Glycerol Monolaurate Affects Growth, Amino Acid Profile, Antioxidant Capacity, Nutrient Apparent Digestibility, and Histological Morphology of Hepatopancreas in Juvenile Pacific White Shrimp (Litopenaeus vannamei)
Abstract
1. Introduction
2. Experimental Procedures and Methodologies
2.1. Dietary Preparation and Formulation
2.2. Experimental Setup
2.3. Digestibility Assessment
2.4. Collection of Samples
2.5. The Analytical Procedures for Composition Determination
2.6. Methods for Biochemical Assays
2.7. Formulae and Statistical Analysis
3. Results
3.1. Growth Performance and Feed Utilization Parameters
3.2. Whole Body and Dorsal Muscle Proximate Composition
3.3. Amino Acid Profiles of Whole Bodies of Litopenaeus vannamei
3.4. Nutrients Apparent Digestibility
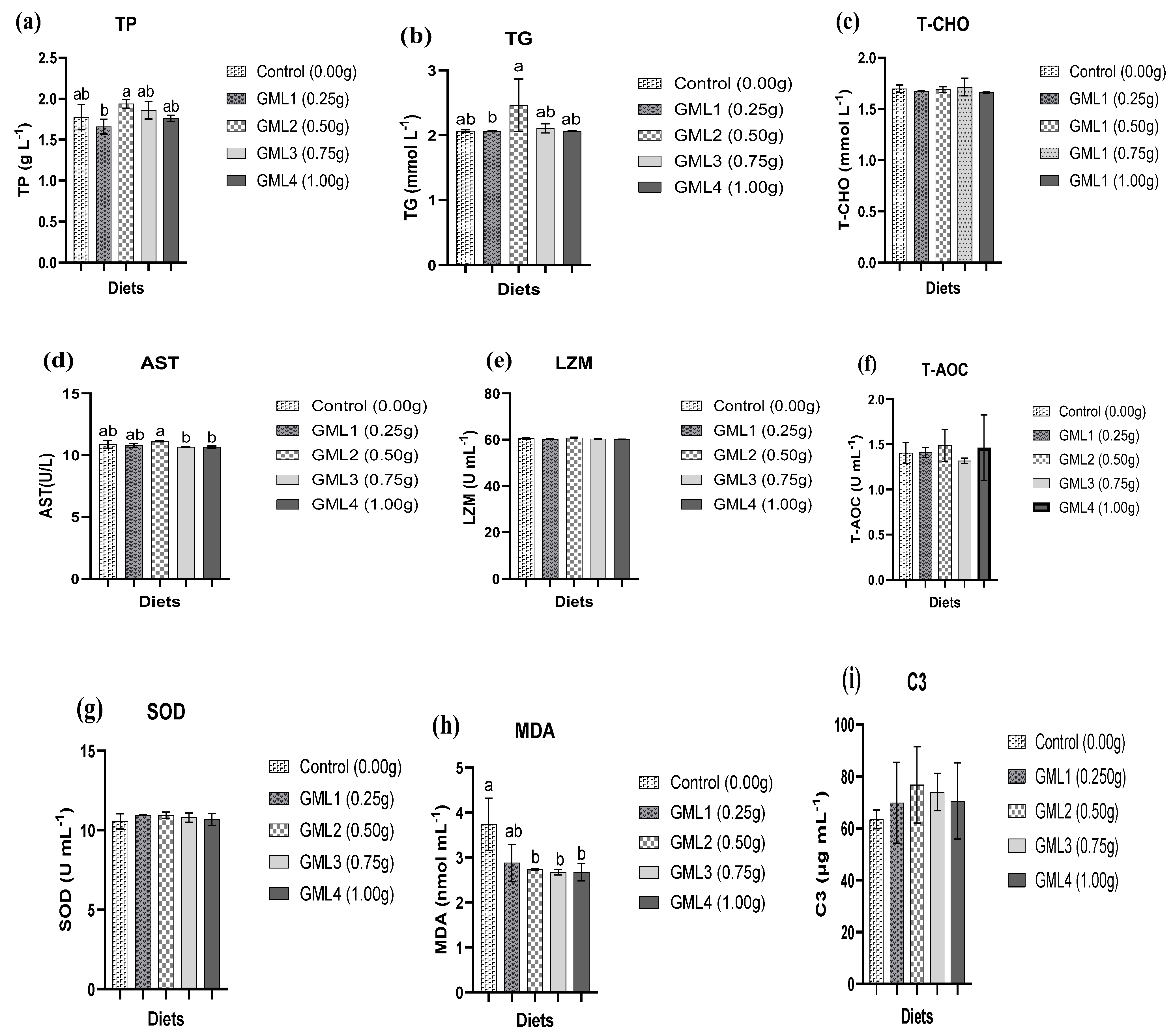
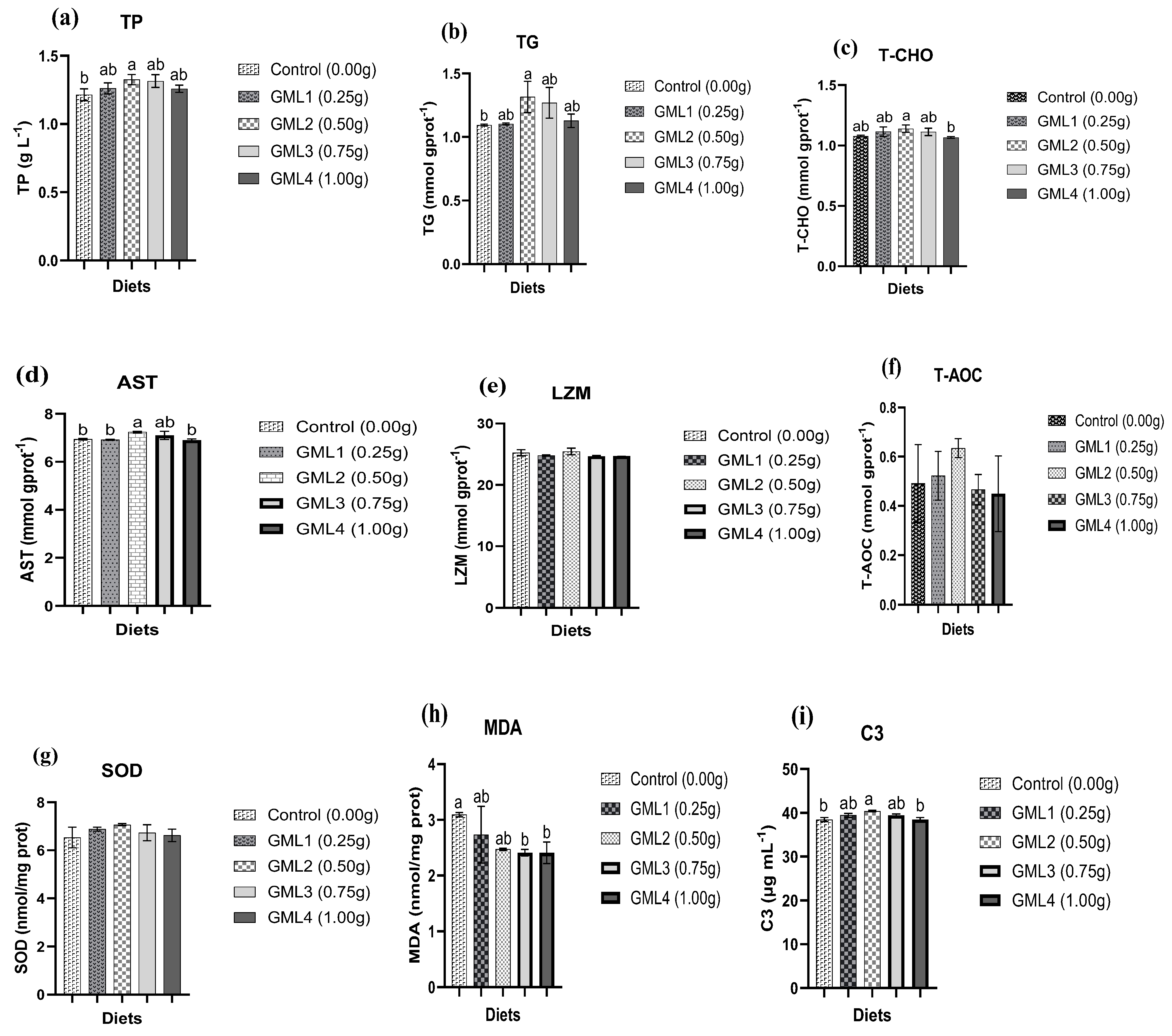
3.5. Serum and Hepatopancreas Antioxidant Parameters
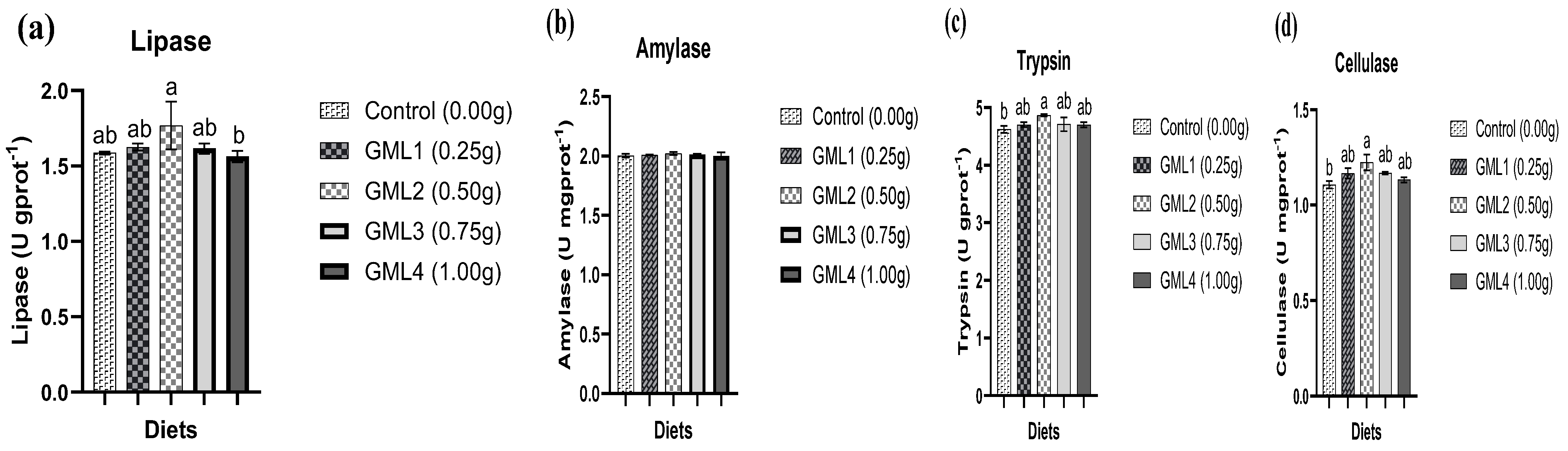
3.6. Digestive Enzymes Activities Hepatopancreas
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, E.; Wang, X.; Chen, K.; Xu, C.; Qin, J.G.; Chen, L. Physiological change and nutritional requirement of Pacific white shrimp Litopenaeus vannamei at low salinity. Rev. Aquac. 2017, 9, 57–75. [Google Scholar] [CrossRef]
- Zhu, M.; Long, X.; Wu, S. Effects of dietary trehalose on the growth performance and nonspecific immunity of white shrimps (Litopenaeus vannamei). Fish Shellfish Immunol. 2018, 78, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zuo, H.; Yang, L.; He, J.-H.; Niu, S.; Weng, S.; He, J.; Xu, X. Long-term influence of cyanobacterial bloom on the immune system of Litopenaeus vannamei. Fish Shellfish Immunol. 2017, 61, 79–85. [Google Scholar] [CrossRef]
- Duan, Y.; Liu, Q.; Wang, Y.; Zhang, J.; Xiong, D. Impairment of the intestine barrier function in Litopenaeus vannamei exposed to ammonia and nitrite stress. Fish Shellfish Immunol. 2018, 78, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Weng, S.; He, J. WSSV–host interaction: Host response and immune evasion. Fish Shellfish Immunol. 2019, 84, 558–571. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, Q.; Xi, L.; Gong, Y.; Su, J.; Han, D.; Zhang, Z.; Liu, H.; Jin, J.; Yang, Y. Effects of replacement of dietary fishmeal by cottonseed protein concentrate on growth performance, liver health, and intestinal histology of largemouth bass (Micropterus salmoides). Front. Physiol. 2021, 12, 764987. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Tang, C.; Zhao, Q.; Zhan, T.; Zhang, K.; Han, Y.; Zhang, J. Effects of dietary supplementation with combinations of organic and medium chain fatty acids as replacements for chlortetracycline on growth performance, serum immunity, and fecal microbiota of weaned piglets. Livest. Sci. 2018, 216, 210–218. [Google Scholar] [CrossRef]
- Zentek, J.; Buchheit-Renko, S.; Ferrara, F.; Vahjen, W.; Van Kessel, A.; Pieper, R. Nutritional and physiological role of medium-chain triglycerides and medium-chain fatty acids in piglets. Anim. Health Res. Rev. 2011, 12, 83–93. [Google Scholar] [CrossRef]
- Sargent, J.R.; Tocher, D.R.; Bell, J.G. 4—The Lipids. In Fish Nutrition, 3rd ed.; Halver, J.E., Hardy, R.W., Eds.; Academic Press: San Diego, CA, USA, 2003; pp. 181–257. [Google Scholar]
- Bergsson, G.; Arnfinnsson, J.; Steingrfmsson, O.; Thormar, H. In vitro killing of Candida albicans by fatty acids and monoglycerides. Antimicrob. Agents Chemother. 2001, 45, 3209–3212. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Li, Y.; Zhang, H.; Wang, J.; Feng, F. Effects of dietary glycerol monolaurate on the growth performance, digestive enzymes, body composition and non-specific immune response of white shrimp (Litopenaeus vannamei). Aquac. Rep. 2020, 18, 100535. [Google Scholar] [CrossRef]
- Zhao, M.; Jiang, Z.; Cai, H.; Li, Y.; Mo, Q.; Deng, L.; Zhong, H.; Liu, T.; Zhang, H.; Kang, J.X. Modulation of the gut microbiota during high-dose glycerol monolaurate-mediated amelioration of obesity in mice fed a high-fat diet. MBio 2020, 11, e00190-20. [Google Scholar] [CrossRef]
- Dierick, N.; Decuypere, J.; Degeyter, I. The combined use of whole Cuphea seeds containing medium chain fatty acids and an exogenous lipase in piglet nutrition. Arch. Anim. Nutr. 2003, 57, 49–63. [Google Scholar] [CrossRef]
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015, 519, 92–96. [Google Scholar] [CrossRef]
- Li, Y.; Liu, T.; Zhang, X.; Zhao, M.; Zhang, H.; Feng, F. Lactobacillus plantarum helps to suppress body weight gain, improve serum lipid profile and ameliorate low-grade inflammation in mice administered with glycerol monolaurate. J. Funct. Foods 2019, 53, 54–61. [Google Scholar] [CrossRef]
- Mo, Q.; Fu, A.; Deng, L.; Zhao, M.; Li, Y.; Zhang, H.; Feng, F. High-dose glycerol monolaurate up-regulated beneficial indigenous microbiota without inducing metabolic dysfunction and systemic inflammation: New insights into its antimicrobial potential. Nutrients 2019, 11, 1981. [Google Scholar] [CrossRef]
- Jackman, J.A.; Hakobyan, A.; Zakaryan, H.; Elrod, C.C. Inhibition of African swine fever virus in liquid and feed by medium-chain fatty acids and glycerol monolaurate. J. Anim. Sci. Biotechnol. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The impact of the gut microbiota on human health: An integrative view. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef]
- Liu, T.; Tang, J.; Feng, F. Glycerol monolaurate improves performance, intestinal development, and muscle amino acids in yellow-feathered broilers via manipulating gut microbiota. Appl. Microbiol. Biotechnol. 2020, 104, 10279–10291. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, H.; Wang, J.; Feng, F. Dietary glycerol monolaurate improved the growth, activity of digestive enzymes and gut microbiota in zebrafish (Danio rerio). Aquac. Rep. 2021, 20, 100670. [Google Scholar] [CrossRef]
- Fortuoso, B.F.; Dos Reis, J.H.; Gebert, R.R.; Barreta, M.; Griss, L.G.; Casagrande, R.A.; de Cristo, T.G.; Santiani, F.; Campigotto, G.; Rampazzo, L. Glycerol monolaurate in the diet of broiler chickens replacing conventional antimicrobials: Impact on health, performance and meat quality. Microb. Pathog. 2019, 129, 161–167. [Google Scholar] [CrossRef]
- Wang, Y.; Du, J.; Li, Y.; Zhang, H.; Feng, F. Effects of glycerol monolaurate on growth, health and nutritional quality of Chinese soft-shelled turtle (Pelodiscus sinensis). Chin. J. Anim. Nutr. 2019, 31, 428–436. [Google Scholar] [CrossRef]
- Jiang, H. The Effect of Glycerol Monolaurate on Growth, Health and Food Quality of Cultured Large Yellow Croaker. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2021. [Google Scholar]
- Das, R.; Sahu, N.P.; Sardar, P.; Jana, P.; Varghese, T.; Deo, A.; Megha, N.D. Chinmayanda. Glycerol monolaurate improves growth, lipid utilization and antioxidative status of white-leg shrimp, Penaeus vannamei fed with varying protein-lipid diets reared in inland saline water. Anim. Feed Sci. Technol. 2023, 306, 115794. [Google Scholar] [CrossRef]
- Alan Ward, D.; Carter, C.; Townsend, A. The use of yttrium oxide and the effect of faecal collection timing for determining the apparent digestibility of minerals and trace elements in Atlantic salmon (Salmo salar L.) feeds. Aquac. Nutr. 2005, 11, 49–59. [Google Scholar] [CrossRef]
- Kavanagh, F. Official Methods of Analysis of the AOAC, 13th ed.; Horwitz, W., Ed.; The Association of Official Analytical Chemists: Arlington, VA, USA, 2010. [Google Scholar]
- Han, D.; Xie, S.; Liu, M.; Xiao, X.; Liu, H.; Zhu, X.; Yang, Y. The effects of dietary selenium on growth performances, oxidative stress and tissue selenium concentration of gibel carp (Carassius auratus gibelio). Aquac. Nutr. 2011, 17, e741–e749. [Google Scholar] [CrossRef]
- Chen, J.; Li, X.; Huan, D.; Yao, W.; Leng, X. Comparative study on the utilization of crystalline methionine and methionine hydroxy analogue calcium by Pacific white shrimp (Litopenaeus vannamei Boone). Aquac. Res. 2018, 49, 3088–3096. [Google Scholar] [CrossRef]
- Yang, Q.; Zhou, X.; Zhou, Q.; Tan, B.; Chi, S.; Dong, X. Apparent digestibility of selected feed ingredients for white shrimp Litopenaeus vannamei, Boone. Aquac. Res. 2009, 41, 78–86. [Google Scholar] [CrossRef]
- Li, S.; Dai, M.; Qiu, H.; Chen, N. Effects of fishmeal replacement with composite mixture of shrimp hydrolysate and plant proteins on growth performance, feed utilization, and target of rapamycin pathway in largemouth bass, Micropterus salmoides. Aquaculture 2021, 533, 736185. [Google Scholar] [CrossRef]
- Liu, T.; Li, C.; Li, Y.; Feng, F. Glycerol monolaurate enhances reproductive performance, egg quality and albumen amino acids composition in aged hens with gut microbiota alternation. Agriculture 2020, 10, 250. [Google Scholar] [CrossRef]
- Rimoldi, S.; Gliozheni, E.; Ascione, C.; Gini, E.; Terova, G. Effect of a specific composition of short-and medium-chain fatty acid 1-Monoglycerides on growth performances and gut microbiota of gilthead sea bream (Sparus aurata). PeerJ 2018, 6, e5355. [Google Scholar] [CrossRef]
- Ullah, S.; Zhang, J.; Xu, B.; Tegomo, A.F.; Sagada, G.; Zheng, L.; Wang, L.; Shao, Q. Effect of dietary supplementation of lauric acid on growth performance, antioxidative capacity, intestinal development and gut microbiota on black sea bream (Acanthopagrus schlegelii). PLoS ONE 2022, 17, e0262427. [Google Scholar] [CrossRef]
- Ullah, S.; Zhang, J.; Feng, F.; Shen, F.; Qiufen, M.; Wang, J.; Ur Rahman, T.; Haleem, A.; Zhao, M.; Shao, Q. Effect of Dietary Supplementation of Glycerol Monolaurate on Growth Performance, Digestive Enzymes, Serum Immune and Antioxidant Parameters, and Intestinal Morphology in Black Sea Bream. Animals 2024, 14, 2963. [Google Scholar] [CrossRef]
- Snoeck, S.d.; Wolf, P.v.d.; Swart, W.; Heliman, E.; Ebbinge, B. The effect of the application of mono-lauric acid with glycerol mono-laurate in weaned piglets, on the use of antimicrobials in sow herds. Agric. Food Sci. 2011, 346–348. [Google Scholar] [CrossRef]
- Liu, M.; Guo, W.; Wu, F.; Qu, Q.; Tan, Q.; Gong, W. Dietary supplementation of sodium butyrate may benefit growth performance and intestinal function in juvenile grass carp (Ctenopharyngodon idellus). Aquac. Res. 2017, 48, 4102–4111. [Google Scholar] [CrossRef]
- Craig, S.R.; Gatlin, D.M., III. Coconut oil and beef tallow, but not tricaprylin, can replace menhaden oil in the diet of red drum (Sciaenops ocellatus) without adversely affecting growth or fatty acid composition. J. Nutr. 1995, 125, 3041–3048. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.-D.; Wu, P.; Tang, R.J.; Liu, Y.; Kuang, S.Y.; Jiang, J.; Tang, L.; Tang, W.N.; Zhang, Y.-A.; Zhou, X.Q. Nutritive values, flavor amino acids, healthcare fatty acids and flesh quality improved by manganese referring to up-regulating the antioxidant capacity and signaling molecules TOR and Nrf2 in the muscle of fish. Food Res. Int. 2016, 89, 670–678. [Google Scholar] [CrossRef]
- Bermudez, R.; Franco, D.; Carballo, J.; Sentandreu, M.A.; Lorenzo, J.M. Influence of muscle type on the evolution of free amino acids and sarcoplasmic and myofibrillar proteins through the manufacturing process of Celta dry-cured ham. Food Res. Int. 2014, 56, 226–235. [Google Scholar] [CrossRef]
- Jewell, J.L.; Guan, K.L. Nutrient signaling to mTOR and cell growth. Trends Biochem. Sci. 2013, 38, 233–242. [Google Scholar] [CrossRef]
- Tang, X.; Keenan, M.M.; Wu, J.; Lin, C.A.; Dubois, L.; Thompson, J.W.; Freedland, S.J.; Murphy, S.K.; Chi, J.T. Comprehensive profiling of amino acid response uncovers unique methionine-deprived response dependent on intact creatine biosynthesis. PLoS Genet. 2015, 11, e1005158. [Google Scholar] [CrossRef]
- Mir, N.A.; Rafiq, A.; Kumar, F.; Singh, V.; Shukla, V. Determinants of broiler chicken meat quality and factors affecting them: A review. J. Food Sci. Technol. 2017, 54, 2997–3009. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, C.; Zhong, H.; Feng, F. Dietary medium-chain α-monoglycerides increase BW, feed intake, and carcass yield in broilers with muscle composition alteration. Poult. Sci. 2021, 100, 186–195. [Google Scholar] [CrossRef]
- Roth, J.A.; Kaeberle, M.L. Effect of glucocorticoids on the bovine immune system. Am. Vet. Med. Assoc. 1982, 180, 894–901. [Google Scholar] [CrossRef]
- Humphrey, S.; Chaloner, G.; Kemmett, K.; Davidson, N.; Williams, N.; Kipar, A.; Humphrey, T.; Wigley, P. Campylobacter jejuni is not merely a commensal in commercial broiler chickens and affects bird welfare. MBio 2014, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, F.; Wang, L.l.; Shao, Q.; Xu, Z.; Xu, J. Dietary protein requirement of juvenile black sea bream, Sparus macrocephalus. J. World Aquac. Soc. 2010, 41, 151–164. [Google Scholar] [CrossRef]
- Huang, Q.; Feng, D.; Liu, K.; Wang, P.; Xiao, H.; Wang, Y.; Zhang, S.; Liu, Z. A medium-chain fatty acid receptor Gpr84 in zebrafish: Expression pattern and roles in immune regulation. Dev. Comp. Immunol. 2014, 45, 252–258. [Google Scholar] [CrossRef]
- Kabara, J.J. Health oils from the tree of life. Nutr. Health Asp. Coconut Oil Indian Coconut J. 2000, 31, 2–8. [Google Scholar]
- Xu, W.-N.; Chen, D.H.; Chen, Q.Q.; Liu, W.B. Growth performance, innate immune responses and disease resistance of fingerling blunt snout bream, Megalobrama amblycephala adapted to different berberine-dietary feeding modes. Fish Shellfish Immunol. 2017, 68, 458–465. [Google Scholar] [CrossRef]
- Seneviratne, K.N.; Sudarshana Dissanayake, D.M. Variation of phenolic content in coconut oil extracted by two conventional methods. Int. J. Food Sci. Technol. 2008, 43, 597–602. [Google Scholar] [CrossRef]
- Witcher, K.J.; Novick, R.P.; Schlievert, P.M. Modulation of immune cell proliferation by glycerol monolaurate. Clin. Diagn. Lab. Immunol. 1996, 3, 10–13. [Google Scholar] [CrossRef]
- Bruce, F. Scientific Evidence Regarding Coconut Oil. The Coconut Oil Miracle: Where is the Evidence? COCOINFO Int. J. 2011, 19, 23–27. [Google Scholar]
- Tezcan, F.; Gultekin Ozguven, M.; Diken, T.; Ozcelik, B.; Erim, F.B. Antioxidant activity and total phenolic, organic acid and sugar content in commercial pomegranate juices. Food Chem. 2009, 115, 873–877. [Google Scholar] [CrossRef]
- Berges, J.; Mulholland, M. Nitrogen in the marine environment. In Nitrogen in the Marine Environment; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Tacon, A.; Cody, J.; Conquest, L.; Divakaran, S.; Forster, I.; Decamp, O. Effect of culture system on the nutrition and growth performance of Pacific white shrimp Litopenaeus vannamei (Boone) fed different diets. Aquac. Nutr. 2002, 8, 121–137. [Google Scholar] [CrossRef]
- Berges, J.A.; Mulholland, M.R. Enzymes and nitrogen cycling. Nitrogen Mar. Environ. 2008, 32, 1385–1444. [Google Scholar] [CrossRef]
- Takada, R.; Kaji, Y.; Saitoh, M.; Mori, T. Effect of dietary medium- and long-chain fats on terminal ileal amino acid digestibility in pigs. J. Jpn. Soc. Anim. Sci. 1994, 65, 432–436. [Google Scholar] [CrossRef]
- Verri, T.; Mandal, A.; Zilli, L.; Bossa, D.; Mandal, P.; Ingrosso, L.; Zonno, V.; Vilella, S.; Ahearn, G.; Storelli, C. D-glucose transport in decapod crustacean hepatopancreas. Comp. Biochem. Physiol. Part A Mol. Integ. Physiol. 2001, 130, 585–606. [Google Scholar] [CrossRef]
- Caceci, T.; Neck, K.F.; Lewis, D.D.H.; Sis, R.F. Ultrastructure of the hepatopancreas of the pacific white shrimp, Penaeus vannamei (Crustacea: Decapoda). J. Mar. Biol. Assoc. UK 1988, 68, 323–337. [Google Scholar] [CrossRef]
- Li, M.; Lai, H.; Li, Q.; Gong, S.; Wang, R. Effects of dietary taurine on growth, immunity and hyperammonemia in juvenile yellow catfish Pelteobagrus fulvidraco fed all-plant protein diets. Aquaculture 2016, 450, 349–355. [Google Scholar] [CrossRef]
- Hansen, A.-C.; Rosenlund, G.; Karlsen, O.; Koppe, W.; Hemre, G.-I. Total replacement of fish meal with plant proteins in diets for Atlantic cod (Gadus morhua L.) I—Effects on growth and protein retention. Aquaculture 2007, 272, 599–611. [Google Scholar] [CrossRef]
- Chiji, H.; Harayama, K.; Kiriyama, S. Effects of feeding rats low protein diets containing casein or soy protein isolate supplemented with methionine or oligo-L-methionine. J. Nutr. 1990, 120, 166–171. [Google Scholar] [CrossRef]
- Oz, H.S.; Chen, T.S.; Neuman, M. Methionine deficiency and hepatic injury in a dietary steatohepatitis model. Dig. Dis. Sci. 2008, 53, 767–776. [Google Scholar] [CrossRef]
| Ingredients | Diets | ||||
|---|---|---|---|---|---|
| Control (0.00 g) | GML1 (0.25 g) | GML2 (0.50 g) | GML3 (0.75 g) | GML4 (1.00 g) | |
| Fishmeal a | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Soybean meal a | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Fermented soybean meal a | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Soybean protein concentrate a | 170.0 | 170.0 | 170.0 | 170.0 | 170.0 |
| Squid liver meal a | 30.0 | 30.0 | 30.0 | 30.0 | 30.0 |
| Shrimp meal a | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 |
| Chicken meal a | 60.0 | 60.0 | 60.0 | 60.0 | 60.0 |
| Fish oil a | 30.0 | 30.0 | 30.0 | 30.0 | 30.0 |
| Beer yeast | 40.0 | 40.0 | 40.0 | 40.0 | 40.0 |
| Wheat flour | 220.0 | 220.0 | 220.0 | 220.0 | 220.0 |
| L-carnitine | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Soybean lecithin | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Carrageenan | 3.7 | 3.7 | 3.7 | 3.7 | 3.7 |
| Ascorbic phosphate ester | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| α-Starch | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| GML b | 0.00 | 0.25 | 0.50 | 0.75 | 1.00 |
| Yarrowia lipolytica | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Carboxymethyl cellulose | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Mineral premix c | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Vitamin premix d | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| Zeolite powder | 21.0 | 21.0 | 21.0 | 21.0 | 21.0 |
| Yttrium oxide (Y2O3) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| L-Lysine | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| Ca(H2PO4)2 | 22.0 | 22.0 | 22.0 | 22.0 | 22.0 |
| α-Cellulose | 8.30 | 8.05 | 7.80 | 7.55 | 7.30 |
| Butyrin | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Total | 1000.0 | 1000.0 | 1000.0 | 1000.0 | 1000.0 |
| Proximate composition | |||||
| Moisture (%) | 10.64 | 11.44 | 12.51 | 13.03 | 15.13 |
| Crude protein (%) | 37.73 | 37.31 | 37.61 | 36.87 | 36.34 |
| Crude lipid (%) | 7.57 | 7.16 | 7.18 | 7.28 | 8.08 |
| Crude ash (%) | 13.58 | 13.51 | 13.61 | 12.87 | 12.93 |
| Gross energy (kJ/g) e | 1603.23 | 1595.49 | 1592.45 | 1596.88 | 1606.37 |
| Dry matter | 98.99 | 98.94 | 98.84 | 98.70 | 98.74 |
| Amino Acids | Diet | ||||
|---|---|---|---|---|---|
| Control (0.00 g) | GML1 (0.25 g) | GML2 (0.50 g) | GML3 (0.75 g) | GML4 (1.00 g) | |
| EAA | |||||
| Valine | 1.733 | 2.602 | 2.083 | 2.589 | 2.943 |
| Threonine | 1.261 | 1.913 | 1.660 | 1.393 | 1.708 |
| Isoleucine | 1.426 | 2.120 | 1.714 | 2.112 | 2.409 |
| Methionine | 0.373 | 0.526 | 0.445 | 0.527 | 0.580 |
| Histidine | 0.844 | 1.254 | 1.006 | 1.238 | 1.412 |
| Leucine | 3.171 | 3.203 | 3.221 | 3.261 | 3.204 |
| Lysine | 2.641 | 3.352 | 2.982 | 3.320 | 3.522 |
| Phenylalanine | 1.614 | 2.409 | 1.927 | 2.387 | 2.721 |
| Arginine | 2.237 | 3.380 | 2.707 | 3.323 | 3.783 |
| NEAA | |||||
| Alanine | 1.845 | 2.769 | 2.236 | 2.729 | 3.122 |
| Aspartate | 1.649 | 2.262 | 1.701 | 3.294 | 4.776 |
| Cysteine | 0.096 | 0.149 | 0.107 | 0.139 | 0.170 |
| Glutamic acid | 0.911 | 1.082 | 1.330 | 0.000 | 0.000 |
| Glycine | 2.058 | 2.957 | 2.365 | 3.037 | 3.474 |
| Serine | 0.536 | 0.807 | 0.702 | 0.898 | 1.785 |
| Proline | 0.199 | 0.195 | 0.231 | 0.228 | 0.350 |
| Tyrosine | 0.763 | 1.125 | 0.879 | 1.093 | 1.245 |
| Parameters | Diets | ||||
|---|---|---|---|---|---|
| Control (0.00) | GML 1 (0.25 g) | GML2 (0.50 g) | GML3 (0.75 g) | GML4 (1 g) | |
| IBW | 1.67 ± 0.24 | 1.67 ± 0.14 | 1.62 ± 0.20 | 1.83 ± 0.09 | 1.81 ± 0.05 |
| FBW | 7.12 ± 0.21 b | 7.27 ± 1.16 b | 8.87 ± 0.30 a | 8.80 ± 0.01 a | 7.13 ± 0.50 b |
| WG (%) | 331.23 ± 44.99 ab | 339.87 ± 94.26 ab | 452.48 ± 56.92 a | 381.44 ± 16.22 ab | 294.25 ± 38.97 b |
| SGR (%/d) | 5.91 ± 0.80 ab | 6.07 ±1.68 ab | 8.08 ± 1.02 a | 6.81 ± 0.29 ab | 5.25 ± 0.70 b |
| MFI (g shrimp−1 day−1) | 0.33 ± 0.01 | 0.32 ± 0.02 | 0.32 ± 0.02 | 0.34 ± 0.01 | 0.33 ± 0.02 |
| FCR | 3.35 ± 0.09 | 3.34 ± 1.06 | 2.48 ± 0.08 | 2.73 ± 0.13 | 3.44 ± 0.39 |
| CF (%) | 1.18 ± 0.08 ab | 1.15 ± 0.04 b | 1.17 ± 0.27 ab | 1.52 ± 0.14 a | 1.12 ± 0.01 b |
| HSI (%) | 3.88 ± 0.44 a | 3.20 ± 0.32 a | 3.18 ± 0.15 a | 2.34 ± 0.43 b | 3.35 ± 0.23 a |
| VSI (%) | 5.14 ± 0.45 b | 4.39 ± 0.36 a | 4.38 ± 0.14 a | 3.62 ± 0.41 a | 4.70 ± 0.13 b |
| SR (%) | 97.33 ± 1.15 | 99.33 ± 1.15 | 98.00 ± 2.00 | 98.00 ± 0.00 | 98.00 ± 2.83 |
| Parameters | Diets | ||||
|---|---|---|---|---|---|
| Control (0.00 g) | GML1 (0.25 g) | GML2 (0.50 g) | GML3 (0.75 g) | GML4 (1.00 g) | |
| Whole body | |||||
| Crude protein | 72.70 ± 1.91 | 73.53 ± 3.15 | 74.83 ± 0.55 | 74.26 ± 1.53 | 72.90 ± 1.054 |
| Crude lipid | 1.60 ± 1.24 | 1.44 ± 0.39 | 1.68 ± 0.57 | 1.30 ± 0.43 | 0.94 ± 0.57 |
| Moisture | 78.58 ± 1.58 | 77.75 ± 0.93 | 77.03 ± 1.75 | 77.16 ± 1.06 | 77.49 ± 0.91 |
| Ash | 15.30 ± 0.36 a | 11.80 ± 0.53 b | 12.53 ± 0.47 b | 12.33 ± 0.50 b | 12.76 ± 0.65 b |
| Phosphorus | 1.25 ± 0.19 | 1.29 ± 0.05 | 1.29 ± 0.08 | 1.30 ± 0.03 | 1.33 ± 0.02 |
| Dorsal Muscle | |||||
| Crude protein | 89.10 ± 0.95 | 89.13 ± 0.49 | 89.16 ± 0.83 | 89.10 ± 0.36 | 88.76 ± 0.95 |
| Crude lipid | 0.39 ± 0.36 | 0.50 ± 0.39 | 0.85 ± 0.69 | 0.91 ± 0.52 | 0.28 ± 0.28 |
| Moisture | 76.25 ± 0.51 | 77.74 ± 1.49 | 76.03 ± 0.37 | 77.36 ± 2.23 | 71.43 ± 8.34 |
| Ash | 5.71 ± 0.76 | 5.98 ± 0.60 | 6.42 ± 0.41 | 6.28 ± 0.58 | 5.82 ± 0.71 |
| Phosphorus | 76.25 ± 0.51 | 77.74 ± 1.49 | 76.03 ± 0.38 | 77.37 ± 2.23 | 71.43 ± 8.34 |
| Amino Acids | Diet | ||||
|---|---|---|---|---|---|
| Control (0.00 g) | GML1 (0.25 g) | GML2 (0.50 g) | GML3 (0.75 g) | GML4 (1.00 g) | |
| EAA | |||||
| Valine | 12.01 ± 1.69 | 11.54 ± 0.59 | 11.03 ± 0.26 | 12.53 ± 1.26 | 10.85 ± 0.77 |
| Threonine | 0.15 ± 0.09 b | 0.8 ± 0.43 ab | 0.71 ± 0.26 ab | 0.92 ± 0.03 a | 0.45 ± 0.39 ab |
| Isoleucine | 5.88 ± 0.49 | 5.90 ± 0.50 | 6.99 ± 0.45 | 6.53 ± 0.25 | 6.10 ± 0.36 |
| Methionine | 3.85 ± 0.19 b | 4.32 ± 0.28 ab | 4.43 ± 0.27 ab | 5.11 ± 0.67 a | 4.35 ± 0.34 ab |
| Histidine | 0.00 ± 0.00 | 0.09 ± 0.16 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Leucine | 13.99 ± 1.73 | 15.77 ± 2.00 | 15.92 ± 2.10 | 17.97 ± 1.67 | 13.94 ± 0.45 |
| Lysine | 18.69 ± 1.06 b | 20.59 ± 1.14 ab | 20.72 ± 0.19 ab | 21.42 ± 0.22 a | 19.14 ± 0.67 b |
| Phenylalanine | 6.43 ± 0.50 | 6.72 ± 0.89 | 7.26 ± 1.23 | 7.94 ± 1.01 | 6.53 ± 2.82 |
| Arginine | 6.39 ± 2.61 | 5.79 ± 0.75 | 4.84 ± 0.39 | 5.46 ± 0.49 | 5.01 ± 1.47 |
| NEAA | |||||
| Alanine | 11.81 ± 0.79 | 11.16 ± 0.33 | 11.34 ± 0.98 | 12.27 ± 1.52 | 10.69 ± 0.22 |
| Aspartate | 19.70 ± 0.88 c | 20.79 ± 0.69 bc | 21.69 ± 0.41 ab | 22.99 ± 0.75 a | 19.92 ± 0.26 c |
| Cysteine | 0.28 ± 0.22 | 0.16 ± 0.08 | 0.03 ± 0.01 | 0.12 ± 0.14 | 0.03 ± 0.02 |
| Glutamic acid | 30.04 ± 0.84 c | 26.83 ± 0.36 d | 32.13 ± 0.12 b | 33.87 ± 0.38 a | 27.09 ± 0.34 d |
| Glycine | 18.94 ± 0.34 b | 22.28 ± 0.33 a | 23.08 ± 0.60 a | 24.90 ± 1.52 a | 18.68 ± 2.05 b |
| Proline | 11.43 ± 0.24 c | 12.90 ± 0.25 b | 14.65 ± 0.29 a | 15.32 ± 0.21 a | 13.15 ± 0.76 b |
| Serine | 6.89 ± 1.45 | 6.42 ± 0.49 | 6.27 ± 0.47 | 7.67 ± 0.88 | 6.47 ± 2.36 |
| Tyrosine | 12.43 ± 0.24 | 12.57 ± 0.45 | 13.32 ± 0.37 | 13.65 ± 0.36 | 13.15 ± 0.76 |
| Parameters (%) | Diets | ||||
|---|---|---|---|---|---|
| Control (0.00 g) | GML1 (0.25 g) | GML2 (0.50 g) | GML3 (0.75 g) | GML4 (1.00 g) | |
| Crude protein | 85.53 ± 0.82 | 86.58 ± 2.71 | 85.41 ± 0.51 | 87.62 ± 3.73 | 83.53 ± 0.86 |
| Crude lipid | 81.68 ± 5.72 | 83.78 ± 2.36 | 81.42 ± 3.91 | 88.33 ± 7.26 | 83.02 ± 2.14 |
| Ash | 3.32 ± 0.11 | 3.19 ± 0.12 | 3.1 ± 0.04 | 3.20 ± 0.00 | 3.25 ± 0.23 |
| Gross energy (kJ/g) | 98.22 ± 1.52 | 98.94 ± 2.36 | 99.66 ± 4.57 | 99.73 ± 1.41 | 101.84 ± 0.91 |
| Dry matter | 81.03 ± 1.06 | 80.98 ± 3.52 | 80.98 ± 0.68 | 83.62 ± 5.18 | 78.16 ± 0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, S.; Liu, B.; Zheng, Y.; Guo, H.; Yang, Y.; Ahmad, M.I.; Lv, S.; Deng, S.; Zhao, M.; Feng, F. Glycerol Monolaurate Affects Growth, Amino Acid Profile, Antioxidant Capacity, Nutrient Apparent Digestibility, and Histological Morphology of Hepatopancreas in Juvenile Pacific White Shrimp (Litopenaeus vannamei). Fishes 2025, 10, 124. https://doi.org/10.3390/fishes10030124
Ullah S, Liu B, Zheng Y, Guo H, Yang Y, Ahmad MI, Lv S, Deng S, Zhao M, Feng F. Glycerol Monolaurate Affects Growth, Amino Acid Profile, Antioxidant Capacity, Nutrient Apparent Digestibility, and Histological Morphology of Hepatopancreas in Juvenile Pacific White Shrimp (Litopenaeus vannamei). Fishes. 2025; 10(3):124. https://doi.org/10.3390/fishes10030124
Chicago/Turabian StyleUllah, Sami, Bingge Liu, Yunyun Zheng, Hongbo Guo, Yarui Yang, Muhammad Ijaz Ahmad, Siyu Lv, Shijie Deng, Minjie Zhao, and Fengqin Feng. 2025. "Glycerol Monolaurate Affects Growth, Amino Acid Profile, Antioxidant Capacity, Nutrient Apparent Digestibility, and Histological Morphology of Hepatopancreas in Juvenile Pacific White Shrimp (Litopenaeus vannamei)" Fishes 10, no. 3: 124. https://doi.org/10.3390/fishes10030124
APA StyleUllah, S., Liu, B., Zheng, Y., Guo, H., Yang, Y., Ahmad, M. I., Lv, S., Deng, S., Zhao, M., & Feng, F. (2025). Glycerol Monolaurate Affects Growth, Amino Acid Profile, Antioxidant Capacity, Nutrient Apparent Digestibility, and Histological Morphology of Hepatopancreas in Juvenile Pacific White Shrimp (Litopenaeus vannamei). Fishes, 10(3), 124. https://doi.org/10.3390/fishes10030124









