Abstract
The degradation of organic matter using TiO2 nanotube photocatalytic technology is limited by the short lifetime and diffusion radius of the generated hydroxyl radicals, decreasing the removal efficiency. This study developed a chlorine radical-coupled photoelectrocatalytic system, significantly enhancing the performance of TiO2 nanotube arrays in removing sulfonamide antibiotics (SAs) from cold-water aquaculture systems. The highest degradation rates were observed at 5 mM NaCl and 15 mM NaNO3. When SA concentrations were 0.1–10 mg/L, degradation efficiency decreased with higher initial concentrations. The best degradation was achieved at an initial pH of 3 for SA. Humic acid and sodium acetate, natural organic matter in the water column, served as low-concentration promoters and high-concentration inhibitors. In our study, three degradation intermediates were identified, and hydrolysis and nitration reactions are proposed as the primary pathways for SA degradation. We confirmed that oxygen radicals play a major role in this system. Furthermore, toxicology experiments revealed the weakening of the toxicity of the degraded products. This study provides an efficient method for treating organic matter in cold-water fish culture water in chloride-containing saline and alkaline waters.
Keywords:
titanium dioxide nanotube arrays; photoelectrocatalysis; chlorine coupling; ecotoxicity; aquaculture; sulfonamide Key Contribution:
Enhanced photoelectrocatalytic ability of TiO2 with certain concentration of chlorinated salts in saline waters and provides a scientific approach for the development of recirculating aquaculture system (RAS) and tailwater treatment in saline areas.
1. Introduction
Antibiotics are a drug class that can inhibit or kill bacteria. They are antimicrobial agents that are widely utilized in the fields of aquaculture and animal husbandry [1]. In 2017, the annual global consumption of antibiotics reached 93 million tons, and will possibly increase to 236 million tons by 2030. However, owing to incomplete metabolism and utilization, more than 50% of antibiotics are released into the environment as pseudo-persistent pollutants, causing chronic human toxic effects [2,3]. Sulfonamide antibiotics (SA) are traditional synthetic antimicrobial drugs that are most widely utilized in the aquaculture industry owing to their low price, chemical stability, and broad-spectrum activity [4,5]. Antibiotic overuse results in the homeostatic imbalance of the aquatic environment and the emergence of antibiotic-resistance genes [6,7]. Furthermore, antibiotics enter the food chain through water sources, soil, and residues in animals, affecting human and animal health [8]. Therefore, effective measures are warranted to impede the spread of antibiotics from the environment and promote global ecological security and sustainable development [9].
Titanium dioxide (TiO2) photoelectrocatalysis is considered a green, sustainable, and efficient advanced oxidation technology that uses sunlight. This technology is widely applied to treat antibiotic-contaminated water [10,11]. It can remove antibiotics in water via oxidation, generating superoxide radicals (•O2−), hydroxyl radicals (•OH), and photogenerated holes (h+) to remove antibiotics in water [12]. This can result in the degradation of most organic and inorganic pollutants in wastewater, ultimately generating CO2, H2O, and other non-toxic substances without causing secondary pollution [13]. TiO2 is widely used for degrading organic pollutants in aqueous environments; however, powdered TiO2 is difficult to recycle and reuse. These limitations have been effectively solved by using TiO2 nanotubes (TNTs) [14,15]. They have higher light transmittance and light-absorbing capacity [16] and can provide a vertical and shortest path for charge transport in the catalytic system process [17]. However, the application of TNT photocatalytic technology for degrading organic matter is impeded by the short lifetime of the generated hydroxyl radicals and a short diffusion radius, decreasing the removal efficiency. He et al. [18] demonstrated that a photoelectrocatalytic coupling system exhibits remarkable efficiency in the synergistic removal of antibiotics and antibiotic-resistant bacteria from aquatic environments. By constructing a photoelectrocatalytic system using Ag/SnO2-S-modified TiO2 nanotubes, they achieved nearly complete degradation of chloramphenicol within 1 h, significantly outperforming conventional photocatalytic methods in terms of degradation efficiency.
Numerous studies have found that degradation performance is significantly enhanced when photoelectrocatalysis is coupled with chlorine radicals. Wang [19] discovered that chlorine radical-mediated TiO2 photocatalytic reactions markedly improved ozone removal efficiency. Under ultraviolet (UV) irradiation, new reactive species, including chlorine radicals, were generated, indicating that chlorination is a process highly coupled with photocatalysis. Cheng [20] and Zhang [21] further demonstrated that the UV-driven TiO2 photocatalytic chlorine activation process enabled the rapid degradation of refractory pollutants such as carbamazepine and dimethyl phthalate. Based on these findings, a novel photoelectrochemical system was proposed. In this system, the catalyst, under light irradiation, activates the generation of chlorine-based reactive species, such as Cl• and ClO• radicals, in addition to HO•. These species exhibit superior oxidative capabilities and can effectively degrade persistent pollutants, including antibiotics [22].
In the present study, to enhance the lifetime of hydroxyl radicals, increase the diffusion radius, and improve the degradation efficiency, we introduced chloride ions into a photoelectrocatalytic system to investigate the effects of different experimental parameters, including initial SA concentration, initial pH, chloride ion concentration, humic acid, and sodium acetate, on SA degradation. Furthermore, we investigated the degradation products, pathways, and mechanisms and evaluated the changes in toxicity during antibiotic treatment via toxicology experiments. This study will provide a reference for enhancing SA degradation in cold-water fish culture water.
2. Materials and Methods
2.1. Experimental Materials
All the reagents involved in the study were analytically pure reagents: titanium sheet, copper sheet, sulfanilamide standard, p-benzoquinone (Aladdin Reagent Co., Ltd., Shanghai, China), sodium acetate, potassium dichromate, isopropanol (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China), humic acid (Shanghai Maclean’s Biochemistry Science and Technology Co., Ltd., Shanghai, China), and L-histidine (Beijing Solepol Technology Co., Ltd., Beijing, China).
2.2. Preparation of TiO2 Nanotubes
Samples were prepared using the anodic oxidation method. First, a titanium plate (thickness 0.5 mm, a × b = 3.5 × 2 cm) and a platinum sheet of the same size were subjected to ultrasonic cleaning using isopropanol, acetone, and ultrapure water. The titanium plate was at the anode and the platinum sheet was at the cathode. A solution of 11.67% (NH4)2SO4 and 3.65% NH4F was used as the electrolyte. Electrolysis was conducted at a constant voltage of 20 V for 2 h. The prepared nanotubes were ultrasonicated with ultrapure water for 15 min and dried, followed by annealing in a muffle furnace at 600 °C for 30 min.
2.3. Methods
2.3.1. LC-MS-Based Method for the Detection of Sulfonamide Antibiotics
TCH was analyzed using a Sciex Exion AD system (Sciex company, Frisco, TX, USA). The chromatographic column employed was a Luna Omega Polar C18 (2.1 × 100 mm, 1.6 μm), with a flow rate of 0.3 mL/min and a column temperature set to 40 °C. The mobile phases consisted of 0.1% formic acid aqueous solution and methanol (with 0.1% formic acid), and the injection volume was 10 μL. Detection of TCH was carried out with an X500R QTOF in IDA acquisition mode, specifically TOFMS-IDA-TOFMSMS. The TOF MS parameters were set to m/z 80–800 with an accumulation time of 0.1 s. Instrument settings included CUR at 30 psi, GS1 at 50 psi, GS2 at 55 psi, ISVF at 5500 V, and TEM at 500 °C. The mass range settings were DP 80 V and CE 35 ±15 V.
2.3.2. Techniques for Characterizing TiO2 Nanotube Materials
The chemical composition and valence band structure of the materials were analyzed using X-ray photoelectron spectroscopy (XPS, Thermo Fisher Scientific, Waltham, MA, USA). The X-ray source utilized Al Kα (1486.6 eV) or Mg Kα (1253.6 eV) radiation, and the energy analyzer was set to a pass energy of 20–100 eV to obtain high-resolution spectra. The analysis chamber was maintained at an ultrahigh vacuum of 10−9 Torr to minimize interference from gas molecules. The crystal structure of the TiO2 nanotubes (TNTs) was investigated using X-ray diffraction (XRD, Rigaku Ultima IV, Japanese, Applied Rigaku Technologies, Cedar Park, TX, USA). Cu Kα radiation (λ = 1.5418 Å) was employed as the X-ray source, with the voltage and current typically set to 40 kV and 40 mA, respectively. The scanning range covered 2θ angles from 10° to 80°, with a step size of 0.02° and a dwell time of 1 s per step. The surface morphology of the samples was examined using scanning electron microscopy (SEM, JSM-7900F, JEOL, Tokyo, Japan). The acceleration voltage was set to 5–10 kV, and the working distance, which is the distance between the sample and the detector, was adjusted to 5–10 mm. The absorption spectra of the TNTs were measured using a UV-vis spectrophotometer (WTW photoLab 7600 UV-vis, Munich, Germany). The scanning range was set from 200 to 800 nm, covering both the ultraviolet and visible light regions. This comprehensive characterization approach provides detailed insights into the chemical, structural, and optical properties of the TiO2 nanotube materials.
2.3.3. Experimental Procedure for the Photocatalytic Degradation of Sulfonamide Antibiotics
A standard stock solution of 100 mg/L SAs was employed and stored at 4 °C away from light. The experiments were conducted in a square quartz reactor (5 × 5 × 5 cm) containing 85 mL of the reaction solution. TNT arrays were used as the anode and a copper sheet as the cathode. A 300 W xenon lamp (PLS-SXE300D, Beijing, China) with a filter (λ < 420 nm) was utilized as the light source, with a rotor inside the quartz reactor. The system was placed on a magnetic stirrer (521 r-min−1) to form a photoelectrocatalytic reaction system (Figure S1). The concentration of the SA degradation reaction solution was 1.0 mg/L (0.1, 5, and 10 mg/L for different SA initial solution concentration groups), the concentration of NaCl was 5 mM (0, 10, and 15 mM for different NaCl concentrations), and the concentration of sodium nitrate was 10 mM (5, 15, and 20 mM for different sodium nitrate concentrations). The initial solution pH was 3 (5, 7, and 9 for different initial pH groups). The water samples for actual aquaculture water testing were collected from salmonid fish farming cages at the Qinghai Provincial Fisheries Environment Monitoring Station. After pretreatment (filtered through 200-mesh sieve cloth), the samples were stored in a refrigerator at 4 °C. Tap water (campus water) was collected from Qinghai University. Reaction solutions (200 mL) with concentrations of 0.5 and 10 mg/L were prepared using different aqueous media (aquaculture water and tap water) without pH adjustment and analyzed after degradation. The tap water complies with the National Standard GB 5749-2022 [23], and the water quality parameters monitored by the Qinghai Provincial Fisheries Environment Monitoring Station are detailed in Table S1.
2.3.4. Stability Experiment of Titanium Dioxide Nanotubes
To evaluate the photoelectrocatalytic stability of TNT, a TNT piece was repeatedly subjected to chlorine radical-coupled photoelectrocatalytic degradation at an SA solution concentration of 1.0 mg/L. After completing one degradation experiment, the titanium and copper pieces were ultrasonically cleaned with ultrapure water and dried for the next use. The stability of the recycled pieces was evaluated by comparing the effect of the degradation rate with the increase in the number of uses via recycling.
2.3.5. Investigation of Degradation Byproducts and Mechanisms of Sulfonamide Antibiotics
HPLC coupled with mass spectrometry (LC-MS, AB SCIEX, Framingham, MA, USA) was performed to determine the byproducts in the SA degradation process. The active substance capture experiment was used to explore the photoelectrocatalytic mechanism. The SA solution concentration was 1.0 mg/L. To investigate the magnitude of the contribution of the active substances to the SA degradation process, 0.5 mL of 15 mg/L potassium dichromate, p-benzoquinone (BQ), L-histidine, and isopropanol were added to the SA solution as the electron hole (h+), superoxide radicals (•O2−), oxygen free radicals, and hydroxyl free radicals (•OH), respectively.
2.3.6. Ecotoxicology Experiments
The luminescent bacteria method [24,25], ring of inhibition method [26], and microalgae acute toxicology experiments [27,28] were used to evaluate the ecotoxicity of the products before and after SA degradation. Text S1 describes the experimental setup.
2.3.7. Data Analysis
The experimental data were processed by Microsoft Excel software and plotted by Origin 2021. The data were statistically analyzed using SPSS software version 20. To determine significant relationships between the data of each group, one-way ANOVA was used. Text S2 describes the methods used for data analysis.
3. Results and Analysis
3.1. Material Characterization
Figure 1a–c illustrate the morphology of the samples observed under a scanning electron microscope (SEM). TNTs were densely arranged with a tube diameter of approximately 120 nm. This improved the photocatalytic activity. Next, X-ray photoelectron spectroscopy (XPS) was used to assess the chemical composition and valence band structure of the materials. The full-scan spectra (Figure 1d) revealed the presence of elements Ti, O, and C. The O1s spectra revealed the existence of O, primarily in the form of Ti-O-Ti and O-H. The TiO2 spectra in Ti 2p (Figure 1f) revealed that the two peaks at 458.5 and 464.2 eV correspond to Ti4+2p3/2 and Ti4+2p1/2, respectively, and 458.9 eV corresponds to Ti3+2p3/2.
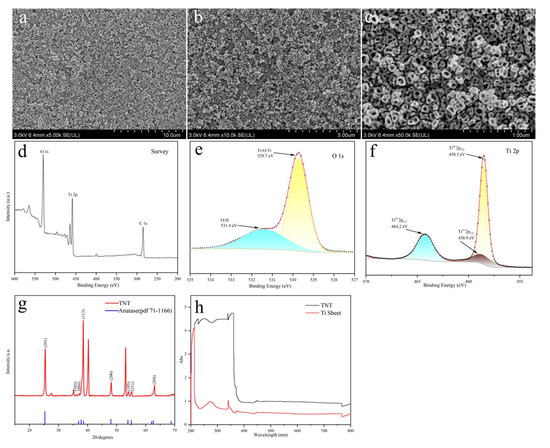
Figure 1.
SEM images (a–c), XPS images (d–f), XRD image (g), and UV-vis image (h) of TNT.
X-ray diffraction analysis (XRD) was performed to assess the crystal structure of TNT. Figure 1g illustrates the crystal faces of TiO2 anatase phases (101), (103), (004), (112), (200), (105), (211), and (204), which correspond to the characteristic peaks 2θ = 25.3°, 36.9°, 37.8°, 38.5°, 48.1°, 53.9°, 55.1°, and 62.7°, respectively. This confirms the TiO2 anatase phase (JCPDS.71-1166), with the characteristic peak at 25.3°, indicating good crystallinity. Comprehensive SEM and XPS analyses collectively verified the special micromorphology, elemental composition, and crystal structure of TNT. Finally, UV-vis absorption spectroscopy was utilized to investigate the optical behavior of TNT (Figure 1h), revealing significant absorption (λ < 380) for the UV region compared to the titanium plates, effectively utilizing UV light for photoelectrocatalysis.
3.2. Effects of Environmental Parameters
3.2.1. Effect of Different Degradation Systems on SA Degradation
Figure 2a illustrates the removal effect of SAs via chlorine radical-induced photoelectrocatalysis: the concentration of the SA solution was 1.0 mg/L. The SAs were not degraded under the dark condition, with a degradation rate of only 26.94% at 120 min. The degradation effect of NaCl and NaNO3 addition in the catalytic system was better than that of without NaCl (photoelectrocatalysis) addition. Furthermore, it was better than the degradation effect without copper flakes (photocatalysis). At 60 min, the degradation rate of the NaCl + NaNO3 system was 100%, the degradation rate without copper flakes was 92.98%, and the degradation rate without NaCl was 94.05%.
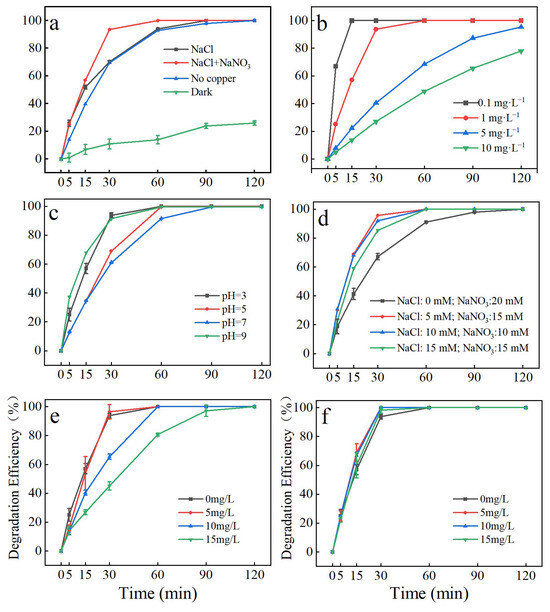
Figure 2.
Degradation curves of the degraded SAs in different systems (a), effect of different initial concentrations on degradation efficiency (b), effect of initial solution pH on degradation efficiency (c), effects of chloride ion (Cl−) and sodium nitrate concentrations on degradation (d), effects of humic acid (e) and sodium acetate (f) on SA degradation.
We noted that the photoelectrocatalytic coupling of chlorine radicals for SA degradation conformed to a first-order reaction kinetic model, with the degradation lnCt/C0 exhibiting a good linear relationship with degradation reaction time. The kinetic constants for photoelectrocatalysis, photoelectrocatalytic addition of NaCl + NaNO3, no copper sheet (photocatalytic), and dark conditions were 0.0464, 0.0924, 0.0458, and 0.0044 min−1, respectively (Table S2).
3.2.2. Effect of Initial Antibiotic Concentration on SA Degradation
The effect of different initial concentrations on the removal rate of SAs by photoelectrocatalysis coupled with chlorine radicals was investigated by varying the initial SA concentrations (Figure 2b). The figure illustrates that the rate of SA degradation by photoelectrocatalysis coupled with chlorine radicals decreased with an increase in the initial concentration. When the SA concentration was increased by 100-fold (from 0.1 mg/L to 10 mg/L), the degradation efficiency decreased from 100% to 77.93%. The reaction rate constant k also decreased from 0.2204 min−1 to 0.012 min−1 (Table S2).
3.2.3. Effect of Initial pH of the Solution
We investigated the effect of pH in the range of 3–9 on the efficiency of photoelectrocatalysis coupled with chlorine radicals for SA degradation. Figure 2c illustrates the more efficient degradation of SAs under acidic conditions (pH = 3). At pH 3, the degradation efficiency reached 100% at 60 min. Furthermore, the degradation efficiency remained above 99% at pH 5, 7, and 9. The reaction rate constants at different initial pH were 0.0924, 0.0558, 0.0519, and 0.0509 min−1, respectively (Table S2). The data indicate that the degradation rate decreases with increasing pH.
3.2.4. Effect of Chloride Ions (Cl−) and Sodium Nitrate
Next, we investigated the effects of different concentrations of chloride ions and sodium nitrate on SA degradation by the chlorine radical-coupled photoelectrocatalytic system (Figure 2d). The degradation rate of SAs was the slowest in the absence of chloride ions, with the degradation efficiency reaching 100% only at 120 min; the reaction rate constant was 0.0428 min−1. The degradation efficiency of SAs increased and then decreased with an increase in chloride ion concentration. Finally, at a chloride ion concentration of 5 mM and sodium nitrate concentration of 15 mM, the maximum degradation efficiency of SAs was 95.65% at 30 min, with a reaction rate constant of 0.1049 min−1 (Table S2).
3.2.5. Effects of Humic Acid and Sodium Acetate
Actual water bodies contain natural organic matter, which may affect SA degradation by TNT. In this experiment, we investigated the effect of natural organic matter on the catalytic reaction using sodium acetate and humic acid as representative examples. Different concentrations of humic acid and sodium acetate injected into the system had different effects on the efficiency of SA degradation.
Figure 2e illustrates the effects of humic acid addition. When the humic acid concentration in the degradation system was increased from 0 mg/L to 5 mg/L, SA removal presented a slight promotion effect, with the degradation rate increasing from 93.77% to 96.49% at 30 min. When the humic acid concentration was further increased from 5 mg/L to 15 mg/L, the degradation rate exhibited a decreasing trend, with the degradation rates of humic acid being 100% at 120 min under the condition of 0–15 mg/L.
Figure 2f illustrates the effects of sodium acetate on SA degradation by the chlorine radical-coupled photoelectrocatalytic system. The SA removal rate reached 93.77%, 100%, 100%, and 98.33%, respectively, when the concentration of sodium acetate was increased from 0 to 15 mg/L at 30 min. Overall, we inferred that sodium acetate does not significantly affect SA degradation in this system.
3.2.6. Effect of Actual Water Bodies
Figure S2 illustrates that the concentrations of SAs in tap water and cultured water were 0.5 and 10 mg/L, respectively, with degradation rates of 100% in both tap water and cultured water at SA concentrations of 0.5 mg/L and 53.54% and 52.31% at SA concentrations of 10 mg/L. The degradation rate constants were 0.0064 and 0.0062 min−1 (Table S2). The degradation profiles of SAs in tap water and cultured water at 10 mg/L were fitted and noted to be consistent with the first-order kinetic profile (Figure 3a).
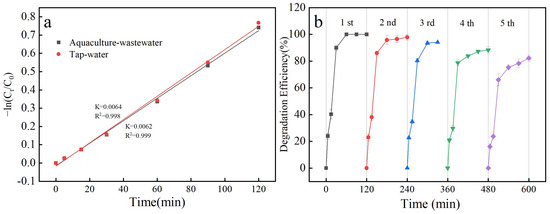
Figure 3.
Primary kinetic curves fitted at an SA concentration of 10 mg/L in tap water and aquaculture wastewater media (a) and degradation rate curves of five cycles (b).
3.3. Cycling Stability Test of TiO2 Nanotubes
In practical terms, the stability or recyclability of a catalyst is a key parameter to evaluate its applicability and economy. In the first degradation experiment, the degradation rate was 100% at 60 min of reaction. After five experimental repetitions, the degradation rate was 82.23% at 120 min (Figure 2b). This indicates the good reusability of the catalyst [29].
3.4. Degradation Mechanisms and Pathways
The degradation efficiencies of SAs with the addition of potassium dichromate, p-benzoquinone, and isopropanol were reduced by 20.1%, 21.6%, and 27.9% (Figure 4a), respectively, in the experimental group compared with the control group with no trapping agent. In the presence of histidine, the degradation efficiency significantly decreased and was only 35.7%. Oxygen radicals were noted to be the most contributing active substance in the chlorine radical-coupled photoelectrocatalytic degradation system, with electron holes (h+), superoxide radicals (•O2−), and hydroxyl radicals (•OH) also playing a role. The order of the contribution rate was as follows: oxygen radicals > •OH > •O2− > h+. Figure 4b illustrates the degradation mechanism.
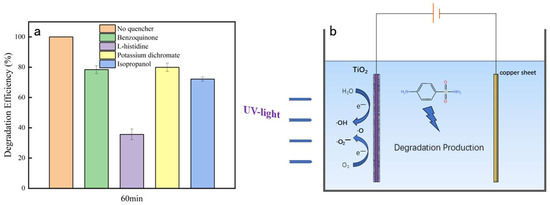
Figure 4.
Free radical trapping (a) and chlorine-coupled photoelectrocatalytic mechanism underlying SA degradation (b).
HPLC–MS analysis was performed to measure three intermediates for SA degradation (Table S3). The possible degradation pathways were further obtained based on the MS data and retention time of each intermediate product during SA degradation (Figure 5). Two main degradation pathways have been hypothesized for the chlorine radical-coupled photoelectrocatalytic degradation of SAs, in which the substance in the dashed line was the compound that was not detected by the instrument, but presumed to be potentially generated. It may have been converted to other degradation products at this time owing to the sampling time. The first pathway began with the hydrolysis of the SAs, generating compound P1 (m/z = 174). This compound was further converted to p-hydroxybenzenesulfonic acid (not detected) under anaerobic conditions. The generated p-hydroxybenzenesulfonic acid was able to be further converted to compound P3 (m/z = 189). The second degradation pathway was the oxidation of -NH2 linked to the benzene ring to -NO2, generating compound P2 (m/z = 201).
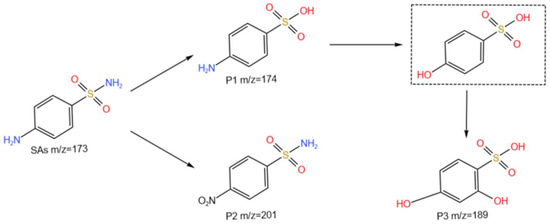
Figure 5.
Possible pathways for the degradation of SAs by the chlorine radical-coupled photoelectrocatalytic system.
3.5. Ecotoxicological Analysis
The luminescent bacterial experiments revealed that the luminescence intensity of Vibrio fischeri under the action of both the SA prodrug and its degradation products at 15 min was greater than that at 0 min (Figure 6a). Furthermore, the luminescence intensity of Vibrio fischeri under the action of the SA degradation products was significantly enhanced compared with that under the action of the SA prodrug during the reaction time. This finding suggests that the photoelectrocatalytically coupled with the chlorine radical system decreases the toxic effect of SAs on Vibrio fischeri.
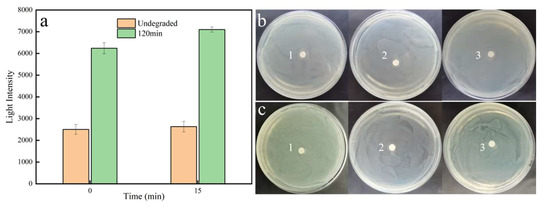
Figure 6.
Effect of the chlorine radical-coupled photoelectrocatalytic degradation of SA products on luminescent bacteria (a), the circle of inhibition formed by the SA prodrugs against E. coli (b), and the circle of inhibition formed after 120 min of degradation (c). Note: b1, b2, and b3 are parallel samples; c1, c2, and c3 are parallel samples.
SAs exhibited a significant inhibitory effect on Escherichia coli DH5α receptor cells, and the diameter of the circle of inhibition formed by the SA prodrug was 12 ± 0.71 mm (Figure 6b). In contrast, the diameter of the circle of inhibition formed by the SA degradation products after catalysis for 120 min was 9.5 ± 1 mm (Figure 6c), which was significantly lower compared with that of the undegraded product (p < 0.001). This indicates that the inhibitory effect of SA degradation products on E. coli DH5α receptor cells was weaker than that of the SA prodrug.
Figure 7 illustrates the effects on the chlorophyll fluorescence (Fv/Fm) of algae. The SA prodrug exhibited higher toxicity to Selenastrum capricornutum and Haematococcus pluvialis, with a significant decrease in the Fv/Fm value (Figure 7c,d). In contrast, no obvious toxic effect was noted on Chlorella and Scenedesmus quadricauda. The Fv/Fm value exhibited a rising tendency and stabilized with an increase in time at the beginning of the experiment (Figure 7a,b). In addition, the SA degradation products also exhibited higher toxicity to Selenastrum capricornutum and Haematococcus pluvialis, with a significant decrease in the Fv/Fm values (Figure 7c,d). Almost no inhibitory effect was noted on the growth of Chlorella and Scenedesmus quadricauda, with the Fv/Fm values exhibiting an increasing tendency with the growth of time (Figure 7a,b).
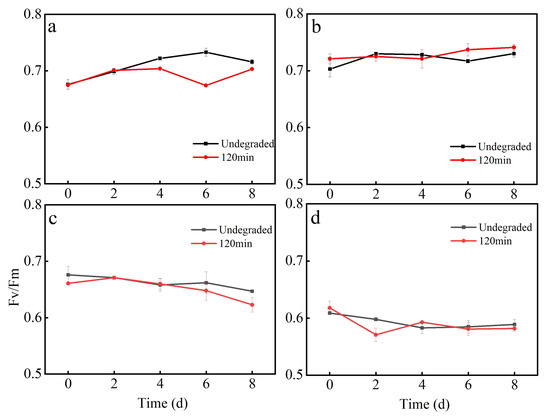
Figure 7.
Effect of the chlorine radical-coupled photoelectrocatalytic degradation products of SAs on the Fv/Fm values of algae: Chlorella (a), Scenedesmus quadricauda (b), Selenastrum capricornutum (c), and Haematococcus pluvialis (d).
Comparison revealed that the SA prodrug and degradation products exhibited different toxicity to the four algae. One-way analysis of variance revealed that the SA prodrug and degradation products (120 min) significantly affected (p < 0.05) the Fv/Fm values of algae. The results of multiple comparisons are presented in Table S4. At the end of the experiment, the Fv/Fm values of Chlorella, Selenastrum capricornutum, and Haematococcus pluvialis were significantly lower in the SA degradation product group than in the SA prodrug experimental group.
(1) Effects on algal growth
4. Discussion
4.1. Key Factors Affecting Sulfonamide Antibiotic (SA) Degradation and the Influence of Chlorinated Byproducts
To solve the problem of difficult degradation of antibiotics and other pollutants in factory aquaculture tailwater treatment, in this study, chlorine radicals were coupled based on photoelectrocatalysis to improve the degradation performance. The introduction of chlorine ions in the photoelectrocatalytic system resulted in a higher degradation performance compared with photocatalysis and photoelectrocatalysis. The highest degradation performance with a rate constant of 0.1049 min−1 was obtained when a certain concentration of sodium chloride and sodium nitrate was added. This indicates that the indirect photoelectrocatalytic reaction generated by the presence of Cl− occupies a significant position, producing chlorine radicals and chlorine gas. Both these products are strongly oxidizing and produce more radicals under the same conditions, thereby improving the degradation performance [30]. Therefore, the improved degradation of SA by photoelectrocatalysis coupled with chlorine radicals is owing to the combined degradation effect of photoelectrocatalysis and chlorine radicals. In this system, the SA degradation efficiency was greatly improved, with a better degradation effect, i.e., fewer byproducts were produced, the degradation was more complete, and byproduct toxicity was weakened. Nevertheless, the degradation pathways and byproducts of the same antibiotic may be different under different degradation systems [31,32].
The initial concentration of the pollutant is the main factor affecting the degradation efficiency. In this study, when the SA concentration was in the range of 0.1–10 mg/L, it was found that the degradation rate gradually decreased with the increase in initial concentration. Numerous studies have found that there is an inverse relationship between the degradation efficiency and the concentration of antibiotics [33,34,35], which is in agreement with the results of this paper. With regard to the blockage of drug breakdown at increased antibiotic concentrations, Cai [36] suggested that more pollutant molecules adsorb to the active site and the production of active substances is inhibited, while Tang [37] suggested that with the increase in initial concentration, the transmittance of light in solution is affected and the conversion efficiency of the catalyst for the absorption of light is suppressed. We support the above view that the photoelectrocatalytically coupled chlorine radical system constructed in this paper can produce only a certain amount of chlorine radicals when the dosage of sodium chloride and sodium nitrate within the system is certain. Also, higher initial concentrations of SAs represent more contaminant molecules, but the number of active sites of titanium dioxide nanotube arrays is limited. The degradation process also produces a number of byproducts and intermediates that compete with antibiotic molecules for active sites and active substances.
The tested concentrations of SAs ranged from 0.1 to 10 mg/L, primarily to better reflect the antibiotic concentrations typically found in real aquatic environments. It is important to note that the experiments were conducted in a 5 cm3 reactor, focusing on investigating the degradation efficiency of sulfonamide antibiotics at varying concentrations using a TiO2 nanotube-based photoelectrocatalytic system coupled with chlorine radicals at the laboratory scale. Zhang [38] found that antibiotic concentrations in pond culture in the middle and lower reaches of the Yangtze River Basin in China ranged from 111.8 to 15,949.05 ng/L. Shi [39] detected antibiotic concentrations ranging from 25.2 to 180 ng/L in recirculating water aquaculture systems in southern China. The range of the effect of initial antibiotic concentrations on degradation rates in this study was much higher than the actual water conditions. In the laboratory, antibiotic solutions with concentrations exceeding those observed in real-world environments were prepared to investigate the influence of concentration factors. At an SA concentration of 1 mg/L, complete removal was achieved after 60 min of treatment. At the same time, in order to be closer to the removal of antibiotics in actual aquaculture water, we used effluent samples of salmon trout recirculating in the aquaculture system, configured with low-concentration (0.5 mg/L) and high-concentration (10 mg/L) SA solutions, which is more capable of examining the degradation effect of the photoelectrocatalytically coupled chlorine radical system for the degradation of antibiotics in practical applications. The degradation rates were 77.96%, 53.54%, and 52.31% after 120 min of treatment with a photoelectrocatalytically coupled chlorine radical system in ultrapure water, tap water, and actual aquaculture water body configured with a 10 mg/L solution of SAs, respectively. Porcar-Santos [40] studied the photocatalytic degradation of SMX using TiO2 to simulate solar irradiation and found that SMX degradation in deionized water was twice as fast as in simulated seawater. The inhibition of the catalytic performance in different media is mainly due to the presence of some organic pollutants and high turbidity in natural waters with a large number of inorganic ions, which significantly affect the catalytic degradation efficiency [41,42], which is the same as in our study. The antibiotics in aquaculture water with SA concentration of 0.5 mg/L could still be completely removed after treatment with the photoelectrocatalytically coupled chlorine radical system. The results showed that the photoelectrocatalytically coupled chlorine radical system can be effectively applied in the degradation of antibiotics in real aquaculture water. After ecotoxicological experiments, it was found that the products degraded by photoelectrocatalytically coupled chlorine radicals had greatly attenuated toxicity compared with the original SAs, which is a safer guarantee for the treatment of tailwater after the degradation of antibiotics in aquaculture water. This system has very good prospects for application in the field of aquaculture.
pH has been reported to affect the degradation rate of antibiotics [43]. In this study, the system was most effective for the degradation of SAs under acidic conditions, although overall the pH variation did not significantly alter the degradation efficiency. The degradation of SAs remained relatively efficient under both acidic and alkaline conditions, consistent with the trends observed in TiO2 photocatalytic degradation of tetracycline hydrochloride [44] and TiO2 photoelectrocatalytic degradation of SAs [45,46]. This can be explained by the pKa values of SAs being 1.85 and 10.1, which makes them more prone to protonation under acidic conditions, resulting in stronger reactivity compared to their unprotonated forms [47]. Under acidic conditions, titanium dioxide nanotube arrays become protonated and the protonated surface is positively charged, facilitating the transfer of photogenerated electrons to the titanium dioxide nanotube array surface, which enhances its photoelectrocatalytic activity [48,49].
In addition to pollutant degradation efficiency, the potential formation of disinfection byproducts (DBPs) during the photoelectrocatalytic process coupled with chlorine radicals is a critical concern. DBP formation is a common risk in chlorinated systems, such as ultraviolet/chlorine (UV/Cl) systems [50]. Studies by Ben [51] and Hua [52] revealed that the concentration of chloroform significantly increased when triclosan was degraded in a UV/Cl system. When amino compounds served as DBP precursors, chlorination and radical oxidation primarily led to the formation of dichloroacetonitrile. The combination of UV/Cl with TiO2 promotes the generation of HO• and Cl•, which may alter the composition of reactive species (e.g., the formation of ClO•) and further influence the pollutant degradation process. Zhang [53] found that in a UV/TiO2/chlorine (UCT) system, the degradation rate of carbamazepine reached 90%, and fewer DBPs were produced compared to the UV/Cl system. This is attributed to the faster degradation of pollutants in the UCT system and the greater consumption of chlorine, which significantly reduces the reaction time between chlorine and DBP precursors, thereby inhibiting DBP formation. The study concluded that the UCT system can effectively mitigate the risk of DBP formation. In this study, chlorine was introduced in the form of sodium chloride at a low concentration. During the photocatalytic process, it reacted with HO• to generate chlorine-based reactive species, thereby accelerating the degradation of sulfonamide antibiotics (SAs). Additionally, the toxicity changes of the SA solution before and after degradation were investigated. The toxicity significantly decreased after degradation by the TiO2 photoelectrocatalytic system coupled with chlorine radicals, and no adverse effects on the normal biological activities of Chlorella, luminescent bacteria, or E. coli were observed compared to the control group. In summary, we infer that the DBP formation mechanism in this system may differ from that of UV/chlorine disinfection. Furthermore, toxicity experiments indicated no inhibitory effects, suggesting that the photoelectrocatalytic system coupled with chlorine radicals can effectively control the risk of DBP formation.
4.2. Degradation Performance Enhancement and Catalyst Improvement
Photoelectrocatalytic technology is a highly efficient method for the environmental treatment of persistent organic pollutants. TiO2 photoelectrocatalysis not only efficiently degrades SAs but also demonstrates strong degradation capabilities for other antibiotics, including tetracycline, sodium cefotaxime, and penicillin [54]. This highlights the broad applicability and versatility of TiO2 nanotube photoelectrocatalysis in various degradation processes. In the current experiment, the degradation rate was 82.23% after five repetitions, which was 17.77% lower than the initial experiment. This decrease in degradation efficiency may be due to the accumulation of SAs within the TiO2 nanotubes, which were not completely removed after several cycles, reducing the availability of adsorption sites [55]. TiO2 nanotubes exhibit excellent catalytic stability and chemical performance, demonstrating significant potential for future engineering applications.
TiO2 as a photocatalyst is known for its high efficiency, stability, low cost, and environmental safety. However, it also has certain limitations, such as a large bandgap that prevents it from absorbing visible light, requiring ultraviolet (UV) radiation for activation. Further optimization of TiO2 photoelectrocatalysis by material doping modification is needed in the future.
4.3. Practical Implications
This study preliminarily reveals a potential synergistic approach for treating aquaculture tailwater in saline-alkaline waters: high concentrations of chloride ions (Cl−) in saline-alkaline environments can be activated in situ into reactive chlorine species (e.g., Cl·, ClO·, and Cl2) within a photoelectrocatalytic system. Laboratory experiments have validated the enhanced degradation mechanism of organic pollutants, such as sulfonamide antibiotics (SAs), by these reactive species [56]. Notably, while the ultraviolet light source excites the photocatalytic response of the catalyst, it may also inhibit the proliferation of pathogenic microorganism s in water through photochemical sterilization [57], offering a synergistic strategy for disease control in aquaculture. Although the current research primarily demonstrates technological innovation at the laboratory level, it provides a novel technical framework for treating aquaculture tailwater in saline-alkaline regions. Future studies should focus on constructing a synergistic photoelectro-chlorine catalytic system, developing specialized catalysts tailored to the chemical characteristics of saline-alkaline water, and conducting technoeconomic analysis and life-cycle assessments to facilitate the practical transformation of this technology into an environmentally friendly aquaculture model.
5. Conclusions
This study developed a photoelectrocatalytic system coupled with chlorine radicals, significantly enhancing the removal efficiency of sulfonamide antibiotics (SAs) in cold-water fish aquaculture environments using TiO2 nanotubes. The main conclusions are as follows.
- System Performance Optimization: The photoelectrocatalytic system coupled with chlorine radicals demonstrated superior degradation efficiency compared to conventional photocatalysis and photoelectrocatalysis. The optimal degradation of SAs was achieved at sodium chloride and sodium nitrate concentrations of 5 mM and 15 mM, respectively.
- Degradation Mechanisms and Pathways: Radical trapping experiments confirmed that oxygen radicals dominated the degradation process (contribution order: oxygen radicals > •OH > •O2− > h+). Analysis of SA degradation byproducts revealed hydrolysis and nitration as the primary degradation pathways.
- Environmental Safety Assessment: The acute toxicity of the degradation products to Vibrio fischeri was significantly reduced compared to the parent compounds, while the ecotoxicity toward Escherichia coli and Chlorella was also markedly diminished. Tests conducted in real water bodies demonstrated the system’s robust anti-interference capability, confirming its potential for practical engineering applications.
This research provides a highly efficient and environmentally friendly technical solution for addressing antibiotic residues in aquaculture tailwater. The system design effectively overcomes the limitations of traditional photocatalysis, such as the short lifespan and restricted diffusion of hydroxyl radicals, offering theoretical support for innovative pollution control technologies in saline-alkaline waters.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/fishes10030116/s1. Text S1: Ecotoxicology experiments; Text S2: Analysis of data; Text S3: Effect of photoelectrocatalytically coupled chlorine radical degradation of sulfonamide products on algal OD680; Table S1: Water quality parameters of Qinghai Provincial Fisheries Environment Monitoring Station; Table S2: Kinetic parameters for the catalytic degradation of SAs by photoelectrically coupled chlorine radicals; Table S3: Identification of intermediates by LC-MS technology; Table S4: Multiple comparisons of the effects of photoelectrocatalytically coupled chlorine radical degradation of sulfonamide products on algal Fv/Fm; Table S5: Multiple comparisons of the effects of photoelectrocatalytically coupled chloride radical degradation of sulfonamide products on algal OD680; Figure S1: Experimental setup diagram; Figure S2: Effects of different water base on sulfonamide degradation: (a) tap water, (b) aquaculture wastewater; Figure S3: Effect of photoelectrocatalytically coupled chlorine radical degradation of sulfonamide products on algal OD680.
Author Contributions
Q.L.: writing—original draft, writing—review and editing, data curation, conceptualization. Y.L.: formal analysis, writing—review and editing, project administration, resources. Y.M.: visualization, software. R.L.: supervision, investigation. Y.J.: validation. J.L.: visualization. F.Z.: writing—review and editing, project administration, resources. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (42107272) and the Qinghai Provincial Science and Technology Department (2022-ZJ-923).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| SA | sulfonamide antibiotics |
| HPLC | high-performance liquid chromatography |
| MS | mass spectrometry |
| SMX | sulfamethoxazole |
| TNTs | titanium dioxide nanotubes |
References
- Coates, A.R.M.; Hu, Y. Novel approaches to developing new antibiotics for bacterial infections. Br. J. Pharmacol. 2007, 152, 1147–1154. [Google Scholar] [CrossRef]
- Adenaya, A.; Berger, M.; Brinkhoff, T.; Ribas-Ribas, M.; Wurl, O. Usage of antibiotics in aquaculture and the impact on coastal waters. Mar. Pollut. Bull. 2023, 188, 114645. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Chen, X.; Ye, Y.; Liao, Y.; Luo, H.; Tang, C.; Liu, G. Enhanced metronidazole removal in seawater using a single-chamber bioelectrochemical system. Water Res. 2024, 252, 121212. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ye, K.X.; Tian, Y.; Liu, Y.; Liang, L.; Li, Q.; Huang, N.; Wang, X. Simultaneous determination of 22 antibiotics in environmental water samples by solid phase extraction-high performance liquid chromatography-tandem mass spectrometry. Se Pu Chin. J. Chromatogr. 2023, 41, 241–249. [Google Scholar] [CrossRef]
- Glinka, M.; Wojnowski, W.; Wasik, A. Determination of aminoglycoside antibiotics: Current status and future trends. TrAC Trends Anal. Chem. 2020, 131, 116034. [Google Scholar] [CrossRef]
- Jian, Z.; Zeng, L.; Xu, T.; Sun, S.; Yan, S.; Yang, L.; Huang, Y.; Jia, J.; Dou, T. Antibiotic resistance genes in bacteria: Occurrence, spread, and control. J. Basic Microbiol. 2021, 61, 1049–1070. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ryu, D.; Houtkooper, R.H.; Auwerx, J. Antibiotic use and abuse: A threat to mitochondria and chloroplasts with impact on research, health, and environment. Bioessays 2015, 37, 1045–1053. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ); Koutsoumanis, K.; Allende, A.; Álvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; Cesare, A.; Herman, L.; et al.; et al. Role played by the environment in the emergence and spread of antimicrobial resistance (AMR) through the food chain. EFSA J. 2021, 19, e06651. [Google Scholar]
- Collignon, P.J.; McEwen, S.A. One health—Its importance in helping to better control antimicrobial resistance. Trop. Med. Infect. Dis. 2019, 4, 22. [Google Scholar] [CrossRef]
- Huang, X.; Yang, D.; Song, L.; Jiang, Y. Degradation of Antibiotics in Aquaculture Seawater: A Treatment Based on Ozone Assisted with Hydrodynamic Cavitation. Water 2025, 17, 566. [Google Scholar] [CrossRef]
- Zhou, Q.; Xing, A.; Zhao, D.; Zhao, K. Tetrabromobisphenol A photoelectrocatalytic degradation using reduced graphene oxide and cerium dioxide comodified TiO2 nanotube arrays as electrode under visible light. Chemosphere 2016, 165, 268–276. [Google Scholar] [CrossRef]
- Bayan, E.M.; Pustovaya, L.E.; Volkova, M.G. Recent advances in TiO2-based materials for photocatalytic degradation of antibiotics in aqueous systems. Environ. Technol. Innov. 2021, 24, 101822. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, J.; Zeng, L.; Zhu, M. Recent progress on the removal of antibiotic pollutants using photocatalytic oxidation process. Crit. Rev. Environ. Sci. Technol. 2022, 52, 1401–1448. [Google Scholar] [CrossRef]
- Sobczak-Kupiec, A.; Drabczyk, A.; Florkiewicz, W.; Glab, M.; Kudlacik-Kramarczyk, S.; Slota, D.; Tomala, A.; Tyliszczak, B. Review of the applications of biomedical compositions containing hydroxyapatite and collagen modified by bioactive components. Materials 2021, 14, 2096. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Mazare, A.; Schmuki, P. One-dimensional titanium dioxide nanomaterials: Nanotubes. Chem. Rev. 2014, 114, 9385–9454. [Google Scholar] [CrossRef]
- Kubiak, A.; Stachowiak, M.; Cegłowski, M. Unveiling the Latest Developments in Molecularly Imprinted Photocatalysts: A State-of-the-Art Review. Polymers 2023, 15, 4152. [Google Scholar] [CrossRef]
- Mohamed, A.E.R.; Barghi, S.; Rohani, S. N-and C-modified TiO2 nanotube arrays: Enhanced photoelectrochemical properties and effect of nanotubes length on photoconversion efficiency. Nanomaterials 2018, 8, 198. [Google Scholar] [CrossRef]
- He, H.; Zhao, T.; Ma, Q.; Yang, X.; Yue, Q.; Huang, B.; Pan, X. Photoelectrocatalytic coupling system synergistically removal of antibiotics and antibiotic resistant bacteria from aquatic environment. J. Hazard. Mater. 2022, 424, 127553. [Google Scholar] [CrossRef]
- Wang, L.; Guan, J.; Han, H.; Yao, M.; Kang, J.; Peng, M.; Wang, D.; Xu, J.; Hao, J. Enhanced photocatalytic removal of ozone by a new chlorine-radical-mediated strategy. Appl. Catal. B Environ. 2022, 306, 121130. [Google Scholar] [CrossRef]
- Cheng, Z.; Ling, L.; Shang, C. Near-ultraviolet light-driven photocatalytic chlorine activation process with novel chlorine activation mechanisms. ACS EST Water 2021, 1, 2067–2075. [Google Scholar] [CrossRef]
- Zhang, H.C.; Liu, Y.L.; Wang, L.; Li, Z.Y.; Lu, X.H.; Yang, T.; Ma, J. Enhanced radical generation in an ultraviolet/chlorine system through the addition of TiO2. Environ. Sci. Technol. 2021, 55, 11612–11623. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, Y.; Bai, J.; Li, J.; Li, L.; Zhou, T.; Chen, S.; Wang, J.; Rahim, M.; Guan, X.; et al. Efficient degradation of N-containing organic wastewater via chlorine oxide radical generated by a photoelectrochemical system. Chem. Eng. J. 2020, 392, 123695. [Google Scholar] [CrossRef]
- GB 5749-2022; Standard for Drinking Water Quality. Ministry of Health of the People’s Republic of China: Beijing, China, 2022.
- Menz, J.; Schneider, M.; Kümmerer, K. Toxicity testing with luminescent bacteria–characterization of an automated method for the combined assessment of acute and chronic effects. Chemosphere 2013, 93, 990–996. [Google Scholar] [CrossRef]
- Pivato, A.; Gaspari, L. Acute toxicity test of leachates from traditional and sustainable landfills using luminescent bacteria. Waste Manag. 2006, 26, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.; Lourenço, O.; Queiroz, J.A.; Domingues, F.C. Bacteriostatic versus bactericidal activity of ciprofloxacin in Escherichia coli assessed by flow cytometry using a novel far-red dye. J. Antibiot. 2011, 64, 321–325. [Google Scholar] [CrossRef]
- Chen, J.Q.; Guo, R.X. Access the toxic effect of the antibiotic cefradine and its UV light degradation products on two freshwater algae. J. Hazard. Mater. 2012, 209, 520–523. [Google Scholar] [CrossRef]
- Fu, L.; Huang, T.; Wang, S.; Wang, X.; Su, L.; Li, C.; Zhao, Y. Toxicity of 13 different antibiotics towards freshwater green algae Pseudokirchneriella subcapitata and their modes of action. Chemosphere 2017, 168, 217–222. [Google Scholar] [CrossRef]
- Assadi, A.A.; Karoui, S.; Trabelsi, K.; Hajjaji, A.; Wlfalleh, W.; Ghorbal, A.; Maghzaoui, M.; Assadi, A.A. Synthesis and characterization of TiO2 nanotubes (TiO2-NTs) with Ag silver nanoparticles (Ag-NPs): Photocatalytic performance for wastewater treatment under visible light. Materials 2022, 15, 1463. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Y.H.; Qiao, Y.; Gau, M.; Carroll, P.; Walsh, P.; Schelter, E. Photocatalytic C–H activation and the subtle role of chlorine radical complexation in reactivity. Science 2021, 372, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Huang, L.; Li, N.; Jin, B.; Zhou, W.; Gao, B.; Yue, Q.; Li, Q. Chlorine dioxide radicals triggered by chlorite under visible-light irradiation for enhanced degradation and detoxification of norfloxacin antibiotic: Radical mechanism and toxicity evaluation. Chem. Eng. J. 2021, 414, 128768. [Google Scholar] [CrossRef]
- Zhang, F.; Li, M.; Li, W.; Feng, C.; Jin, Y.; Guo, X.; Cui, J. Degradation of phenol by a combined independent photocatalytic and electrochemical process. Chem. Eng. J. 2011, 175, 349–355. [Google Scholar] [CrossRef]
- Alfred, M.O.; Omorogie, M.O.; Bodede, O.; Moodley, R.; Ogunlaja, A.; Adeyemi, O.G.; Günter, C.; Taubert, A.; Iermak, I.; Eckert, H.; et al. Solar-active clay-TiO2 nanocomposites prepared via biomass assisted synthesis: Efficient removal of ampicillin, sulfamethoxazole and artemether from water. Chem. Eng. J. 2020, 398, 125544. [Google Scholar] [CrossRef]
- Ioannidou, E.; Frontistis, Z.; Antonopoulou, M.; Venieri, D.; Konstantinou, I.; Kondarides, D.I.; Mantzavinos, D. Solar photocatalytic degradation of sulfamethoxazole over tungsten—Modified TiO2. Chem. Eng. J. 2017, 318, 143–152. [Google Scholar]
- Sarafraz, M.; Sadeghi, M.; Yazdanbakhsh, A.; Amini, M.M.; Sadani, M.; Eslami, A. Enhanced photocatalytic degradation of ciprofloxacin by black Ti3+/N-TiO2 under visible LED light irradiation: Kinetic, energy consumption, degradation pathway, and toxicity assessment. Process Saf. Environ. Prot. 2020, 137, 261–272. [Google Scholar] [CrossRef]
- Cai, Q.; Hu, J. Decomposition of sulfamethoxazole and trimethoprim by continuous UVA/LED/TiO2 photocatalysis: Decomposition pathways, residual antibacterial activity and toxicity. J. Hazard. Mater. 2017, 323, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Zhang, W.; Meng, Y.; Xie, B.; Ni, Z.; Xia, S. Investigation onto the performance and mechanism of visible light photodegradation of methyl orange catalyzed by M/CeO2 (M = Pt, Ag, Au). Mater. Res. Bull. 2021, 144, 111497. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, H.; Wang, C.; Cheng, Y.; Li, Y.; Wang, Z. Distribution and ecological risk assessment of antibiotics in different freshwater aquaculture ponds in a typical agricultural plain, China. Chemosphere 2024, 361, 142498. [Google Scholar] [CrossRef]
- Shi, B.; Cheng, X.; Chen, H.; Xie, J.; Zhou, Z.; Jiang, S.; Peng, X.; Zhu, D.; Lu, Z. Occurrence, source tracking and removal of antibiotics in recirculating aquaculture systems (RAS) in southern China. J. Environ. Manag. 2022, 324, 116311. [Google Scholar] [CrossRef]
- Porcar-Santos, O.; Cruz-Alcalde, A.; López-Vinent, N.; Zanganas, D.; Sans, C. Photocatalytic degradation of sulfamethoxazole using TiO2 in simulated seawater: Evidence for direct formation of reactive halogen species and halogenated by-products. Sci. Total Environ. 2020, 736, 139605. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Li, R.; Guo, J.; Li, Y.; Zhu, J.; Xie, X. TiO2 supported on reed straw biochar as an adsorptive and photocatalytic composite for the efficient degradation of sulfamethoxazole in aqueous matrices. Chemosphere 2017, 185, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Li, S.; Zhang, H.; Wang, Z.; Huang, H. Promoting charge separation of biochar-based Zn-TiO2/pBC in the presence of ZnO for efficient sulfamethoxazole photodegradation under visible light irradiation. Sci. Total Environ. 2019, 659, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, G.; Meng, D.; Yang, F. Photoelectrocatalytic activation of sulfate for sulfamethoxazole degradation and simultaneous H2 production by bifunctional N, P co-doped black-blue TiO2 nanotube array electrode. Chem. Eng. J. 2024, 485, 149828. [Google Scholar] [CrossRef]
- Almomani, F.; Bhosale, R.; Kumar, A.; Khraiseh, M. Potential use of solar photocatalytic oxidation in removing emerging pharmaceuticals from wastewater: A pilot plant study. Sol. Energy 2018, 172, 128–140. [Google Scholar] [CrossRef]
- Bu, J.; Deng, Z.; Liu, H.; Li, T.; Yang, Y.; Zhong, S. Bimetallic modified halloysite particle electrode enhanced electrocatalytic oxidation for the degradation of sulfanilamide. J. Environ. Manag. 2022, 312, 114975. [Google Scholar] [CrossRef] [PubMed]
- Zafar, Z.; Fatima, R.; Kim, J.O. Experimental studies on water matrix and influence of textile effluents on photocatalytic degradation of organic wastewater using Fe–TiO2 nanotubes: Towards commercial application. Environ. Res. 2021, 197, 111120. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Lu, X.; Song, F.; Yao, Y.; Han, E. Electrocatalytic degradation of sulfamethazine on IrO2-RuO2 composite electrodes: Influencing factors, kinetics and modeling. J. Environ. Chem. Eng. 2021, 9, 105301. [Google Scholar] [CrossRef]
- Wu, F.; Yao, C.; Xie, Y.B. Electrolyte-Dependent Capacitance of Titanium Dioxide Nanotube Array Electrode Substrate. J. Nano Res. 2022, 75, 71–80. [Google Scholar] [CrossRef]
- Bavykin, D.V.; Friedrich, J.M.; Walsh, F.C. Protonated titanates and TiO2 nanostructured materials: Synthesis, properties, and applications. Adv. Mater. 2006, 18, 2807–2824. [Google Scholar] [CrossRef]
- Yang, X.; Sun, J.; Fu, W.; Shang, J.; Li, Y.; Chen, Y.; Gan, W.; Fang, J. PPCP degradation by UV/chlorine treatment and its impact on DBP formation potential in real waters. Water Res. 2016, 98, 309–318. [Google Scholar] [CrossRef]
- Ben, W.; Sun, P.; Huang, C.-H. Effects of combined UV and chlorine treatment on chloroform formation from triclosan. Chemosphere 2016, 150, 715–722. [Google Scholar] [CrossRef]
- Hua, Z.; Li, J.; Zhou, Z.; Zheng, S.; Zhang, Y.; Fang, J. Exploring pathways and mechanisms for dichloroacetonitrile formation from typical amino compounds during UV/chlorine treatment. Environ. Sci. Technol. 2022, 56, 9712–9721. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Z.; Zhou, X.; Lu, X.; Gu, H.; Ma, J. Insight into the performance of UV/chlorine/TiO2 on carbamazepine degradation: The crucial role of chlorine oxide radical (ClO•). Sci. Total Environ. 2022, 853, 158345. [Google Scholar] [CrossRef] [PubMed]
- Brillas, E.; Garcia-Segura, S. Recent progress of applied TiO2 photoelectrocatalysis for the degradation of organic pollutants in wastewaters. J. Environ. Chem. Eng. 2023, 11, 109635. [Google Scholar] [CrossRef]
- Xia, T.; Yan, N.; Li, S.; Su, T. Adsorption of tylosin and sulfamethazine by carbon nanotubes and titanium dioxide nanoparticles: pH-dependent mechanisms. Colloids Surf. A Physicochem. Eng. Asp. 2019, 581, 123851. [Google Scholar] [CrossRef]
- Lei, Y.; Lei, X.; Westerhoff, P.; Zhang, X.; Yang, X. Reactivity of chlorine radicals (Cl• and Cl2•−) with dissolved organic matter and the formation of chlorinated byproducts. Environ. Sci. Technol. 2020, 55, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huang, R.; Zhang, T.; Wang, B.; Li, N.; Sun, Y.; Ma, H.; Zhang, Q.; Zhang, J.; Liu, Y. Study on the inactivation and reactivation mechanism of pathogenic bacteria in aquaculture by UVC-LED. Front. Mar. Sci. 2023, 10, 1139713. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).