Antiviral Effect and Metabolic Regularity of a Phenylpropanoid- Based Compound as Potential Immunopotentiator
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish, Cells, and Virus
2.2. In Vitro Inhibition
2.3. Horizontal Transmission
2.4. In Vivo Inhibition
2.5. RNA Extraction
2.6. Histopathology
2.7. High-Performance Liquid Chromatography (HPLC)
2.8. Statistical Analysis
3. Results
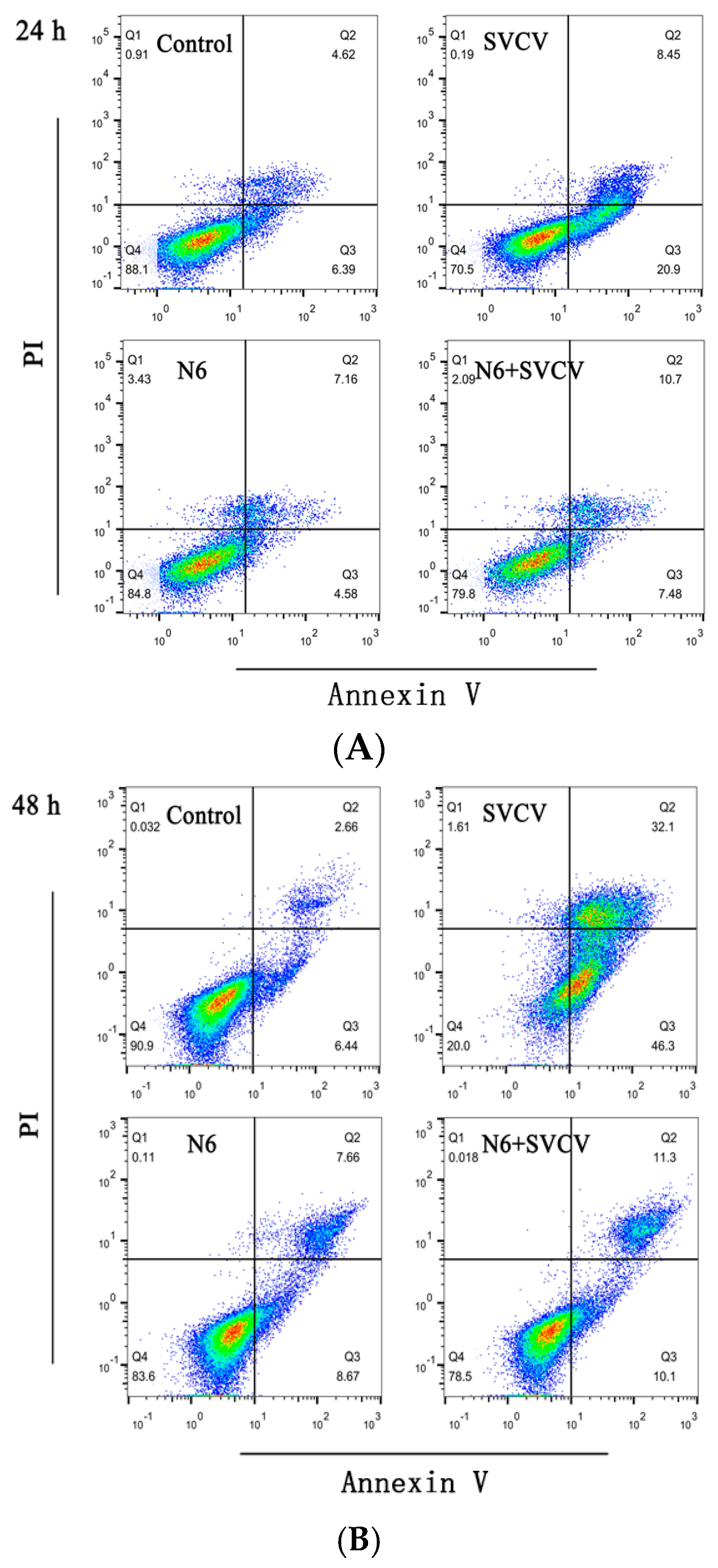
3.1. Anti-Apoptotic Effect of N6 on SVCV-Infected Cells
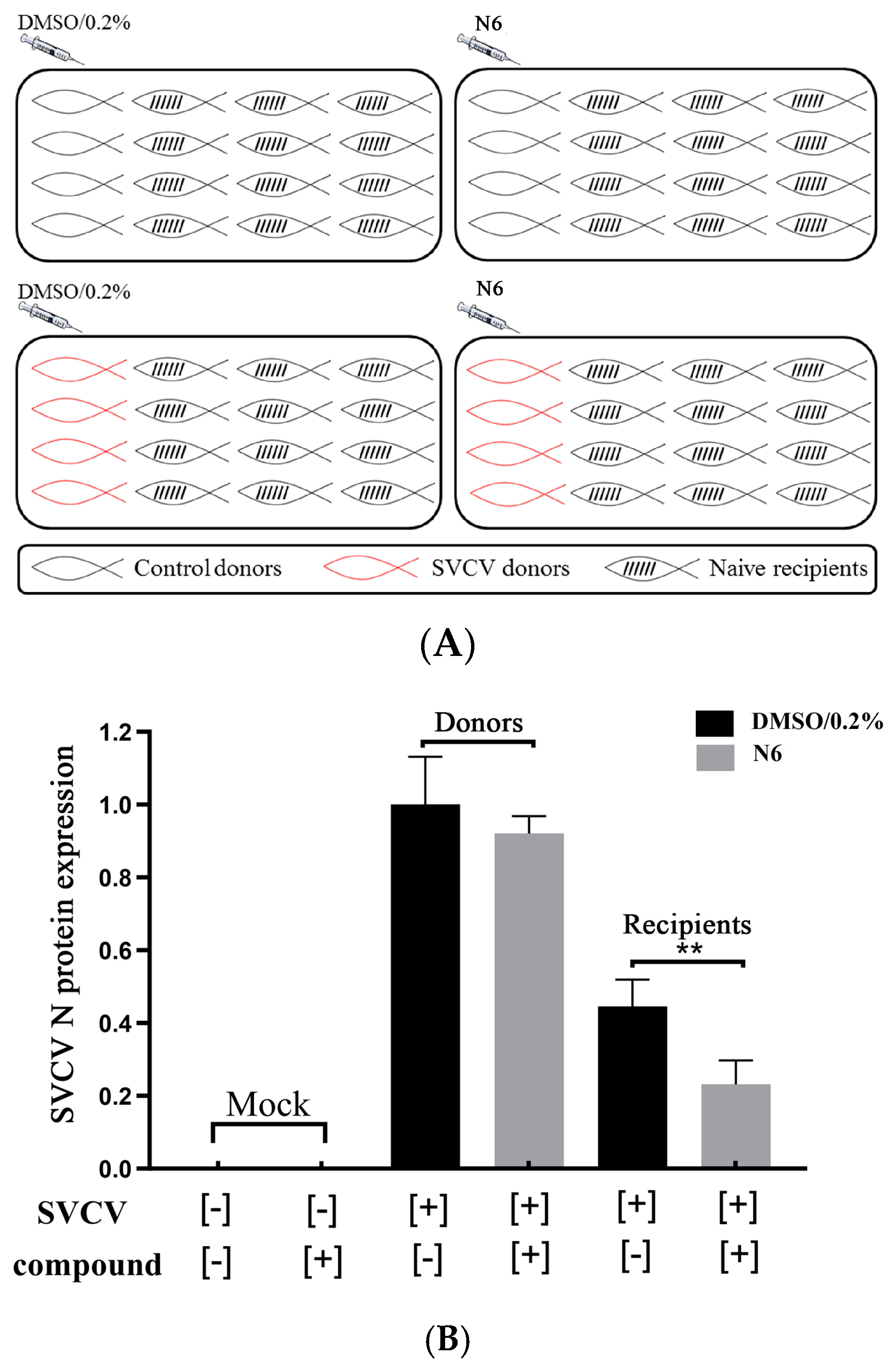
3.2. N6 Reduced Horizontal Transmission of SVCV
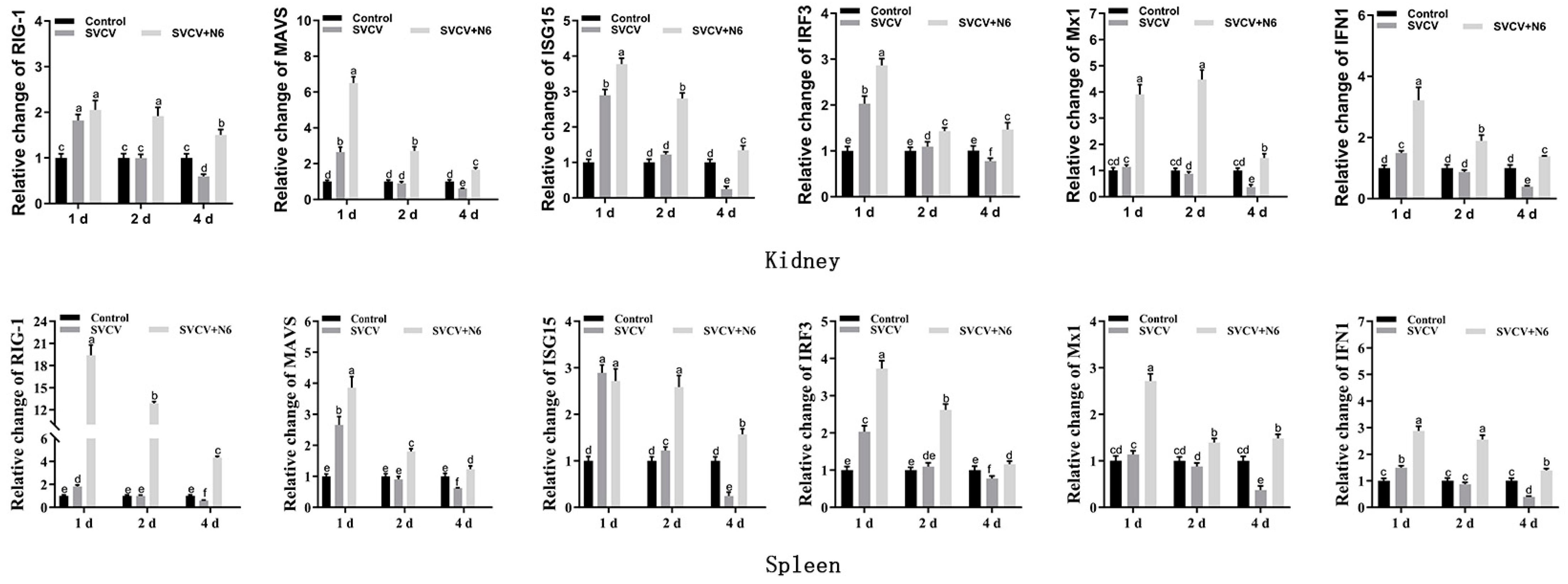
3.3. Antiviral Responses of Common Carp Under N6-Treatment
3.4. Histopathology
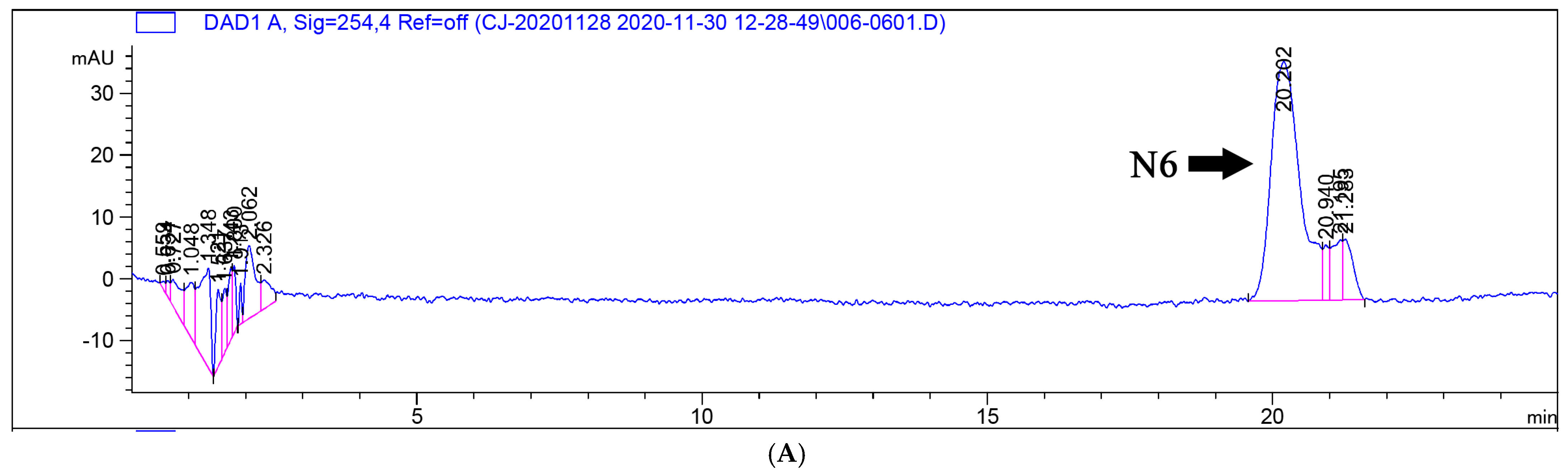
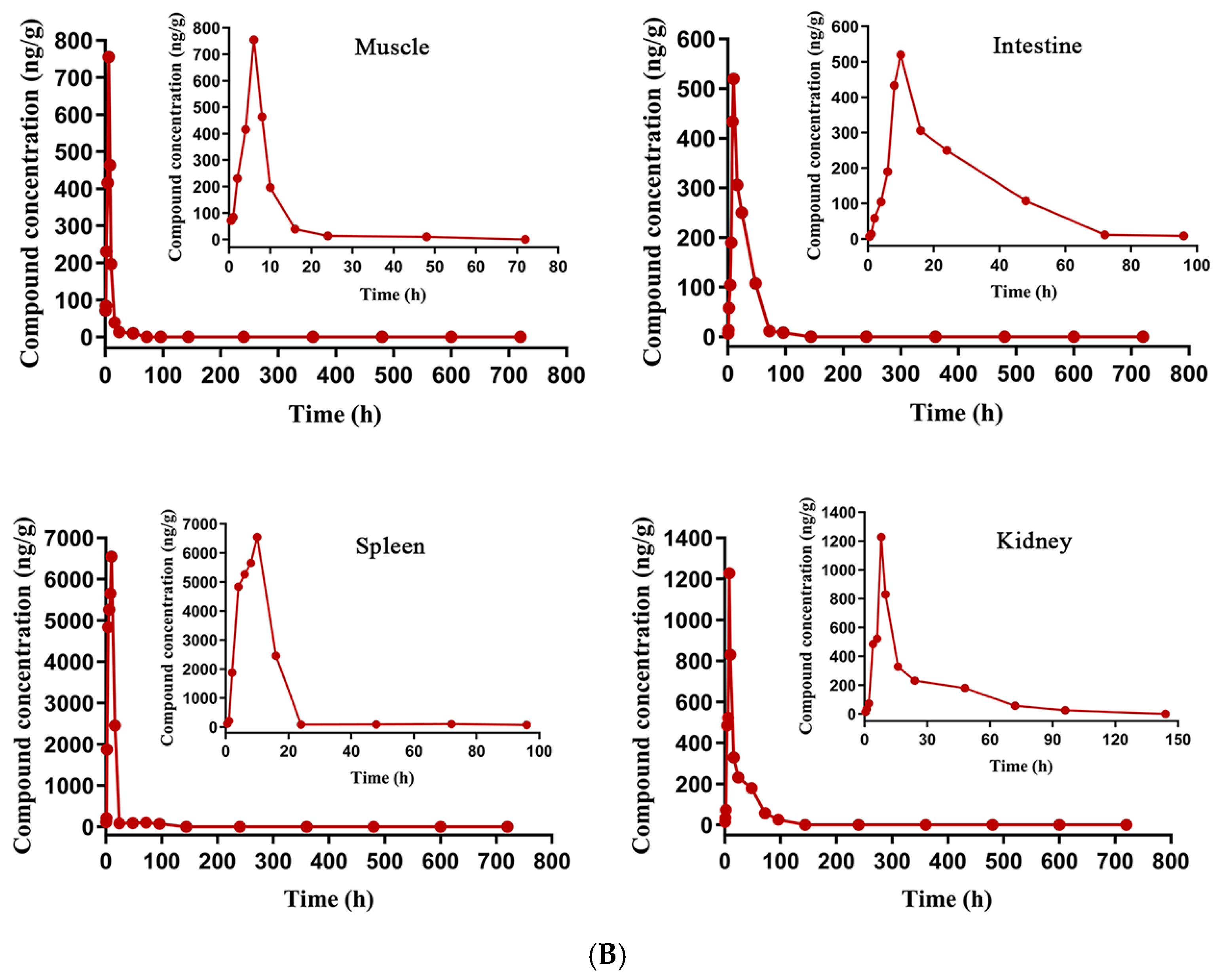
3.5. Metabolism of N6
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture; FAO: Rome, Italy, 2024. [Google Scholar]
- Toranzo, A.E.; Magariños, B.; Romalde, J.L. A review of the main bacterial fish diseases in mariculture systems. Aquaculture 2005, 246, 37–61. [Google Scholar] [CrossRef]
- Crane, M.; Hyatt, A. Viruses of fish: An overview of significant pathogens. Viruses 2011, 3, 2025–2046. [Google Scholar] [CrossRef]
- Alvarez-Pellitero, P. Fish immunity and parasite infections: From innate immunity to immunoprophylactic prospects. Vet. Immunol. Immunop. 2008, 126, 171–198. [Google Scholar] [CrossRef] [PubMed]
- Baudouy, A.; Danton, M.; Merle, G. SVCV infection of Carp (author’s transl). Ann. Rech. Vet. 1980, 11, 245–249. [Google Scholar]
- OIE. Aquatic Animal Health Code, Chapter 1.3. Diseases Listed by the OIE. 2024. Available online: https://www.woah.org/en/what-we-do/standards/codes-and-manuals/aquatic-code-online-access/?id=169&L=1&htmfile=chapitre_diseases_listed.htm (accessed on 20 December 2024).
- Veselý, T.; Pokorová, D.; Reschová, S.; Piačková, V. Experimental infection of common carp (Cyprinus carpio) with spring viremia of carp virus. In Proceedings of the Ninth International Symposium on Viruses of Lower Vertebrates, Malaga, Spain, 1–4 October 2014; pp. 213–214. [Google Scholar]
- Emmenegger, E.J.; Sanders, G.E.; Conway, C.M.; Binkowski, F.P.; Winton, J.R.; Kurath, G. Experimental infection of six North American fish species with the North Carolina strain of spring viremia of carp virus. Aquaculture 2016, 450, 273–282. [Google Scholar] [CrossRef]
- Teng, Y.; Liu, H.; Lv, J.Q.; Fan, W.H.; Zhang, Q.Y.; Qin, Q.W. Characterization of complete genome sequence of the spring viremia of carp virus isolated from common carp (Cyprinus carpio) in China. Arch. Virol. 2007, 152, 1457–1465. [Google Scholar] [CrossRef]
- Fijan, N. Spring viraemia of carp and other viral diseases and agents of warm water fish. In Fish Diseases and Disorders; Woo, P.T.K., Bruno, D.W., Eds.; CAB International: London, UK, 1999; Volume 3, pp. 177–244. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Yarovaya, O.I.; Salakhutdinov, N.F. Mono- and sesquiterpenes as a starting platform for the development of antiviral drugs. Russ. Chem. Rev. 2021, 90, 488–510. [Google Scholar] [CrossRef]
- Ge, H.; Wang, Y.F.; Xu, J.; Gu, Q.; Liu, H.B.; Xiao, P.G.; Zhou, J.; Liu, Y.; Yang, Z.; Su, H. Anti-influenza agents from Traditional Chinese Medicine. Nat. Prod. Rep. 2010, 27, 1758–1780. [Google Scholar] [CrossRef]
- Natella, F.; Nardini, M.; Di Felice, M.; Scaccini, C. Benzoic and cinnamic acid derivatives as antioxidants: Structure activity relation. J. Agr. Food Chem. 1999, 47, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, L.; Li, B.Y.; Shen, Y.F.; Wang, G.X.; Zhu, B. Synthesis of arctigenin derivatives against infectious hematopoietic necrosis virus. Eur. J. Med. 2019, 163, 183–194. [Google Scholar] [CrossRef]
- Kashman, Y.; Gustafson, K.R.; Fuller, R.W.; Cardellina, J.H.I.; Mcmahon, J.B.; Currens, M.J.; Buckheit, R.W.J.; Hughes, S.H.; Cragg, G.M.; Boyd, M.R. Cheminform abstract: HIV inhibitory natural products. Part 7. The calanolides, a novel HIV-inhibitory class of coumarin derivatives from the tropical rainforest tree, Calophyllum lanigerum. J. Med. Chem. 1992, 35, 2735–2743. [Google Scholar] [CrossRef]
- Charlton James, L. Antiviral Activity of Lignans. J. Nat. Prod. 1998, 61, 1447–1451. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.C.; Zhang, Y.; Golub, L.M.; Johnson, F.; Simon, S.R. Inhibition of anthrax lethal factor by curcumin and chemically modiffed curcumin derivatives. J. Enzym. Inhib. Med. Ch. 2014, 29, 663–669. [Google Scholar] [CrossRef]
- Song, D.W.; Liu, L.; Shan, L.P.; Qiu, T.X.; Chen, J.; Chen, J.P. Rhabdoviral clearance effect of a phenylpropanoid medicine against spring viraemia of carp virus infection in vitro and in vivo. Aquaculture 2020, 526, 735412. [Google Scholar] [CrossRef]
- Song, D.W.; Liu, L.; Fu, X.Y.; Liu, G.L.; Hu, Y.; Chen, J. Immune responses and protective efffcacy on 4-(2-methoxyphenyl)-3,4-dihydro-2H-chromeno [4,3-d] pyrimidine-2,5 (1H)-dione against spring viremia of carp virus in vivo. Aquaculture 2021, 540, 736694. [Google Scholar] [CrossRef]
- Qiu, T.X.; Wang, H.; Hu, Y.; Shan, L.P.; Liu, G.L.; Liu, L.; Zhang, X.; Chen, J. Inhibition of fish rhabdovirus demonstrates application prospect of two methylimidazole phenylpropanoid-based small molecules in aquaculture. Aquaculture 2024, 595, 741636. [Google Scholar] [CrossRef]
- Liu, L.; Shan, L.P.; Xue, M.Y.; Lu, J.F.; Hu, Y.; Liu, G.L.; Chen, J. Potential application of antiviral coumarin in aquaculture against IHNV infection by reducing viral adhesion to the epithelial cell surface. Antivir. Res. 2021, 195, 105192. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.P.; Zhou, Y.; Yan, M.C.; Liu, L.; Chen, J.; Chen, J.P. A novel antiviral coumarin derivative as a potential agent against WSSV infection in shrimp seedling culture. Virus Res. 2021, 297, 198387. [Google Scholar] [CrossRef]
- Koutna’, M.; Veselý, T.; Psikal, I.; Hůlova’, J. Identiffcation of spring viraemia of carp virus (SVCV) by combined RT-PCR and nested PCR. Dis. Aquat. Org. 2003, 55, 229–235. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Liu, H.; Li, Z.Q.; Wang, M.; Zhang, Q.Y. Detection of viral pathogen from diseased common carp (Cyprinus carpio) by infectious tests. J. Fish. Sci. China 2006, 13, 617–623. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using realtime quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Roulston, A.; Marcellus, R.C.; Branton, P.E. Viruses and apoptosis. Annual. Rev. Microbiol. 1999, 53, 577–628. [Google Scholar] [CrossRef]
- Liang, R.; Song, H.X.; Huang, J.L.; Fei, R.M.; Zhang, J.Q. PEDV epidemic strain JS2013 induced apoptosis of Vero cell. J. Nanjing Agric. Univ. 2021, 44, 514–520. [Google Scholar]
- Suleva, P.C.; Manuel, O.V.; Patricia, D.; La, C.O.; Marina, V.P. Dynamic reorganization of the cytoskeleton during apoptosis: The two coffins hypothesis. Int. J. Mol. Sci. 2017, 18, 2393. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Hu, Y.; Shen, Y.F.; Wang, G.X.; Zhu, B. Evaluation on antiviral activity of coumarin derivatives against spring viraemia of carp virus in epithelioma papulosum cyprini cells. Antivir. Res. 2017, 144, 173–185. [Google Scholar] [CrossRef]
- Liu, L.; Song, D.W.; Liu, G.L.; Shan, L.P.; Qiu, T.X.; Chen, J. Hydroxycoumarin efffciently inhibits spring viraemia of carp virus infection in vitro and in vivo. Zool. Res. 2020, 41, 395–409. [Google Scholar] [CrossRef]
- Chen, C.; Shen, Y.F.; Hu, Y.; Liu, L.; Chen, W.C.; Wang, G.X.; Zhu, B. Highly efficient inhibition of spring viraemia of carp virus replication in vitro mediated by bavachin, a major constituent of psoralea corlifonia Lynn. Virus Res. 2018, 255, 24–35. [Google Scholar] [CrossRef]
- Xiao, Y.; Shao, L.; Zhang, C.; An, W. Genomic evidence of homologous recombination in spring viremia of carp virus: A negatively single stranded RNA virus. Virus Res. 2014, 189, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Secombes, C.J. Teleost fish interferons and their role in immunity. Dev. Comp. Immunol. 2011, 35, 1376–1387. [Google Scholar] [CrossRef] [PubMed]
- Forlanza, M.; Dias, J.D.; Vesel, T. Transcription of signal-3 cytokines, IL-12 and IFNα/β, coincides with the timing of CD8α/β up-regulation during viral infection of common carp (Cyprinus carpio L.). Mol. Immunol. 2008, 45, 1531–1547. [Google Scholar] [CrossRef] [PubMed]
- Adamek, M.A.; Rakus, K.; Chyb, J.A.; Brogden, G.; Huebner, A.; Irnazarow, I.; Steinhagen, D. Interferon type I responses to virus infections in carp cells: In vitro studies on Cyprinid herpesvirus 3 and Rhabdovirus carpio infections. Fish Shellfish Immun. 2012, 33, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Qiu, T.X.; Song, D.W.; Shan, L.P.; Liu, G.L.; Liu, L. Potential prospect of a therapeutic agent against spring viraemia of carp virus in aquaculture. Aquaculture 2020, 515, 734558. [Google Scholar] [CrossRef]
- Rodriguez, J.; Li, T.; Xu, Y.R.; Sun, Y.Y.; Zhu, C.L. Role of apoptosis-inducing factor in perinatal hypoxic-ischemic brain injury. Neural Regen. Res. 2020, 16, 205–213. [Google Scholar]
- Tafalla, C.; Sanchez, E.; Lorenzen, N.; DeWitte-Orr, S.J.; Bols, N.C. Effects of viral hemorrhagic septicemia virus (VHSV) on the rainbow trout (Oncorhynchus mykiss) monocyte cell line RTS-11. Mol. Immunol. 2008, 45, 1439–1448. [Google Scholar] [CrossRef]
- Lopez, M.A.; Roca, F.J.; Meseguer, J.; Mulerol, V. New insights into the evolution of IFNs: Zebrafish group II IFNs induce a rapid and transient expression of IFN-dependent genes and display powerful antiviral activities. J. Immunol. 2009, 182, 3440–3449. [Google Scholar] [CrossRef]
- Kawai, T.; Takahashi, K.; Sato, S.; Coban, C.; Akira, S. IPS-1, an adaptor triggering RIG-I and MDA5 mediated type I interferon induction. Nat. Immunol. 2005, 6, 981–988. [Google Scholar] [CrossRef]

| Primer | Sequences (from 5′to 3′) | |
|---|---|---|
| SVCV nucleoprotein (N) | Forward | AACAGCGCGTCTTACATGC |
| Reverse | CTAAGGCGTAAGCCATCAGC | |
| RIG-I | Forward | AAACTGTGACTTAGACGAGGCT |
| Reverse | GTTGCTGCTCATCAGCATGT | |
| Mx1 | Forward | ATGAATCCTGGAAGCCCTC |
| Reverse | GAACTTCGGGAAGAATTTGC | |
| ISG15 | Forward | AAGCCATATTCAGCGAAGC |
| Reverse | AACCGTTATCGGCAGACAG | |
| MAVS | Forward | TCACACTCACTGATAGGGAAGAG |
| Reverse | TAGCTCTCATCTCATTAGCCAGT | |
| IRF3 | Forward | GGAGACCACTCTGTTTGGAAG |
| Reverse | CGGCATCGTTCTTGTTGTC | |
| IFN1 | Forward | ACCAAACCCAAATGTGGACGTG |
| Reverse | CCACTCATTTCCCGAAGCAGA | |
| Fish actin | Forward | GATGATGAAATTGCCGCACTG |
| Reverse | ACCAACCATGACACCCTGATGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, D.; Cai, X.; Shao, Q.; Tong, X.; Zhao, Z.; Liu, L.; Liu, G. Antiviral Effect and Metabolic Regularity of a Phenylpropanoid- Based Compound as Potential Immunopotentiator. Fishes 2025, 10, 77. https://doi.org/10.3390/fishes10020077
Song D, Cai X, Shao Q, Tong X, Zhao Z, Liu L, Liu G. Antiviral Effect and Metabolic Regularity of a Phenylpropanoid- Based Compound as Potential Immunopotentiator. Fishes. 2025; 10(2):77. https://doi.org/10.3390/fishes10020077
Chicago/Turabian StyleSong, Dawei, Xue Cai, Qianhao Shao, Xinhui Tong, Zhe Zhao, Lei Liu, and Guanglu Liu. 2025. "Antiviral Effect and Metabolic Regularity of a Phenylpropanoid- Based Compound as Potential Immunopotentiator" Fishes 10, no. 2: 77. https://doi.org/10.3390/fishes10020077
APA StyleSong, D., Cai, X., Shao, Q., Tong, X., Zhao, Z., Liu, L., & Liu, G. (2025). Antiviral Effect and Metabolic Regularity of a Phenylpropanoid- Based Compound as Potential Immunopotentiator. Fishes, 10(2), 77. https://doi.org/10.3390/fishes10020077






