Effects of CO2 and O2 in Modified Atmosphere Packaging on Water Retention, Protein Stability, and Microbial Growth in Atlantic Salmon Fillets
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Modified Atmosphere Packaging
2.2. Microbiological Analysis
2.3. Total Volatile Basic Nitrogen (TVB-N) Analysis
2.4. Cooking Loss Analysis
2.5. Protein Oxidation Determination
2.6. SDS–Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.7. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.8. Low-Field Nuclear Magnetic Resonance (LF-NMR) Analysis
2.9. Statistical Analysis
3. Results
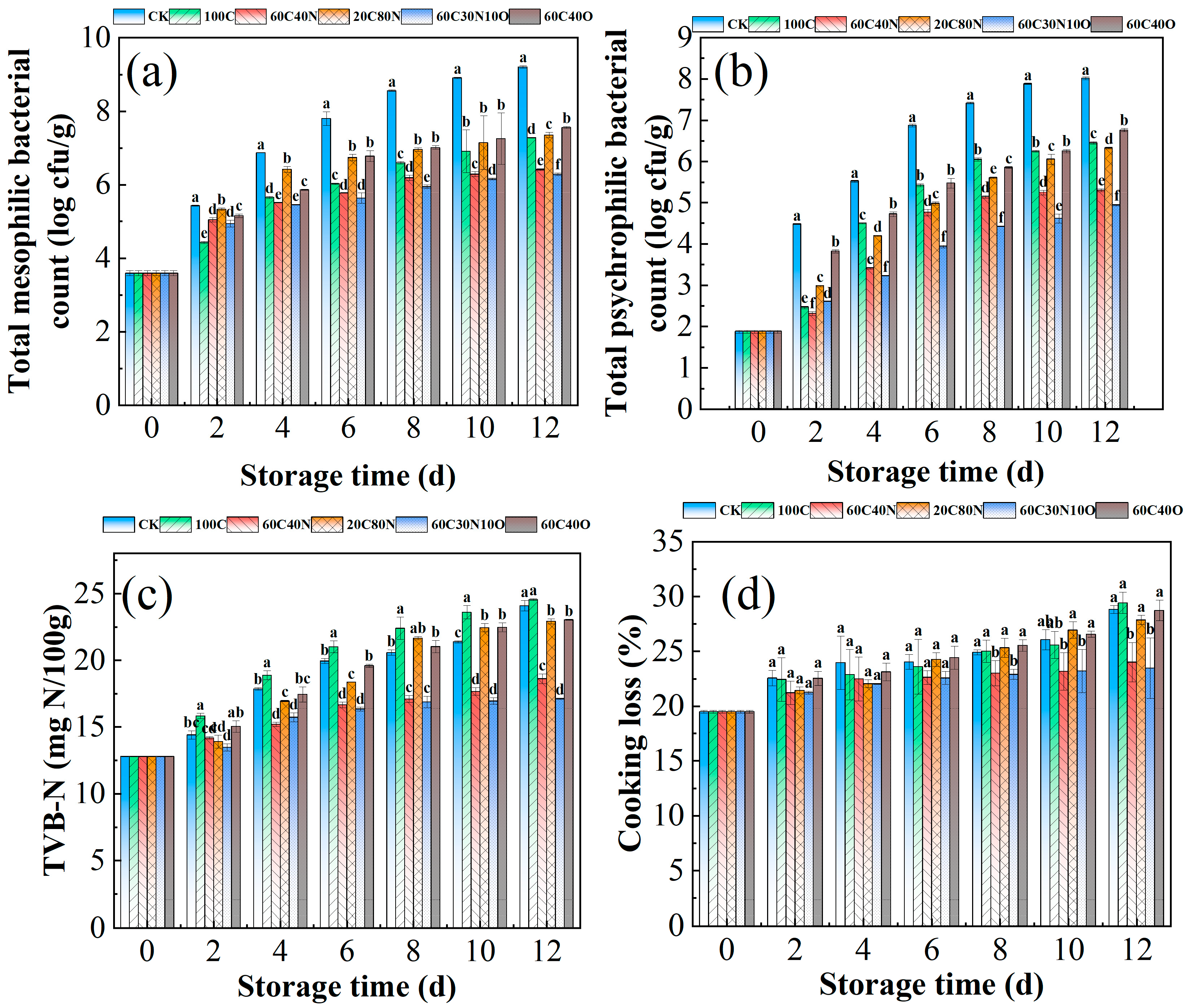
3.1. Microbial Growth
3.2. Changes in TVB-N Content
3.3. Changes in Cooking Loss
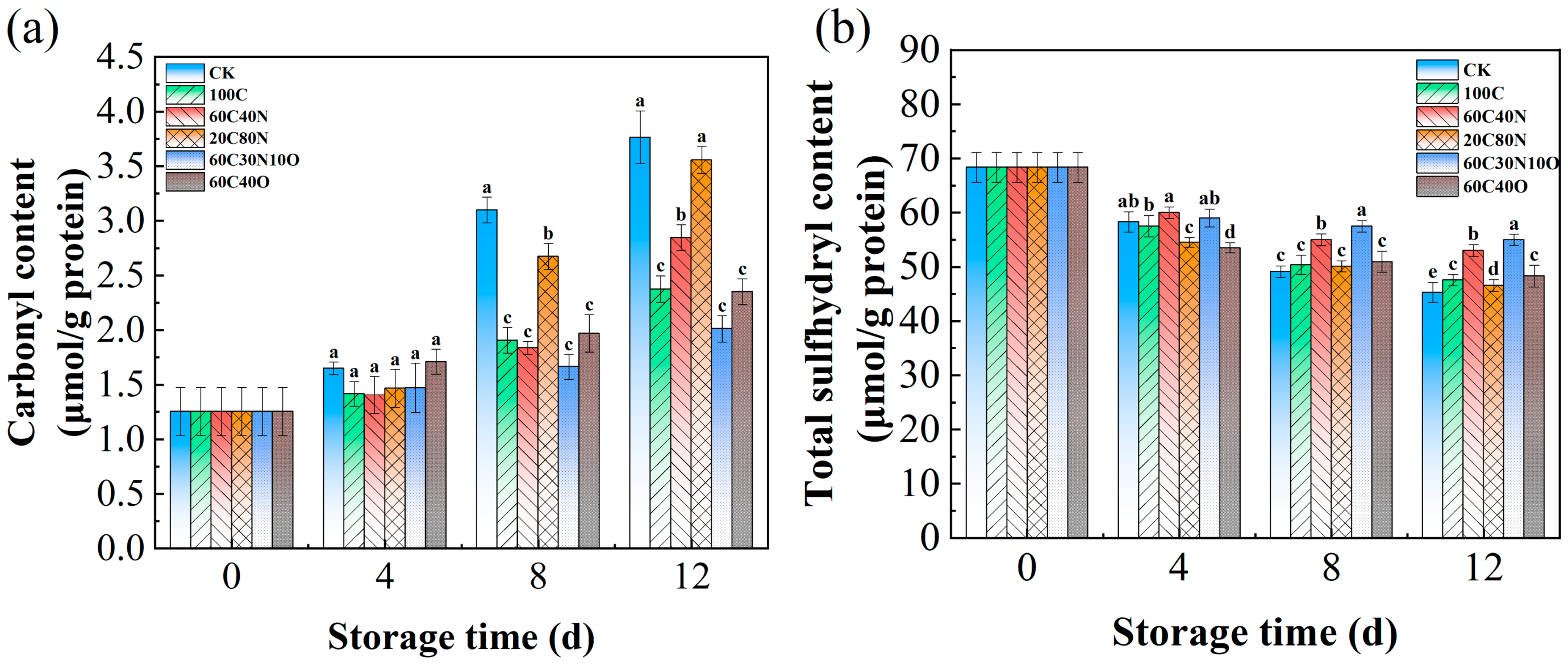
3.4. Changes of Carbonyl Content and Total Sulfhydryl Content
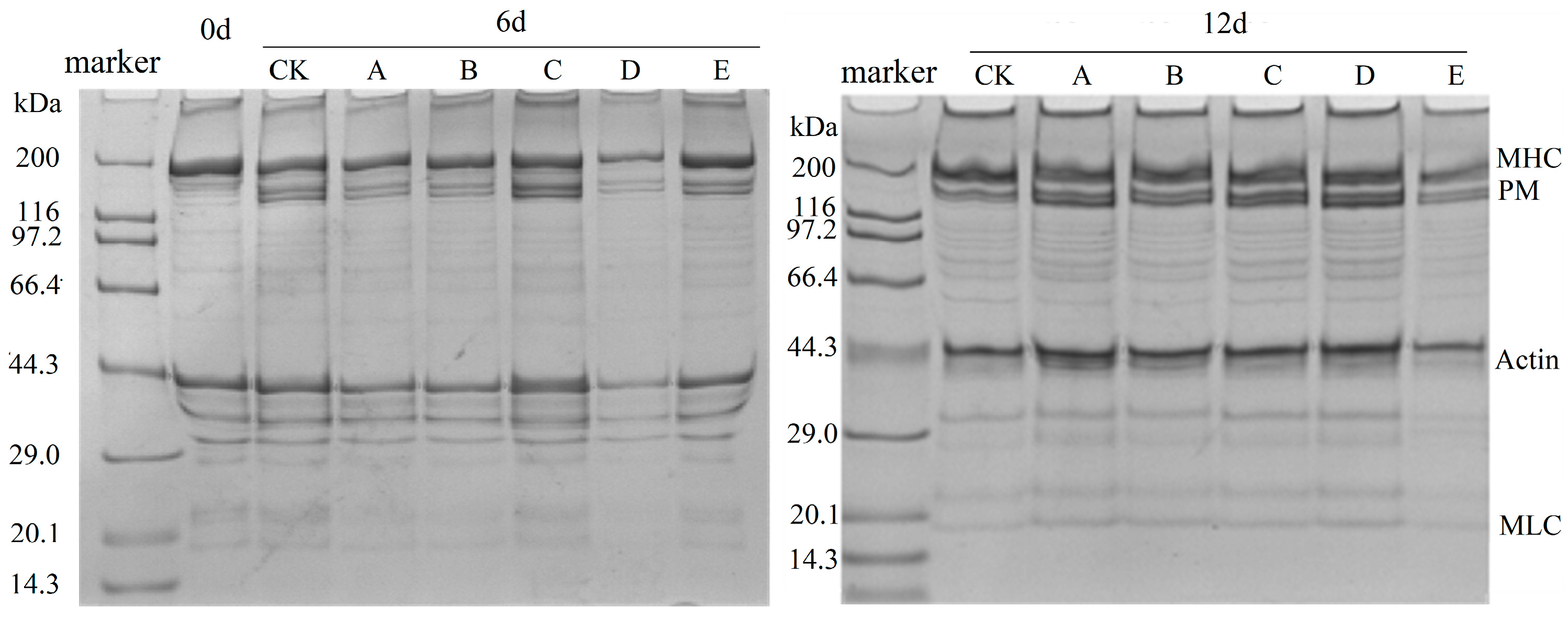
3.5. SDS-PAGE
3.6. Secondary Structure
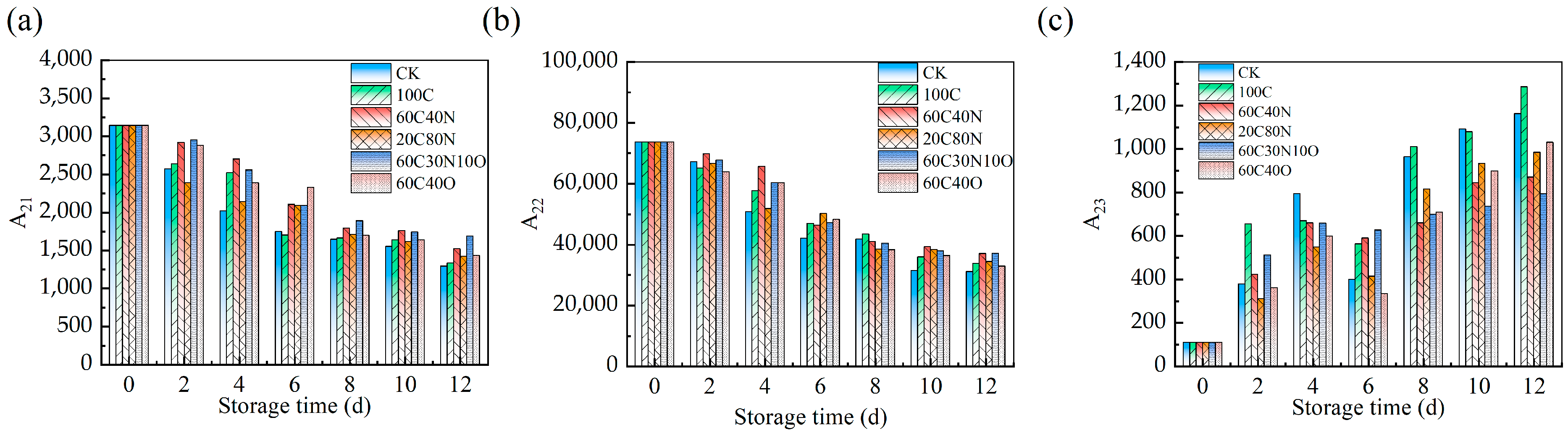
3.7. LF-NMR
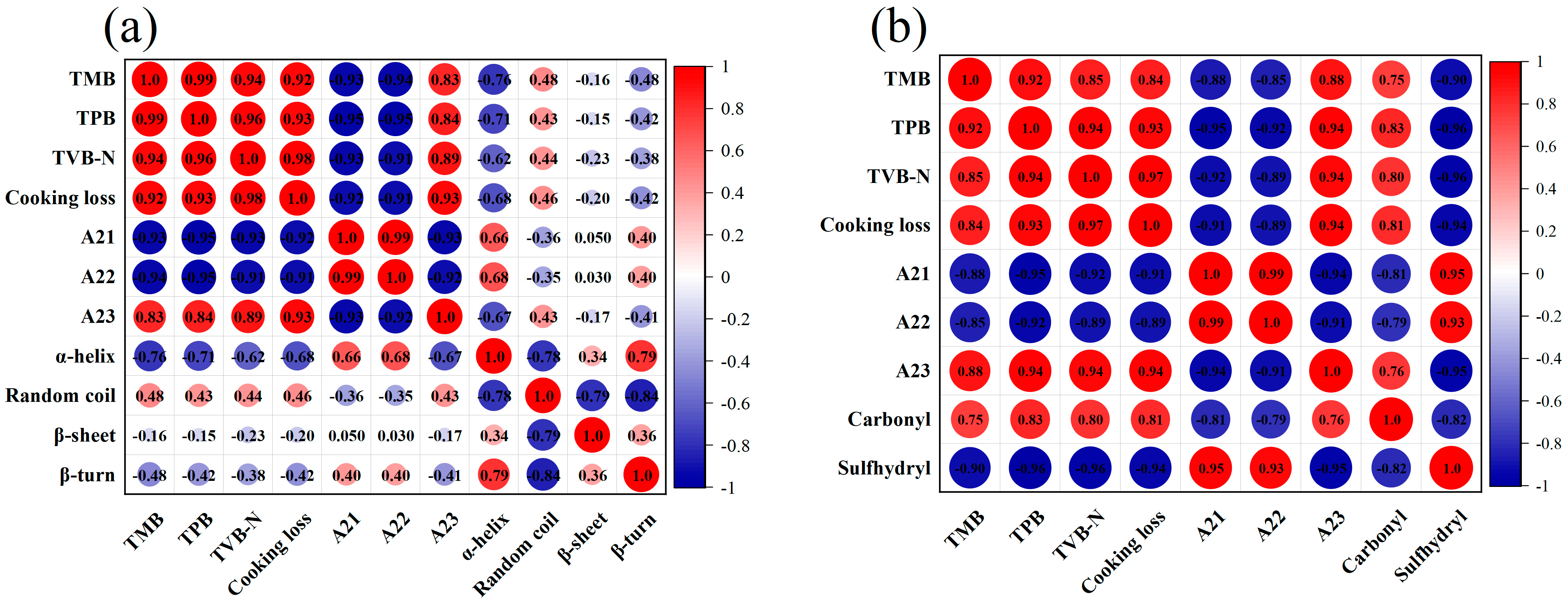
3.8. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chan, S.S.; Roth, B.; Jessen, F.; Løvdal, T.; Jakobsen, A.N.; Lerfall, J. A comparative study of Atlantic salmon chilled in refrigerated seawater versus on ice: From whole fish to cold-smoked fillets. Sci. Rep. 2020, 10, 17160. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Majumdar, R.K.; Mehta, N.K.; Ngasotter, S.; Gaurav, K.; Tripathi, M. Evaluating the efficacy of citrus fruit peel extract in preserving the quality of silver carp (Hypophthalmichthys molitrix) surimi during frozen storage. J. Agric. Food Res. 2024, 18, 101440. [Google Scholar] [CrossRef]
- Chan, S.S.; Roth, B.; Skare, M.; Hernar, M.; Jessen, F.; Løvdal, T.; Jakobsen, A.N.; Lerfall, J. Effect of chilling technologies on water holding properties and other quality parameters throughout the whole value chain: From whole fish to cold-smoked fillets of Atlantic salmon (Salmo salar). Aquaculture 2020, 526, 735381. [Google Scholar] [CrossRef]
- Nawaz, A.; Irshad, S.; Ali Khan, I.; Khalifa, I.; Walayat, N.; Muhammad Aadil, R.; Kumar, M.; Wang, M.; Chen, F.; Cheng, K.-W.; et al. Protein oxidation in muscle-based products: Effects on physicochemical properties, quality concerns, and challenges to food industry. Food Res. Int. 2022, 157, 111322. [Google Scholar] [CrossRef]
- Bao, Y.; Boeren, S.; Ertbjerg, P. Myofibrillar protein oxidation affects filament charges, aggregation and water-holding. Meat Sci. 2018, 135, 102–108. [Google Scholar] [CrossRef]
- Nikoo, M.; Benjakul, S.; Ahmadi Gavlighi, H.; Xu, X.; Regenstein, J.M. Hydrolysates from rainbow trout (Oncorhynchus mykiss) processing by-products: Properties when added to fish mince with different freeze-thaw cycles. Food Biosci. 2019, 30, 100418. [Google Scholar] [CrossRef]
- Bai, X.; Yin, F.; Ru, A.; Li, M.; Tian, W.; Zhang, G.; Chen, Q.; Chai, R.; Liu, Y.; Cui, W.; et al. Myosin heavy chain isoform expression and meat quality characteristics of different muscles in yak (Bos grunniens). Meat Sci. 2024, 209, 109414. [Google Scholar] [CrossRef]
- Cao, L.; Rasco, B.A.; Tang, J.; Niu, L.; Lai, K.; Fan, Y.; Huang, Y. Effects of freshness on the cook loss and shrinkage of grass carp (Ctenopharyngodon idellus) fillets following pasteurization. Int. J. Food Prop. 2016, 19, 2297–2306. [Google Scholar] [CrossRef]
- Van Haute, S.; Raes, K.; Devlieghere, F.; Sampers, I. Combined use of cinnamon essential oil and MAP/vacuum packaging to increase the microbial and sensorial shelf life of lean pork and salmon. Food Packag. Shelf Life 2017, 12, 51–58. [Google Scholar] [CrossRef]
- Wang, G.; Ma, F.; Zeng, L.; Bai, Y.; Wang, H.; Xu, X.; Zhou, G. Modified atmosphere packaging decreased Pseudomonas fragi cell metabolism and extracellular proteolytic activities on meat. Food Microbiol. 2018, 76, 443–449. [Google Scholar] [CrossRef]
- Liang, Z.; Veronica, V.; Huang, J.; Zhang, P.; Fang, Z. Combined effects of plant food processing by-products and high oxygen modified atmosphere packaging on the storage stability of beef patties. Food Control 2022, 133, 108586. [Google Scholar] [CrossRef]
- Tan, N.; Shao, Y.; Xu, Y.; Li, Z.; Huang, Z.; Zhang, W.; Deng, S.; Zhang, B.; Zhang, L.; Yuan, P. Tandem mass tag-based quantitative proteomics analysis of modified atmosphere packaging in large yellow croaker (Pseudosciaena crocea) fillets during refrigerated storage. Food Chem. 2025, 463, 141744. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hu, S.; Gao, Y.; Ye, C.; Wang, H. Effect of collagen-lysozyme coating on fresh-salmon fillets preservation. LWT-Food Sci. Technol. 2017, 75, 59–64. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Q.; Hong, H.; Luo, Y. Prevention of protein oxidation and enhancement of gel properties of silver carp (Hypophthalmichthys molitrix) surimi by addition of protein hydrolysates derived from surimi processing by-products. Food Chem. 2020, 316, 126343. [Google Scholar] [CrossRef] [PubMed]
- Niamnuy, C.; Devahastin, S.; Soponronnarit, S. Changes in protein compositions and their effects on physical changes of shrimp during boiling in salt solution. Food Chem. 2008, 108, 165–175. [Google Scholar] [CrossRef]
- Eranda, D.H.U.; Chaijan, M.; Uysal-Unalan, I.; Panpipat, W.; Naik, A.S.; Dib, A.L.; Karnjanapratum, S.; Gagaoua, M. Biopreservation of pre-processed fresh fish by bio-based coatings: A single strategy with multiple benefits towards waste prevention. Food Biosci. 2024, 58, 103696. [Google Scholar] [CrossRef]
- Chen, B.; Mei, J.; Xie, J. Effects of packaging methods and temperature variations on the quality and microbial diversity of grouper (Epinephelus Lanceolatus) during cold storage. Food Biosci. 2024, 60, 104315. [Google Scholar] [CrossRef]
- Ozogul, F.; Durmuş, M.; Kosker, A.R.; Özkütük, A.S.; Kuley, E.; Yazgan, H.; Yazgan, R.; Simat, V.; Ozogul, Y. The impact of marine and terrestrial based extracts on the freshness quality of modified atmosphere packed sea bass fillets. Food Biosci. 2023, 53, 102545. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Liu, X.; Lei, Y.; Regenstein, J.M.; Luo, Y. Characterization of the microbial composition and quality of lightly salted grass carp (Ctenopharyngodon idellus) fillets with vacuum or modified atmosphere packaging. Int. J. Food Microbiol. 2019, 293, 87–93. [Google Scholar] [CrossRef]
- Qian, Y.; Liu, C.; Zhang, J.; Ertbjerg, P.; Yang, S. Effects of modified atmosphere packaging with varied CO2 and O2 concentrations on the texture, protein, and odor characteristics of salmon during cold storage. Foods 2022, 11, 3560. [Google Scholar] [CrossRef]
- Yang, Z.; Yan, J.; Xie, J. Effect of vacuum and modified atmosphere packaging on moisture state, quality, and microbial communities of grouper (Epinephelus coioides) fillets during cold storage. Food Res. Int. 2023, 173, 113340. [Google Scholar] [CrossRef]
- Lerfall, J.; Bjørge Thomassen, G.M.; Jakobsen, A.N. Quality of fresh saithe (Pollachius virens) in modified atmosphere packages as affected by the gas composition. Food Packag. Shelf Life 2018, 18, 147–156. [Google Scholar] [CrossRef]
- McSharry, S.; Koolman, L.; Whyte, P.; Bolton, D. An investigation of the survival and/or growth of Clostridioides (Clostridium) difficile in beef stored under aerobic, anaerobic and commercial vacuum packaging conditions at 2 °C and 20 °C. Food Control 2021, 119, 107475. [Google Scholar] [CrossRef]
- Bekhit, A.E.-D.A.; Holman, B.W.B.; Giteru, S.G.; Hopkins, D.L. Total volatile basic nitrogen (TVB-N) and its role in meat spoilage: A review. Trends Food Sci. Technol. 2021, 109, 280–302. [Google Scholar] [CrossRef]
- Vargas Baldi, S.C.; Parisi, G.; Bonelli, A.; Balieiro, J.C.C.; Lapa Guimarães, J.; Macedo Viegas, E.M. Effects of different stunning/slaughter methods on frozen fillets quality of cobia (Rachycentron canadum). Aquaculture 2018, 486, 107–113. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Wang, H.; Zhou, F.; Wu, Y.; Ma, H.; Zhao, R.; He, J.; Gu, Z. Effect of ultrasonic thawing temperature on the quality of quick-frozen small yellow croaker (Larimichthys polyactis) and its possible mechanisms. LWT-Food Sci. Technol. 2023, 179, 114620. [Google Scholar] [CrossRef]
- Sun, B.; Zhao, Y.; Yu, J.; Ling, J.; Shang, H.; Liu, Z. The combined efficacy of superchilling and high CO2 modified atmosphere packaging on shelf life and quality of swimming crab (Portunus trituberculatus). J. Aquat. Food Prod. Technol. 2017, 26, 655–664. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, J.; Zhang, R.; Zhou, L.; Ni, L.; Zhang, W. Study on the effects of pre-slaughter transport stress on water holding capacity of pork: Insights from oxidation, structure, function, and degradation properties of protein. Food Chem. X 2024, 24, 101913. [Google Scholar] [CrossRef]
- He, Y.; Huang, H.; Li, L.; Yang, X.; Hao, S.; Chen, S.; Deng, J. The effects of modified atmosphere packaging and enzyme inhibitors on protein oxidation of tilapia muscle during iced storage. LWT-Food Sci. Technol. 2018, 87, 186–193. [Google Scholar] [CrossRef]
- Vate, N.K.; Benjakul, S. Combined effect of squid ink tyrosinase and tannic acid on heat induced aggregation of natural actomyosin from sardine. Food Hydrocol. 2016, 56, 62–70. [Google Scholar] [CrossRef]
- Li, P.; Zhou, Q.; Chu, Y.; Lan, W.; Mei, J.; Xie, J. Effects of chitosan and sodium alginate active coatings containing ε-polysine on qualities of cultured pufferfish (Takifugu obscurus) during cold storage. Int. J. Biol. Macromol. 2020, 160, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tan, Y.; Luo, Y.; Li, X.; Hong, H. Evidence of myofibrillar protein oxidation and degradation induced by exudates during the thawing process of bighead carp fillets. Food Chem. 2024, 434, 137396. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Hu, J.; Gao, B.; Zhang, B.; Li, P.; Shang, N. Effects of the interaction between Aeromonas sobria and Macrococcus caseolyticus on protein degradation of refrigerated sturgeon fillets: Novel perspective on fish spoilage. LWT-Food Sci. Technol. 2023, 183, 114908. [Google Scholar] [CrossRef]
- Martínez, M.A.; Velazquez, G.; Cando, D.; Núñez-Flores, R.; Borderías, A.J.; Moreno, H.M. Effects of high pressure processing on protein fractions of blue crab (Callinectes sapidus) meat. Innov. Food Sci. Emerg. Technol. 2017, 41, 323–329. [Google Scholar] [CrossRef]
- Chan, S.S.; Roth, B.; Jessen, F.; Jakobsen, A.N.; Lerfall, J. Water holding properties of Atlantic salmon. Compr. Rev. Food Sci. Food Saf. 2022, 21, 477–498. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, Y.-F.; Shi, C.-J.; Liu, C.-C.; Zhang, J.-J.; Yang, S.-P. Effects of CO2 and O2 in Modified Atmosphere Packaging on Water Retention, Protein Stability, and Microbial Growth in Atlantic Salmon Fillets. Fishes 2025, 10, 141. https://doi.org/10.3390/fishes10040141
Qian Y-F, Shi C-J, Liu C-C, Zhang J-J, Yang S-P. Effects of CO2 and O2 in Modified Atmosphere Packaging on Water Retention, Protein Stability, and Microbial Growth in Atlantic Salmon Fillets. Fishes. 2025; 10(4):141. https://doi.org/10.3390/fishes10040141
Chicago/Turabian StyleQian, Yun-Fang, Cheng-Jian Shi, Cheng-Cheng Liu, Jing-Jing Zhang, and Sheng-Ping Yang. 2025. "Effects of CO2 and O2 in Modified Atmosphere Packaging on Water Retention, Protein Stability, and Microbial Growth in Atlantic Salmon Fillets" Fishes 10, no. 4: 141. https://doi.org/10.3390/fishes10040141
APA StyleQian, Y.-F., Shi, C.-J., Liu, C.-C., Zhang, J.-J., & Yang, S.-P. (2025). Effects of CO2 and O2 in Modified Atmosphere Packaging on Water Retention, Protein Stability, and Microbial Growth in Atlantic Salmon Fillets. Fishes, 10(4), 141. https://doi.org/10.3390/fishes10040141





