Functional Analysis of NdBCO-like4 Gene in Pigmentation of Neocaridina denticulata sinensis
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Experimental Animals
2.3. Tissue Collection
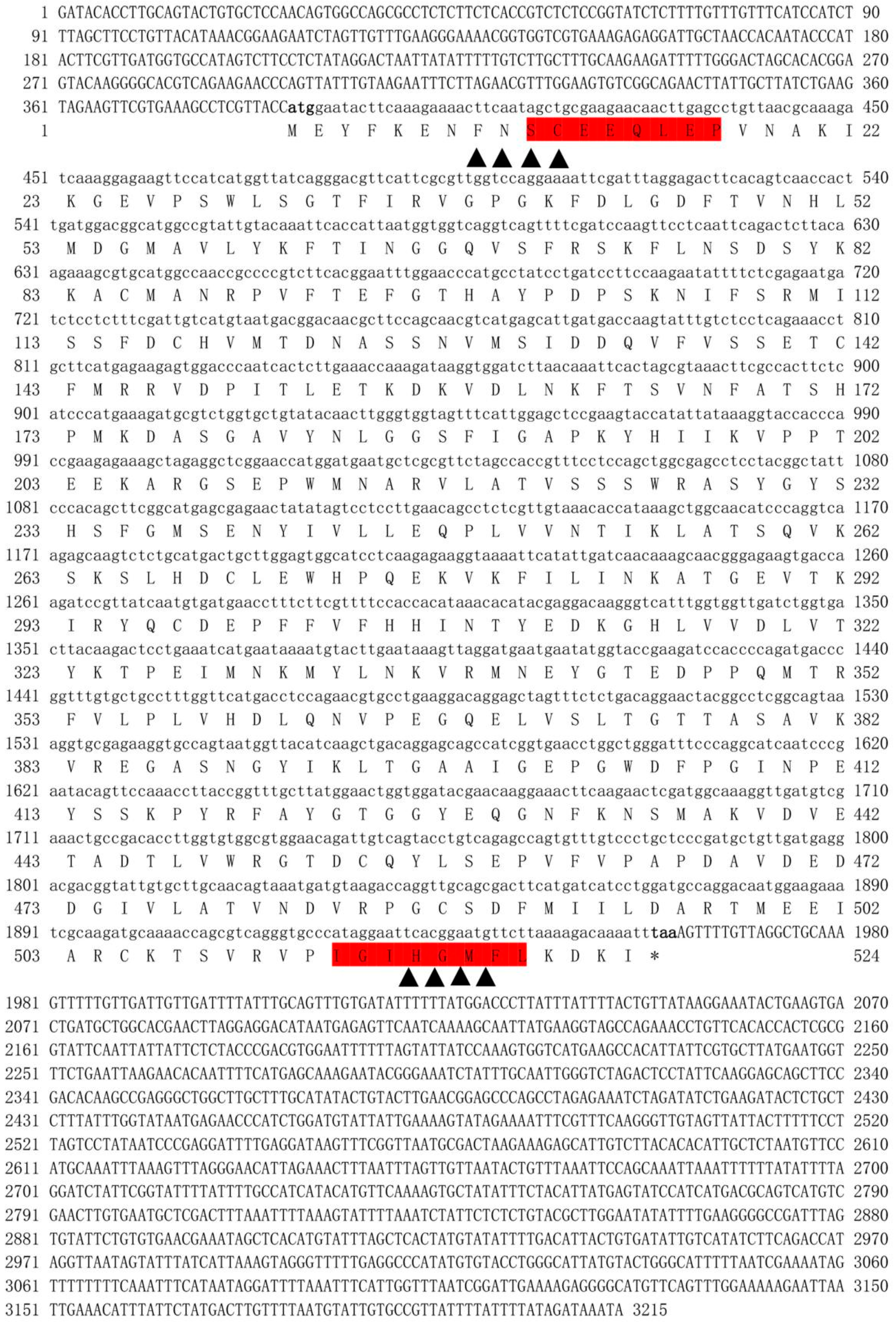
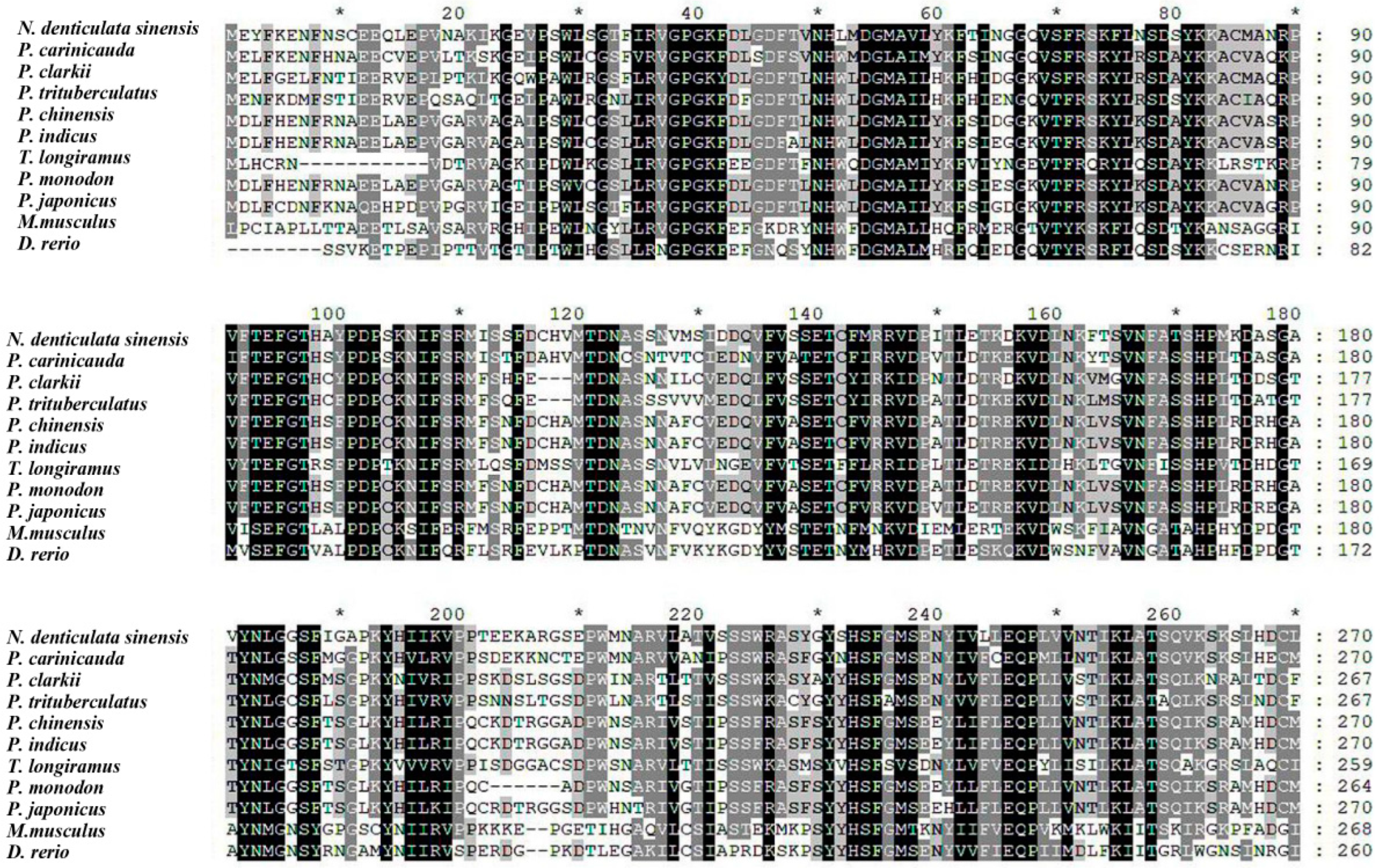
2.4. Full-Length cDNA Verification and Bioinformatics Analysis of NdBCO-like4
2.5. Collection of Embryos at Different Developmental Stages
2.6. Total RNA Extraction, cDNA Synthesis, and Quantitative Polymerase Chain Reaction (qPCR)
2.7. Double-Stranded RNA (dsRNA) Generations
2.8. Functional Analysis of NdBCO-like4 Gene Was Performed by RNA Interference (RNAi)
2.9. Single Nucleotide Polymorphism (SNP) Identification
2.10. Genotyping of Candidate SNPs
2.11. Prediction of RNA Secondary Structure
2.12. Data Analysis
3. Results
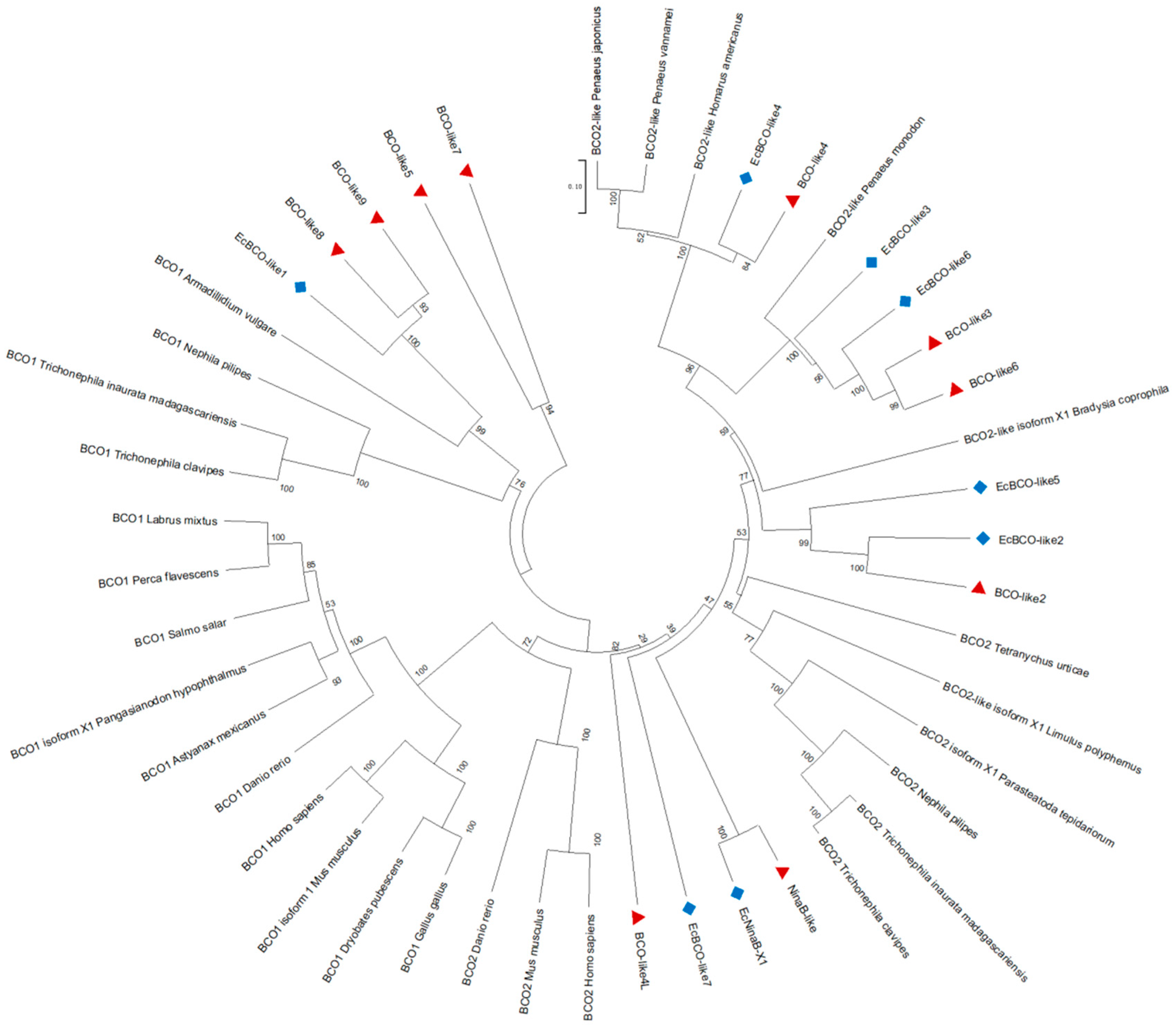
3.1. Molecular Features and Sequence Analysis of the NdBCO-like4 Gene
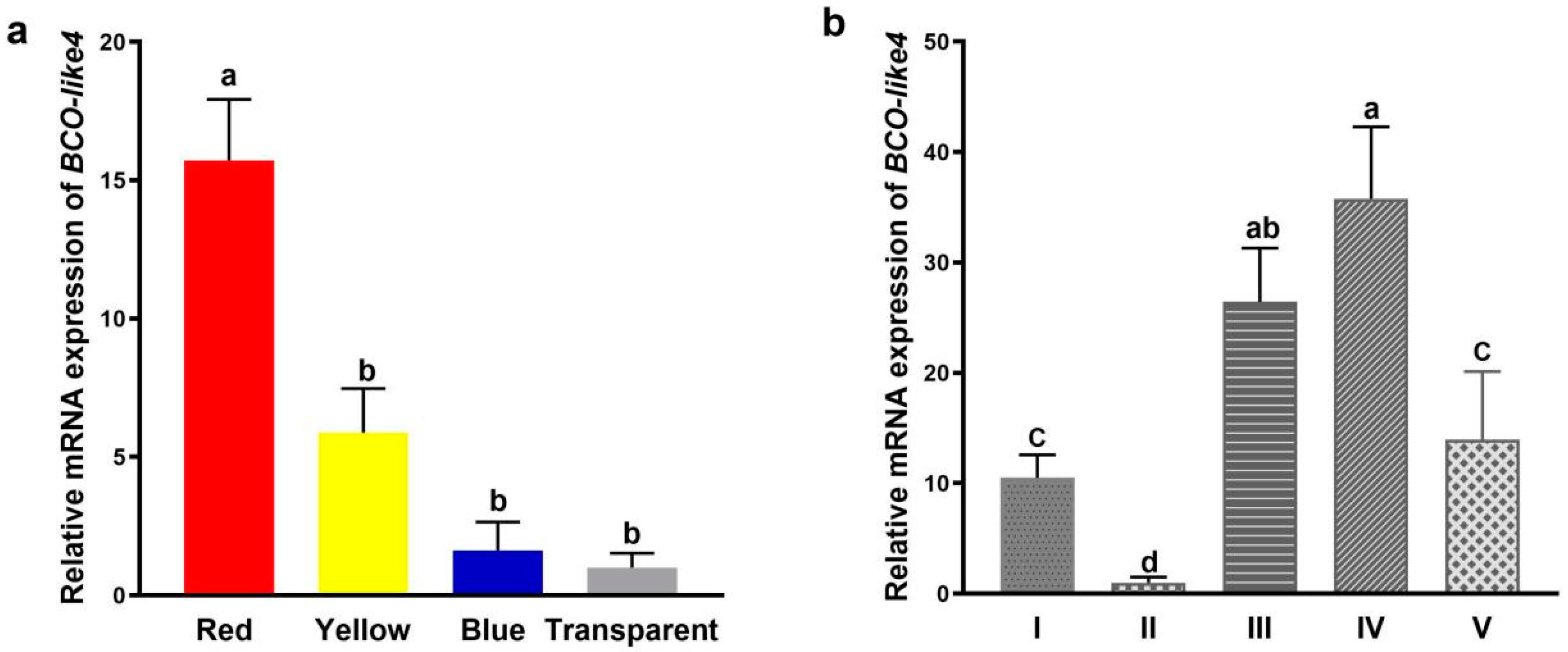
3.2. Expression of NdBCO-like4 Gene in Various Strains and Developmental Stages
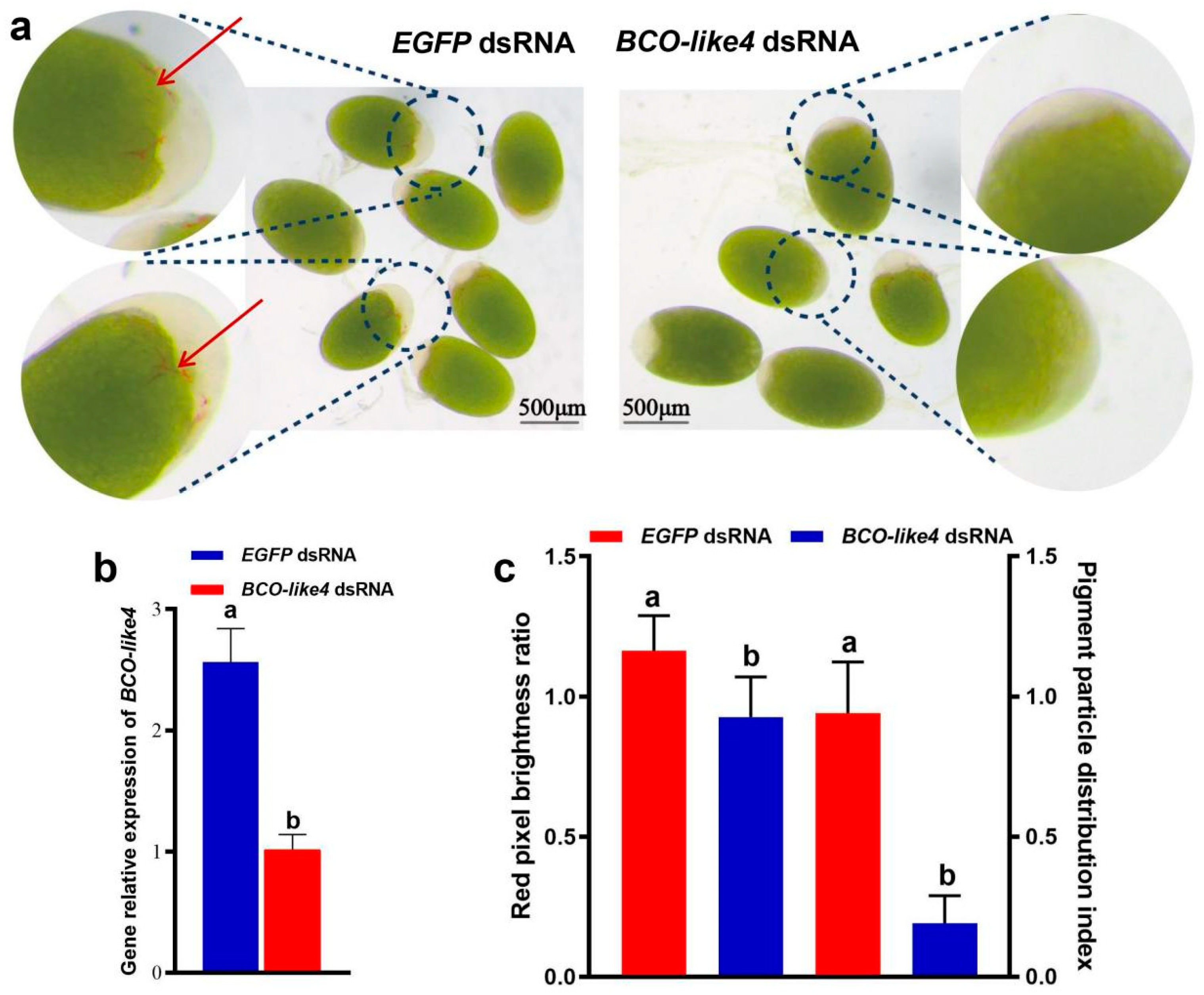
3.3. Functional Analyses of NdBCO-like4 Gene via RNA Interference
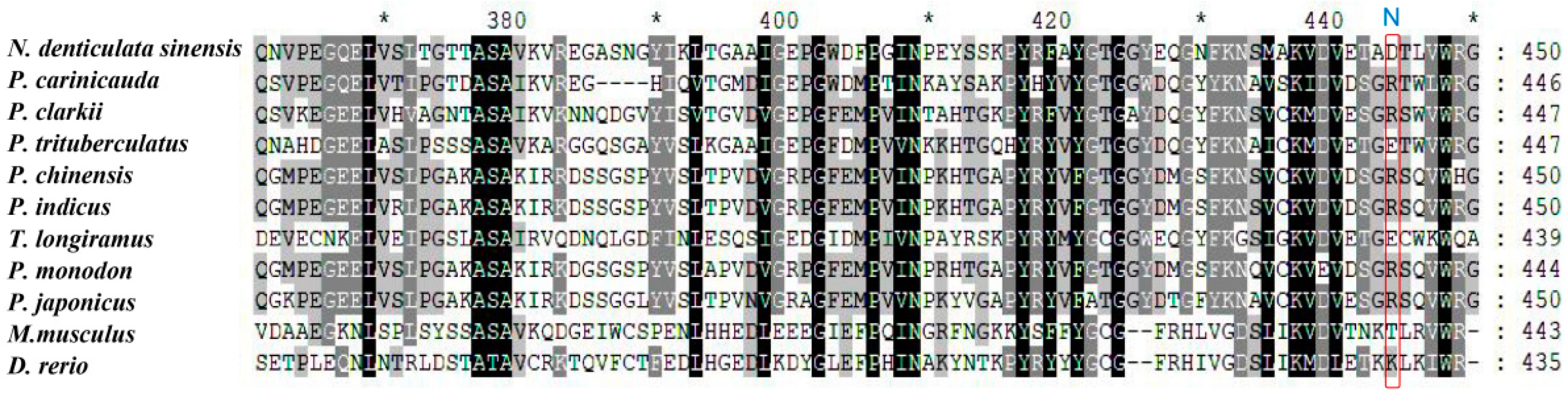
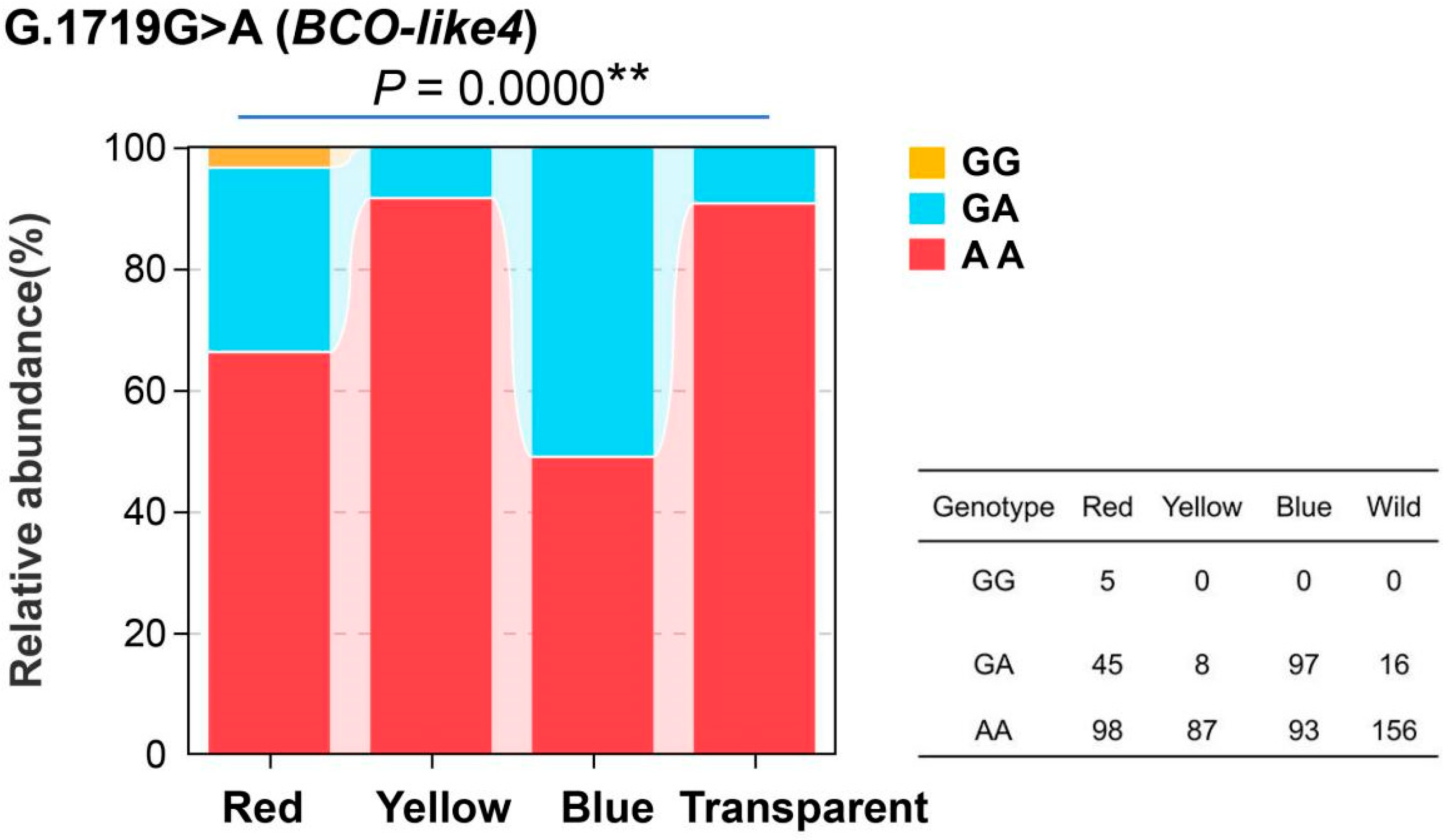
3.4. Genotyping and Correlation Analyses
3.5. Comparison of RNA Secondary Structure
4. Discussion
4.1. The Expression Pattern of NdBCO-like4 Revealed by qPCR in Different Strains and Development Stages
4.2. Validation of the NdBCO-like4 Function via RNAi at the Metanauplius Stage
4.3. Genotyping and RNA Secondary Structure
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BCO2 | β, β-carotene 9′ 10 ′-dioxygenase |
| SNP | Single-nucleotide polymorphism |
| CCD | Carotenoid cracking dioxygenase |
| BCO1 | β-carotene oxygenase 1 |
| qPCR | Quantitative PCR |
| RNAi | RNA interference |
| ORF | Open reading frame |
| NJ | Neighbor joining |
| dsRNA | Double-stranded RNA |
| EGFP | Enhanced green fluorescent protein |
| TG | Treated group |
| CG | Control group |
| AFI | Allele frequency imbalance |
| RPB | Red pigment brightness |
| PDS | Pigment distribution scale |
| GATK | Genome Analysis ToolKit |
| BCO | β-carotene oxygenase |
| H | Heterozygosity |
| FST | F-statistics |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
Appendix A
References
- Flores, E.E.; Chien, Y.-H. Chromatosomes in three phenotypes of Neocaridina denticulata Kemp, 1918: Morphological and chromatic differences measured non-invasively. J. Crustac. Biol. 2011, 31, 590–597. [Google Scholar] [CrossRef]
- Mykles, D.L.; Hui, J.H. Neocaridina denticulata: A decapod crustacean model for functional genomics. Integr. Comp. Biol. 2015, 55, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Bjerkeng, B. Carotenoids in Aquaculture: Fish and Crustaceans. In Carotenoids: Volume 4: Natural Functions; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser: Basel, Switzerland, 2008; pp. 237–254. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Twomey, E.; Johnson, J.D.; Castroviejo-Fisher, S.; Van Bocxlaer, I. A ketocarotenoid-based colour polymorphism in the Sira poison frog Ranitomeya sirensis indicates novel gene interactions underlying aposematic signal variation. Mol. Ecol. 2020, 29, 2004–2015. [Google Scholar] [CrossRef]
- von Lintig, J.; Moon, J.; Lee, J.; Ramkumar, S. Carotenoid metabolism at the intestinal barrier. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2020, 1865, 158580. [Google Scholar] [CrossRef]
- dela Seña, C.; Narayanasamy, S.; Riedl, K.M.; Curley, R.W.; Schwartz, S.J.; Harrison, E.H. Substrate specificity of purified recombinant human β-carotene 15,15′-oxygenase (BCO1). J. Biol. Chem. 2013, 288, 37094–37103. [Google Scholar] [CrossRef]
- Amengual, J.; Widjaja-Adhi, M.A.K.; Rodriguez-Santiago, S.; Hessel, S.; Golczak, M.; Palczewski, K.; Von Lintig, J. Two Carotenoid Oxygenases Contribute to Mammalian Provitamin A Metabolism. J. Biol. Chem. 2013, 288, 34081–34096. [Google Scholar] [CrossRef]
- Raghuvanshi, S.; Reed, V.; Blaner, W.S.; Harrison, E.H. Cellular localization of β-carotene 15,15′ oxygenase-1 (BCO1) and β-carotene 9′,10′ oxygenase-2 (BCO2) in rat liver and intestine. Arch. Biochem. Biophys. 2015, 572, 19–27. [Google Scholar] [CrossRef]
- Wu, L.; Guo, X.; Lyu, Y.; Clarke, S.L.; Lucas, E.A.; Smith, B.J.; Hildebrand, D.; Wang, W.; Medeiros, D.M.; Shen, X. Targeted metabolomics reveals abnormal hepatic energy metabolism by depletion of β-carotene oxygenase 2 in mice. Sci. Rep. 2017, 7, 14624. [Google Scholar] [CrossRef]
- Guo, X.; Wu, L.; Lyu, Y.; Chowanadisai, W.; Clarke, S.L.; Lucas, E.A.; Smith, B.J.; He, H.; Wang, W.; Medeiros, D.M. Ablation of β, β-carotene-9′, 10′-oxygenase 2 remodels the hypothalamic metabolome leading to metabolic disorders in mice. J. Nutr. Biochem. 2017, 46, 74–82. [Google Scholar] [CrossRef]
- Palczewski, G.; Widjaja-Adhi, M.A.; Amengual, J.; Golczak, M.; von Lintig, J. Genetic dissection in a mouse model reveals interactions between carotenoids and lipid metabolism. J. Lipid Res. 2016, 57, 1684–1695. [Google Scholar] [CrossRef] [PubMed]
- Asson-Batres, M.A.; Rochette-Egly, C. Biochemistry of Retinoid Signaling II; Springer: Dordrecht, The Netherlands, 2016. [Google Scholar]
- Lehnert, S.; Christensen, K.; Vandersteen, W.; Sakhrani, D.; Pitcher, T.; Heath, J.; Koop, B.; Heath, D.; Devlin, R. Carotenoid pigmentation in salmon: Variation in expression at BCO2-l locus controls a key fitness trait affecting red coloration. Proc. R. Soc. B 2019, 286, 20191588. [Google Scholar] [CrossRef] [PubMed]
- Von Lintig, J. Colors with functions: Elucidating the biochemical and molecular basis of carotenoid metabolism. Annu. Rev. Nutr. 2010, 30, 35–56. [Google Scholar] [CrossRef]
- Hill, G.E.; Hood, W.R.; Ge, Z.; Grinter, R.; Greening, C.; Johnson, J.D.; Park, N.R.; Taylor, H.A.; Andreasen, V.A.; Powers, M.J.; et al. Plumage redness signals mitochondrial function in the house finch. Proc. R. Soc. B 2019, 286, 20191354. [Google Scholar] [CrossRef] [PubMed]
- Kedishvili, N.Y. Enzymology of retinoic acid biosynthesis and degradation: Thematic Review Series: Fat-Soluble Vitamins: Vitamin A. J. Lipid Res. 2013, 54, 1744–1760. [Google Scholar] [CrossRef]
- Lim, J.Y.; Liu, C.; Hu, K.-Q.; Smith, D.E.; Wang, X.-D. Ablation of carotenoid cleavage enzymes (BCO1 and BCO2) induced hepatic steatosis by altering the farnesoid X receptor/miR-34a/sirtuin 1 pathway. Arch. Biochem. Biophys. 2018, 654, 1–9. [Google Scholar] [CrossRef]
- Wu, L. Functional Impact of β,β-Carotene-9′,10′-Oxygenase 2 (Bco2) in Hepatic Mitochondria. Bachelor’s Thesis, Nanjing Agricultural University, Nanjing, China, 2015. [Google Scholar]
- Huang, Y.; Zhang, L.; Wang, G.; Huang, S. De novo assembly transcriptome analysis reveals the genes associated with body color formation in the freshwater ornamental shrimps Neocaridina denticulate sinensis. Gene 2022, 806, 145929. [Google Scholar] [CrossRef]
- Lin, S. Discovery and Identification of Pigment-Related Genes in Serratus Sinensis. Master’s Thesis, Jimei University, Xiamen, China, 2017. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Aiyar, A. The use of CLUSTAL W and CLUSTAL X for multiple sequence alignment. In Bioinformatics Methods and Protocols; Humana: Totowa, NJ, USA, 1999; pp. 221–241. [Google Scholar]
- Fan, C. Gonadal Development and Germ Cell Genesis in Neocaridina Denticulata Sinensis. Master’s Thesis, Hebei University, Baoding, China, 2007. [Google Scholar]
- Xie, J.; Huang, H.; Wei, X.; Tan, P.; Ouyang, L.; Wang, L.; Liu, D.; Wang, F.; Wang, Z.; Tu, P. Boswellia carterii n-hexane extract suppresses breast cancer growth via induction of ferroptosis by downregulated GPX4 and upregulated transferrin. Sci. Rep. 2024, 14, 14307. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, L.; Wang, G.; Huang, S.; Zhang, Y.; Xie, Y. The occurrence process of chromatophores in three body color strains of the ornamental shrimp Neocaridina denticulata sinensis. Zoomorphology 2022, 141, 283–295. [Google Scholar] [CrossRef]
- Wang, G.; Li, N.; Zhang, L.; Zhang, L.; Zhang, Z.; Wang, Y. IGFBP7 is involved in abalone metamorphosis. Aquaculture 2016, 451, 377–384. [Google Scholar] [CrossRef]
- McLean, D. Adobe photoshop and illustrator techniques. J. Audiov. Media Med. 2002, 25, 79–81. [Google Scholar] [CrossRef]
- McNamara, J.C.; Milograna, S.R. Adaptive Color Change and the Molecular Endocrinology of Pigment Translocation in Crustacean Chromatophores. In The Natural History of the Crustacea; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Van der Auwera, G.A.; Carneiro, M.O.; Hartl, C.; Poplin, R.; Del Angel, G.; Levy-Moonshine, A.; Jordan, T.; Shakir, K.; Roazen, D.; Thibault, J. From FastQ data to high-confidence variant calls: The genome analysis toolkit best practices pipeline. Curr. Protoc. Bioinform. 2013, 43, 11.10.1–11.10.33. [Google Scholar] [CrossRef]
- Wang, K.; Li, M.; Hakonarson, H. ANNOVAR: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010, 38, e164. [Google Scholar] [CrossRef]
- Narum, S.R.; Hess, J.E. Comparison of FST outlier tests for SNP loci under selection. Mol. Ecol. Resour. 2011, 11, 184–194. [Google Scholar] [CrossRef]
- Campbell, N.R.; Harmon, S.A.; Narum, S.R. Genotyping-in-Thousands by sequencing (GT-seq): A cost effective SNP genotyping method based on custom amplicon sequencing. Mol. Ecol. Resour. 2015, 15, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, G.; Zhang, L.; Liang, Q. Analysis of biochemical, genetic, and morphological adaptation focused on metamorphosis from Heliocidaris crassispina based on the transcriptomic and accurate genotyping strategy. Aquaculture 2024, 585, 740740. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, G.; Zhang, L.; Xu, W.; Zhang, G.; Ge, H. A genome-wide association study and an accurate genotyping strategy to identify and validate the SNPs related to the larval period in bullfrog Aquarana catesbeiana. Aquac. Int. 2024, 32, 8267–8286. [Google Scholar] [CrossRef]
- Park, H.M. Comparing Group Means: T-Tests and One-Way ANOVA Using Stata, SAS, R, and SPSS; The Trustees of Indiana University: Indianapolis, IN, USA, 2009. [Google Scholar]
- Krueger, F. Trim Galore!: A Wrapper Around Cutadapt and FastQC to Consistently Apply Adapter and Quality Trimming to FastQ Files, with Extra Functionality for RRBS Data; Babraham Institute: Babraham, UK, 2015. [Google Scholar]
- Masella, A.P.; Bartram, A.K.; Truszkowski, J.M.; Brown, D.G.; Neufeld, J.D. PANDAseq: Paired-end assembler for illumina sequences. BMC Bioinform. 2012, 13, 31. [Google Scholar] [CrossRef]
- Wan, S.-M.; Xiong, X.-M.; Tomljanović, T.; Chen, Y.-L.; Liu, H.; Treer, T.; Gao, Z.-X. Identification and mapping of SNPs associated with number of intermuscular bone in blunt snout bream. Aquaculture 2019, 507, 75–82. [Google Scholar] [CrossRef]
- Jin, Y.; Yu, Y.; Zhang, C.; Li, S.; Zhang, X.; Li, F. Characterization and function analysis of the beta-carotene oxygenase-like genes in carotenoids metabolism of the ridgetail white prawn Exopalaemon carinicauda. Front. Physiol. 2020, 11, 745. [Google Scholar] [CrossRef] [PubMed]
- Gross, J. Carotenoids. In Pigments in Vegetables: Chlorophylls and Carotenoids; Springer: New York, NY, USA, 1991; pp. 75–278. [Google Scholar]
- Lopes, R.J.; Johnson, J.D.; Toomey, M.B.; Ferreira, M.S.; Araujo, P.M.; Melo-Ferreira, J.; Andersson, L.; Hill, G.E.; Corbo, J.C.; Carneiro, M. Genetic basis for red coloration in birds. Curr. Biol. 2016, 26, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Mundy, N.I.; Stapley, J.; Bennison, C.; Tucker, R.; Twyman, H.; Kim, K.-W.; Burke, T.; Birkhead, T.R.; Andersson, S.; Slate, J. Red carotenoid coloration in the zebra finch is controlled by a cytochrome P450 gene cluster. Curr. Biol. 2016, 26, 1435–1440. [Google Scholar] [CrossRef]
- Hill, G.E. Condition-dependent traits as signals of the functionality of vital cellular processes. Ecol. Lett. 2011, 14, 625–634. [Google Scholar] [CrossRef]
- Johnson, J.D.; Hill, G.E. Is carotenoid ornamentation linked to the inner mitochondria membrane potential? A hypothesis for the maintenance of signal honesty. Biochimie 2013, 95, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Guo, X.; Wong, S.Y.; Lu, P.; Hartson, S.D.; Medeiros, D.M.; Wang, W.; Clarke, S.L.; Lucas, E.A.; Smith, B.J. Deficiency of β-carotene oxygenase 2 induces mitochondrial fragmentation and activates the STING-IRF3 pathway in the mouse hypothalamus. J. Nutr. Biochem. 2021, 88, 108542. [Google Scholar] [CrossRef]
- Guo, X.; Wu, L.; Soh, T.; Chowanadisai, W.; Clarke, S.; Lucas, E.; Smith, B.; Wang, W.; Medeiros, D.; Lin, D. Altered fatty acid metabolism remodels the hypothalamic metabolome to stimulate feeding behavior in BCO2 knockout mice. FASEB J. 2016, 30, 271.6. [Google Scholar] [CrossRef]
- Våge, D.I.; Boman, I.A. A nonsense mutation in the beta-carotene oxygenase 2 (BCO2) gene is tightly associated with accumulation of carotenoids in adipose tissue in sheep (Ovis aries). BMC Genet. 2010, 11, 10. [Google Scholar] [CrossRef]
- Guo, L.; Kruglyak, L. Genetics and biology of coloration in reptiles: The curious case of the Lemon Frost geckos. Comp. Physiol. Genom. 2023, 55, 479–486. [Google Scholar] [CrossRef]
- Poon, Z.W.J.; Shen, X.; Uichanco, J.A.; Terence, C.; Chua, S.W.G.; Domingos, J.A. Comparative transcriptome analysis reveals factors involved in the influence of dietary astaxanthin on body colouration of Malabar Snapper (Lutjanus malabaricus). Aquaculture 2023, 562, 738874. [Google Scholar] [CrossRef]
- Xie, W.; Du, G.; Deng, H.; Ma, Y.; Gao, M.; Duan, H.; Yik Yeong, S.; Sui, L. Transcriptomes of Litopenaeus vannamei reveal modulation of antioxidant system induced by dietary archaeal carotenoids. J. Oceanol. Limnol. 2023, 41, 2010–2019. [Google Scholar] [CrossRef]
- Wan, S.; Li, Q.; Yu, H.; Liu, S.; Kong, L. Transcriptome analysis based on dietary beta-carotene supplement reveals genes potentially involved in carotenoid metabolism in Crassostrea gigas. Gene 2022, 818, 146226. [Google Scholar] [CrossRef] [PubMed]
- Judan Cruz, K.G.; Landingin, E.P.; Gajeton, M.B.; Fernando, S.I.D.; Watanabe, K. Carotenoid coloration and coloration-linked gene expression in red tilapia (Oreochromis sp.) tissues. BMC Vet. Res. 2021, 17, 314. [Google Scholar] [CrossRef]
- Ahi, E.P.; Lecaudey, L.A.; Ziegelbecker, A.; Steiner, O.; Glabonjat, R.; Goessler, W.; Hois, V.; Wagner, C.; Lass, A.; Sefc, K.M. Comparative transcriptomics reveals candidate carotenoid color genes in an East African cichlid fish. BMC Genom. 2020, 21, 54. [Google Scholar] [CrossRef] [PubMed]
- Gazda, M.A.; Araújo, P.M.; Lopes, R.J.; Toomey, M.B.; Andrade, P.; Afonso, S.; Marques, C.; Nunes, L.; Pereira, P.; Trigo, S. A genetic mechanism for sexual dichromatism in birds. Science 2020, 368, 1270–1274. [Google Scholar] [CrossRef]
- Sun, Y.; Yan, C.; Liu, M.; Liu, Y.; Wang, W.; Cheng, W.; Yang, F.; Zhang, J. CRISPR/Cas9-mediated deletion of one carotenoid isomerooxygenase gene (EcNinaB-X1) from Exopalaemon carinicauda. Fish Shellfish. Immunol. 2020, 97, 421–431. [Google Scholar] [CrossRef]
- Clugston, R.D. Carotenoids and fatty liver disease: Current knowledge and research gaps. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2020, 1865, 158597. [Google Scholar] [CrossRef]
- Silva, L.S.e.; de Miranda, A.M.; de Brito Magalhães, C.L.; Dos Santos, R.C.; Pedrosa, M.L.; Silva, M.E. Diet supplementation with beta-carotene improves the serum lipid profile in rats fed a cholesterol-enriched diet. J. Physiol. Biochem. 2013, 69, 811–820. [Google Scholar] [CrossRef]
- Kuhn, B.N. Phenotypic Effects of Knocking Out the Pigmentation Related Genes, abcg2, bco1, and bco1-like in Atlantic Salmon. Master’s Thesis, Norwegian University of Life Sciences, Ås, Norway, 2022. [Google Scholar]
- Praud, C.; Al Ahmadieh, S.; Voldoire, E.; Le Vern, Y.; Godet, E.; Couroussé, N.; Graulet, B.; Duval, E.L.B.; Berri, C.; Duclos, M.J. Beta-carotene preferentially regulates chicken myoblast proliferation withdrawal and differentiation commitment via BCO1 activity and retinoic acid production. Exp. Cell Res. 2017, 358, 140–146. [Google Scholar] [CrossRef]
- Eroglu, A.; Harrison, E.H. Carotenoid metabolism in mammals, including man: Formation, occurrence, and function of apocarotenoids: Thematic Review Series: Fat-Soluble Vitamins: Vitamin A. J. Lipid Res. 2013, 54, 1719–1730. [Google Scholar] [CrossRef]
- Black, S.D. Membrane topology of the mammalian P450 cytochromes. FASEB J. 1992, 6, 680–685. [Google Scholar] [CrossRef]
- Urlacher, V.B.; Girhard, M. Cytochrome P450 monooxygenases in biotechnology and synthetic biology. Trends Biotechnol. 2019, 37, 882–897. [Google Scholar] [CrossRef]
- Reboul, E. Mechanisms of carotenoid intestinal absorption: Where do we stand? Nutrients 2019, 11, 838. [Google Scholar] [CrossRef] [PubMed]
- Hill, F. Xanthophyll pigmentation in sheep fat. Nature 1962, 194, 865–866. [Google Scholar] [CrossRef] [PubMed]
- Berry, S.; Davis, S.; Beattie, E.; Thomas, N.; Burrett, A.; Ward, H.; Stanfield, A.; Biswas, M.; Ankersmit-Udy, A.; Oxley, P. Mutation in bovine β-carotene oxygenase 2 affects milk color. Genetics 2009, 182, 923–926. [Google Scholar] [CrossRef] [PubMed]
- Strychalski, J.; Gugołek, A.; Brym, P.; Antoszkiewicz, Z.; Chwastowska-Siwiecka, I. Polymorphism of the BCO2 gene and the content of carotenoids, retinol, and α-tocopherol in the liver and fat of rabbits. Rev. Bras. Zootec. 2019, 48, e20180243. [Google Scholar] [CrossRef]
- Eriksson, J.; Larson, G.; Gunnarsson, U.; Bed’Hom, B.; Tixier-Boichard, M.; Strömstedt, L.; Wright, D.; Jungerius, A.; Vereijken, A.; Randi, E. Identification of the yellow skin gene reveals a hybrid origin of the domestic chicken. PLoS Genet. 2008, 4, e1000010. [Google Scholar] [CrossRef]
- Shi, X.; Zhu, W.; Guo, J.; Lin, K.; Fu, J.; Wang, L.; Dong, Y.; Luo, M.; Dong, Z. Genome-wide association study reveals candidate genes critical for skin pigmentation in common carp (Cyprinus carpio) strains including koi. Aquaculture 2024, 590, 741075. [Google Scholar] [CrossRef]
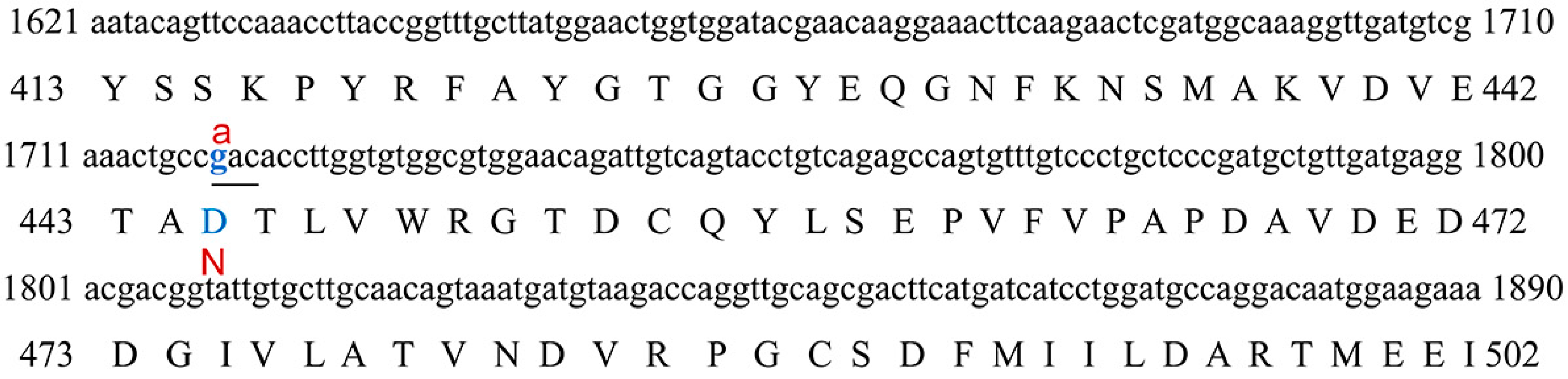

| Primer Name | Primer Sequences (5′–3′) | Application |
|---|---|---|
| NdBCO-like4 Fq | GAACTCGCGCTTATCCTGAC | qPCR |
| NdBCO-like4 Rq | CATTCACAGCGCAGTTGTCT | qPCR |
| NdBCO-like4 Fd | GCCCAGTACATAATGCCCAG | dsRNA generation |
| NdBCO-like4 Rd | CGCAGTCATGTCGAACTTGT | dsRNA generation |
| NdBCO-like4 F1 | CATTCTCCGGGAAGTGGTAA | Target sequence amplification |
| NdBCO-like4 R1 | ACTTACCCGTTAACGCCACA | Target sequence amplification |
| NdBCO-like4 F2 | CGACAGGTTCAGAGTTCTACAGCCGACGATCCATTCTCCGGGAAGTGGTAA | Add adapter |
| NdBCO-like4 R2 | GCTCGTCGTGACGCCATGACGGACTTACCCGTTAACGCCACA | Add adapter |
| ds-EGFP F | GGTGAACTTCAAGATCCGCC | dsRNA generation |
| ds-EGFP R | CTTGTACAGCTCGTCCATGC | dsRNA generation |
| GADPH Fq | CGGTGCTGCTCAGAATATCA | qPCR |
| GADPH Rq | TTACCAAGGCGAACGGTAAG | qPCR |
| T7F | TAATACGACTCACTATAGGG | Adapter |
| T7R | CCCTATAGTGAGTCGTATTA | Adapter |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huo, Z.; Li, H.; Wang, G.; Zhao, T. Functional Analysis of NdBCO-like4 Gene in Pigmentation of Neocaridina denticulata sinensis. Fishes 2025, 10, 134. https://doi.org/10.3390/fishes10030134
Huo Z, Li H, Wang G, Zhao T. Functional Analysis of NdBCO-like4 Gene in Pigmentation of Neocaridina denticulata sinensis. Fishes. 2025; 10(3):134. https://doi.org/10.3390/fishes10030134
Chicago/Turabian StyleHuo, Zhipeng, Haifan Li, Guodong Wang, and Tanjun Zhao. 2025. "Functional Analysis of NdBCO-like4 Gene in Pigmentation of Neocaridina denticulata sinensis" Fishes 10, no. 3: 134. https://doi.org/10.3390/fishes10030134
APA StyleHuo, Z., Li, H., Wang, G., & Zhao, T. (2025). Functional Analysis of NdBCO-like4 Gene in Pigmentation of Neocaridina denticulata sinensis. Fishes, 10(3), 134. https://doi.org/10.3390/fishes10030134





