Influence of the Silkworm-Derived (Bombyx mori) Functional Substance (Silkrose-BM) on the Fish Meat Quality of Yellowtail (Seriola quinqueradiata)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Preparation of Experimental Diets
2.3. Fish Culture
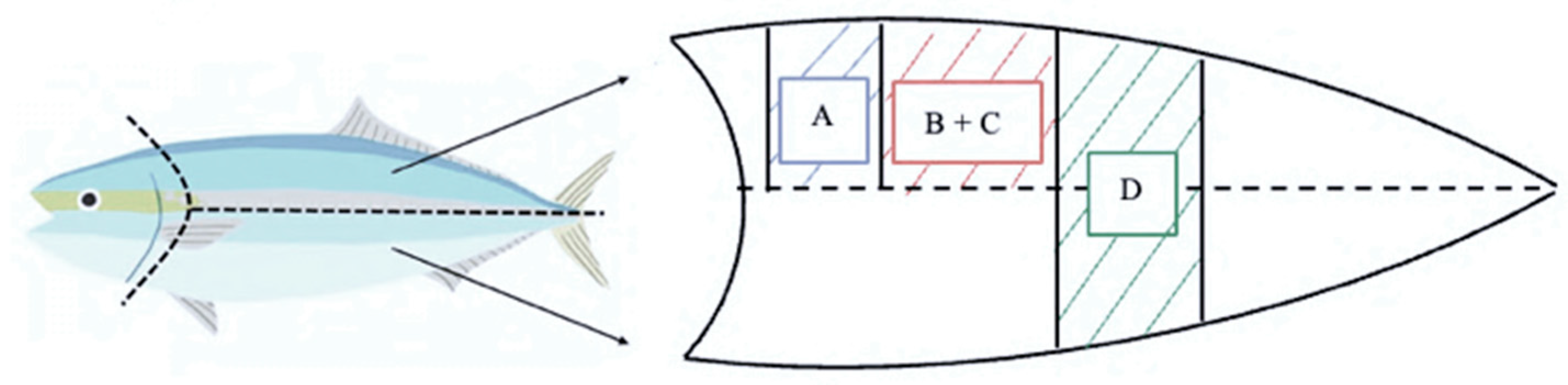
2.4. Sampling Techniques
2.5. Proximate Analysis
2.6. Color Change Analysis
- ΔE*ab = total color difference (calculated for each observation 0, 24, and 48 h)
- L1 = mean L* value of silkrose-BM
- L0 = mean L* value of EP feed
- a1 = mean a* value silkrose-BM
- a0 = mean a* value EP feed
- b1 = mean b* value of silkrose-BM
- b0 = mean b* value of EP feed
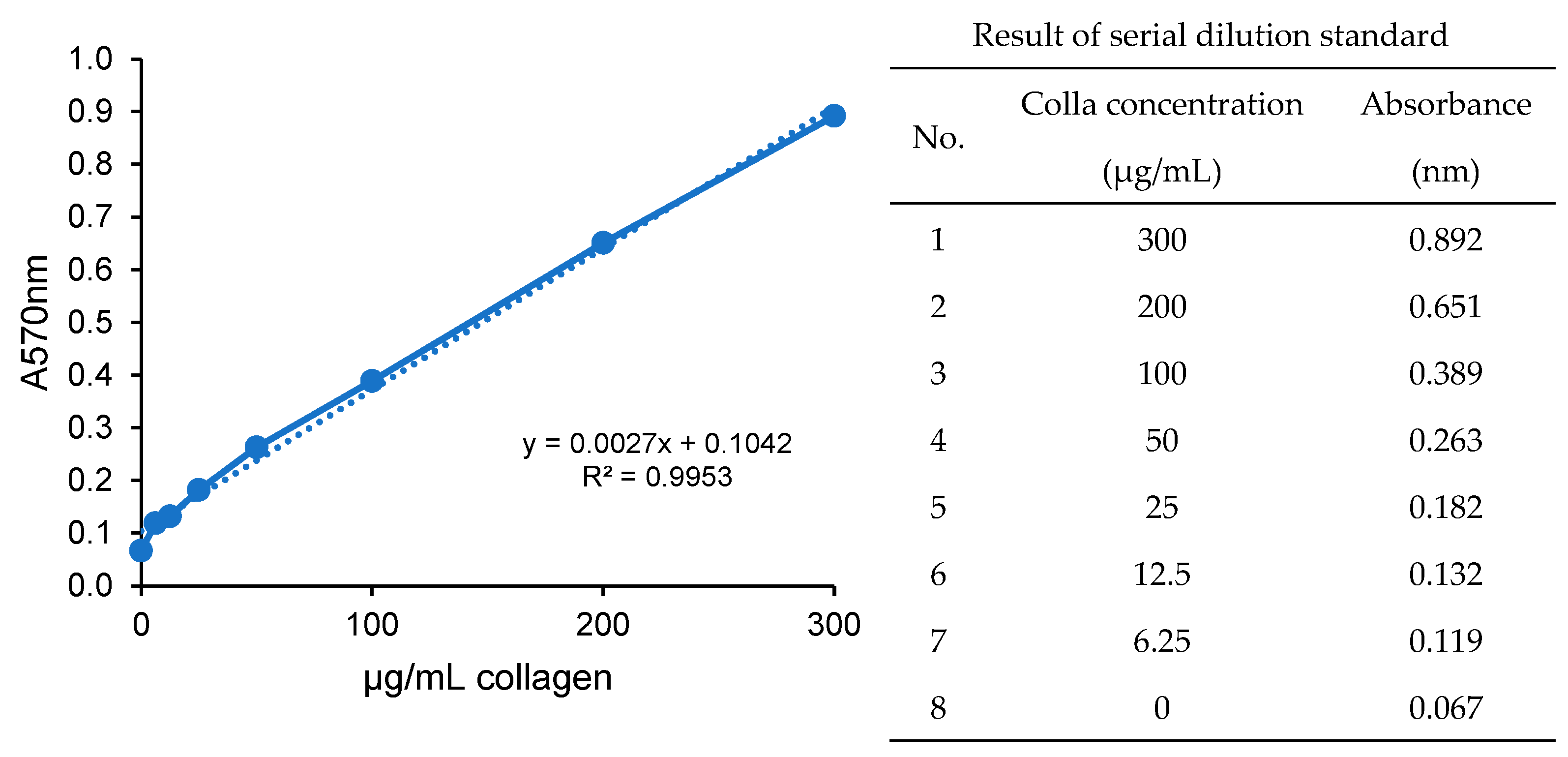
2.7. Total Collagen Determination
2.8. Drip Loss Analysis
- W0 = weight of fillet before storage time (g)
- W1 = weight of fillet after storage time (g)
2.9. Histological Analysis
2.10. Analysis of Gene Expression (Total RNA Isolation and qRT-PCR)
2.11. Statistical Analysis
3. Results and Discussion
3.1. Proximate Composition
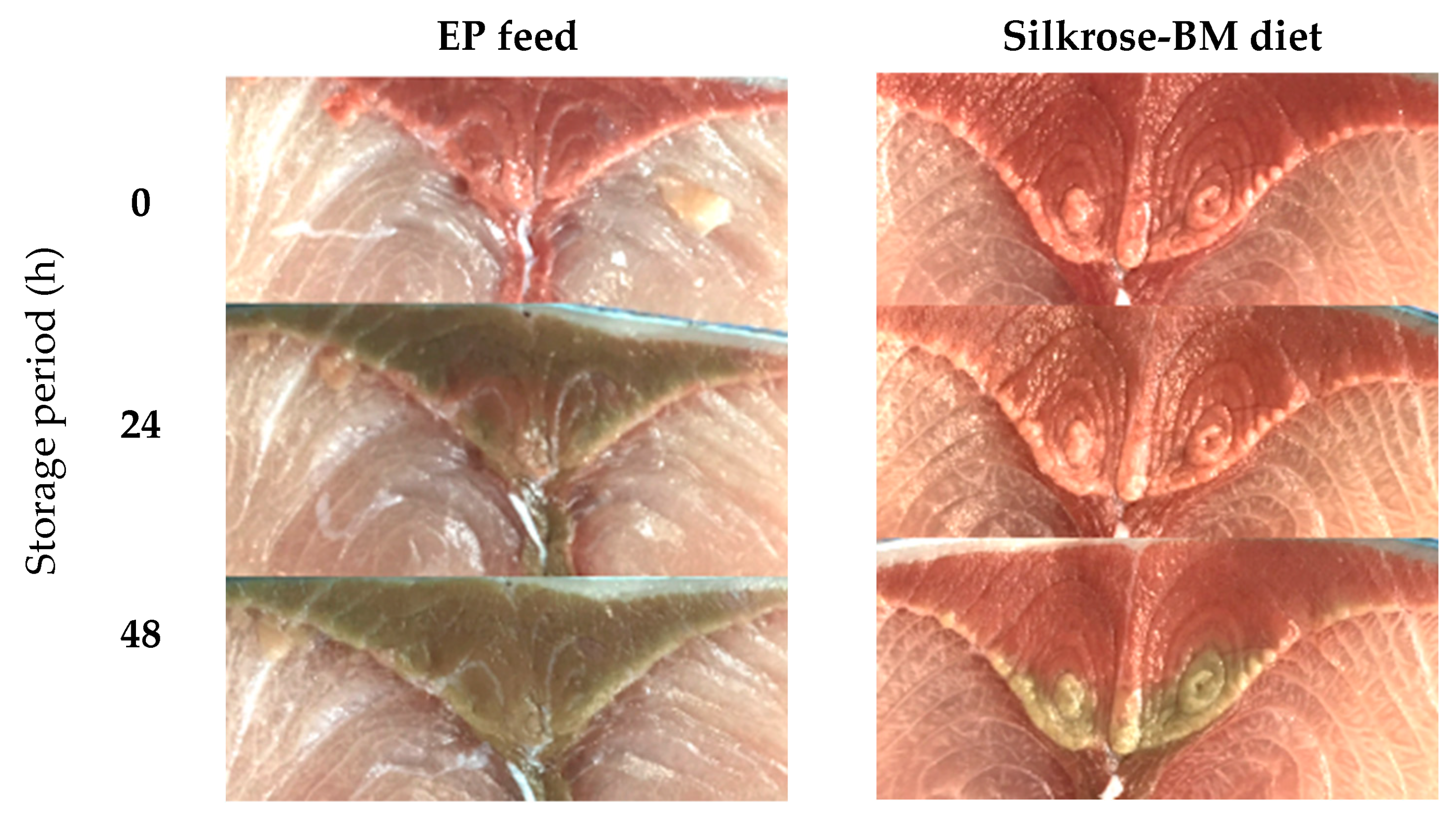
3.2. Discoloration in Yellowtail Red Muscle
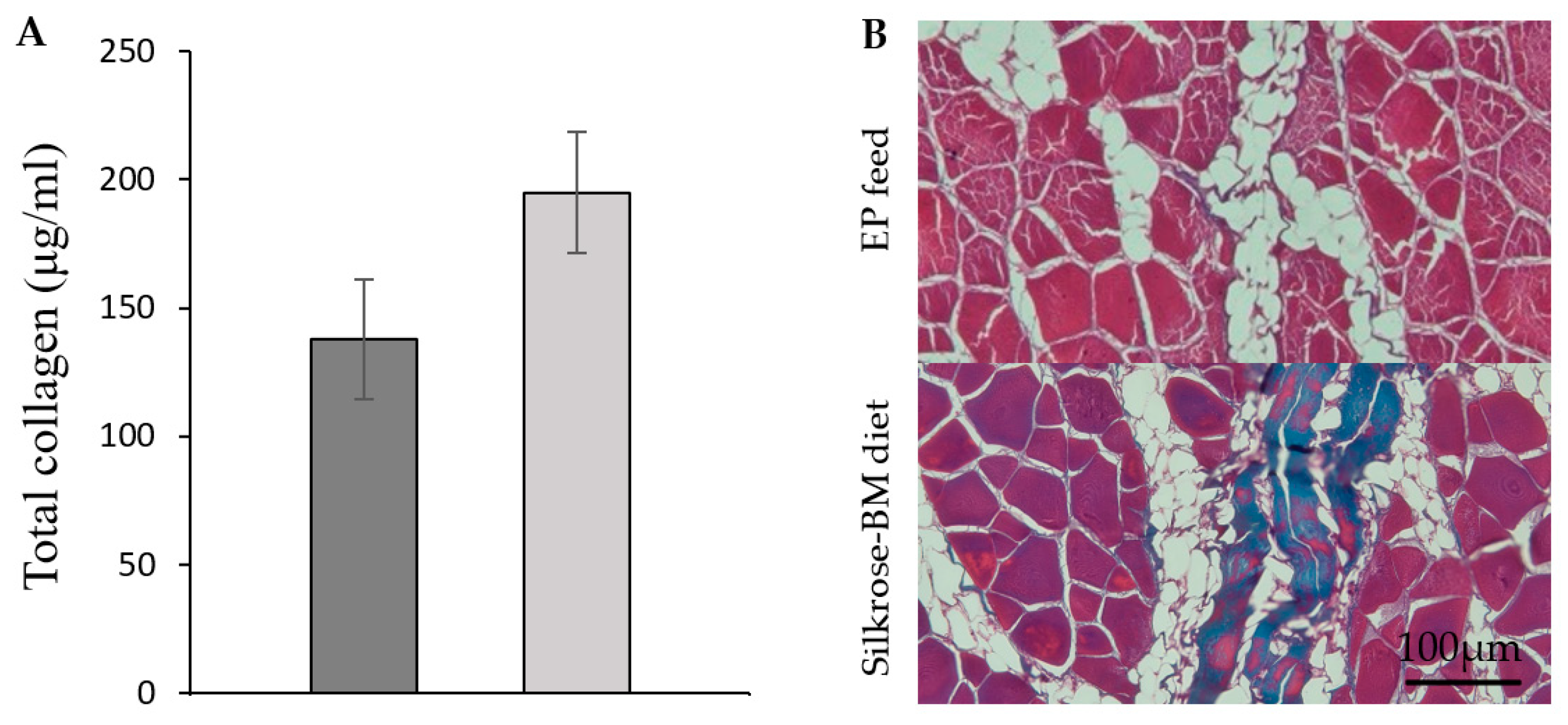
3.3. Quantification of Total Collagen
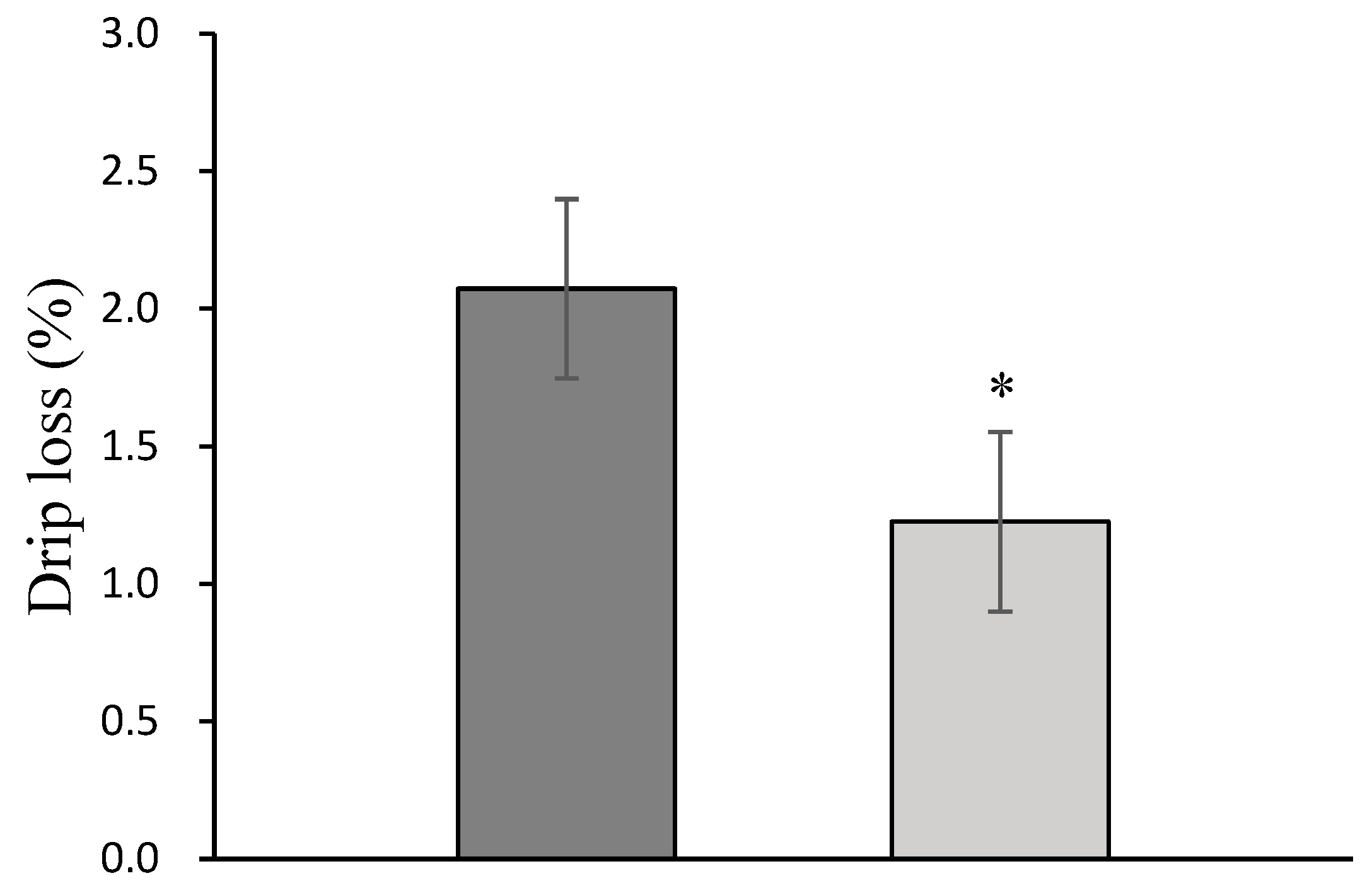
3.4. Drip Loss
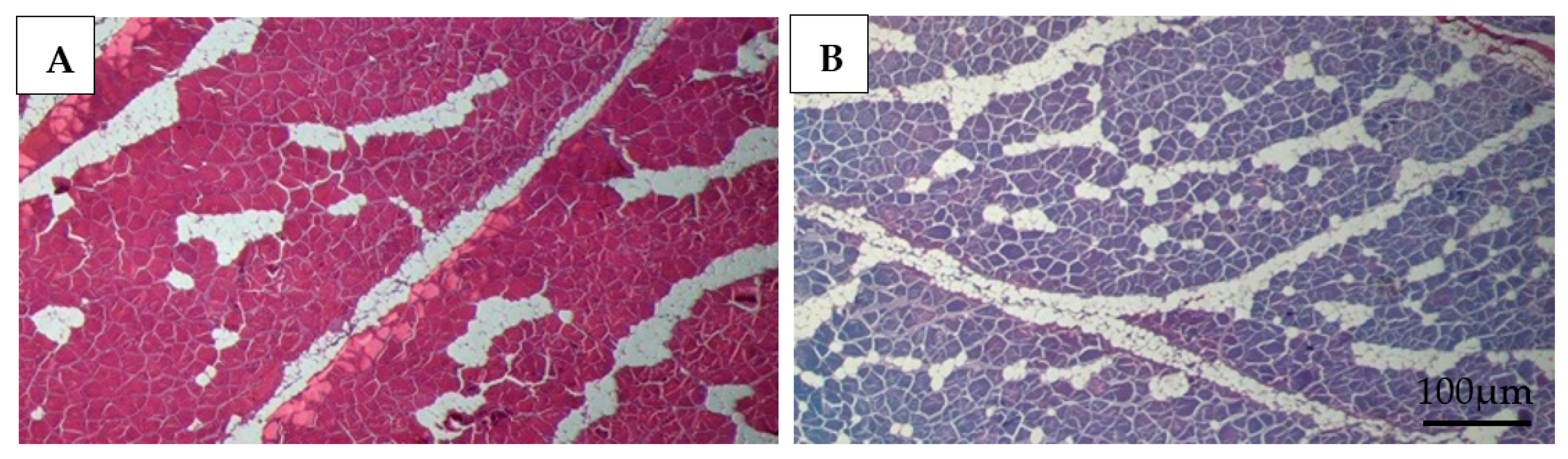
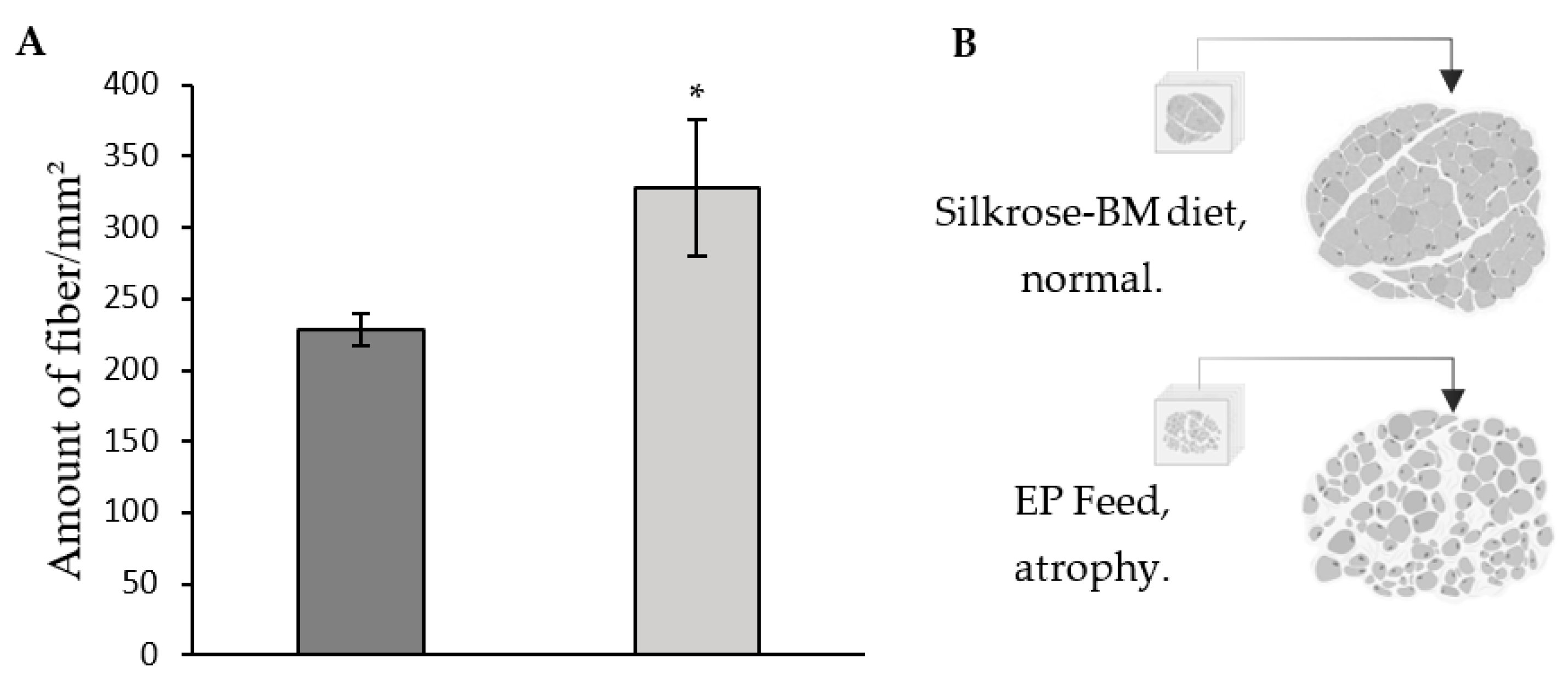
3.5. Histology
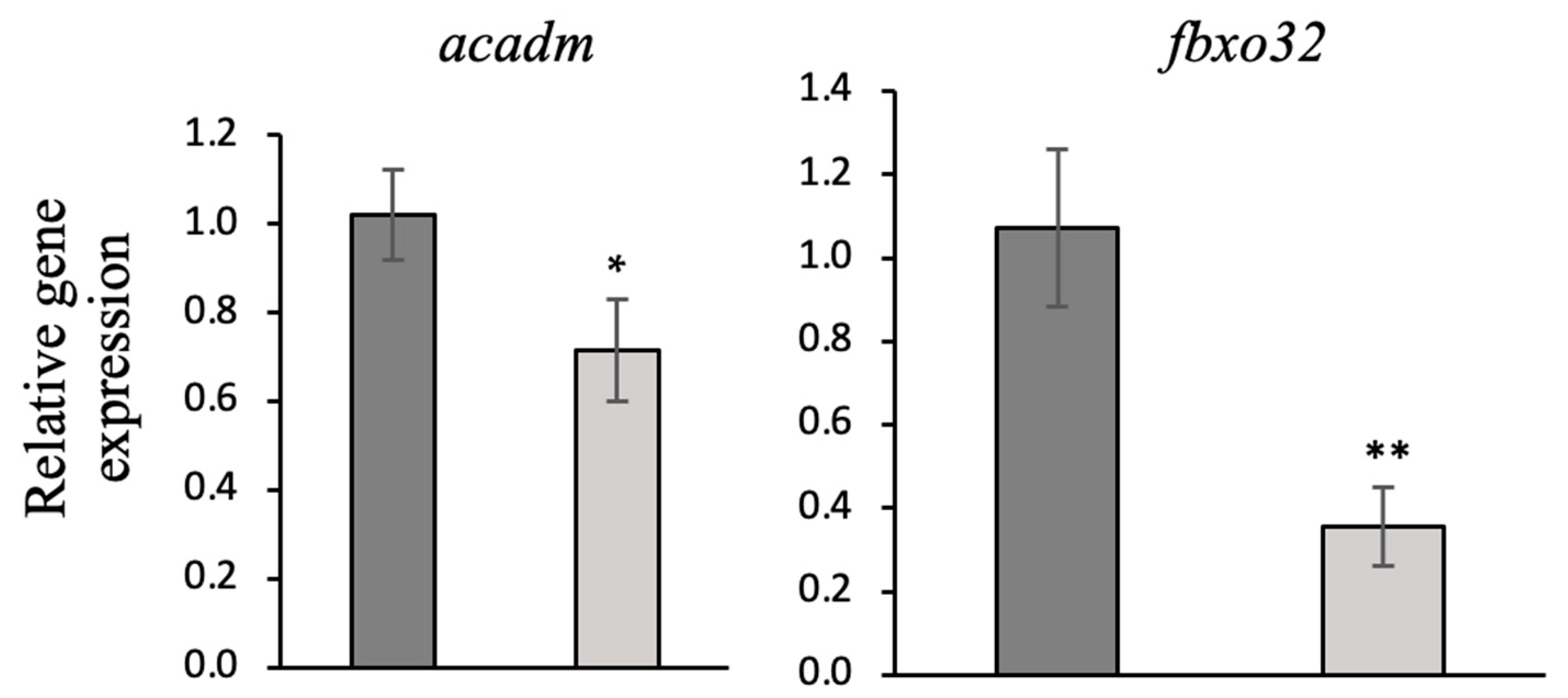
3.6. Gene Expression Analysis (qRT-PCR)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, J.H.; Sun, D.W.; Han, Z.; Zeng, X.A. Texture and Structure Measurements and Analyses for Evaluation of Fish and Fillet Freshness Quality: A Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Suzuki, T.; Ichimaida, K.; Hattori, T.; Ueda, R. Do Consumers Actually Sense that Sashimi Made from Frozen-Thawed Fish Tastes Worse than Non-Frozen One? Int. J. Refrig. 2020, 111, 94–102. [Google Scholar] [CrossRef]
- Kiessling, A.; Ruohonen, K.; Bjornevik, M. Muscle Fibre Growth and Quality in Fish. Arch. Tierz. 2006, 49, 137–146. [Google Scholar]
- Ali, M.F.Z.; Nakahara, S.; Otsu, Y.; Ido, A.; Miura, C.; Miura, T. Effects of Functional Polysaccharide from Silkworm as an Immunostimulant on Transcriptional Profiling and Disease Resistance in Fish. J. Insects Food Feed 2021, 8, 1221–1233. [Google Scholar] [CrossRef]
- Miura, T.; Nishikawa, M.; Otsu, Y.; Ali, M.F.Z.; Hashizume, A.; Miura, C. The Effects of Silkworm-Derived Polysaccharide (Silkrose) on Ectoparasitic Infestations in Yellowtail (Seriola quinqueradiata) and White Trevally (Pseudocaranx dentex). Fishes 2022, 7, 14. [Google Scholar] [CrossRef]
- Zou, X.; Liu, M.; Li, X.; Pan, F.; Wu, X.; Fang, X.; Zhou, F.; Peng, W.; Tian, W. Applications of insect nutrition resources in animal production. J. Agric. Food Res. 2024, 15, 100966. [Google Scholar] [CrossRef]
- Nishiguchi, H.; Suryadi, I.B.B.; Ali, M.F.Z.; Miura, C.; Miura, T. Dietary Black Soldier Fly (Hermetia illucens)-Dipterose-BSF-Enhanced Zebrafish Innate Immunity Gene Expression and Resistance to Edwardsiella tarda Infection. Insects 2024, 326, 326. [Google Scholar] [CrossRef]
- Ali, M.F.Z.; Yasin, I.A.; Ohta, T.; Hashizume, A.; Ido, A.; Takahashi, T.; Miura, C.; Miura, T. The Silkrose of Bombyx mori Effectively Prevents Vibriosis in Penaeid Prawns via The Activation of Innate Immunity. Sci. Rep. 2018, 8, 8836. [Google Scholar] [CrossRef]
- Bharath, K.B.; Chandrashekaer, S.; Pallavi. Silkworm Pupae: A Goldmine Waste. J. Enomology Zool. Stud. 2024, 12, 231–236. [Google Scholar] [CrossRef]
- Ohta, T.; Ido, A.; Kusano, K.; Miura, C.; Miura, T. A Novel Polysaccharide in Insects Activities the Innate Immune System in Mouse Macrophage RAW264 Cells. PLoS ONE 2014, 9, e114823. [Google Scholar] [CrossRef]
- Ohta, T.; Kusano, K.; Miura, C.; Miura, T. Silkrose: A Novel Acidic Polysaccharide from The Silkmoth that can Stimulate The Innate Immune Response. Carbohydr. Polym. 2016, 136, 995–1001. [Google Scholar] [CrossRef]
- Yamada, S.; Sugahara, K.; Ozbeck, S. Evolution of Glycosaminoglycans, Comparative Biochemical Study. Commun. Intergrative Biol. 2011, 4, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Sahu, B.; Sharma, D.D.; Jayakumar, G.C.; Madhan, B.; Zameer, F. A Review on an Imperative By-Product: Glycosaminoglycans-A Holistic Approach. Carbohydr. Polym. Technol. Appl. 2023, 5, 100275. [Google Scholar] [CrossRef]
- Ali, M.F.Z.; Kameda, K.; Kondo, F.; Iwai, T.; Kurniawan, R.A.; Ohta, T.; Ido, A.; Takahashi, T.; Miura, C.; Miura, T. Effects of Dietary Silkrose of Antheraea yamamai on Gene Expression Profiling and Disease Resistance to Edwardsiella tarda in Japanese Medaka (Oryzias latipes). Fish Shellfish Immunol. 2021, 114, 207–217. [Google Scholar] [CrossRef]
- Climate and Average Weather Year Round in Uwajima, Japan. 2024. Available online: https://weatherspark.com/y/143164/Average-Weather-in-Uwajima-Japan-Year-Round (accessed on 25 December 2024).
- Mabuchi, R.; Adachi, M.; Kikutani, H.; Tanimoto, S. Discriminants Analysis of Muscle Tissue Type in Yellowtail Seriola quinqueradiata Muscle Based on Metabolic Component Profiles. Food Sci. Technol. Res. 2018, 24, 883–891. [Google Scholar] [CrossRef]
- Ando, M.; Mok, W.J.; Maeda, Y.; Miki, R.; Fukuda, T.; Tsukamasa, Y. Quality Assessment of Yellowtail (Seriola quinqueradiata) Meat Cultured in an Offshore Floating Flexible Facility. Food Sci. Nutr. 2022, 10, 3024–3033. [Google Scholar] [CrossRef]
- Shioya, I.; Takemura, S.; Ishizuka, R.; Yamaguchi, T. Variations in the Proximate Composition of Mucle in Cultured Yellowtail Seriola quinqueradiata at Different Anatomical Portions. Food Sci. Technol. 2012, 78, 725–733. [Google Scholar] [CrossRef]
- Date, K.; Yamamoto, Y. Seasonal Variations with Growth in Nutritive Components in Meat of Cultured Yellowtail Seriola quinqueradiata. Nippon Suisan Gakkaishi 1988, 54, 1041–1047. [Google Scholar] [CrossRef]
- Saeki, K.; Kumagai, H. Muscle Components of Wild and Cultured yellowtail. Shokuhin Eiseigaku Zasshi 1979, 20, 101–105. [Google Scholar] [CrossRef]
- Shimizu, Y.; Tada, M.; Endo, K. Seasonal Variations in Chemical Constituents of Yellowtail Muscle 1. Water, Lipid and Crude Protein. Nippon Suisan Gakkaishi 1973, 39, 993–999. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemist (AOAC). Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemist Inc.: Rockville, MD, USA, 2005. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using real-time quantitative PCR and the 2 (-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, A.; Nabeshima, H.; Umeda, S.; Hasegawa, I. Gonad Maturation and Dynamics of Crude Lipid Content in The Body of Yellowtail During the Spawning Season. Nippon Suisan Gakkaishi 1980, 46, 407–412. [Google Scholar] [CrossRef]
- Langer, R.O.S.; Simoes, G.S.; Soares, A.L.; Oba, A.; Rossa, A.; Shimokomaki, M.; Ida, E.I. Broiler Transportation Conditions in A Brazilian Commercial Line and The Accurence of Breast PSE (Pale, Soft, Exudative) Meat and DFD-Like (Dark, Firm, Dry) Meat. Braz. Arch. Biol. Technol. 2010, 53, 1161–1167. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Jia, G.Q.; Zhang, Y.; Lei, J.; Ren, L.; Feng, D.Y. Effects of Constant and Cyclic Heat Stress on Muscle Metabolism and Meat Quality of Broiler Breast Fillet and Thigh Meat. Poult. Sci. 2012, 91, 2931–2937. [Google Scholar] [CrossRef]
- Gong, H.; Wang, B.; Jiang, H.; Li, W.; Zhang, Y.; Ji, C.; Lin, X.; Zhang, S. Quality Assessment and Microbial Community Succession of Finely Chopped Common Carp (Cyprinus Carpio) Under Low-Temperature Storage: Implication for Nutritional Composition and Spoilage Indicators. Food Control 2025, 171. [Google Scholar] [CrossRef]
- Wu, H.; Tatiyaborworntham, N.; Hajimohammad, M.; Decker, E.A.; Richards, M.P.; Undeland, I. Model Systems for Studying Lipid Oxidation Associated with Muscle Foods: Methods, Challenges, and Prospects. Crit. Rev. Food Sci. Nutr. 2024, 64, 153–171. [Google Scholar] [CrossRef]
- Faustman, C.; Sun, Q.; Mancini, R.; Suman, S.P. Myoglobin and Lipid Oxidation Interactions: Mechanistic Bases and Control. Meat Sci. 2010, 86, 86–94. [Google Scholar] [CrossRef]
- Bayram, I.; Decker, E.A. Underlying Mechanisms of Synergistic Antioxidant Iteractions During Lipid Oxidation. Trends Food Sci. Technol. 2023, 133, 219–230. [Google Scholar] [CrossRef]
- Cao, J.; Yan, H.; Ye, B.; Shen, Y.; Liu, L. Effects of Mailard Reaction Products on Myoglobin-Mediated Lipid Oxidation During Refrigerated Storage of Carp. Food Chem. 2024, 434, 137465. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms of Antioxidants in the Oxidation of Foods. Compr. Rev. Food Sci. Food Saf. 2009, 8, 345–358. [Google Scholar] [CrossRef]
- Pazos, M.; Gonzalez, M.J.; Gallardo, J.M.; Torres, J.L.; Medina, I. Preservation of The Endogeneous Antioxidant System of Fish Muscle by Grape Polyphenols During Frozen Storage. Eur. Food Res. Technol. 2005, 220, 514–519. [Google Scholar] [CrossRef]
- Chatham, J.C.; Zhang, J.; Wende, A.R. Role of O-linked N-acetylglucosamine (O-glcNAc) Protein Modification in Cellular (patho)physiology. Am. Physiol. Soc. Physiol. Rev. 2021, 101, 427–493. [Google Scholar] [CrossRef]
- Adhikari, E.; Liu, Q.; Burton, C.; Mockabee-Macias, A.; Lester, D.K. L-fucose, A Sugary Regulator of Antitumor Immunity and Immunotherapies. Mol. Carciogenesis 2022, 61, 439–453. [Google Scholar] [CrossRef]
- Listrat, A.; Lebret, B.; Louveau, I.; Astruc, T.; Bonnet, M.; Lefaucheur, L.; Picard, B.; Bugeon, J. How Muscle Structure and Composition Influence Meat and Flesh Quality. Sci. World J. 2016, 2016, 3182746. [Google Scholar] [CrossRef]
- Sato, K.; Yoshinaka, R.; Sato, M.; Shimizu, Y. Collagen content in the muscle of fishes in association with their swimming movement and meat texture. Bull. Jpn. Soc. Sci. Fish. 1986, 52, 1595–1600. [Google Scholar] [CrossRef]
- Puri, S.; Coulson-Thomas, Y.M.; Gesteira, T.F.; Coulson-Thomas, V.J. Distribution and Function of Glycosaminoglycans and Proteoglycans in the Development Homeostasis and Pathology of the Ocular Surface. Front. Cell Dev. Biol. 2020, 8, 731. [Google Scholar] [CrossRef]
- Brooke, L.F.; Melrose, J. The Glycosaminoglycan Side Chains and Modular Core Proteins of Heparan Sulphate Proteoglycans and the Varied Ways They Provide Tissue Protection by Regulating Physiological Processes and Cellular Behaviour. Int. J. Mol. Sci. 2023, 24, 14101. [Google Scholar] [CrossRef]
- Rini, S.R.; Sugiharo; Mahfudz, L.D. The Effect of Different Rearing Temperatures on the Physical Quality of Broiler Meat in the Finisher Period (in Bahasa Indonesia). J. Sains Peternak. Indones. 2019, 14, 387–395. [Google Scholar] [CrossRef]
- Wada, S.; Ogawa, Y. High Pressure Effects on Fish Lipid Degradation: Myoglobin Change and Water Holding Capacity. Prog. Biotechnol. 1996, 13, 351–356. [Google Scholar] [CrossRef]
- Nkukwana, T.T.; Muchenje, V.; Masika, P.J.; Pieterse, E.; Hoffman, L.C.; Dzama, K. Proximate composition and variation in colour, drip loss and pH of breast meat from broilers supplemented with Moringa oleifera leaf meal over time. Anim. Prod. Sci. 2015, 56, 1208–1216. [Google Scholar] [CrossRef]
- Kale, L.D.; Eikevik, T.M.; Rustad, T.; Nordtvedt, T.S. Changes in Water Holding Capacity and Driploss of Atlantic Salmon (Salmo salar) Muscle during Superchilled Storage. LWT-Food Sci. Technol. 2014, 55, 528–535. [Google Scholar] [CrossRef]
- Mkrtchyan, M.; Safronov, D.; Tokarev, A.; Makavchik, S.; Orlova, D. Determination The Quality of Meat, Manufactured Meat, and Meat Products Via The Histological Method. Int. Trans. J. Eng. Manag. Appl. Sci. Technol. 2020, 11, 1115. [Google Scholar]
- Bodine, S.C.; Latres, E.; Baumhueter, S.; Lai, V.K.; Nunez, L.; Clarke, B.A.; Poueymirou, W.T.; Panaro, F.J.; Na, E.; Dharmarajan, K.; et al. Identification of Ubiquitin Ligases Required for Skeletal Muscle Atrophy. Science 2001, 294, 1704–1708. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Komatsu, T.; Yamazaki, M.; Abe, H. Effects of Fasting and Refeeding on Expression of Proteolytic-Related Genes in Skeletal Muscle of Chicks. J. Nutr. Sci. Vitaminol. 2005, 51, 248–253. [Google Scholar] [CrossRef]
- Tacchi, L.; Bickerdike, R.; Scombes, C.J.; Pooley, N.J.; Urquhart, K.L.; Collet, B.; Martin, S.A.M. Ubiquitin E3 ligase atrogin-1 (Fbox-32) in Atlantic salmon (Salmo salar): Sequence Analysis, Genomic Structure and Modulation of Expression. Comp. Biochem. Physiol. Part B 2010, 157, 364–373. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, X.; Zhu, H.; Wang, Y.; Hou, Y.; Liu, Y. Dietary Fish Oil Supplementation Alters Liver Gene Expressions to Protect Against LPS-Induced Liver Injury in Weanling Piglets. Innate Immun. 2019, 25, 60–72. [Google Scholar] [CrossRef]
- Ranjithkumar, K.; Sudhan, C.; Roy, U.; Rao, M.B. Ribosomal RNA and Their Applications in Species Identification. J. Aquac. Trop. 2018, 33, 91–99. [Google Scholar] [CrossRef]
- He, L.; Shi, X.; Han, K.; Huang, W.; Chen, D.; Lian, Z.; Ruan, S. Molecular Characterization of Adenosine Monophosphate Deaminase 1 and the Correlation Analysis between its mRNA Expression Levels and Inosine Monophosphate Content in Large Yellow Croaker (Larimichthys crocea). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2024, 272. [Google Scholar] [CrossRef]
- Pinel, K.; Heraud, C.; Morin, G.; Dias, K.; Marcé, A.; Beauclair, L.; Fontagné-Dicharry, S.; Masagounder, K.; Klünemann, M.; Seiliez, I.; et al. Are the Main Methionine Sources Equivalent? A Focus on DL-Methionine and DL-Methionine Hydroxy Analog Reveals Differences on Rainbow Trout Hepatic Cell Lines Functions. I. J. Mol. Sci. 2022, 23, 2935. [Google Scholar] [CrossRef]
- Persson, B.; Hedlund, J.; Jornvall, H. Medium- and short-chain Dehydrogenase/Reductase Gene and Protein Families: The MDR Superfamily. Cell. Mol. Life Sci. 2008, 65, 3879–3894. [Google Scholar] [CrossRef]
- Mason, E.; Hindmarch, C.C.T.; Dunham-Snary, K.J. Medium-chain Acyl-CoA Dehydrogenase Deficiency: Pathogenesis, Diagnosis, and Treatment. Endocrinol. Diabetes Metab. 2022, 6, 1–11. [Google Scholar] [CrossRef]
- Xu, C.; Wu, G.; Zohar, Y.; Du, S. Analysis of Myostatin Gene Structure, Expression and Function in Zebrafish. J. Exp. Biol. 2003, 206, 4067–4079. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, B.M.; Evenhuis, J.P. Molecular Characterization of Atrogin-1/F-box protein-32 (FBXO32) and F-box protein-25 (FBXO25) in Rainbow Trout (Oncorhynchus mykiss): Expression Across Tissues in Response to Feed Deprivation. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2010, 157, 248–257. [Google Scholar] [CrossRef] [PubMed]
| Ingredients (%) | Diet | |
|---|---|---|
| EP Feed | Silkrose-BM diet | |
| Animal meal (fish meal, krill meal) | 50.0 | 50.0 |
| Grain (flour, starch) | 15.0 | 14.9 |
| Vegetable meal (soybean meal, corn gluten meal) | 9.0 | 9.0 |
| Vegetable oil | 1.0 | 1.0 |
| Others (fish meal oil, calcium phosphate, marigold petal extract, licorice extract, rice germ extract) | 25.0 | 25.0 |
| Silkrose-BM | 0.1 | |
| Proximate analysis (%) | ||
| Crude protein | ±40.0 or more | |
| Crude fat | ±28.0 or more | |
| Crude fiber | ±3.0 or less | |
| Ash | ±13.0 or less | |
| Calcium | ±1.0 or more | |
| Phosphate | ±1.0 or more | |
| Total feed intake (kg) | 11,726 | 12,709 |
| Time | Parameter | ||||
|---|---|---|---|---|---|
| Salinity (%) | Temperature (°C) | DO 1 (%) | DOM 2 (mg/L C) | Turbidity (ntu 3) | |
| April | 34.47 ± 0.02 | 16.74 ± 0.02 | 108.55 ± 2.05 | 8.68 ± 0.09 | 0.36 ± 0.03 |
| May | 34.48 ± 0.04 | 16.69 ± 0.01 | 113.68 ± 0.17 | 8.96 ± 0.01 | 0.32 ± 0.01 |
| June | 34.21 ± 0.04 | 18.20 ± 0.01 | 104.63 ± 0.49 | 8.02 ± 0.04 | 0.45 ± 0.03 |
| July | 33.87 ± 0.02 | 20.28 ± 0.11 | 104.11 ± 0.89 | 7.70 ± 0.06 | 0.32 ± 0.02 |
| Parameters | Compositions Level (%) | p-Value | |
|---|---|---|---|
| EP Feed Group | Silkrose-BM Diet Group | ||
| Moisture | 58.39 ± 1.81 | 60.60 ± 4.39 | 1 |
| Crude fat | 20.18 ± 2.08 | 20.59 ± 3.12 | 0.84 |
| Crude protein | 20.07 ± 0.46 | 19.56 ± 1.90 | 0.31 |
| Crude ash | 1.63 ± 0.04 | 1.38 ± 0.13 | 0.09 |
| Color | EP Feed Group | Silkrose-BM Diet Group |
|---|---|---|
| L* | Increased drastically over 48 h of storage | Decreased over 48 h of storage |
| a* | Decreased drastically every 24 h during the 48 h storage period | Decreased drastically after 24 h of storage, but the decrease was less drastic after 48 h |
| b* | Increased drastically every 24 h during the 48 h storage period | Increased drastically after 48 h of storage. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Athira, A.; Nishiguchi, H.; Hayashi, D.; Otsu, Y.; Miura, C.; Suryadi, I.B.B.; Ali, M.F.Z.; Miura, T. Influence of the Silkworm-Derived (Bombyx mori) Functional Substance (Silkrose-BM) on the Fish Meat Quality of Yellowtail (Seriola quinqueradiata). Fishes 2025, 10, 130. https://doi.org/10.3390/fishes10030130
Athira A, Nishiguchi H, Hayashi D, Otsu Y, Miura C, Suryadi IBB, Ali MFZ, Miura T. Influence of the Silkworm-Derived (Bombyx mori) Functional Substance (Silkrose-BM) on the Fish Meat Quality of Yellowtail (Seriola quinqueradiata). Fishes. 2025; 10(3):130. https://doi.org/10.3390/fishes10030130
Chicago/Turabian StyleAthira, Athira, Haruki Nishiguchi, Daichi Hayashi, Yuki Otsu, Chiemi Miura, Ibnu Bangkit Bioshina Suryadi, Muhammad Fariz Zahir Ali, and Takeshi Miura. 2025. "Influence of the Silkworm-Derived (Bombyx mori) Functional Substance (Silkrose-BM) on the Fish Meat Quality of Yellowtail (Seriola quinqueradiata)" Fishes 10, no. 3: 130. https://doi.org/10.3390/fishes10030130
APA StyleAthira, A., Nishiguchi, H., Hayashi, D., Otsu, Y., Miura, C., Suryadi, I. B. B., Ali, M. F. Z., & Miura, T. (2025). Influence of the Silkworm-Derived (Bombyx mori) Functional Substance (Silkrose-BM) on the Fish Meat Quality of Yellowtail (Seriola quinqueradiata). Fishes, 10(3), 130. https://doi.org/10.3390/fishes10030130







