Methyltransferase HsdM Regulates the Pathogenicity of Streptococcus agalactiae to Nile Tilapia (Oreochromis niloticus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Cells
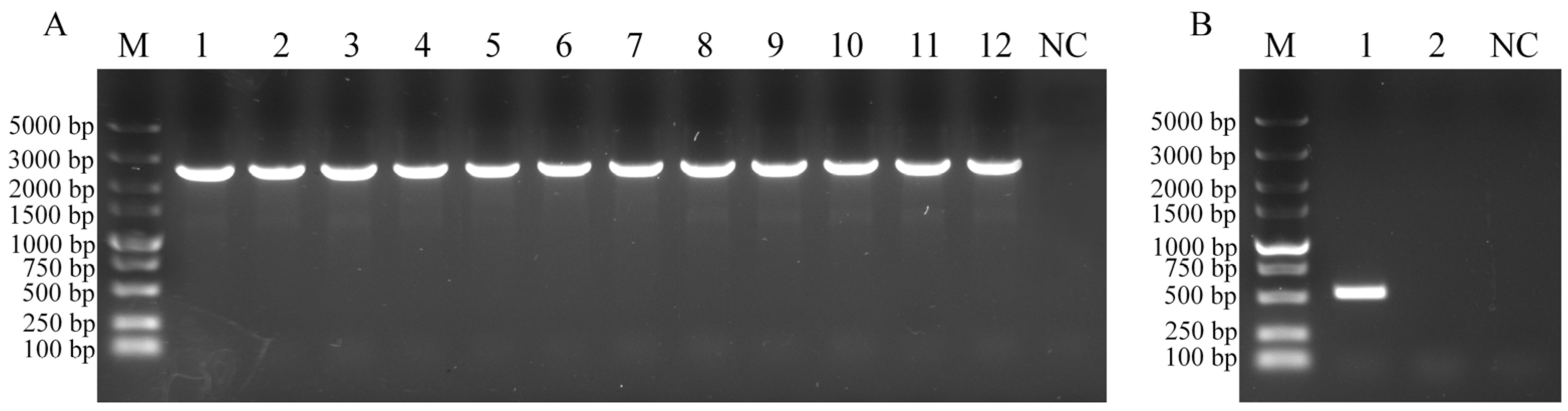
2.2. Construction of the hsdM Mutant
2.3. Phenotype of Mutant WC1535ΔhsdM
2.3.1. Growth Curve
2.3.2. Hemolytic Activity Assay
2.3.3. Capsular Staining and Transmission Electron Microscopy Observation
2.4. Survival Assay in Whole Blood
2.5. Cell Adhesion Assay
2.6. Phagocytosis Assay
2.7. Challenge Test
2.8. Dynamic Distribution Assay
2.9. Reverse-Transcription Quantitative Polymerase Chain Reaction (RT-qPCR) Assay
2.10. Statistics
3. Results
3.1. Construction of WC1535ΔhsdM
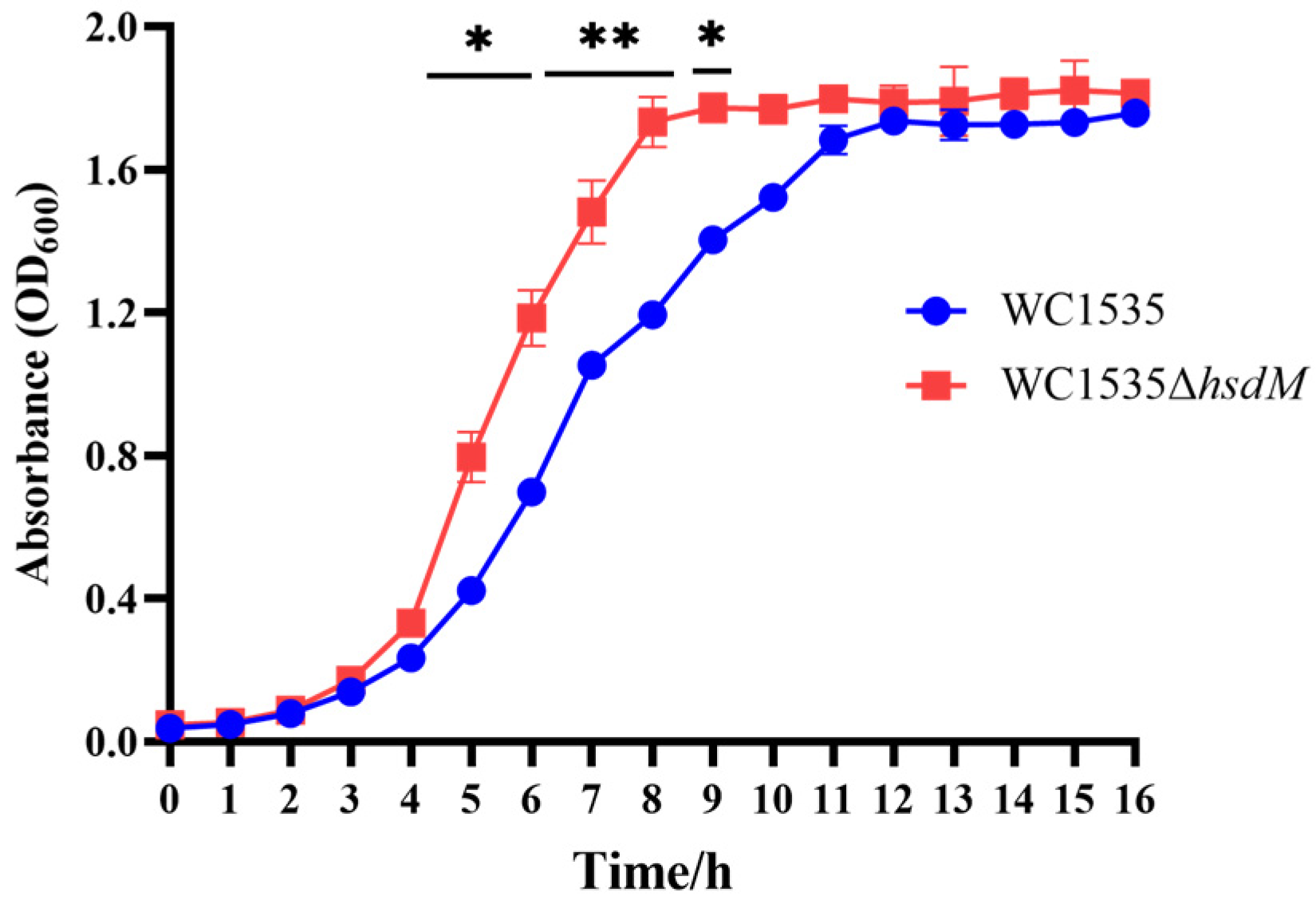
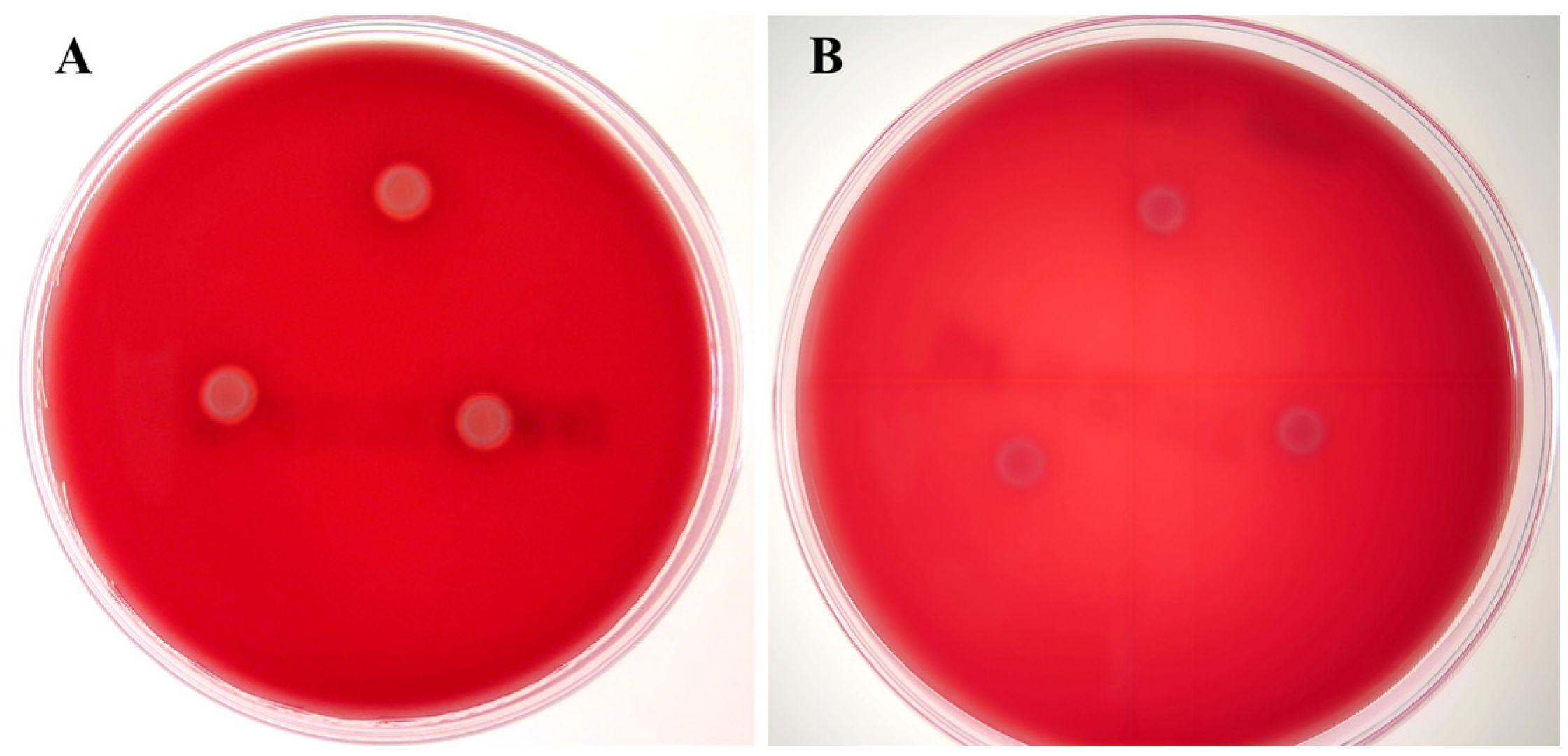
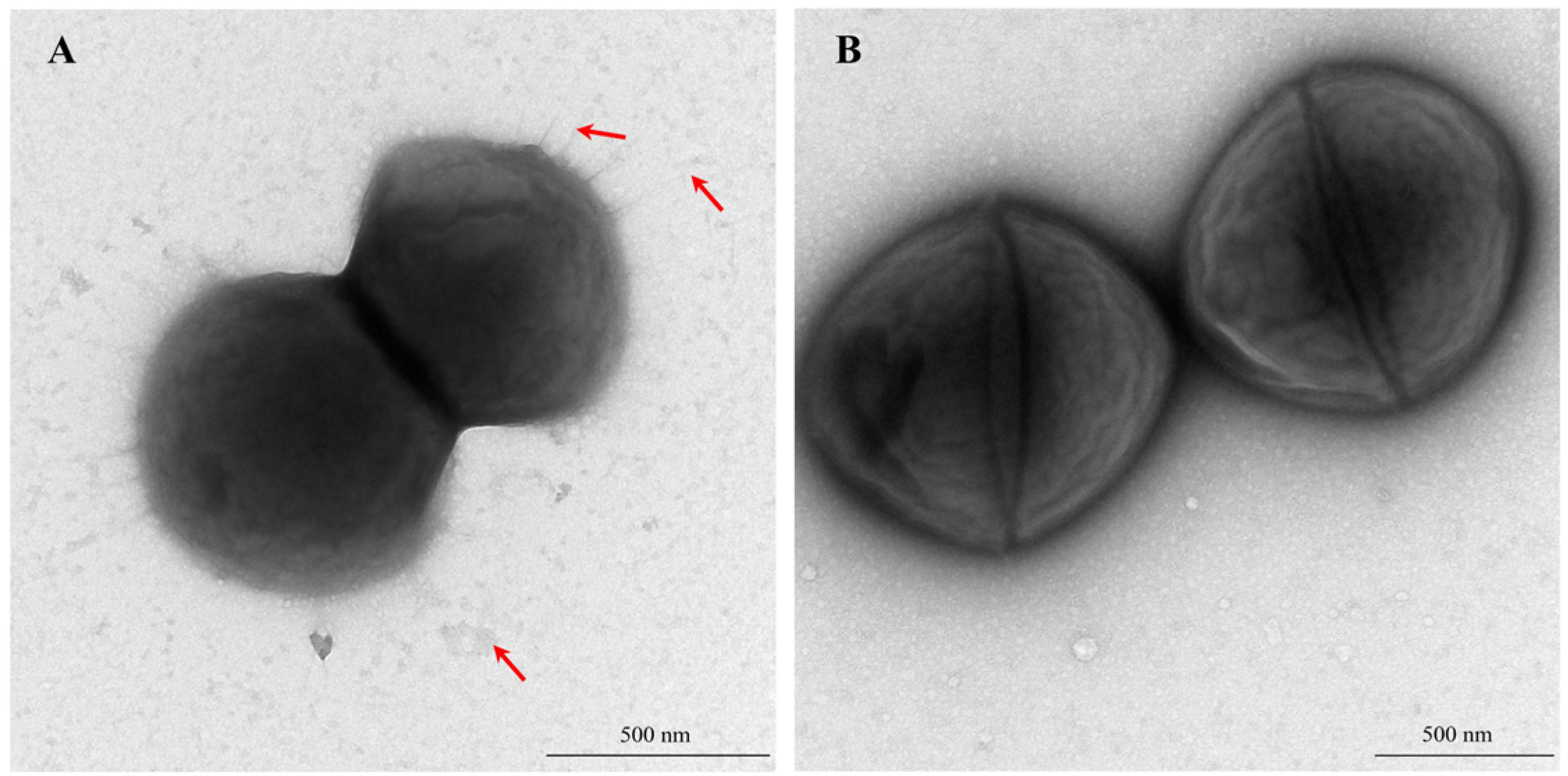
3.2. Biological Characteristics of WC1535ΔhsdM
3.2.1. Growth Curves
3.2.2. Hemolytic Activity
3.2.3. Bacterial Morphology and Capsule Characteristics
3.3. Survival in Whole Blood
3.4. Cell Adhesion and Anti-Macrophage Phagocytosis
3.5. Pathogenicity
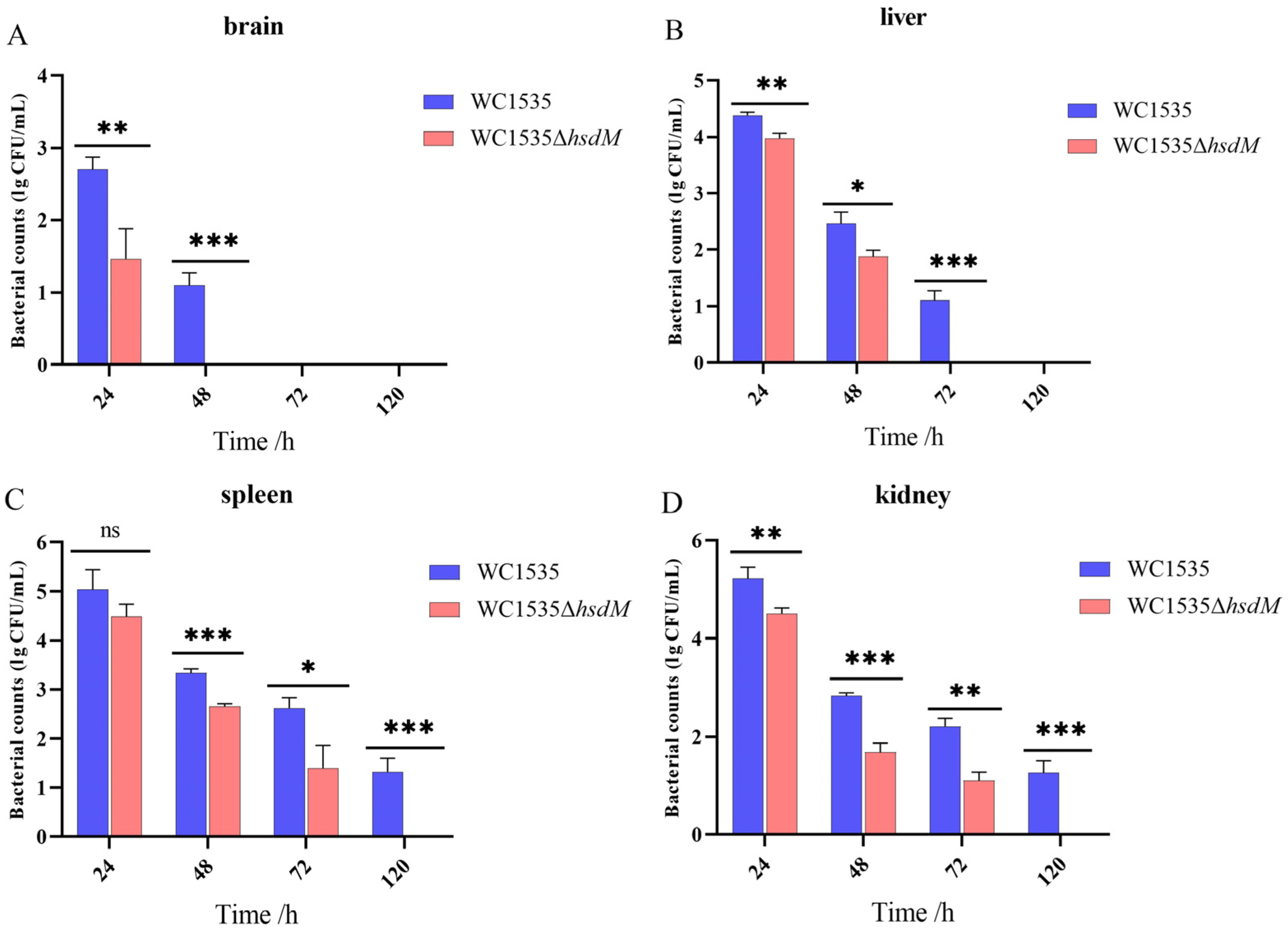
3.6. Dynamic Distribution In Vivo
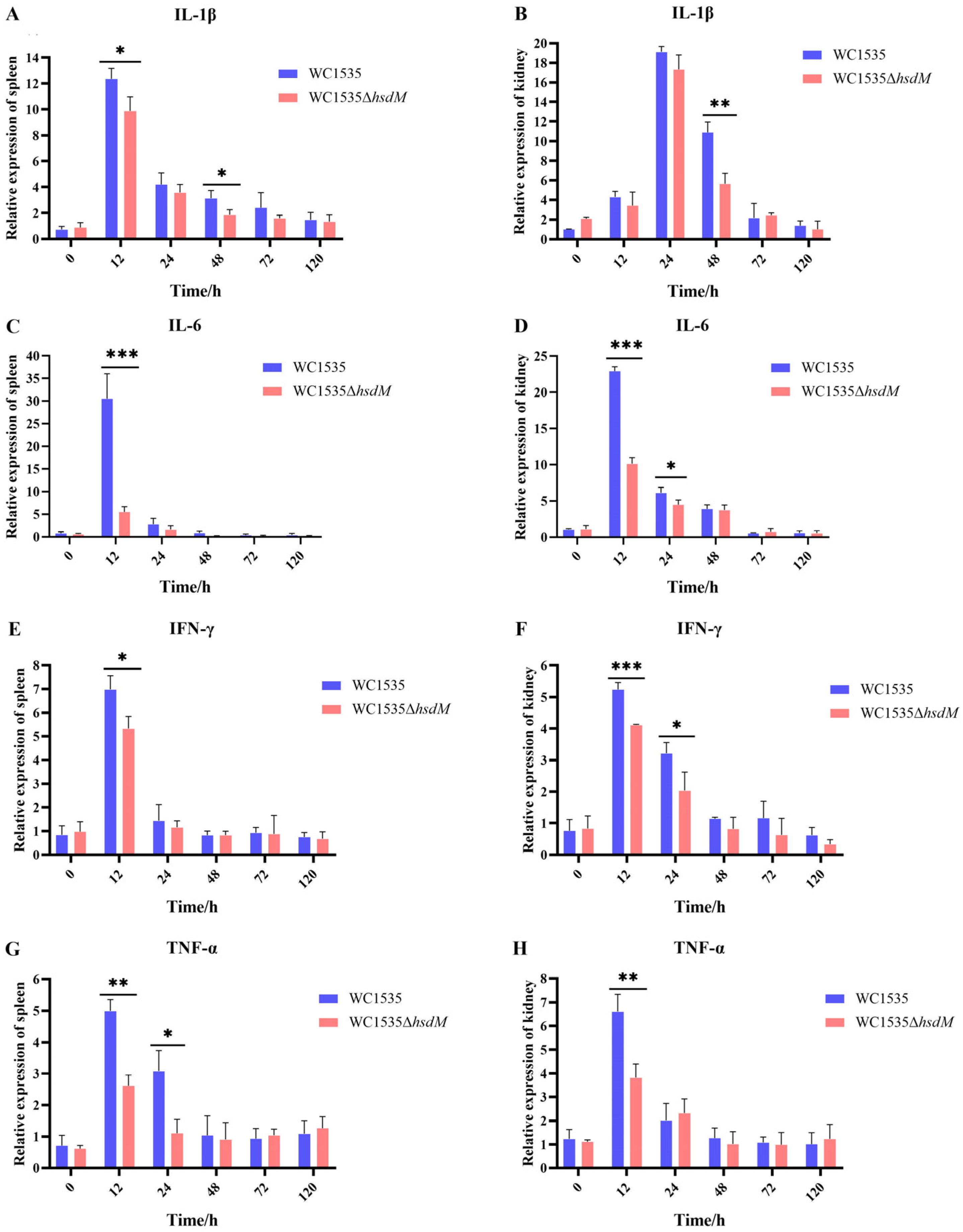
3.7. Expression Levels of Immune-Related Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piamsomboon, P.; Srisuwatanasagul, S.; Kongsonthana, K.; Wongtavatchai, J. Streptococcus agalactiae infection caused spinal deformity in juvenile red tilapia (Oreochromis sp.). J. Fish Dis. 2022, 45, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.M.; Wong, W.K.; Lee, W.Y.; Tan, Z.B.; Tay, Y.H.; Teo, X.H.; Chee, L.D.; Fernandez, C.J. Streptococcus agalactiae outbreaks in cultured golden pomfret, Trachinotus blochii (Lacepede), in Singapore. J. Fish Dis. 2017, 40, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.F.; Ke, X.; Liu, L.; Lu, M.; Shi, C.; Liu, Z. Streptococcus agalactiae from tilapia (Oreochromis sp.) transmitted to a new host, bighead carp (Aristichthys nobilis), in China. Aquac. Int. 2018, 26, 885–897. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Z.; Ren, Y.; Wang, Y.; Pan, H.; Liang, D.; Bei, W.; Chang, O.; Wang, Q.; Shi, C. Epidemiological characteristics of Streptococcus agalactiae in tilapia in China from 2006 to 2020. Aquaculture 2022, 549, 885–897. [Google Scholar] [CrossRef]
- Abdallah, E.S.H.; Metwally, W.G.M.; Abdel-Rahman, M.A.M.; Albano, M.; Mahmoud, M.M. Streptococcus agalactiae infection in Nile tilapia (Oreochromis niloticus): A review. Biology 2024, 13, 914. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, J. Group B Streptococcus: Virulence factors and pathogenic mechanism. Microorganisms 2022, 10, 2483. [Google Scholar] [CrossRef]
- Burton, N.O.; Greer, E.L. Multigenerational epigenetic inheritance: Transmitting information across generations. Semin. Cell Dev. Biol. 2022, 127, 132–212. [Google Scholar] [CrossRef] [PubMed]
- Lyko, F. The DNA methyltransferase family: A versatile toolkit for epigenetic regulation. Nat. Rev. Genet. 2018, 19, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Romero, M.A.; Casadesús, J. The bacterial epigenome. Nat. Rev. Microbiol. 2020, 18, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Casadesús, J.; Low, D. Epigenetic gene regulation in the bacterial world. Microbiol. Mol. Biol. Rev. 2006, 70, 830–856. [Google Scholar] [CrossRef]
- Heusipp, G.; Fälker, S.; Schmidt, M.A. DNA adenine methylation and bacterial pathogenesis. Int. J. Med. Microbiol. 2007, 297, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Heithoff, D.M.; Sinsheimer, R.L.; Low, D.A.; Mahan, M.J. An essential role for DNA adenine methylation in bacterial virulence. Science 1999, 284, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Willemse, N.; Schultsz, C. Distribution of type I restriction–modification systems in Streptococcus suis: An outlook. Pathogens 2016, 5, 62. [Google Scholar] [CrossRef] [PubMed]
- Croix, M.D.S.; Vacca, I.; Kwun, M.J.; Ralph, J.D.; Bentley, S.D.; Haigh, R.; Croucher, N.J.; Oggioni, M.R. Phase-variable methylation and epigenetic regulation by type I restriction–modification systems. FEMS Microbiol. Rev. 2017, 41, S3–S15. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Li, H.; Wang, D.; Tang, Y.Q.; Tang, H.Q.; Ma, X.; Liu, Z. The effect of DNA methyltransferase hsdM gene on the motility of Aeromonas veronii. J. Biol. 2022, 39, 28–32. (In Chinese) [Google Scholar] [CrossRef]
- Chu, H.; Hu, Y.; Zhang, B.; Sun, Z.; Zhu, B. DNA Methyltransferase HsdM induce drug resistance on Mycobacterium tuberculosis via multiple effects. Antibiotics 2021, 10, 1544. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhou, X.; Yin, T.; Chen, K.; Hu, Y.; Zhu, B.; Mi, K. The mycobacterial DNA methyltransferase HsdM decreases intrinsic isoniazid susceptibility. Antibiotics 2021, 11, 1323. [Google Scholar] [CrossRef]
- DebRoy, S.; Shropshire, W.C.; Tran, C.N.; Hao, H.; Gohel, M.; Galloway-Peña, J.; Hanson, B.; Flores, A.R.; Shelburne, S.A.; Fey, P.D. Characterization of the type I restriction modification system broadly conserved among group A Streptococci. mSphere 2021, 6, e00799-21. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Zhang, P.; Li, W.; Liu, R.; Tang, J.; Fan, H. hsdS, belonging to the type I restriction-modification system, contributes to the Streptococcus suis serotype 2 survival ability in phagocytes. Front. Microbiol. 2017, 8, 1524. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ke, X.; Liu, Z.; Cao, J.; Su, Y.; Lu, M.; Gao, F.; Wang, M.; Yi, M.; Qin, F. Capsular polysaccharide of Streptococcus agalactiae is an essential virulence factor for infection in Nile tilapia (Oreochromis niloticus Linn.). J. Fish Dis. 2019, 42, 293–302. [Google Scholar] [CrossRef]
- Fijan, N.; Sulimanović, D.; Bearzotti, M.; Muzinić, D.; Zwillenberg, L.O.; Chilmonczyk, S.; Vautherot, J.F.; Kinkelin, P. Some properties of the Epithelioma papulosum cyprini (EPC) cell line from carp Cyprinus carpio. Ann. Virol. (Inst. Pasteur) 1983, 134, 207–220. [Google Scholar] [CrossRef]
- Li, H.; Cao, J.; Han, Q.; Li, Z.; Zhuang, J.; Wang, C.; Wang, H.; Luo, Z.; Wang, B.; Li, A. Protease SfpB plays an important role in cell membrane stability and immune system evasion in Streptococcus agalactiae. Microb. Pathog. 2024, 192, 106683. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Yang, B.; Kang, Y.; Cong, W. Critical roles of VipB protein on virulence and oxidative stress tolerance in Aeromonas veronii. J. Fish Dis. 2023, 46, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Liu, M.; Zhou, H.; Fan, H. Screening for phagocytosis resistance-related genes via a transposon mutant library of Streptococcus suis serotype 2. Virulence 2020, 11, 825–838. [Google Scholar] [CrossRef]
- Li, Q.; Yu, X.; Ye, L.; Hou, T.; Liu, Y.; Liu, G.; Wang, Q.; Zhang, D. Hypermucoviscous multidrug-resistant Klebsiella variicola strain LL2208 isolated from Chinese longsnout catfish (Leiocassis longirostris): Highly similar to human K. variicola strains. Pathogens 2024, 13, 647. [Google Scholar] [CrossRef] [PubMed]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, H.; Mo, S.; Li, J.; Ma, X.; Tang, Y.; Li, H.; Liu, Z. Acquisition of type I methyltransferase via horizontal gene transfer increases the drug resistance of Aeromonas veronii. Microb. Genom. 2023, 9, 001107. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, M.; Qi, Y.; Wang, J.; Shi, Q.; Xie, X.; Zhou, C.; Ma, L. hsdSA regulated extracellular vesicle-associated PLY to protect Streptococcus pneumoniae from macrophage killing via LAPosomes. Microbiol. Spectr. 2024, 12, e0099523. [Google Scholar] [CrossRef]
- Nye, T.M.; Jacob, K.M.; Holley, E.K.; Nevarez, J.M.; Dawid, S.; Simmons, L.A.; Watson, M.E. DNA methylation from a type I restriction modification system influences gene expression and virulence in Streptococcus pyogenes. PLoS Pathog. 2019, 15, e1007841. [Google Scholar] [CrossRef]
- Huang, Q.Q.; Liu, S.L.; Huang, J.H.; Wang, F.; Zhao, Z.C.; Deng, H.W.; Lin, C.; Guo, W.L.; Zhong, Z.H.; Li, J.L.; et al. Transcriptome analysis of tilapia Streptococcus agalactiae in response to baicalin. Genes Genom. 2025, 47, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Huang, J.; Yu, J.; Xu, Z.; Liu, L.; Song, Y.; Sun, X.; Zhang, A.; Jin, M. HP1330 contributes to Streptococcus suis virulence by inducing toll-like receptor 2- and ERK1/2-dependent pro-inflammatory responses and influencing in vivo S. suis loads. Front. Immunol. 2017, 8, 869. [Google Scholar] [CrossRef]

| Primer | Sequence (5′-3′) | Length (bp) | Annealing Temperature (°C) |
|---|---|---|---|
| hsdMCm-dF | TTAAACTTCAGGGAGCAGGATC | 2460 | 53 |
| hsdMCm-dR | AACTCTCCGTCGCTATTGTAACC | ||
| hsdM-inF | CTGCTATGGAATCTGGAACAC | 522 | 55 |
| hsdM-inR | TAATAGTCGTAGGGATGCTTG | ||
| Spe-dF | TTGGTACTTACATGTTTGGATC | 612 | 52 |
| Spe-dR | CTCCAAGATAACTACGAACTGC |
| Primer | Sequence (5′-3′) | Annealing Temperature (°C) | Genbank Accession No. |
|---|---|---|---|
| IL-1β-F | CGCTCCAGTTTGACCTTCTTA | 60 | XM_019365842.2 |
| IL-1β-R | TGATGCTACCTTGTGATGTAACT | ||
| IFN-γ-F | CCAACAACTCAGGCTCGCTA | 60 | KF294754.1 |
| IFN-γ-R | TGCTCATGGTAGCGGTGTTT | ||
| TNF-α-F | AGGGTGATCTGCGGGAATACT | 60 | AY428948.1 |
| TNF-α-R | GCCCAGGTAAATGGCGTTGT | ||
| IL-6-F | ACAGAGGAGGCGGAGATG | 60 | XM_019350387.2 |
| IL-6-R | GCAGTGCTTCGGGATAGA | ||
| EF-1α-F | CAGGGCATCCATCAACAAGA | 60 | NM_001279647.1 |
| EF-1α-R | GCATAAGCCAGTCCTTGAGTATAG | ||
| β-actin-F | GCGGAATCCACGAAACCACC | 60 | XM_003443127.5 |
| β-actin-R | CTGTCAGCGATGCCAGGGTA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, D.; Wang, B.; Ren, Y.; Mo, X.; Yu, M.; Zhang, D. Methyltransferase HsdM Regulates the Pathogenicity of Streptococcus agalactiae to Nile Tilapia (Oreochromis niloticus). Fishes 2025, 10, 86. https://doi.org/10.3390/fishes10020086
Jiang D, Wang B, Ren Y, Mo X, Yu M, Zhang D. Methyltransferase HsdM Regulates the Pathogenicity of Streptococcus agalactiae to Nile Tilapia (Oreochromis niloticus). Fishes. 2025; 10(2):86. https://doi.org/10.3390/fishes10020086
Chicago/Turabian StyleJiang, Dongdong, Bei Wang, Yan Ren, Xubing Mo, Meiling Yu, and Defeng Zhang. 2025. "Methyltransferase HsdM Regulates the Pathogenicity of Streptococcus agalactiae to Nile Tilapia (Oreochromis niloticus)" Fishes 10, no. 2: 86. https://doi.org/10.3390/fishes10020086
APA StyleJiang, D., Wang, B., Ren, Y., Mo, X., Yu, M., & Zhang, D. (2025). Methyltransferase HsdM Regulates the Pathogenicity of Streptococcus agalactiae to Nile Tilapia (Oreochromis niloticus). Fishes, 10(2), 86. https://doi.org/10.3390/fishes10020086








