Abstract
A number of viral diseases have significantly impeded the growth of the aquaculture industry. Antiviral drugs represent an effective means of controlling infection. However, the efficacy of the entire therapeutic process is contingent upon the availability of an efficient delivery system. This study selected three common antiviral drugs and constructed corresponding drug delivery systems utilising single-walled carbon nanotubes (SWCNTs) as carriers. The reliability of carbon nanotubes as delivery carriers was evaluated by detecting the therapeutic effect on infectious splenic and renal necrosis virus (ISKNV). The findings demonstrated that SWCNTs can effectively enhance the absorption of the three drugs into the body, prolong their metabolic half-life, and improve the survival rate of fish infected with ISKNV. The Ribavirin-SWCNTs (RBV-SWCNTs) group exhibited the most pronounced protective effect, with a mortality rate of less than 25%. It was observed that SWCNTs facilitated the rapid transportation of ribavirin, with the drug content in the RBV-SWCNTs group being approximately double that of the free ribavirin group. Furthermore, this system markedly diminished the viral load, augmented enzyme activities, and elevated antiviral gene expression. This study indicated that carbon nanotubes are optimal carriers for antiviral drugs, which have considerable potential as a delivery vehicle for antiviral drugs to prevent viral infections in aquaculture.
Keywords:
carbon nanotubes; drug delivery system; ribavirin; infectious spleen and kidney necrosis virus; mandarin fish Key Contribution:
The utilisation of nano delivery systems has emerged as a promising approach to enhance the efficiency of drug delivery to fish, thereby stimulating heightened immune responses. This strategy offers a novel potential treatment modality for fish diseases.
1. Introduction
Antiviral drugs have become crucial tools for controlling viruses due to their ability to inhibit viral replication, reduce viral loads, and enhance immune responses [1,2]. However, a significant challenge remains in the low bioavailability of many drugs, with a considerable proportion of the administered dose being metabolised and excreted from the body before it can exert its antiviral effects [3,4,5]. It is therefore imperative to develop appropriate drug delivery systems that can increase the amount of drug entering the body and prolong its metabolic duration, thereby enhancing its antiviral efficacy [6]. Inorganic nanomaterials, due to their excellent biocompatibility, drug-loading capacity, and enhanced permeability and retention effect, can carry a significant number of drugs and remain in the body for an extended period, substantially improving drug efficacy [7,8,9,10]. Carbon nanotubes, the novel nanomaterial with high penetration and biocompatibility, have been shown to be safe and efficient for delivering drugs by immersion treatment [2,11,12,13]. Previous studies have suggested that single-walled carbon nanotube delivery systems (SWCNTs) can significantly increase the content of substances entering the body, thereby reducing the dosage of drugs and improving the therapeutic effect [14,15,16].
Antiviral agents, including ribavirin (RBV, 1-(β-D-ribofuranosyl)-1,2,4-triazole-3-carboxamide), cytarabine (Ara-C, cytosine β-D-arabinofuranoside), and the antiviral agent acyclovir (ACV, 9-[(2-hydroxyethoxy)methyl]guanine), have been employed to control a range of viruses, including herpesviruses and cytomegaloviruses [17,18]. Ribavirin has been employed in clinical settings due to its efficacy against a multitude of human infectious diseases. It is a synthetic guanosine nucleotide analogue with antiviral activity against both DNA and RNA viruses [19,20]. However, the mechanisms of antiviral interplay remain poorly understood. One such mechanism that ribavirin has demonstrated is the ability to undergo rapid phosphorylation upon entering virus-infected cells, competitively inhibiting protein synthesis and thereby impeding viral replication and dissemination [21,22,23,24,25,26]. Ribavirin exhibited a good antiviral effect that effectively inhibited the replication of viral hemorrhagic septicemia virus (VHSV) and Micropterus salmoides rhabdovirus (MSRV), and it can be used to control viral diseases in aquaculture [3,27,28,29]. Cytarabine, a pyrimidine nucleoside analogue, is usually used against herpes simplex virus and cytomegalovirus [1,30,31]. After being phosphorylated, cytarabine inhibits DNA polymerase activity and triggers apoptosis [32,33]. Acyclovir, a nucleoside analogue, is usually used against a cytomegalovirus (CMV) infection and has been the major antiviral therapy for herpesvirus infections [8,34,35]. Its mechanism works through inhibiting viral replication by interfering with viral DNA polymerase or interrupting the elongation of DNA strands [7,34,36,37]. Acyclovir is proven to have a strong antiviral effect as a safe and effective candidate antiviral agent, which can effectively inhibit virus replication while maintaining normal cell morphology and structure [38,39].
Infectious spleen and kidney necrosis virus (ISKNV) is a viral disease that primarily infects mandarin fish (Siniperca chuatsi), which holds significant economic value in the aquaculture industry of China [40,41]. According to the virus classification report, ISKNV, a double strand DNA macromolecular virus, is the type species of Megalocytivirus genus in the family of Iridoviridae [42]. The main species infected with ISKNV is the mandarin fish, with a mortality rate of over 90% after two weeks of culture at 28 °C. This is accompanied by characteristic pathological changes and necrosis of the spleen and kidneys, as indicated by the name of the virus [43]. Currently, there are no approved drugs on the market; therefore, the development of effective anti-ISKNV measures in Siniperca chuatsi would contribute to the development of mandarin fish aquaculture [44]. Our study selected three common antiviral drugs to construct a drug delivery system that uses carbon nanotubes as carriers. Using ISKNV as the viral model, we evaluated whether SWCNTs could serve as an ideal drug delivery carrier through assessing the metabolism of the drug delivery system in vivo and its antiviral efficacy. The findings of this research will provide credible evidence for the application of carbon nanotubes in drug delivery systems and viral therapy.
2. Materials and Methods
2.1. Compounds, Virus, and Experimental Fish
Ribavirin (CAS No.: 36791-04-5), Cytarabine (CAS No.: 147-94-4), Acyclovir (CAS No.: 59277-89-3), and other chemicals agents were purchased from Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). The fish used in our experiments were obtained from a local fishery in Jiangmen (Guangdong, China). The raw SWCNTs were purchased from Time Nano Co., Ltd. (Chengdu, China).
Mandarin fish (3.0 ± 0.5 g) tested without ISKNV [45] were cultivated in tanks with a flow-through system and feed twice daily. The viruses (ISKNV-QY) were kindly provided by Professor Xiao-Zhe Fu (Pearl River Fisheries Research Institute, Guangdong, China) and propagated in a previous study [10]. All of the experimental animals were handled in accordance with the regulations of the Experiment Animal Center, Northwest A&F University (Approval Code: DK2021132; Approval Date: 5 March 2021).
2.2. Preparation and Characterization of Drug Delivery Systems
Oxidized SWCNTs were prepared through the previous study [46], 4 g of oxidized SWCNTs and 3 g of N-Hydroxy succinimide (NHS, Macklin, Shanghai, China, CAS No.: 6066-82-6) were added into 300 mL of N,N-Dimethylformamide (DMF, Macklin, China, CAS No.: 68-12-2) and stirred for 30 min. Then, 4.0 g of RBV and 1.0 mL of N,N’-Diisopropylcarbodiimide (DIC, Macklin, China, CAS No.: 693-13-0) were added and stirred at room temperature for 48 h. The reaction products were freeze-dried to obtain the drug delivery system (RBV-SWCNTs). Ara-C-SWCNTs and ACV-SWCNTs were obtained following the same procedure. All three linkage products were characterised by means of scanning electron microscopy (SEM, Zeiss Gemini 300, Oberkochen, Germany) observations, with the objective of determining the drug structure when forming carbon nanotube linkages.
2.3. Drugs Loadings in Delivery System
Drug loading was calculated by the content of the drug and the mass of the delivery system. Standard samples were prepared by spiking the blank mobile phase with different quantities (1, 5, 10, 20, 40, 80, 200 μg/μL) of drugs. Standard curves were prepared by a linear regression analysis involving comparing the peak area of drugs against the concentration of drugs. The delivery systems with a fixed mass were precisely weighed, then extracted and cleaned using acetonitrile. The product was dried at 60 °C and dissolved in the mobile phase with a 0.22 μm membrane filter. The mobile phase of RBV was consisted by H2O and methanol (30:70, V/V). The mobile phase of Ara-C was consisted by acetonitrile and a 0.01 mol/L phosphate solution (5:95, V/V). The mobile phase of ACV was consisted by H2O and methanol (10:90, V/V). Then, the contents of the drug in the delivery system were determined by an Agilent 1260 infinity II HPLC System (Agilent Technologies (USA) Co., Ltd., Santa Clara, CA, USA). A thermostatted column compartment was used to maintain the column temperature at 25 °C. The C18 analytical column (4.6 mm × 250 mm, 5 μm) was used with a 1.0 mL/min flow rate. The variable wavelength detector of the HPLC system was used to test the RBV at 207 nm, the Ara-C was tested at 280 nm, and the ACV was tested at 252 nm. Drug loading efficiencies were calculated as shown below.
Drug loading efficiencies = (Drug content detected/Total drug inputs) × 100%
2.4. Drugs Metabolism in Kidney Tissue
The mandarin fish were randomly divided into drug (RBV, Ara-C, and ACV) and delivery system (RBV-SWCNTs, Ara-C-SWCNTs, and ACV-SWCNTs) groups (40 mg/L, 180 fish per group, three replicates per group) for 6 h, then washed by fresh water and transferred to new tanks with fresh water. From the beginning of drug treatment, the kidney tissues from 5 fish in each group were sampled at a specific observation time according to the previous study [47]. Subsequently, the tissue samples were processed in the same way as before to examine drug metabolism, and the same assay and conditions were detected by the HPLC System.
Then, the test method for the metabolism of drugs was validated and QC (quality control) samples were prepared by spiking the blank mobile phase with different quantities (60, 80, and100 μg/mL) of RBV. The QC samples were used to validate the intra-day precision (counted a single run), inter-day precision (among different runs), and the recovery of the analytical method [48,49].
2.5. Infection of Mandarin Fish with ISKNV and Drug Treatment
Before the experiments, to infect the ISKNV-free mandarin fish, they were placed in tanks with live ISKNV (6 × 107 TCID50/L) for 6 h, then randomly transferred to 8 groups (180 fish per groups with three replicates) for drug treatment, including a control group (PBS), an SWCNTs group (40 mg/L SWCNTs alone), drug groups (40 mg/L), and delivery system groups (40 mg/L).
After 6 h of drug treatment via baths, the fish were replaced in new tanks, and monitored (43 fish per groups) after the drug treatments. The survival of the mandarin fish following drug treatments was monitored and documented on a daily basis up to the ninth day. Thereafter, the cumulative mortality rate of the mandarin fish in the various treatment groups was calculated statistically.
2.6. qPCR Analysis of Infection Rates of ISKNV and Expression of Immune-Related Genes
After drug treatments, liver, spleen and kidney samples were taken from 11 fish per group. DNA from the samples was extracted using the by TAINamp Marine Animals DNA Kits (Tiangen biotech (China) Co., Ltd., Beijing, China), then qPCR was used to detect the presence of ISKNV and analyse the infection rates.
Spleen and kidney tissues (5 fish per group) were collected and extracted from the total RNA by a Trizol reagent (Takara, China) following the instructions of the manufacturer. Then, cDNA synthesis using the All-in-One First-Strand Synthesis MasterMix kit (Jiangsu Yugong Biotech Co., Ltd., Lianyungang, China) was performed following the manufacturer’s instructions. The product of the cDNA was used to resolve the expression of immune-related genes (I-IFN, type I interferon; Mx, Mx protein; TNF-α, tumour necrosis factor α; MHC I, major histocompatibility complex class I; Viperin, virus inhibitory protein; and IRF-1, interferon-regulatory factor 1) by qPCR, which was performed by the StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) with three replicates. The samples were mixed well and placed in a real-time fluorescence quantitative PCR instrument, and the reaction was carried out according to the following procedure program: 95 °C pre-denaturation for 30 s; 95 °C denaturation for 10 s; 60 °C annealing for 30 s; and repeat for 40 cycles. The levels of expression were statistied by the 2−ΔΔCt method [50]. The primers for the immune-related genes and virus (ISKNV-MCP) are listed in Table 1.

Table 1.
Primers used for the analysis of mRNA expression by qPCR.
2.7. Measurement of Enzyme Activities
After treatment, 5 fish from each group were collected to obtain the serums by standing the blood overnight and centrifuging it for 15 min with 5000 g. The serums were used to analyse the immune indexes including superoxide dismutase (SOD), acid phosphatase (ACP), alkaline phosphatase (AKP) activities, and alternative complement (C3) content; these were measured following the instructions of and using the assay kits (Nanjing Jiancheng Bioengineering Institute Co., Ltd., Nanjing, China).
2.8. Statistical Analysis
All statistical analyses were performed using SPSS 24.0 software (IBM Inc., USA). To assess the exposure data, all data are expressed as mean ± standard deviation (SD), and were analysed by one-way ANOVA. In all cases, different letters showed significantly different p-values (p < 0.05) among the treatments.
3. Results
3.1. Synthesis and Characterization of Drug Delivery Systems
The drug delivery system is a black powdery solid, which is fluffy and disperses quickly in water, and does not easily gather and settle at the bottom of the water. SEM observation revealed that the SWCNTs retained their tubular elongated structure. Furthermore, the drug was observed to be connected to the surface of the drug delivery systems in all groups after treatment (Figure 1).
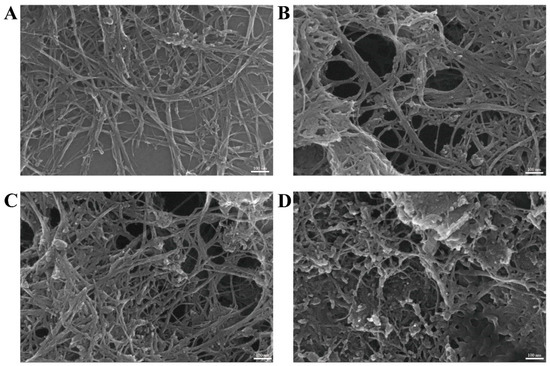
Figure 1.
Drug delivery system SEM observations: (A) SWCNTs; (B) RBV-SWCNTs; (C) Ara-C-SWCNTs; and (D) ACV-SWCNTs.
3.2. Drug Loading in Delivery Systems and Drugs Metabolism in Kidney Tissues
The correlation coefficients of the regression equations were all higher than 0.95, they showed the peak area of the standard samples, and the drugs showed a good linearity (Figure 2). So, we used the regression equation as the standard curve to calculate the drug loadings of the delivery systems. The loading efficiencies of the RBV, Ara-C, and ACV were 20.49%, 21.46%, and 21.6%, respectively.
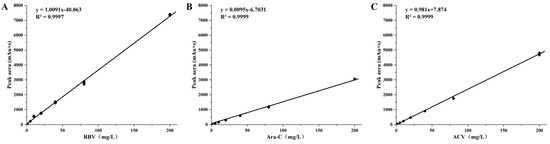
Figure 2.
Standard curves of ribavirin, cytarabine, and acyclovir: (A) RBV, ribavirin; (B) Ara-C, cytarabine; and (C) ACV, acyclovir.
The results for the drug metabolisms in the kidney tissues (Figure 3) showed that concentrations of the drug increased over time during the drug treatment (0 h to 6 h) and reached the highest concentration (125.12 μg/g) at 6 h, then gradually reduced after the transfer to clean water (8 h to 216 h). The concentration of RBV in the RBV group was 62.48 μg/g at 6 h and decreased to an undetectable concentration at 168 h, which in the RBV-SWCNTs group was 123.57 μg/g at 6 h, and low concentrations (3.44 μg/g) could still be detected at 216 h. The concentration of Ara-C in the Ara-C group was 49.84 μg/g at 6 h and decreased to an undetectable concentration at 144 h, which in the Ara-C-SWCNTs group was 86.88 μg/g at 6 h and decreased to an undetectable concentration at 216 h. The concentration of ACV in the ACV group was 43.15 μg/g at 6 h and reduced to undetectable levels at 144 h, which in the ACV-SWCNTs group was 78.94 μg/g at 6 h and reduced to undetectable levels at 168 h. In addition, the recovery rate of the samples was above 90%, and the precision was less than 5% (Table 2); all the values were within the range required by the test.
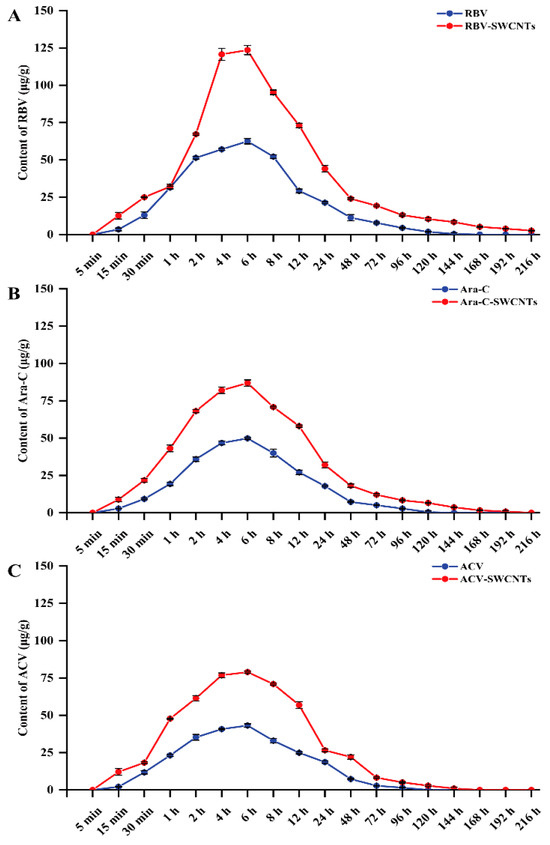
Figure 3.
The metabolism of ribavirin, cytarabine, and acyclovir in mandarin fish. Mandarin fish were immersed in drug (RBV, Ara-C, and ACV) groups or delivery system (RBV-SWCNTs, Ara-C-SWCNTs, and ACV-SWCNTs) groups at 40 mg/L for 6 h, and then maintained in filtered tap water for 216 h. At the specific observation time, 5 fish were sampled to determine the drugs metabolism by HPLC System, with three replicates. (A) RBV, RBV -SWCNTs; (B) Ara-C, Ara-C-SWCNTs; and (C) ACV, ACV-SWCNTs. Values are presented as mean ± SD.

Table 2.
Precision and recovery of the assay for RBV (60, 80, and 100 μg/mL).
3.3. Infection Rate of Virus
After infection by ISKNV, the mandarin fish were treated with drugs and delivery systems were used; the infection rates of the virus were detected to evaluate the effect of the drugs against ISKNV (Figure 4). The results showed that all of the drug and delivery system groups were effective against viral infection, and the RBV-SWCNTs group had the best antiviral effect for which the virus infection rate was less than 10%. The infection rate of the RBV group was almost three times that of the RBV-SWCNTs group, but was significantly lower than the Ara-C and ACV groups, and even the ACV-SWCNTs group. The infection rates of all three delivery system groups were also significantly lower than that of the drug groups; the RBV-SWCNTs group reduced the infection rates by up to about 20% more than the RBV group.
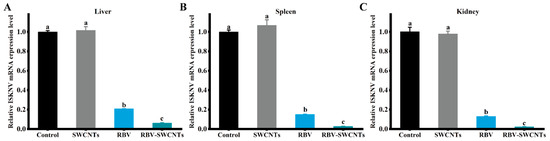
Figure 4.
Virus infection rate of mandarin fish. (A) Viral load in the liver, (B) Viral load in the spleen, and (C) Viral load in the kidney. Values are presented as mean ± SD, which with different letters showed significantly different p-values (p < 0.05) among treatments.
3.4. Cumulative Mortality
After being treated by different drugs, cumulative mortalities were counted and are shown in Figure 5. The mortality of the control group and the SWCNTs group were 100% at 9 d after treatment, and all the drug and delivery system groups can reduce mortality. The highest protection was observed in the RBV-SWCNTs group, with a mortality rate of less than 25%, which was significantly higher than that of the RBV group and the other delivery system groups. The mortality rate of the RBV group was almost two times that of the RBV-SWCNTs group, but was significantly lower than that of the Ara-C and ACV groups, as well as the Ara-C-SWCNTs and ACV-SWCNTs groups.
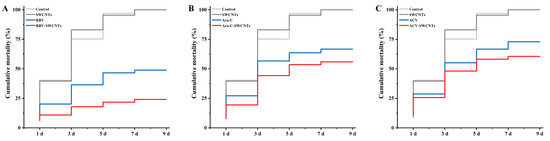
Figure 5.
Cumulative mortality of mandarin fish. (A) Cumulative mortality of mandarin fish after treatment with RBV and RBV-SWCNTs. (B) Cumulative mortality of mandarin fish after treatment with Ara-C and Ara-C-SWCNTs. (C) Cumulative mortality of mandarin fish after treatment with ACV and ACV-SWCNTs. The mortalities were recorded daily and calculated at the end of the monitoring period.
3.5. Expression of Immune-Related Genes
The expression of the immune-related genes of the RBV and RBV-SWCNTs groups were examined and are shown in Figure 6. All examined genes were significantly up-regulated in the RBV and RBV-SWCNTs groups compared to the control group, and reached a peak on the third day, then gradually decreased to be insignificantly different from the control group at 9 d. The expression of Mx and TNF-α in the RBV-SWCNTs group at 3 d was above 10 times that of the control group, which was more than four times that of the RBV group. Furthermore, the immune-related genes of the RBV-SWCNTs groups were up-regulated almost twice as much as the RBV group at 3 d.
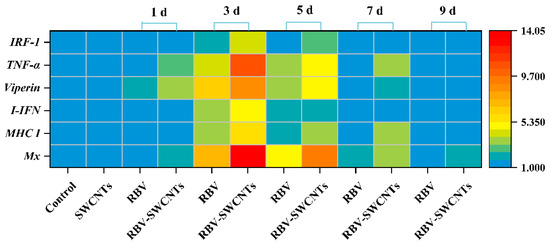
Figure 6.
Heat map showing the fold changes of immune-related genes expression in treated fish. Data are representative of three different independent experiments (mean ± SD). The colour scale is shown on the right, with blue colour indicating low fold changes and red colour indicating high fold changes.
3.6. Evaluation of Non-Specific Immune Responses
As shown in Figure 7, all examined enzyme activity and C3 content were significantly up-regulated in the RBV and RBV-SWCNTs groups compared to the control group, and reached a peak on the third day, then gradually decreased. The SOD activity of the RBV-SWCNTs group at 1 d was above 1.5 times that of the control group, which was about 1.6 times that of the control group at 3 d; the rate of up-regulation for this group was faster than that of the other enzyme activities. In addition, these enzyme activities and the C3 content of the RBV-SWCNTs group were higher than the RBV group at 3 d.
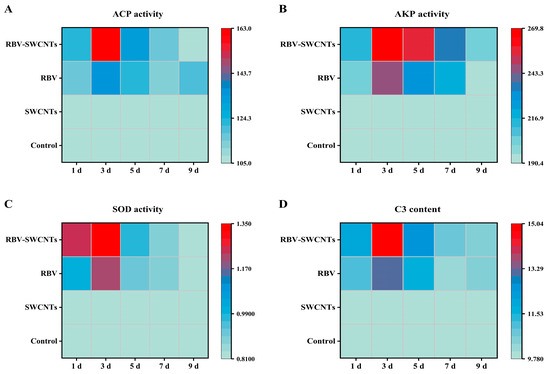
Figure 7.
Heat map showing the fold changes of immune-related physiological indexes in treated fish. (A) ACP activity, (B) AKP activity, (C) SOD activity, and (D) C3 content. Data are representative of three different independent experiments (mean ± SD). The colour scale is shown on the right, with the blue haze colour indicating low fold changes and the red colour indicating high fold changes.
4. Discussion
Aquaculture has gradually become a very important part of human food, and the output of aquaculture exceeded that of wild fishing fisheries few years ago [51,52]. However, a fishery usually increases the density of fish to achieve a higher yield, resulting in long-term high-stress levels for the fish and, with a decreased anti-infection ability, various viral diseases have seriously affected the development of the aquaculture industry. There is no available drug against ISKNV infection, to control the infection, searching for the antiviral drugs that have been proven to be effective against multiple viruses is considered to be a feasible option. When treating infected fish with drugs, the drugs are usually directly added to the aquaculture water or feed with poor bioavailability and high toxicity. A drug delivery system can reduce the degradation rate of drugs in water, enabling a sustained release and high bioavailability to achieve a desired therapeutic response [53,54]. In this study, drug delivery systems were constructed with SWCNTs as carriers and of the three common antiviral agents, RBV-SWCNTs had the best antiviral activity against ISKNV, which had the highest drug absorption concentration (123.57 μg/g), longest drug metabolism time (above 216 h), and highest survival rate (75.97%).
Most drugs can interfere with or block some cellular processes, such as binding, entry, uncoating, and a replication of the viral cycle, and were often used to treat viral diseases, include infectious diseases [55]. Ribavirin plays a role in antiviral effects by inducing the transcription and expression of SAT1, and reducing the virus titter in different cells [56]. In the experimental treatment for Human respiratory syncytial virus (HRSV), during viral replication, ribavirin has shown a higher mutation rate of polymerase and caused more mutations, thereby reducing the viral load and lessening the burden of a disease [57]. For hepatitis B virus (HBV), ribavirin, as an inhibitor of inosine 5′-monophosphate dehydrogenase, has been shown to have significant antiviral effects against HBV by causing a depletion of intracellular dGTP levels [58]. Among the many antiviral drugs for human viruses, some of them have also shown similar antiviral effects against some fish viruses. Lertwanakarn et al. (2021) demonstrated that ribavirin when taken in high doses to fight against tilapia lake virus (TiLV) can significantly reduce cytopathic effects and improve cell viability and survival rates [59]. The significant inhibition of the replicative viral cycle and the more than 90% reduction in mortality showed that ribavirin was able to fight against infectious salmon anaemia virus (ISAV); the viral load was significantly reduced, and viral proteins were not detectable after ribavirin treatment [60]. Similar results were shown in our study, ribavirin reduced the infection rate of mandarin fish by more than 70% in the fish infected with ISKNV when compared with the control group. The in vivo experiments showed that ribavirin could increase the survival rate by more than 50%, which was significantly higher than cytarabine and acyclovir. Although the antiviral effect against ISKNV was not obvious, both cytarabine and acyclovir have been shown to be effective for human DNA viruses, and acyclovir has been shown to be effective for aquatic viruses [38,61]. The pharmacological function and mechanism between antiviral drugs and various viruses have not been fully understood, and there are still many gaps that need further research and discovery.
A drug delivery system offers protection and the timed-release of drugs to achieve the expected effect in a host; it has been widely used on laboratory animal models and reared fish. Nanoparticles, as carriers to deliver drugs in fish, have been widely studied, such as polymers, inorganic compounds, and metal derivates [4,10,62]. In this study, we used carbon nanotubes as drug carriers and the results showed that SWCNTs allow the RBV to enter the fish more quickly with a peak drug concentration in the fish after 6 h of treatment and a similar concentration at 4 h. And SWCNTs nearly doubled the amount of drug that went into the fish in the RBV-SWCNTs group, prolonging the drug retention time by more than 72 h. The RBV was undetectable in the RBV group at 168 h post treatment, while a low concentration of the drug was still detectable in the RBV-SWCNT group at 216 h. SWCNTs are ideal carriers in biomedical and pharmaceutical applications because of their unique hollow nanotube structure with an extremely strong drug loading capacity, which can be attached to the surface by encapsulation or used to fill the tubular structure with the drug [63]. CNTs can use for drug delivery without external stimulation. Guven et al. (2017) demonstrated that the SWCNTs loaded in a CDDP system exhibited a stronger tumour growth inhibition effect by prolonging the circulation time over 44 h, making the drug accumulate more in the tumour cells to enhance the drug delivery effect [64]. Due to their efficient drug delivery capability, SWCNTs have been used to control infectious diseases in aquaculture. Zhu et al. (2021) used SWCNTs as the carriers of amantadine to construct a drug delivery system against nervous necrosis virus (NNV) that could reach a maximum absorption 12 h earlier, which was nearly three times that of the free amantadine group [65]. With the transport of amantadine occurring in a fast manner, the drug delivery system could extend the drug retention time by 62 h, and increase the survival rate of the NNV-infected group by nearly 50% compared to the control group. Carbon nanotubes have significant potential as delivery vehicles for antiviral drugs to prevent viral infections, but further research and exploration are still needed for commercial exploitation in aquaculture.
5. Conclusions
The drug delivery systems used SWCNTs as carriers and increased the content of the drugs that entered the body, prolonging the retention time to above 72 h, and improving the anti-ISKNV effect and immune responses. This study shows a bright future for using drug delivery systems with SWCNTs to control viral fish diseases in aquaculture.
Author Contributions
Y.J.: performing the experiments; processing the data; writing and revising the manuscript; Z.Z.: data analysis; writing and revising the manuscript; L.C.: data analysis; Y.L.: performing the experiments; B.Z.: formulating or evolving the overarching research goals and aims. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support for this study was provided by Key Research and Development Program of Shaanxi (Program No.2024NC-YBXM-095).
Institutional Review Board Statement
All of the experimental animals were handled in accordance with the regulations of Experiment Animal Center, Northwest A&F University Approval Code: DK2021132 2021-03-05.
Data Availability Statement
All data generated or analysed during this study are included in this published article.
Acknowledgments
The authors would like to thank Xiao-Zhe Fu, Ning-Qiu Li, Zhi-Bin Huang, and Qiang Lin for technical advice and assistance in the experiments.
Conflicts of Interest
The authors have declared no conflicts of interest.
References
- De, R.; Mahata, M.K.; Kim, K.T. Structure-Based Varieties of Polymeric Nanocarriers and Influences of Their Physicochemical Properties on Drug Delivery Profiles. Adv. Sci. 2022, 9, e2105373. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yang, J.; Asakura, Y.; Shuai, Q.; Yamauchi, Y. Nanoarchitectonics of Hollow Covalent Organic Frameworks: Synthesis and Applications. ACS Nano 2023, 17, 8918–8934. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Filipczak, N.; Torchilin, V.P. Cell penetrating peptides: A versatile vector for co-delivery of drug and genes in cancer. J. Control. Release 2021, 330, 1220–1228. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, J.; Pei, X.; Chen, J.; Wei, X.; Liu, Y.; Xia, P.; Wan, Q.; Gu, Z.; He, Y. Blue-ringed octopus-inspired microneedle patch for robust tissue surface adhesion and active injection drug delivery. Sci. Adv. 2023, 9, eadh2213. [Google Scholar] [CrossRef]
- Liu, J.; Cabral, H.; Mi, P. Nanocarriers address intracellular barriers for efficient drug delivery, overcoming drug resistance, subcellular targeting and controlled release. Adv. Drug Deliv. Rev. 2024, 207, 115239. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Gowd, V.; Saha, P.; Rashid, S.; Ahmad Chaudhary, A.; Mohamed, M.Y.A.; Alawam, A.S.; Khan, R. Biologically inspired stealth—Camouflaged strategies in nanotechnology for the improved therapies in various diseases. Int. J. Pharm. 2023, 631, 122407. [Google Scholar] [CrossRef]
- Waheed, S.; Li, Z.; Zhang, F.; Chiarini, A.; Armato, U.; Wu, J. Engineering nano-drug biointerface to overcome biological barriers toward precision drug delivery. J. Nanobiotechnol. 2022, 20, 395. [Google Scholar] [CrossRef] [PubMed]
- Xia, K.; Yatabe, T.; Yonesato, K.; Kikkawa, S.; Yamazoe, S.; Nakata, A.; Ishikawa, R.; Shibata, N.; Ikuhara, Y.; Yamaguchi, K.; et al. Ultra-stable and highly reactive colloidal gold nanoparticle catalysts protected using multi-dentate metal oxide nanoclusters. Nat. Commun. 2024, 15, 851. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Song, X.; Liu, Y.; Fu, Y.; Ye, L.; Wang, N.; Wang, F.; Li, L.; Mohammadniaei, M.; Zhang, M.; et al. Synthesis of graphene quantum dots and their applications in drug delivery. J. Nanobiotechnol. 2020, 18, 142. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Xiong, Y.; Zhang, C.; Jia, Y.J.; Qiu, D.K.; Wang, G.X.; Zhu, B. Optimization of the efficacy of a SWCNTs-based subunit vaccine against infectious spleen and kidney necrosis virus in mandarin fish. Fish Shellfish Immunol. 2020, 106, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Liu, G.L.; Ling, F.; Song, L.S.; Wang, G.X. Development toxicity of functionalized carbon nanotubes on rare minnow embryos and larvae. Nanotoxicology 2015, 9, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhu, B.; Huang, A.; Hu, Y.; Wang, G.; Ling, F. Toxicological effects of multi-walled carbon nanotubes on Saccharomyces cerevisiae: The uptake kinetics and mechanisms and the toxic responses. J. Hazard. Mater. 2016, 318, 650–662. [Google Scholar] [CrossRef]
- Yu, X.B.; Chen, X.H.; Ling, F.; Hao, K.; Wang, G.X.; Zhu, B. Moroxydine hydrochloride inhibits grass carp reovirus replication and suppresses apoptosis in Ctenopharyngodon idella kidney cells. Antivir. Res. 2016, 131, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Luo, F.; Li, J.; Zhu, B.; Wang, G.X. Biocompatibility assessment of single-walled carbon nanotubes using Saccharomyces cerevisiae as a model organism. J. Nanobiotechnol. 2018, 16, 44. [Google Scholar] [CrossRef]
- Zhu, S.; Huang, A.G.; Luo, F.; Li, J.; Li, J.; Zhu, L.; Zhao, L.; Zhu, B.; Ling, F.; Wang, G.X. Application of virus targeting nanocarrier drug delivery system in virus-induced central nervous system disease treatment. ACS Appl. Mater. Interfaces 2019, 11, 19006–19016. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, G.X.; Zhu, B. Application of antigen presenting cell-targeted nanovaccine delivery system in rhabdovirus disease prophylactics using fish as a model organism. J. Nanobiotechnol. 2020, 18, 24. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.E.; Knudsen, J.L.; Lease, E.D.; Jerome, K.R.; Rakita, R.M.; Boeckh, M.; Limaye, A.P. Risk factors and outcomes of ganciclovir-resistant cytomegalovirus infection in solid organ transplant recipients. Clin. Infect. Dis. 2017, 65, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Meister, T.L.; Brüggemann, Y.; Nocke, M.K.; Ulrich, R.G.; Schuhenn, J.; Sutter, K.; Gömer, A.; Bader, V.; Winklhofer, K.F.; Broering, R.; et al. A ribavirin-induced ORF2 single-nucleotide variant produces defective hepatitis E virus particles with immune decoy function. Proc. Natl. Acad. Sci. USA 2022, 119, e2202653119. [Google Scholar] [CrossRef]
- Pfeiffer, J.K.; Kirkegaard, K. A single mutation in poliovirus RNA-dependent RNA polymerase confers resistance to mutagenic nucleotide analogs via increased fidelity. Proc. Natl. Acad. Sci. USA 2003, 100, 7289–7294. [Google Scholar] [CrossRef] [PubMed]
- Leyssen, P.; Balzarini, J.; De Clercq, E.; Neyts, J. The predominant mechanism by which ribavirin exerts its antiviral activity invitro against flaviviruses and paramyxoviruses is mediated by inhibition of IMP dehydrogenase. J. Virol. 2005, 79, 1943–1947. [Google Scholar] [CrossRef] [PubMed]
- Wray, S.K.; Gilbert, B.E.; Noall, M.W.; Knight, V. Mode of action of ribavirin: Effect of nucleotide pool alterations on influenza virus ribonucleoprotein synthesis. Antivir. Res. 1985, 5, 29–37. [Google Scholar] [CrossRef] [PubMed]
- McCormick, J.B.; King, I.J.; Webb, P.A.; Scribner, C.L.; Craven, R.B.; Johnson, K.M.; Elliott, L.H.; Belmont-Williams, R. Lassa fever. Eff. Ther. Ribavirin. N. Engl. J. Med. 1986, 314, 20–26. [Google Scholar] [CrossRef]
- Stein, D.S.; Creticos, C.M.; Jackson, G.G.; Bernstein, J.M.; Hayden, F.G.; Schiff, G.M.; Bernstein, D.I. Oral ribavirin treatment of influenza A and B. Antimicrob. Agents Chemother. 1987, 31, 1285–1287. [Google Scholar] [CrossRef]
- Graci, J.D.; Cameron, C.E. Mechanisms of action of ribavirin against distinct viruses. Rev. Med. Virol. 2006, 16, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Olivencia, G.; Estébanez, M.; Membrillo, F.J.; Ybarra, M.D.C. Use of ribavirin in viruses other than hepatitis, C. A review of the evidence. Enfermedades Infecc. Microbiol. Clin. 2019, 37, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Takayama-Ito, M.; Saijo, M. Antiviral drugs against severe fever with thrombocytopenia syndrome virus infection. Front. Microbiol. 2020, 11, 150. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Shen, Y.; Li, B.; Wang, G.X.; Zhu, B. Evaluation on the antiviral activity of ribavirin against infectious hematopoietic necrosis virus in epithelioma papulosum cyprini cells. Virus Res. 2019, 263, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Song, K.; Zhang, Z.; Chen, C.; Wang, G.; Yao, J.; Ling, F. Evaluation on the antiviral activity of ribavirin against Micropterus salmoides rhabdovirus (MSRV) in vitro and in vivo. Aquaculture 2021, 543, 736975. [Google Scholar] [CrossRef]
- Baek, E.J.; Kim, M.J.; Kim, K.I. In vitro and in vivo evaluation of the antiviral activity of arctigenin, ribavirin, and ivermectin against viral hemorrhagic septicemia virus infection. Fish Shellfish Immunol. 2023, 132, 108456. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, D.; He, W.; Zhang, H.; Li, Z.; Luan, Y. Nanoassemblies from amphiphilic cytarabine prodrug for leukemia targeted therapy. J. Colloid Interface Sci. 2017, 487, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Geethakumari, D.; Bhaskaran Sathyabhama, A.; Raji Sathyan, K.; Mohandas, D.; Somasekharan, J.V.; Thavarool Puthiyedathu, S. Folate functionalized chitosan nanoparticles as targeted delivery systems for improved anticancer efficiency of cytarabine in MCF-7 human breast cancer cell lines. Int. J. Biol. Macromol. 2022, 199, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Kripp, M.; Hofheinz, R.D. Treatment of lymphomatous and leukemic meningitis with liposomal encapsulated cytarabine. Int. J. Nanomed. 2008, 3, 397–401. [Google Scholar]
- Ping, L.; Ruxian, J.; Mengping, Z.; Pei, J.; Zhuoya, L.; Guosheng, L.; Zhenyu, W.; Hailei, W. Whole-cell biosynthesis of cytarabine by an unnecessary protein-reduced Escherichia coli that coexpresses purine and uracil phosphorylase. Biotechnol. Bioeng. 2022, 119, 1768–1780. [Google Scholar] [CrossRef]
- Chen, F.; Xu, H.; Liu, J.; Cui, Y.; Luo, X.; Zhou, Y.; Chen, Q.; Jiang, L. Efficacy and safety of nucleoside antiviral drugs for treatment of recurrent herpes labialis: A systematic review and meta-analysis. J. Oral Pathol. Med. 2017, 46, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Quenelle, D.C.; Birkmann, A.; Goldner, T.; Pfaff, T.; Zimmermann, H.; Bonsmann, S.; Collins, D.J.; Rice, T.L.; Prichard, M.N. Efficacy of pritelivir and acyclovir in the treatment of herpes simplex virus infections in a mouse model of herpes simplex encephalitis. Antivir. Res. 2018, 149, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Elion, G.B.; Furman, P.A.; Fyfe, J.A.; de Miranda, P.; Beauchamp, L.; Schaeffer, H.J. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc. Natl. Acad. Sci. USA 1977, 74, 5716–5720. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.H.; Miller, R.L. Phosphorylation of acyclovir (acycloguanosine) monophosphate by GMP kinase. J. Biol. Chem. 1980, 255, 7204–7207. [Google Scholar] [CrossRef] [PubMed]
- Quijano Cardé, E.M.; Yazdi, Z.; Yun, S.; Hu, R.; Knych, H.; Imai, D.M.; Soto, E. Pharmacokinetic and efficacy study of acyclovir against cyprinid herpesvirus 3 in cyprinus carpio. Front. Vet. Sci. 2020, 7, 587952. [Google Scholar] [CrossRef]
- Hao, K.; Yuan, S.; Yu, F.; Chen, X.H.; Bian, W.J.; Feng, Y.H.; Zhao, Z. Acyclovir inhibits channel catfish virus replication and protects channel catfish ovary cells from apoptosis. Virus Res. 2021, 292, 198249. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.F.; He, J.; Zeng, R.Y.; Li, Z.M.; Luo, Z.Y.; Pan, W.Q.; He, J.G. Deletion of the infectious spleen and kidney necrosis virus ORF069L reduces virulence to mandarin fish Siniperca chuatsi. Fish Shellfish Immunol. 2019, 95, 328–335. [Google Scholar] [CrossRef]
- He, J.H.; Xia, Q.; Weng, S.; He, J.; Xu, X. Identification of infectious spleen and kidney necrosis virus (ISKNV)-encoded microRNAs. Virus Genes 2020, 56, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Chinchar, V.G.; Hick, P.; Ince, I.A.; Jancovich, J.K.; Marschang, R.; Qin, Q.; ICTV Report Consortium. ICTV virus taxonomy profile: Iridoviridae. J. Gen. Virol. 2017, 98, 890–891. [Google Scholar] [CrossRef] [PubMed]
- Fusianto, C.; Hick, P.M.; Becker, J.A. Stability of infectious spleen and kidney necrosis virus and susceptibility to physical and chemical disinfectants. Aquaculture 2019, 506, 104–111. [Google Scholar] [CrossRef]
- Zhu, Z.M.; Duan, C.; Li, Y.; Huang, C.L.; Weng, S.P.; He, J.G.; Dong, C.F. Pathogenicity and histopathology of infectious spleen and kidney necrosis virus genotype II (ISKNV-II) recovering from mass mortality of farmed Asian seabass, Lates calcarifer, in southern China. Aquaculture 2020, 534, 736326. [Google Scholar] [CrossRef]
- Lin, Q.; Fu, X.; Liu, L.; Liang, H.; Guo, H.; Yin, S.; Li, N. Application and development of a TaqMan real-time PCR for detecting infectious spleen and kidney necrosis virus in Siniperca chuatsi. Microb. Pathog. 2017, 107, 98–105. [Google Scholar] [CrossRef]
- Zhu, B.; Liu, G.L.; Ling, F.; Wang, G.X. Carbon nanotube-based nanocarrier loaded with ribavirin against grass carp reovirus. Antivir. Res. 2015, 118, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Xu, S.; Hu, L.; Su, X.; Ou, J.; Zou, H.; Guo, Z.; Zhang, Y.; Guo, B. Using oxidized carbon nanotubes as matrix for analysis of small molecules by MALDI-TOF MS. J. Am. Soc. Mass Spectrom. 2005, 16, 883–892. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, G.; Liu, J.; Yan, Z.; Zhang, Z. Bioaccumulation, distribution and metabolism of BDE-153 in the freshwater fish Carassius auratus after dietary exposure. Ecotoxicol. Environ. Saf. 2014, 108, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Rigo-Bonnin, R.; Padullés, A.; Corral-Comesaña, S.; Cerezo, G.; Grinyó, J.M.; Colom, H.; Alía-Ramos, P.; Lloberas, N. Measurement of ganciclovir concentration in human plasma by ultra-performance liquid chromatography-tandem mass spectrometry. Clin. Chim. Acta 2014, 427, 58–64. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−△△CT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- FAO. The State of World Fisheries and Aquaculture; Food and Agriculture Oraganization of the United Nations: Rome, Italy, 2014. [Google Scholar]
- Embregts, C.W.; Forlenza, M. Oral vaccination of fish: Lessons from humans and veterinary species. Dev. Comp. Immunol. 2016, 64, 118–137. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, I.; Byrne, J.; Shakur, R.; Langer, R. Engineered drug delivery devices to address Global Health challenges. J. Control. Release 2021, 331, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Pereiro, P.; Figueras, A.; Novoa, B. Compilation of antiviral treatments and strategies to fight fish viruses. Rev. Aquac. 2021, 13, 1223–1254. [Google Scholar] [CrossRef]
- Tate, P.M.; Mastrodomenico, V.; Mounce, B.C. Ribavirin induces polyamine depletion via nucleotide depletion to limit virus replication. Cell Rep. 2019, 28, 2620–2633.e4. [Google Scholar] [CrossRef] [PubMed]
- Aljabr, W.; Touzelet, O.; Pollakis, G.; Wu, W.; Munday, D.C.; Hughes, M.; Hertz-Fowler, C.; Kenny, J.; Fearns, R.; Barr, J.N.; et al. Investigating the influence of ribavirin on human respiratory syncytial virus rna synthesis by using a high-resolution transcriptome sequencing approach. J. Virol. 2016, 90, 4876–4888. [Google Scholar] [CrossRef] [PubMed]
- Ying, C.; De Clercq, E.; Neyts, J. Ribavirin and mycophenolic acid potentiate the activity of guanine- and diaminopurine-based nucleoside analogues against hepatitis B virus. Antivir. Res. 2000, 48, 117–124. [Google Scholar] [CrossRef]
- Lertwanakarn, T.; Trongwongsa, P.; Yingsakmongkol, S.; Khemthong, M.; Tattiyapong, P.; Surachetpong, W. Antiviral activity of ribavirin against tilapia tilapinevirus in fish cells. Pathogens 2021, 10, 1616. [Google Scholar] [CrossRef]
- Rivas-Aravena, A.; Vallejos-Vidal, E.; Cortez-San Martin, M.; Reyes-Lopez, F.; Tello, M.; Mora, P.; Sandino, A.M.; Spencer, E. Inhibitory effect of a nucleotide analog on infectious salmon anemia virus infection. J. Virol. 2011, 85, 8037–8045. [Google Scholar] [CrossRef]
- Troszok, A.; Kolek, L.; Szczygieł, J.; Wawrzeczko, J.; Borzym, E.; Reichert, M.; Kamińska, T.; Ostrowski, T.; Jurecka, P.; Adamek, M.; et al. Acyclovir inhibits Cyprinid herpesvirus 3 multiplication in vitro. J. Fish Dis. 2018, 41, 1709–1718. [Google Scholar] [CrossRef]
- Nasr-Eldahan, S.; Nabil-Adam, A.; Shreadah, M.A.; Maher, A.M.; El-Sayed Ali, T. A review article on nanotechnology in aquaculture sustainability as a novel tool in fish disease control. Aquac. Int. 2021, 29, 1459–1480. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, N.G.; Bao, H.; Pan, Y.; Pal, M.; Kakran, M.; Cheng, H.K.; Li, L.; Tan, L.P. Functionalized carbon nanomaterials as nanocarriers for loading and delivery of a poorly water-soluble anticancer drug: A comparative study. Chem. Commun. 2011, 47, 5235–5237. [Google Scholar] [CrossRef]
- Guven, A.; Villares, G.J.; Hilsenbeck, S.G.; Lewis, A.; Landua, J.D.; Dobrolecki, L.E.; Wilson, L.J.; Lewis, M.T. Carbon nanotube capsules enhance the in vivo efficacy of cisplatin. Acta Biomater. 2017, 58, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Luo, F.; Zhu, B.; Ling, F.; Wang, E.L.; Liu, T.Q.; Wang, G.X. A nanobody-mediated virus-targeting drug delivery platform for the central nervous system viral disease therapy. Microbiol. Spectr. 2021, 9, e0148721. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).