A.E.; Martins, I.S.; McGill, B.; McGlinn, D.J.; Sagouis, A.; et al. Local biodiversity change reflects interactions among changing abundance, evenness, and richness. Ecology 2022, 103, e3820. [Google Scholar] [CrossRef] [PubMed]
- Laine, A.-L.; Tylianakis, J.M. The coevolutionary consequences of biodiversity change. Trends Ecol. Evol. 2024, 39, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Weiskopf, S.R.; Rubenstein, M.A.; Crozier, L.G.; Gaichas, S.; Griffis, R.; Halofsky, J.E.; Hyde, K.J.; Morelli, T.L.; Morisette, J.T.; Muñoz, R.C. Climate change effects on biodiversity, ecosystems, ecosystem services, and natural resource management in the United States. Sci. Total Environ. 2020, 733, 137782. [Google Scholar] [CrossRef]
- Valente, S.; Moro, S.; Di Lorenzo, M.; Milisenda, G.; Maiorano, L.; Colloca, F. Mediterranean fish communities are struggling to adapt to global warming. Evidence from the western coast of Italy. Mar. Environ. Res. 2023, 191, 106176. [Google Scholar] [CrossRef]
- Global Ocean Biogeochemistry Hindcast Product (GLOBAL_MULTIYEAR_BGC_001_029). E.U. Copernicus Marine Service Information (CMEMS). Marine Data Store (MDS). Available online: https://data.marine.copernicus.eu/product/GLOBAL_MULTIYEAR_BGC_001_029/download?dataset=cmems_mod_glo_bgc_my_0.25deg_P1M-m_202406 (accessed on 15 December 2023).
- Luo, Z.; Yang, C.; Wang, L.; Liu, Y.; Shan, B.; Liu, M.; Chen, C.; Guo, T.; Sun, D. Relationships between fish community structure and environmental factors in the nearshore waters of Hainan Island, South China. Diversity 2023, 15, 901. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication. Philos. Rev. 1949, 60, 117. [Google Scholar]
- Ulanowicz, R.E. Information theory in ecology. Comput. Chem. 2001, 25, 393–399. [Google Scholar] [CrossRef]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Xing, S.; Zhang, C.; Chen, L.; Gong, X. Fish community structure of Qingcaosha Reservoir in the Yangtze River Estuary in 2021. J. Shanghai Ocean Univ. 2024, 33, 114–123. Available online: https://kns.cnki.net/kcms2/article/abstract?v=QuBpG80dbeB1fRBjMT66bow791HFyE2sYCJneWYSi5fJF6vkLfTrt6Co_B9nRslzFlXR9Y2PMW_u9kfDjisae7DyqzhpGJzwvTPRsgIYMy8ZVoZGrr94uOKJ2-JB5b0D7zKkUT2gRjJmiC2cJDx33Q==&uniplatform=NZKPT&language=CHS (accessed on 1 March 2024).
- Mantel, N. The detection of disease clustering and a generalized regression approach. Cancer Res. 1967, 27, 209–220. [Google Scholar]
- Zhao, G.; Ding, W.; Tian, J.; Liu, J.; Gu, Y.; Shi, S.; Wang, R.; Sun, N. Spearman rank correlations analysis of the elemental, mineral concentrations, and mechanical parameters of the Lower Cambrian Niutitang shale: A case study in the Fenggang block, Northeast Guizhou Province, South China. J. Pet. Sci. Eng. 2022, 208, 109550. [Google Scholar] [CrossRef]
- FAO. ASFIS List of Species for Fishery Statistics Purposes. 2025. Available online: http://www.fao.org/fishery/collection/asfis/en (accessed on 24 February 2025).
- Hampton, J. Tuna Fisheries Status and Management in the Western and Central Pacific Ocean. Ocean. Fish. Prog. 2010, 23, 1–23. Available online: https://d2ouvy59p0dg6k.cloudfront.net/downloads/background_paper___status_and_management_of_tuna_in_the_wcpfc.pdf (accessed on 1 March 2024).
- Barrier, N.; Lengaigne, M.; Rault, J.; Person, R.; Ethé, C.; Aumont, O.; Maury, O. Mechanisms underlying the epipelagic ecosystem response to ENSO in the equatorial Pacific ocean. Prog. Oceanogr. 2023, 213, 103002. [Google Scholar] [CrossRef]
- Hu, X.; Wang, X.; Zhao, S.; Cao, L.; Pan, Y.; Li, F.; Li, F.; Lu, J.; Li, Y.; Song, G.; et al. Uncovering the dynamic evolution of microbes and n-alkanes: Insights from the Kuroshio Extension in the Northwest Pacific Ocean. Sci. Total Environ. 2023, 875, 162418. [Google Scholar] [CrossRef]
- Lin, H.; Wang, J.; Chen, X.; Jiang, M.; Xu, Z.A.; Lei, L.; Lyu, Z. Spatial-temporal changes in western and central Pacific warm pool and their impact on distribution of Katsuwonus pelamis. South China Fish. Sci. 2023, 19, 173–180. [Google Scholar]
- Holbrook, N.J.; Hernaman, V.; Koshiba, S.; Lako, J.; Kajtar, J.B.; Amosa, P.; Singh, A. Impacts of marine heatwaves on tropical western and central Pacific Island nations and their communities. Glob. Planet. Change 2022, 208, 103680. [Google Scholar] [CrossRef]
- Moyano, G.; Plaza, G.; Cerna, F.; Muñoz, A.A. Local and global environmental drivers of growth chronologies in a demersal fish in the south-eastern pacific ocean. Ecol. Indic. 2021, 131, 108151. [Google Scholar]
- Mediodia, H.J.P. Effects of sea surface temperature on tuna catch: Evidence from countries in the Eastern Pacific Ocean. Ocean Coast. Manag. 2021, 209, 105657. [Google Scholar] [CrossRef]
- Leroy, B.; Phillips, J.S.; Nicol, S.; Pilling, G.M.; Harley, S.; Bromhead, D.; Hoyle, S.; Caillot, S.; Allain, V.; Hampton, J. A critique of the ecosystem impacts of drifting and anchored FADs use by purse-seine tuna fisheries in the Western and Central Pacific Ocean. Aquat. Living Resour. 2013, 26, 49–61. [Google Scholar] [CrossRef]
- Pons, M.; Kaplan, D.; Moreno, G.; Escalle, L.; Abascal, F.; Hall, M.; Restrepo, V.; Hilborn, R. Benefits, concerns, and solutions of fishing for tunas with drifting fish aggregation devices. Fish Fish. 2023, 24, 979–1002. [Google Scholar] [CrossRef]
- Lezama Ochoa, N. Biodiversity and Habitat Preferences of the By-Catch Communities from the Tropical Tuna Purse-Seine Fishery in the Pelagic Ecosystem: The Case of the Indian, Pacific and Atlantic Ocean. Ph.D. Thesis, University of the Basque Country, Leioa, Spain, 2016. [Google Scholar]
- Schaefer, K.M.; Fuller, D.W.; Chaloupka, M. Performance evaluation of a shallow prototype versus a standard depth traditional design drifting fish-aggregating device in the equatorial eastern Pacific tuna purse-seine fishery. Fish. Res. 2021, 233, 105763. [Google Scholar] [CrossRef]
- Pratihary, A.; Lavik, G.; Naqvi, S.W.A.; Shirodkar, G.; Sarkar, A.; Marchant, H.; Ohde, T.; Shenoy, D.; Kurian, S.; Uskaikar, H.; et al. Chemolithoautotrophic denitrification intensifies nitrogen loss in the Eastern Arabian Sea Shelf waters during sulphidic events. Prog. Oceanogr. 2023, 217, 103075. [Google Scholar] [CrossRef]
- Lorrain, A.; Graham, B.S.; Popp, B.N.; Allain, V.; Olson, R.J.; Hunt, B.P.V.; Potier, M.; Fry, B.; Galván-Magaña, F.; Menkes, C.E.R.; et al. Nitrogen isotopic baselines and implications for estimating foraging habitat and trophic position of yellowfin tuna in the Indian and Pacific Oceans. Deep-Sea Res. Part II 2015, 113, 188–198. [Google Scholar] [CrossRef]
- Nagatomo, Y.; Horii, S.; Hirai, J.; Hashihama, F.; Sado, T.; Fukuchi, T.; Miya, M.; Takahashi, K. Geographic distribution of micronektonic fish communities in the subtropical North Pacific: The effect of primary productivity and nitrogen fixation. Prog. Oceanogr. 2023, 217, 103086. [Google Scholar] [CrossRef]
- Nataniel, A.; Lopez, J.; Soto, M. Modelling seasonal environmental preferences of tropical tuna purse seine fisheries in the Mozambique Channel. Fish. Res. 2021, 243, 106073. [Google Scholar] [CrossRef]
- Yen, K.-W.; Wang, G.; Lu, H.-J. Evaluating habitat suitability and relative abundance of skipjack (Katsuwonus pelamis) in the Western and Central Pacific during various El Niño events. Ocean Coast. Manag. 2017, 139, 153–160. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Zhu, J. Oscillation mode analysis on the time series of the abundance of unassociated school skipjack tuna (Katsuwonus pelamis) in the Western and Central Pacific Ocean. Mar. Fish. 2024, 46, 266–274. [Google Scholar] [CrossRef]
- Mohammadi, M.; Mahboobi-Soofiani, N.; Farhadian, O.; Malekpouri, P. Metabolic and NH4 excretion rate of fresh water species, Chondrostoma regium in response to environmental stressors, different scenarios for temperature and pH. Sci. Total Environ. 2019, 648, 90–101. [Google Scholar] [CrossRef]
- Zbinden, Z.D. A needle in the haystack? Applying species co-occurrence frameworks with fish assemblage data to identify species associations and sharpen ecological hypotheses. J. Fish Biol. 2022, 100, 339–351. [Google Scholar] [CrossRef]
- Peoples, B.K.; Frimpong, E.A. Biotic interactions and habitat drive positive co-occurrence between facilitating and beneficiary stream fishes. J. Biogeogr. 2016, 43, 923–931. [Google Scholar] [CrossRef]
- Holland, K.N.; Kleiber, P.; Kajiura, S.M. Different residence times of yellowfin tuna, Thunnus albacares, and bigeye tuna, T. obesus, found in mixed aggregations over a seamount. Fish. Bull. 1999, 97, 392–395. [Google Scholar]
- Schaefer, K.M.; Fuller, D.W. Simultaneous behavior of skipjack (Katsuwonus pelamis), bigeye (Thunnus obsesus), and yellowfin (T. albacares) tunas, within large multi-species aggregations associated with drifting fish aggregating devices (FADs) in the equatorial eastern Pacific Ocean. Mar. Biol. 2013, 160, 3005–3014. [Google Scholar] [CrossRef]
- Artetxe-Arrate, I.; Fraile, I.; Marsac, F.; Farley, J.H.; Rodriguez-Ezpeleta, N.; Davies, C.R.; Clear, N.P.; Grewe, P.; Murua, H. A review of the fisheries, life history and stock structure of tropical tuna (skipjack Katsuwonus pelamis, yellowfin Thunnus albacares and bigeye Thunnus obesus) in the Indian Ocean. In Advances in Marine Biology; Elsevier: Amsterdam, The Netherlands, 2021; Volume 88, pp. 39–89. [Google Scholar] [CrossRef]
- Hutchinson, M.; Coffey, D.M.; Holland, K.; Itano, D.; Leroy, B.; Kohin, S.; Vetter, R.; Williams, A.J.; Wren, J. Movements and habitat use of juvenile silky sharks in the Pacific Ocean inform conservation strategies. Fish. Res. 2019, 210, 131–142. [Google Scholar] [CrossRef]
- Wang, S.; Xu, L.; Wang, X. A review of impacts of fish aggregation devices (FADs) on feeding patterns for tropical tunas. Acta Ecol. Sin. 2014, 34, 3490–3498. [Google Scholar] [CrossRef][Green Version]
- Buckley, T.W.; Miller, B.S. Feeding habits of yellowfin tuna associated with fish aggregation devices in American Samoa. Bull. Mar. Sci. 1994, 55, 445–459. [Google Scholar]
- Weng, J.-S.; Lee, M.-A.; Liu, K.-M.; Hsu, M.-S.; Hung, M.-K.; Wu, L.-J. Feeding ecology of juvenile yellowfin tuna from waters southwest of Taiwan inferred from stomach contents and stable isotope analysis. Mar. Coast. Fish. 2015, 7, 537–548. [Google Scholar] [CrossRef]
- Jaquemet, S.; Potier, M.; Ménard, F. Do drifting and anchored Fish Aggregating Devices (FADs) similarly influence tuna feeding habits? A case study from the western Indian Ocean. Fish. Res. 2011, 107, 283–290. [Google Scholar] [CrossRef]
- Perez, I.; Guéry, L.; Authier, M.; Gaertner, D. Assessing the effectiveness of dFADs fishing moratorium in the Eastern Atlantic Ocean for conservation of juvenile tunas from AOTTP data. Fish. Res. 2022, 253, 106360. [Google Scholar] [CrossRef]
- Fei, J.; Li, C.; Zhang, J.; Teng, Y.; Wu, Y.; Shi, J. Effects of seamount characteristics in Central and Western Pacific Ocean on CPUEs of yellowfin tuna (Thunnus albacares) in longline and purse seine fisheries. South China Fish. Sci. 2024, 20, 1–10. Available online: https://schinafish.cn/en/article/Y2024/I2/1 (accessed on 5 April 2024).
- Jiang, M.; Chen, X.; Xu, Z.; Lin, H.; Lv, Z.; Lei, L.; He, H.; Jia, H.; Wang, J. Spatial Clustering Characteristics of Katsuwonus pelamis in West and Central Pacific Ocean and Its Relationship with ENSO. Period. Ocean. Univ. China 2023, 53, 47–54. [Google Scholar] [CrossRef]
- Chiang, W.-C.; Musyl, M.K.; Sun, C.-L.; DiNardo, G.; Hung, H.-M.; Lin, H.-C.; Chen, S.-C.; Yeh, S.-Z.; Chen, W.-Y.; Kuo, C.-L. Seasonal movements and diving behaviour of black marlin (Istiompax indica) in the northwestern Pacific Ocean. Fish. Res. 2015, 166, 92–102. [Google Scholar] [CrossRef]
- Farchadi, N.; Hinton, M.G.; Thompson, A.R.; Yin, Z.-Y. Habitat Preferences of Blue Marlin (Makaira nigricans) and Black Marlin (Istiompax indica) in the Eastern Pacific Ocean. Master’s Thesis, University of San Diego, San Diego, CA, USA, 2018. [Google Scholar]
- Perryman, R.J.Y. Social Organisation, Social Behaviour and Collective Movements in Reef Manta Rays. Ph.D. Thesis, Macquarie University, Sydney, Australia, 2022. [Google Scholar]
- Salvetat, J.; Lebourges-Dhaussy, A.; Travassos, P.; Gastauer, S.; Roudaut, G.; Vargas, G.; Bertrand, A. In situ target strength measurement of the black triggerfish Melichthys niger and the ocean triggerfish Canthidermis sufflamen. Mar. Freshw. Res. 2020, 71, 1118. [Google Scholar] [CrossRef]
- Forget, F. Behaviour and Trophic Ecology of Oceanic Triggerfish (Canthidermis maculata) and Rainbow Runner (Elagatis bipinnulata) Associated with Floating Objects in the Open Ocean. Ph.D. Thesis, Rhodes University, Grahamstown, South Africa, 2016. [Google Scholar]
- Forget, F.; Cowley, P.D.; Capello, M.; Filmalter, J.D.; Dagorn, L. Drifting along in the open-ocean: The associative behaviour of oceanic triggerfish and rainbow runner with floating objects. Mar. Environ. Res. 2020, 161, 104994. [Google Scholar] [CrossRef]
- Hart, R.; Calver, M.; Dickman, C. The index of relative importance: An alternative approach to reducing bias in descriptive studies of animal diets. Wildl. Res. 2002, 29, 415–421. [Google Scholar] [CrossRef]
- Mingist, M.; Gebremedhin, S. Diversity and abundance of fishes in Aveya River, Blue Nile Basin, Ethiopia. Int. J. Curr. Res. 2014, 6, 6466–6473. [Google Scholar]


| Marine Species Classifications | Family | Scientific Name | English Name | Code | FSCs | DFADs |
|---|---|---|---|---|---|---|
| Billfish and Barracudas | Istiophoridae | Istiompax indica | Black marlin | BLM | 2.04 × 10−2 | 3.46 × 10−2 |
| Kajikia audax | Striped marlin | MLS | 6.72 × 10−6 | |||
| Makaira nigricans | Blue marlin | BUM | 4.46 × 10−4 | 1.05 × 10−2 | ||
| Tetrapturus angustirostris | Shortbill spearfish | SSP | 2.46 × 10−6 | |||
| Xiphiidae | Xiphias gladius | Swordfish | SWO | 5.04 × 10−7 | ||
| Bottom-Dwelling Fish | Arcidae | Anadara spp. | Anadara clams nei | BLS | 2.02 × 10−7 | |
| Bothidae | Monolene antillarum | Slim flounder | BML | 3.07 × 10−6 | ||
| Gobiidae | Cryptocentrus filifer | / | YTF | 1.81 × 10−6 | ||
| Macrouridae | Coryphaenoides nasutus | Largenose grenadier | MHN | 3.17 × 10−7 | ||
| Percophidae | Percophis brasiliensis | Brazilian flathead | FLA | 2.34 × 10−6 | ||
| Soleidae | Dicologlossa cuneata | Wedge sole | CET | 2.96 × 10−7 | ||
| Dolphins and Whales | Delphinidae | Globicephala macrorhynchus | Short-finned pilot whale | SHW | 5.76 × 10−7 | |
| Globicephala macrorhynchus | Short-finned pilot whale | SHW | 5.76 × 10−7 | |||
| Grampus griseus | Risso’s dolphin | DRR | 3.84 × 10−7 | |||
| Grampus griseus | Risso’s dolphin | DRR | 3.84 × 10−7 | |||
| Pseudorca crassidens | False killer whale | FAW | 5.40 × 10−4 | |||
| Pseudorca crassidens | False killer whale | FAW | 5.40 × 10−4 | |||
| Stenella longirostris | Spinner dolphin | DSI | 2.30 × 10−6 | |||
| Stenella longirostris | Spinner dolphin | DSI | 2.30 × 10−6 | |||
| Steno bredanensis | Rough-toothed dolphin | RTD | 2.05 × 10−4 | 8.59 × 10−5 | ||
| Steno bredanensis | Rough-toothed dolphin | RTD | 2.05 × 10−4 | 8.59 × 10−5 | ||
| Tursiops truncatus | Bottlenose dolphin | DBO | 1.47 × 10−5 | |||
| Tursiops truncatus | Bottlenose dolphin | DBO | 1.47 × 10−5 | |||
| Others | Loricariidae | Otocinclus affinis | Golden otocinclus | OCA | 1.34 × 10−6 | |
| Megalopidae | Megalops cyprinoides | Indo-Pacific tarpon | TAI | 2.54 × 10−7 | ||
| Molidae | Masturus lanceolatus | Sharptail mola | MRW | 2.02 × 10−7 | ||
| Mola mola | Ocean sunfish | MOX | 9.75 × 10−6 | |||
| Ranzania laevis | Slender sunfish | RZV | 2.13 × 10−7 | |||
| Stomiidae | Eustomias lipochirus | / | FAI | 4.52 × 10−7 | ||
| Rays and Mantas | Dasyatidae | Pteroplatytrygon violacea | Pelagic stingray | PLS | 4.23 × 10−5 | |
| Mobulidae | Mobula birostris | Giant manta | RMB | 5.09 × 10−3 | 1.02 × 10−2 | |
| Mobula spp. | Mobula nei | RMV | 3.94 × 10−5 | 5.48 × 10−4 | ||
| Mobulidae | Mantas, devil rays nei | MAN | 4.46 × 10−5 | 4.27 × 10−5 | ||
| Urolophidae | Urolophus aurantiacus | Sepia stingray | RUU | 9.60 × 10−7 | ||
| Reef and Coastal Fish | Balistidae | Balistidae | Triggerfishes, durgons nei | TRI | 1.21 × 10−2 | |
| Canthidermis maculata | Rough triggerfish | CNT | 1.11 × 10−1 | |||
| Carangidae | Caranx sexfasciatus | Bigeye trevally | CXS | 1.28 × 10−5 | ||
| Decapterus macarellus | Mackerel scad | MSD | 8.58 × 10−1 | |||
| Elagatis bipinnulata | Rainbow runner | RRU | 2.09 | |||
| Gnathanodon speciosus | Golden trevally | GLT | 7.84 × 10−7 | |||
| Selar crumenophthalmus | Bigeye scad | BIS | 1.62 × 10−5 | |||
| Seriola lalandi | Yellowtail amberjack | YTC | 4.44 × 10−6 | |||
| Uraspis secunda | Cottonmouth jack | USE | 2.02 × 10−7 | |||
| Coryphaenidae | Coryphaena hippurus | Common dolphinfish | DOL | 6.99 × 10−4 | 2.03 × 10−2 | |
| Ephippidae | Platax teira | Longfin batfish | BAO | 1.82 × 10−6 | ||
| Kyphosidae | Kyphosus cinerascens | Blue sea chub | KYC | 1.85 × 10−4 | ||
| Monacanthidae | Aluterus monoceros | Unicorn leatherjacket filefish | ALM | 1.85 × 10−6 | ||
| Sea Turtles | Cheloniidae | Caretta caretta | Loggerhead turtle | TTL | 9.24 × 10−6 | |
| Chelonia mydas | Green turtle | TUG | 2.68 × 10−4 | 9.76 × 10−7 | ||
| Lepidochelys olivacea | Olive ridley turtle | LKV | 1.35 × 10−5 | |||
| Natator depressus | Flatback turtle | FBT | 1.73 × 10−6 | |||
| Sharks | Alopiidae | Alopias pelagicus | Pelagic thresher | PTH | 1.92 × 10−7 | |
| Alopias superciliosus | Bigeye thresher | BTH | 3.86 × 10−6 | |||
| Carcharhinidae | Carcharhinus falciformis | Silky shark | FAL | 4.57 × 10−1 | 2.89 | |
| Carcharhinus longimanus | Oceanic whitetip shark | OCS | 3.57 × 10−4 | 5.90 × 10−3 | ||
| Galeocerdonidae | Galeocerdo cuvier | Tiger shark | TIG | 1.92 × 10−7 | ||
| Lamnidae | Isurus oxyrinchus | Shortfin mako | SMA | 4.54 × 10−6 | ||
| Rhincodontidae | Rhincodon typus | Whale shark | RHN | 1.79 × 10−2 | 1.57 × 10−4 | |
| Sphyrnidae | Sphyrna lewini | Scalloped hammerhead | SPL | 8.92 × 10−5 | ||
| Sphyrna mokarran | Great hammerhead | SPK | 2.22 × 10−6 | |||
| Tunas | Scombridae | Acanthocybium solandri | Wahoo | WAH | 7.27 × 10−3 | |
| Auxis thazard | Frigate tuna | FRI | 2.13 × 10−7 | |||
| Euthynnus affinis | Kawakawa | KAW | 1.18 × 10−6 | |||
| Katsuwonus pelamis | Skipjack tuna | SKJ | 8.02 × 103 | 1.85 × 104 | ||
| Thunnus albacares | Yellowfin tuna | YFT | 1.92 × 102 | 3.01 × 102 | ||
| Thunnus obesus | Bigeye tuna | BET | 4.97 × 10−2 | 3.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fei, J.; Zhang, J.; Wang, X.; Wu, Y.; Teng, Y. A Catch Community Diversity Analysis of Purse Seine in the Tropical Western and Central Pacific Ocean. Fishes 2025, 10, 164. https://doi.org/10.3390/fishes10040164
Fei J, Zhang J, Wang X, Wu Y, Teng Y. A Catch Community Diversity Analysis of Purse Seine in the Tropical Western and Central Pacific Ocean. Fishes. 2025; 10(4):164. https://doi.org/10.3390/fishes10040164
Chicago/Turabian StyleFei, Jiaojiao, Jian Zhang, Xiao Wang, Yuntao Wu, and Yuxiu Teng. 2025. "A Catch Community Diversity Analysis of Purse Seine in the Tropical Western and Central Pacific Ocean" Fishes 10, no. 4: 164. https://doi.org/10.3390/fishes10040164
APA StyleFei, J., Zhang, J., Wang, X., Wu, Y., & Teng, Y. (2025). A Catch Community Diversity Analysis of Purse Seine in the Tropical Western and Central Pacific Ocean. Fishes, 10(4), 164. https://doi.org/10.3390/fishes10040164





